Abstract
Osteosarcoma is the most common primary malignancy of bone in children and young adults, the highest incidence peak is during adolescence and doesn’t have any gender predominance. The main site of metastasis are the lungs and extrapulmonary cases are occasional. The incidence of metastasis in the Central Nervous System (CNS) is 2–6.5%, increase to 10–15% in patients with pulmonary metastases. Therefore, metastatic disease of the CNS is rare and the information on such patients is limited. Here, we describe a case of a 20-year old patient diagnosed with osteosarcoma in the left distal femur stage IIB, he developed pulmonary disease, during palliative chemotherapy experienced relapse to the brain classified as recursive partitioning analysis (RPA) class II, and was treated with external radiotherapy (30 Gy in 10 fractions) and later he had a poor evolution and died.
Keywords: Osteosarcoma, Brain metastasis, Radiation therapy
1. Introduction
Osteosarcoma is a malignant primary bone tumor caused by the production of osteoid material by malignant cells; it is a rare neoplasm [1]. Osteosarcoma represents 4.5% of the total neoplasms in pediatric populations in Mexico City [2]; other age group affected (albeit notably less) are older adults over 80 years with a previous history of radiotherapy [3]; regarding incidence, there are no racial or gender differences. Younger patients and lesions located in extremities rather than in the pelvis or spine have better prognosis [1].
Many patients with osteosarcoma, particularly children, have a genetic predisposition, with Rb1 gene mutation (related to hereditary retinoblastoma) and p53 mutation (related to Li-Fraumeni syndrome) being the most frequent ones [4]. In adult cases, the main risk factor is radiotherapy, with an interval between irradiation and the appearance of osteosarcoma ranging from 12 to 16 years [5], another risk factor is Paget's disease [6].
Previous to the advent of chemotherapy, patients were treated with surgery and/or radiotherapy and developed metastasis in 80% of cases. Adjuvant chemotherapy currently used in the treatment has been demonstrated to increase overall survival from 16% to 70% [7]. The main site of metastasis is the lungs (40% at diagnosis), extrapulmonary metastasis are rare and curable in less than 5% of patients [8,9]; the incidence of CNS metastasis is 2–6.5% [9,10], in patients with a pulmonary metastases it increases to 10–15%, but these are rarely symptomatic by themselves at diagnosis [11,12].
Metastasis to CNS is rare, but this may be changing with overall survival improvement in the modern chemotherapy era [13,14], we present a patient diagnosed with metastatic osteosarcoma to the lungs, that developed metastasis to the CNS during palliative treatment with chemotherapy.
1.1. Case presentation
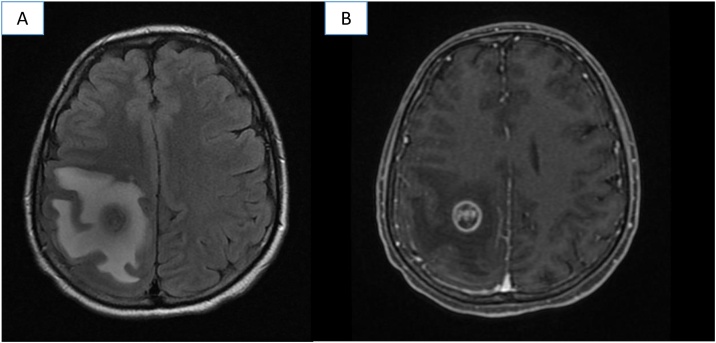
20-Year old male was diagnosed with osteoblastic osteosarcoma of left distal femur stage IIB (T2, G2, N0, M0), he received three cycles of platinum-based neoadjuvant chemotherapy and subsequent supracondylar amputation, hystolopathologyical report was Huvos IIA. One month after surgery, he developed lung metastasis. Palliative treatment with Docetaxel (75 mg/m2 D1) and Gemcitabine (1000 mg/m2 D1 and D8) for three cycles was started. Later, he was admitted to the emergency room for left body hemiparesis and sudden holocraneal cephalea with 24 h of evolution. Motor examination revealed left paresis (power left upper limb and left lower limb 3/5) and osteo-tendinous reflexes ++. He was further evaluated with computed tomography (CT) which revealed a right fronto-parietal lesion with perilesional edema. Brain magnetic resonance imaging (MRI) showed a fronto-parietal lesion hypointense on T1, heterogeneous on T2, reinforced in a ring shape with contrast, and perilesional edema in FLAIR sequence (Fig. 1). The patient was classified as RPA class II (for extracranial metastasis) and received external holocraneal radiotherapy 30 Gy in 10 fractions, showing clinical improvement. Afterwards, he was subjected to right posterolateral thoracotomy with resection of six pulmonary lesions and started treatment with Ifosfamide/Etoposide for one cycle, which caused hematological toxicity grade 4, hypovolemic shock for hemoptysis and the deterioration of the performance status. After this event, the patient remained with palliative care equipment and died 14 months after diagnosis.
