Abstract
Aim
The present retrospective study was to compare toxicity and survival outcomes in a group of low-risk PCa patients treated with either the preoperative planning technique (145 Gy) or the real-time IoP technique (160 Gy).
Background
The two most common permanent seed implantation techniques are preoperative planning (PP) with 145 Gy and real-time intraoperative planning (IoP) with 160 Gy. Although IoP has largely replaced PP at many centres in recent years, few studies have directly compared these two techniques.
Materials and methods
Retrospective study of 408 patients with low-risk PCa treated with permanent seed implant brachytherapy at our institution between October 2003 and December 2014. Of these, 187 patients were treated with PP at a dose of 145 Gy while 221 received real-time IoP with 160 Gy.
Results
At a median follow up of 90 months, 5- and 8-year rates of biochemical relapse-free survival (BRFS) were 94.8% and 86% with the IoP technique versus 90.8% and 83.9%, respectively, with PP. The maximum dose to the urethra was <217 Gy with both techniques. Despite the higher dose, IoP did not cause any significant increase in toxicity (p = 0.11).
Conclusions
The present study shows that real-time intraoperative brachytherapy at a dose of 160 Gy yield better biochemical control than preoperative planning at 145 Gy. In addition, urinary toxicity did not increase, despite the dose escalation, probably because the dose constraints to the urethra were met despite the increased dose escalation. These findings support the use of real-time IoP.
Keywords: Cancer prostate, Brachytherapy, Permanent implant, Seed I125
1. Background and Aim
Prostate cancer (PCa) is the third most common cancer among males in Spain, and, as in other European countries, the incidence is growing. According to the latest International Agency for Research in Cancer (IARC) report,1 44,100 patients in Spain were diagnosed with PCa between the years 2005 and 2009. In Spain, 22,000 new cases of PCa are diagnosed each year, with early-stage disease accounting for 90% of cases; only 4% of cases present with metastatic disease.2 Worldwide, most patients (60–70%) are diagnosed with organ-confined disease. Treatment options3 for patients with organ-confined disease include radical prostatectomy, external beam radiotherapy (EBRT), interstitial brachytherapy, hormonotherapy, and/or ``watchful waiting’’.
In patients with localized disease, permanent seed implant brachytherapy delivers outcomes that are comparable to prostatectomy and EBRT.4 However, seed brachytherapy offers numerous practical advantages. For these reasons, the number of patients treated with this technique continues to increase.
The two most common permanent seed implantation techniques are preoperative planning (PP) with 145 Gy and real-time intraoperative planning (IoP) with 160 Gy. Although IoP has largely replaced PP at many centres in recent years, few studies have directly compared these two techniques. Given this context, the aim of the present retrospective study was to compare toxicity and survival outcomes in a group of low-risk PCa patients treated with either the preoperative planning technique (145 Gy) or the real-time IoP technique (160 Gy).
2. Material and methods
2.1. Patients
Between October 2003 and December 2014, a total of 786 PCa patients were treated with permanent seed iodine 125 (125-I) brachytherapy at the Department of Radiation Oncology at our hospital (Hospital La Fe, Valencia, Spain). The treatment was delivered as monotherapy. In the present work, we retrospectively evaluated all patients diagnosed with low-risk disease (408 of the 786 patients) according to the National Comprehensive Cancer Network (NCCN) guidelines.3
Inclusion criteria for treatment with this technique were as follows: positive biopsy for prostate adenocarcinoma; Gleason score ≤6; pre-treatment prostate-specific antigen (PSA) value <10; clinical stage ≤T2a; prostate volume <60 cc.; International Prostate Symptoms Score (IPSS) ≤15; Qmax >12 mL/s; life expectancy ≥5 years; and prior transurethral resection (if any) performed ≥6 months prior to brachytherapy.
In most cases, patients with a prostate volume ≥50 cc (91 patients; 22.3%) received neoadjuvant cytoreductive hormone therapy.
2.2. Procedure
The treatment was performed in accordance with the recommendations of the American Brachytherapy Society (ABS)5 and the GEC-ESTRO.6 During the 11 year study period, the brachytherapy technique used at our institution changed from preoperative planning (145 Gy) to real-time IoP. As a result, 187 patients underwent PP through September 2007 after which all subsequent patients (221 patients) received IoP with a higher dose (160 Gy). The characteristics of both groups are shown in Table 1.
Table 1.
Patient characteristics.
| Factors | Total | Pre-planning 145 Gy |
Intraoperative planning 160 Gy |
|---|---|---|---|
| Patients, n | 408 | 187 (43.3%) | 221 (56.7%) |
| Mean age, years | 66 | 67 | 65 |
| <55 | 25 (6.1%) | 6 (3.2%) | 19 (8.6%) |
| 55–65 | 161 (39.5%) | 69 (36.9%) | 92 (41.6%) |
| >65 | 222 (54.4%) | 112 (59.9%) | 110 (49.8%) |
| T stage | |||
| T1c | 237 (58.2%) | 122 (65.6%) | 115 (52%) |
| T2a | 170 (41.8%) | 64 (34.4%) | 106 (48%) |
| Median PSA (1st–3rd quartiles) |
6.4 (5.4–7.7) | 6.21 (5.2–7.5) | 6.51 (5.6–7.81) |
| Gleason score | |||
| ≤4 | 79 (19.4%) | 50 (26.7%) | 29 (13.1%) |
| 5 | 82 (20.1) | 40 (21.4%) | 42 (19%) |
| 6 | 247 (60.5) | 97 (51.9%) | 150 (67.9%) |
| Prior TUR | 23 (5.7%) | 15 (8.2%) | 8 (3.5%) |
| Median pre-treatment IPSS | 3 | 3 | 3 |
| Median prostate volume (1 st–3rd quartiles) |
37 (30–46) | 37 (30–46.5) | 37 (30–46) |
| Median number of seeds (1 st–3rd quartiles) |
64 (54–67) | 73 (60–88.5) | 59 (50.25–69) |
| Follow up Median (months) (1 st–3rd quartiles) |
64 (38–94) | 93 (69–111) | 46 (30–46) |
PSA = prostate-specific antigen; TUR = transurethral resection; IPSS = International Prostate Symptom Score.
Importantly, unlike most published studies, we maintained the dose constraint limitation (<217 Gy) to the urethra even after switching to the higher-dose IoP approach. This dose constraint represents 150% of the total dose (145 Gy) in the PP technique and 135% of the IoP dose (160 Gy).
2.2.1. Preoperative planning
From October 2003 to September 2007, 187 patients underwent ultrasound (US)-guided dosimetry pre-planning. In most cases (130 patients), this was performed 2 weeks before the implant; however, in 57 patients, the US was performed in the operating room immediately prior to the implant in order to improve patient positioning. Patients were placed in the lithotomy position for the transrectal ultrasound. All images were acquired with 5-mm slices. The SIMUPLAN planning systems was used for dosimetric planning. The total prescribed dose was 145 Gy. Stranded seeds were inserted through US-guided (endorectal approach) needles.
2.2.2. Real-time intraoperative planning
In October 2007, we switched to the IoP technique. From October 2007 through December 2014, a total of 221 patients were treated with this technique. All patients treated during this period received a dose of 160 Gy. Stranded seeds were used in all cases, except for the last 45 patients in whom loose seeds were used. We used the SPOT PRO software from Elekta/Nucletron.
In both treatment techniques, volume definition was performed in accordance with ICRU Report 58 and the recommendations of the ESTRO/EAU/EORTC.7 Since the GTV can only be defined for tumors higher than stage T1c, we did not contour the GTV in these low risk cases; rather, we used the clinical target volume (CTV), which includes the entire gland. Because set up errors are not significant in brachytherapy, we assumed that the CTV was the same as the planning target volume (PTV). The source distribution was performed in a modified peripheral pattern.