Fig. 1.
MRI with right frontoparietal lesion with perilesional edema: A. T2 FLAIR sequence B. With gadolinium.
2. Discussion
Metastases to CNS in osteosarcoma patients are rare [10], we collected information of all cases reported in the literature, we searched in Pubmed/MEDLINE and Google Scholar with the keywords “Osteosarcoma” AND “brain metastasis” OR “CNS metastasis”, exclusion criteria were: no complete information and brain primary, we found 35 cases and seven were excluded. 28 cases were evaluated in the literature and our case. Table 1 shows the characteristics of those patients.
Table 1.
Clinical characteristics, treatment and outcome of patients with brain metastases.
| Year | Author | Age | Gender | Primary site | Initial clinical stage | Initial treatmetnt | Other sites of metastases | Time to brain relapse (mo) | Treatment to brain metastases | Overall survival (mo) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1979 | Danziger [12] | 20 | F | Right distal femur | Metastasic | – | No | Initially | CT, RT | ND |
| 1979 | Danziger [12] | 15 | F | Right distal femur | Locally | CT, RT, surgery | Lung, bone | 24 | Surgery | 33 |
| 1979 | Danziger [12] | 18 | F | Distal femur | Metastasic | – | Lung, breast | 6 | CT, RT | 6 |
| 1983 | Ozarda [15] | 23 | M | Rigth femur | Localy | ND | No | 8 | CT, RT | ND |
| 1990 | Niedeggen [16] | 7 | M | Left femur | Locally | Surgery, CT | Lung | 76 | Surgery, RT | 13 |
| 1993 | Marina [10] | 3 | M | Left upper humerus | Locally | Surgery | Lung | 4 | CT | 4 |
| 1993 | Marina [10] | 10 | F | Left distal femur | Locally | Surgery | Lung, bone | 20 | None | 20 |
| 1993 | Marina [10] | 25 | M | Proximal tibia | Locally | Surgery | Lung, bone | 38 | None | 38 |
| 1993 | Marina [10] | 12 | M | Left distal femur | Locally | CT | Lung | 14 | None | 14 |
| 1993 | Marina [10] | 15 | M | Left proximal humerus | Locally | Surgery | Lung | 11 | None | 12 |
| 1993 | Marina [10] | 18 | M | Left distal femur | Locally | Surgery | Lung | 41 | None | 41 |
| 1993 | Marina [10] | 9 | M | Rigth proximal humerus | Locally | Surgery, CT | Lung | 15 | None | 16 |
| 1993 | Marina [10] | 4 | M | Left proximal humerus | Locally | Surgery | Lung | 10 | CT | 103 (NED) |
| 1993 | Marina [10] | 19 | F | Left proximal tibia | Locally | Surgery, MIOS | Lung | 45 | CT | 47 |
| 1993 | Marina [10] | 17 | M | Left proximal femur | Locally | Surgery, MIOS | Lung | 15 | None | 16 |
| 1993 | Marina [10] | 19 | M | Left distal femur | Locally | Surgery, CT | Lung, local | 12 | None | 12 |
| 1993 | Marina [10] | 7 | F | Right distal femur | Locally | Surgery, CT | Lung, local | 18 | None | 18 |
| 1993 | Marina [10] | 17 | M | Distal humerus | Locally | Surgery, CT | Local | 8 | None | 8 |
| 1993 | Marina [10] | 16 | M | Distal femur | Locally | Surgery, CT | Lung, local | 12 | None | 15 |
| 1993 | Wexler [13] | 10 | F | Neck of right femur | Locally | Surgery, CT | Lung | 53 | Surgery. RT | 120 |
| 1994 | Chang [17] | 20 | M | Bilateral femur | Metastasic | – | ND | Inically | Surgery, RT | 5 |