On the treatment day (day 0), a computed tomography (CT) scan was performed to verify implant quality. At one month post-treatment, the following imaging tests were performed: chest and abdominal X-ray (to assess possible seed migration), CT, and t2 MRI (for post-planning) (Table 2).
Table 2.
Dosimetric data.
| Parameter | Total dose, 145 Gy | Total dose, 160 Gy |
|---|---|---|
| D90 | >145 Gy | >160 Gy |
| V100 | >95% | >95% |
| V150 | <50% | <50% |
| Dmax urethra/D0.5 cc | <217 Gy (150%) | <217 Gy (135%) |
| Dmax rectum/D0.5 cc | <145 Gy (100%) | <145 Gy (90%) |
V100 and V150 indicate the percentage of the prostate volume receiving 100% and 150%, respectively, of the prescribed dose; D90, the minimum dose received by 90% of the prostate; Dmax urethra/D0.5 cc indicate, respectively, the maximum dose received by the urethra/maximum dose allowed to 0.5 cc of the urethra; Dmax rectum/D0.5 cc, the maximum dose received by the rectum/maximum dose allowed to 0.5 cc of the rectum.
2.3. Follow up
All patients were prescribed alpha-blockers for at least one month after implant.
The patients were monitored by clinical interview and serum PSA testing every 3–4 months during the first year, then every 6 months until the fifth year, and annually thereafter. Biochemical relapse was defined according to the Phoenix criteria (nadir + 2 ng/mL).8 When necessary, the pertinent clinical tests were performed to confirm the type of relapse.
The Radiotherapy Oncology Group (RTOG) toxicity scales9 and the Common Terminology Criteria for Adverse Effects (CTCAE v3.0)10 were used to assess toxicity.
2.4. Data analysis
The variables of interest are reported as means (standard deviation) or medians (interquartile range; IQR) for continuous variables and as absolute and relative frequencies for categorical variables. The level of statistical significance was set at p = 0.05. The log-rank test was used to compare the Kaplan Meier curves. The ``R" statistical software program (v. 3.2.3) was used for the statistical analysis and to prepare the figures.11
3. Results
3.1. Disease control
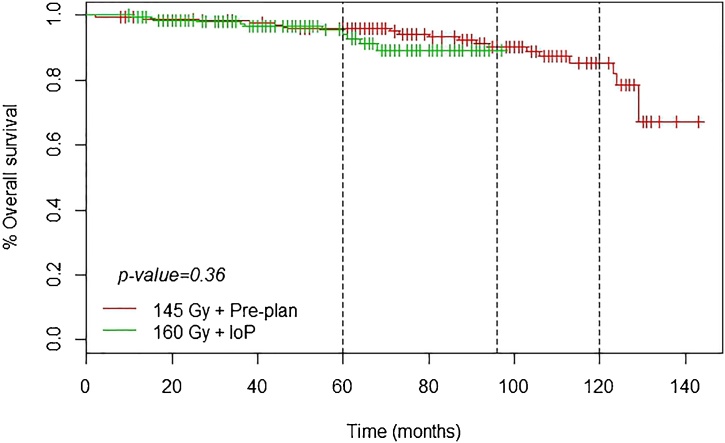
At a median follow up of 90 months, overall survival (408 patients) was as follows: 5 years, 95.2%; 8 years, 89.1%; and 10 years, 84.2%. None of the patients died of prostate cancer. The 5-, 8- and 10-year BRFS rates were, respectively, 92.7%, 85.0%, and 82.2%. There were 36 (8.8%) recurrences, of which 25 (6.1%) were local recurrence confirmed by biopsy or MRI; additionally, there were 6 regional and 3 metastatic recurrences (Table 3).
Table 3.
Recurrences.
| Recurrence | Biochemical |
Local |
Nodal |
Metastatic |
||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | No | Yes | No | Yes | |
| Pre-planning (145 Gy) | 161 | 26 | 168 | 19 | 182 | 5 | 185 | 2 |
| IoP (160 Gy) | 211 | 10 | 215 | 6 | 220 | 1 | 220 | 1 |
| p value | 0.0016 | 0.0035 | 0.14 | 0.88 | ||||
IoP = real-time, intraoperative brachytherapy.
The median maximum nadir was 0.10 (range, <0.04–0.36) with a median of 35.6 months until reaching the nadir. The relation between PSA nadir and time until biochemical relapse was evaluated with the Cox regression model, which showed that the higher the PSA nadir, the greater the risk of biochemical relapse (p < 0.001).
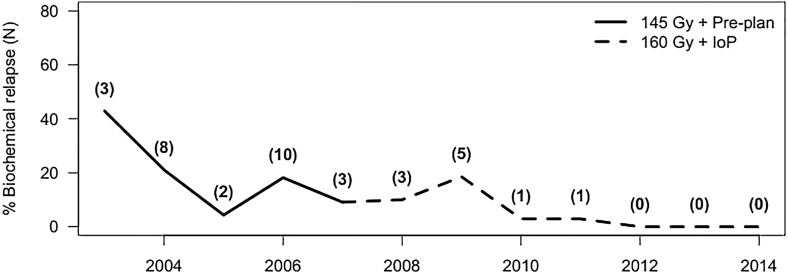
Fig. 1 shows the percentage of patients with a recurrence in each year (absolute numbers per year are shown in parentheses). As the figure shows, there was an initial spike followed by a progressive decrease in recurrences. The two intermediate peaks in 2006 and 2009 may be associated with the learning curve for our team.
Fig. 1.
Percentage of patients with recurrence in each year. Absolute numbers per year are shown in parentheses.
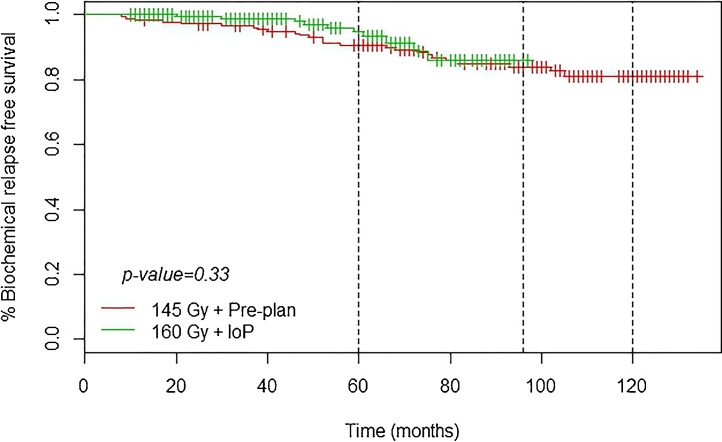
Fig. 2, Fig. 3 show that the IoP technique (160 Gy) resulted in better BRFS curves than the PP (145 Gy) technique.
Fig. 2.
BRF.
Fig. 3.
Overall survival.
In the IoP group, the 45 patients treated with loose seeds had no recurrences, whereas there were 10 recurrences in the 176 patients treated with stranded seeds.