| 2002 | Hettmer [18] | 16 | M | Left proximal tibia | Locally | CT, surgery | Lung | 84 | CT | 84 |
| 2003 | Yonemoto [19] | 14 | F | ND | Locally | CT, surgery | Lung | 12 | Surgery, RT | 114 |
| 2005 | Weil [20] | 26 | M | Rigth tibia | Metastasic | CT, surgery | Lung | 36 | Surgery, CT, RT | 4 |
| 2009 | Niazi [11] | 16 | M | Metaphysis of the rigth femur | Metastasic | ND | ND | Initially | ND | ND |
| 2012 | Onodena [21] | 14 | F | Left femur | Locally | Surgery, CT | No | 12 | Surgery | ND |
| 2013 | Rabah [22] | 10 | F | Right humerus | Metastasic | – | No | Initially | CT, RT | 14 |
| 2017 | Doval [23] | 36 | F | Left femur | Locally | Surgery, CT | Lung | 84 | Surgery | ND |
| 2018 | Present case | 20 | M | Left distal femur | Locally | CT, surgery | Lung | 7 | RT | 14 |
F: Female, M: Male, ND: No data, mo: months, CT: Chemotherapy, RT: Radiotherapy, MIOS: Multi-institutional Osteosarcoma study, NED: No evidence of disease.
Median age was 15.72 years (3–36 years), 37.9% were females, the most frequent primary site was femur (58.1%), 70.3% (23 patients) were diagnosed for local disease and six (20.68%) for metastatic disease, treatment was heterogeneous, with only four (13.79%) patients treated with neoadjuvant chemotherapy; 75.86% of patients who developed brain metastases had lung metastasis, median time to brain metastasis was 26.60 months (4–84), eight patients were treated with surgery ± radiotherapy, eight patients with chemotherapy ± radiotherapy, one patient with radiotherapy only, and eleven patients did not receive any treatment, median overall survival was 32.12 months(4–120).
More cases have been described with the introduction of chemotherapy [14,19], Marina et al. described 254 patients with osteosarcoma, 13 with brain metastasis, showing that patients diagnosed after 1982 (advent of chemotherapy) have an increased risk of brain metastasis (p = 0.007), but not with a different frequency (15.5% vs. 4.5% p = 0.125) [10]. In our analysis 15 patients (51.72%) received chemotherapy and two were included in the MIOS trial.
The dissemination route is presumably hematogenous, through lung metastases [19]. It has been described that brain lesions caused by osteosarcoma are hypervascularized and mimic multiform glioblastoma [11]. Generally, they are located in the cerebral cortex, although some cases in the cerebellum have also been reported [18,24]. In the patient described in this case, metastases were found in the right frontoparietal region of the cerebral cortex.
Baram et al. presented 87 patients with osteosarcoma, 39 of them had pulmonary metastases and five with brain metastases; clinical manifestations were catastrophic: two with massive hemorrhages and three with epileptic status [9]; we described the patient with hemiparesis and cephalea.
Approximately 30–40% of patients with localized tumors develop lung metastasis and 10–15% of those patients experienced relapse, they may be at risk of brain metastasis [18], we found 75.86% of patients with lung metastases.