3.2. Toxicity
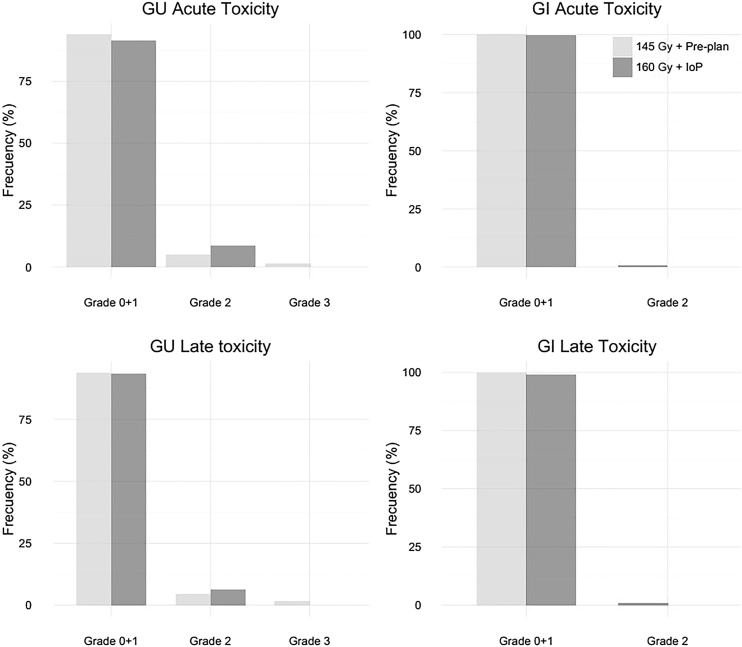
Acute and chronic toxicity was grade (G) 0 or 1 in most (95%) patients in the sample. Toxicity was <G2 in 96% of patients in the PP group versus 93% in the IoP group. Fig. 4 shows the toxicity outcomes according to implant technique.
Fig. 4.
Toxicity outcomes (percentage of patients affected) according to implant technique.
3.2.1 Genitourinary (GU) toxicity
3.2.1.1 Pre-planning
In the PP group, 8 patients (4%) developed G2 toxicity (hematuria and dysuria) and two developed G3 toxicity (urethral stenosis). Chronic G2 GU toxicity was observed in two cases and G3 in two others (who required at least 3 transurethral resections with toxicity still present >5 years post-treatment).
3.2.1.2 IoP
In the IoP group, 19 patients (8%) developed acute G2 GU toxicity (hematuria and dysuria) and 14 (6%) chronic G2 GU toxicity.
3.2.2 Gastrointestinal (GI) toxicity
In the PP group, one patient developed acute G2 GI toxicity (diarrhea and rectal bleeding) while two patients in the IoP group developed chronic G2 GI toxicity.
None of the patients in the IoP group developed G3 GI or GU toxicity (see Fig. 4).
Most patients (88%) in both groups who were sexually active prior to treatment maintained sexual function after seed implantation.
4. Discussion
The main aim of the present study was to compare the results obtained in a group of low-risk PCa patients treated with either the preoperative planning technique (145 Gy) or the real-time intraoperative planning approach (160 Gy). Overall, BRFS rates were excellent: 92.7%, 85% and 82% at 5-, 8- and 10-years, respectively. These findings are in line with 5-year outcomes reported in the largest series, including that of Zelefsky et al.12 (2012), who reported a 98% rate in 840 patients and Dickinson et al.13 (2013), who reported a 94% BRFS at 5-years in a series of 1038 patients (4% with stage T2b disease). Similarly, our results are comparable to the findings reported by Martínez et al.14 (although it is worth noting that 9% of their sample consisted of intermediate risk patients) and to those described by Prada et al.15 and Silvester et al.16
In terms of toxicity, the vast majority (95%) of patients in our sample presented only mild toxicity (G0–G1), a finding that is consistent with other published series.15, 17, 18, 19 The most common adverse effects were those related to urinary irritation–obstruction. The mean time elapsed from the implant until the emergence of the maximum toxicity grade was 9.5 months (all of our patients, even those treated towards the end of the study period, presented a minimum follow up >10 months). Four patients developed G3 GU toxicity (two were acute and resolved within 6 months while the other two were chronic). Given that only a few patients presented G3 toxicity, it is difficult to reach any definite conclusions, except to emphasize that treatment was well-tolerated by most patients. Note that the 4 patients with G3 toxicity all had large prostates and were all treated with the PP technique.
To our knowledge, few studies have directly compared two different permanent implant techniques and doses as we have done with this study. However, Table 4 summarizes the results of recent studies that also compared dose and/or implant technique.
Table 4.
Summary of results of recent studies that compared dose and/or implant technique.
| Author (year) | Patients, N | Dose, Gy | Technique | BRFS | |
|---|---|---|---|---|---|
| Matzkin et al. (2013)20 | 128 905 |
145 | PP IoP |
77% (5 yr) 97% (5 yr) |
70% (7 yr) 95% (7 yr) |
| Guinot et al. (2015)21 | 250 250 |
145 | PP IoP |
91% (5 yr) 97% (5 yr) |
|
| Ishiyama et al. (2015)22 | 27 86 192 |
145/160 | PP Pre-plan IoP IoP |
100% (8 yr) 90% (8 yr) 97% (8 yr) |
|
| Current study (Pons et al.) (2016) | 187 221 |
145 160 |
PP IoP |
90% (5 yr) 94.8% (5 yr) |
83.9%(7 yr) 86% (7 yr) |
Matzkin et al.20 compared IoP to PP in low risk patients, finding that although IoP achieved better outcomes than PP, the results were not comparable due to the lack of homogeneity between groups. However, the authors performed a secondary analysis to assess two subgroups (132 patients in each subgroup) with similar characteristics. In the subgroup analysis, they found that the outcomes in the IoP group were superior to those in the PP group, with 5- and 7-year BRFS rates of 77% and 70% in the IoP group versus only 96% and 94% in the PP group. However, these differences were not statistically significant.
Guinot et al.21 compared two groups of 250 patients each, but, unlike our study, patients treated with EBRT were also included. Although they obtained better results for the IoP group (Table 4), the differences were not statistically significant. Ishiyama et al.22 compared three different groups: PP, intraoperative (without vector correction), and real-time IoP with vector correction. The intraoperative technique yielded the poorest results among the three techniques, whereas both the real time IoP and the PP technique achieved similarly good results, without any statistically significant differences between them.
In our series, BRFS rates were (non-significantly) better both at 5 and 10 years for the real-time IoP group (versus PP) at the end of follow up (median, 90 months). However, we cannot definitively attribute this improvement to the technique, although the results suggest that IoP was superior. Due to the short follow up and excellent results in both groups, no definitive conclusions can be made. Nonetheless, our results confirm the conclusions reached by other authors: the IoP technique appears to yield better results, but without statistical confirmation.
In terms of reported toxicity, in most cases and regardless of the specific technique, rectal and urinary complications were low-grade (G0 or G1), a finding that is consistent with previous reports.20, 22 Overall, the IoP technique produced slightly more toxicity (5.8% vs. 4.8%), a finding that is in line with other studies.20, 21, 22 Despite this statistical increase in toxicity, it is important to note that this was within acceptable levels in our study and in studies described above. In our study, the symptom with the highest toxicity grade was urinary retention, which required catheter insertion and a transurethral resection in 2 patients. No cases of G4 toxicity were observed in any of the patients in our sample, regardless of the technique. Only slightly more than 2% of PP patients experienced G3 toxicity versus 0% in the IoP group, a remarkable finding given that other studies reported both G3 and G4 toxicity with the intraoperative technique.21, 22 Importantly, in the IoP group, we found no difference in toxicity regardless of whether stranded or loose seeds were used.