Baram et.al. found two patients (2/5) with brain metastases and both had surgery with a transient clinical improvement [9], lesions potentially treatable with surgery are single lesions, long survival has been reported only in isolated cases [16,18]. We found only eight (27.58%) patients treated with surgery ± radiotherapy with a median overall survival of 48.16 months.
The prognosis of patients with intracranial metastasis is poor, mean interval to brain metastases from initial diagnosis is approximately 20 months in soft tissue sarcomas and survival is no longer than several months [18,25], we reported a median time to brain relapse of 24.51 months and overall survival was 32.1.
3. Conclusion
Brain metastasis is an unusual event in osteosarcoma, currently there is no accurate information on the incidence of this complication, as we previously mentioned, there are case reports of this complication with different experiences in clinical management. The prognosis of these patients is very poor, we reported. Some authors suggest periodical neuroimaging studies in patients with lung metastatic disease, although this statement is controversial.
Financial disclosure
None declared.
Conflict of interest
None declared.
References
- 1.Ng V.Y., Scharschmidt T.J., Mayerson J.L., Fisher J.L. Incidence and survival in sarcoma in the United States: a focus on musculoskeletal lesions. Anticancer Res. 2013;33:2597–2604. [PubMed] [Google Scholar]
- 2.Rodriguez J., Tecualt R., Amaya R., Atencio A., Cario A., González R. Epidemiologic behavior of osteosarcoma in Mexican population from 2005 to 2014. Revista de la Asociación Argentina de Ortopedia y Traumatología. 2016;81(3):219–226. [Google Scholar]
- 3.van der Graaf W.T., Orbach D., Judson I.R., Ferrari A. Soft tissue sarcomas in adolescents and young adults: a comparison with their paediatric and adult counterparts. Lancet Oncol. 2017;18(3):e166–e175. doi: 10.1016/S1470-2045(17)30099-2. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J., Walsh M.F., Wu G. Germline mutations in predisposition genes in pediatric cancer. N Engl J Med. 2015;373(24):2336–2346. doi: 10.1056/NEJMoa1508054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs B. Etiology of osteosarcoma. Clin Orthop Relat Res. 2002;(397):40–52. doi: 10.1097/00003086-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Hadjipavlou A., Lander P., Srolovitz H., Enker I.P. Malignant transformation in Paget disease of bone. Cancer. 1992;70(12):2802. doi: 10.1002/1097-0142(19921215)70:12<2802::aid-cncr2820701213>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 7.Anninga J.K., Gelderblom H., Fiocco M. Chemotherapeutic adjuvant treatment for osteosarcoma: where do we stand? Eur J Cancer. 2011;47(16):2431–2445. doi: 10.1016/j.ejca.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 8.Picci P. Osteosarcoma (osteogenic sarcoma) Orphanet J Rare Dis. 2007;23:6. doi: 10.1186/1750-1172-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baram T.Z., van Tassel P., Jaffe N.A. Brain metastases in osteosarcoma: incidence, clinical and neuroradiological findings and management options. J Neurooncol. 1988;6(1):47–52. doi: 10.1007/BF00163540. [DOI] [PubMed] [Google Scholar]
- 10.Marina N.M., Pratt C.B., Shema S.J., Brooks T., Rao B., Meyer W.H. Brain metastases in osteosarcoma. Report of a long-term survivor and review of the St. Jude Children's Research Hospital experience. Cancer. 1993;71(11):3656–3660. doi: 10.1002/1097-0142(19930601)71:11<3656::aid-cncr2820711130>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 11.Niazi T.N., Forester C., Afify Z., Riva-Cambrin J. Osteosarcoma presenting as hemorrhagic cerebellar metastasis. Childs Nerv Syst. 2009;25(12):1643–1647. doi: 10.1007/s00381-009-0987-3. [DOI] [PubMed] [Google Scholar]
- 12.Danziger J., Wallace S., Handel S.F., deSantos L.A. Metastatic osteogenic sarcoma to the brain. Cancer. 1979;43(2):707–710. doi: 10.1002/1097-0142(197902)43:2<707::aid-cncr2820430245>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Wexler L.H., DeLaney T.F., Saris S., Horowitz M.E. Long-term survival after central nervous system relapse in a patient with osteosarcoma. Cancer. 1993;72(4):1203–1208. doi: 10.1002/1097-0142(19930815)72:4<1203::aid-cncr2820720412>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 14.Bacci G., Ruggieri P., Picci P. Changing pattern of relapse in osteosarcoma of the extremities treated with adjuvant and neoadjuvant chemotherapy. J Chemother. 1995;7(3):230–239. doi: 10.1179/joc.1995.7.3.230. [DOI] [PubMed] [Google Scholar]
- 15.Ozarda A.T., Legaspi J.R., Haynie T.P. Detection of a brain metastasis from osteosarcoma with 99mTc-methylene diphosphonate bone scanning. Eur J Nucl Med. 1983;8(12):552–554. doi: 10.1007/BF00251620. [DOI] [PubMed] [Google Scholar]
- 16.Niedeggen A., Weis J., Mertens R., Röther J., Bröcheler J. Unusually long survival time after resection and irradiation of a brain metastasis from osteosarcoma. Neurosurg Rev. 1990;13(3):247–252. doi: 10.1007/BF00313027. [DOI] [PubMed] [Google Scholar]
- 17.Chang J.W., Howng S.L., Sun Z.M., Kuo T.H., Duh C.C. An unusual intracranial metastasis of osteosarcoma. Gaoxiong Yi Xue Ke Xue Za Zhi. 1994;10(12):700–704. [PubMed] [Google Scholar]
- 18.Hettmer S., Fleischhack G., Hasan C., Kral T., Meyer B., Bode U. Intracranial manifestation of osteosarcoma. Pediatr Hematol Oncol. 2002;19(5):347–354. doi: 10.1080/08880010290057363. [DOI] [PubMed] [Google Scholar]
- 19.Yonemoto T., Tatezaki S., Ishii T., Osato K., Takenouchi T. Long term survival after surgical removal of solitary brain metastasis from osteosarcoma. Int J Clin Oncol. 2003;8(5):340–342. doi: 10.1007/s10147-003-0341-9. [DOI] [PubMed] [Google Scholar]
- 20.Weil R.J., Lonser R.R., Quezado M.M. CNS manifestations of malignancies: case 2. Skull and brain metastasis from tibial osteosarcoma. J Clin Oncol. 2005;23(18):4226–4229. doi: 10.1200/JCO.2005.05.055. [DOI] [PubMed] [Google Scholar]
- 21.Onodera H., Yoshida Y., Sakakibara Y. A case of intracerebral metastasis in osteosarcoma without active pulmonary metastasis. Br J Neurosurg. 2012;26(1):91–93. doi: 10.3109/02688697.2011.581771. [DOI] [PubMed] [Google Scholar]
- 22.Rabah F., Al-Mashaikhi N., Beshlawi I. Brain is not always the last fortress, osteosarcoma with large brain metastasis. J Pediatric Hematol/Oncol. 2013;35(2):e91–e93. doi: 10.1097/MPH.0b013e318271cb0f. [DOI] [PubMed] [Google Scholar]
- 23.Doval D.C., Chacko M., Sinha R. A rare case of brain metastasis in a patient with osteosarcoma. South Asian J Cancer. 2017;6(1):36–37. doi: 10.4103/2278-330X.202572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashkan K., Pollock J., D’Arrigo C., Kitchen D. Intracranial osteosarcomas: report of four cases and review of the literature. J Neurooncol. 1998;40:87. doi: 10.1023/a:1006007411312. [DOI] [PubMed] [Google Scholar]
- 25.Mateos M.E., López-Laso E., Garrido C., Torres M.J., López J., Simón De Las Heras R. Osteosarcoma and brain metastasis. An Esp Pediatr. 2002;56(5):462–465. [PubMed] [Google Scholar]