The technological advances that led to the change from PP to IoP were also accompanied by a dose escalation to 160 Gy. Previous studies have shown that dosimetric calculations indicate that the dose to the prostate gland can be safely increased without substantially increasing the dose to the rectum or urethra.23 To avoid increased toxicity in the bladder neck—the area most commonly associated with urinary toxicity—we maintained the dose constraint to the urethra (Dmax <217 Gy). The fact that the peripheral area of the prostate gland is where most relapses occur (rather than the transition or central zones, which are located closer to the urethra) supports the use of dose escalation to minimize recurrence in that area. By modifying the dose to the peripheral area, we are able to deliver an extremely conformal dose distribution. This dose conformity is further improved by the use of real-time IoP with image-guidance, which eliminates glandular motion because the 125-I implant is performed within the prostate tissue. As a result, this allows us to avoid increasing the complication rate, even with the increased dose. Nevertheless, although the toxicity graphs appear to suggest that acute toxicity in the urethra was greater with the IoP technique, statistically, urethral toxicity was the same for both techniques, despite the use of higher doses with the IoP technique. Nevertheless, other groups have found that the use of 160 Gy increases complications, especially urinary toxicity. Matzkin et al.24 analyzed urinary toxicity in two groups, one treated with the PP technique at 145 Gy and the other with IoP with 160 Gy, finding increased toxicity in the IoP group. The Mount Sinai Medical Center group25 assessed 643 patients treated with D90 doses of ≥180 Gy, finding excellent results (5-year BRFS, 97.3%) and only 10.7% urinary retention rates. Gómez-Iturriaga et al.26 also obtained good results with 180 Gy: 5-year BRFS of 96.8% and only 1.5% of cases with late G3 urinary retention. Overall, our findings and those of the authors mentioned above support dose escalation because toxicity does not increase significantly while G3 toxicity is rare.
There are several reasons that may explain why the IoP results in improved outcomes versus PP, even though most of the steps in both techniques are similar. In the PP technique, planning is performed by US up to several days (with all the uncertainty that this implies) before the implant. In addition, the seeds are inserted through 20-cm needles in precise and pre-defined positions according to the pre-plan. The limitation of this system is that the vector positions cannot be modified (that is, the needle position cannot be updated). Moreover, needle removal must be done very carefully to avoid altering the position of the strand. Moreover, once seeded, the seeds cannot be removed. Therefore, if any cold spots remain, new seeds need to be implanted. Finally, the use of stranded seeds does not allow for intermediate positioning of the seeds since these are separated by 1 cm in the strand.
Another disadvantage of the PP technique is that the prostate volume can vary by 30% to 90% from day 0 until the edema is resolved, and this variation is not considered in the PP dose plan.27, 28 By contrast, when real-time volumetry is performed, the dosimetry can be adjusted more reliably to the actual volume, although it is still not possible to accurately control for the edema in the prostate that occurs during the dose release period. The IoP technique allows us to modify the seed implants to match the real-time prostate volume; in addition, the use of loose seeds allows more flexibility in seed positioning but also requires greater surgical skill. Prior research with I-125 seeds has shown that variability in seed location ranges from 0.5 mm to 10 mm in the post-plans; in addition, results show that uncertainty of 2 mm in seed location can produce a deviation of up to 5% in the prostate D90 while uncertainty of 10 mm can result in a decrease in the D90 of >30 Gy.29
In an attempt to identify predictors of relapse, we evaluated two variables: prostate volume and the number of seeds. However, neither was associated with a greater risk of relapse in our study. In the literature, multiple studies have demonstrated the prognostic importance of Gleason <7 and PSAi <10, both of which are criteria for low-risk prostate cancer. We found that the PSA nadir value was associated with biochemical control, such that a lower nadir seems to indicate an increased likelihood of better biochemical control.
5. Conclusions
In conclusion, the present study demonstrates that real-time intraoperative planning with 160 Gy appears to yield better biochemical outcomes versus pre-planning with 145 Gy. However, because several variables (technique, dose, and type of seed [loose vs. stranded]) were modified, it is not possible to definitively attribute improved outcomes specifically to the dose escalation or technique. Of the known predictors of relapse (Gleason score and PSA value), the only variable significantly associated with biochemical disease control in our study was the nadir PSA value, with lower values resulting in better control.
Chronic toxicity was also better with IoP, with no G3 toxicity and only a slight increase in G2 toxicity. A strong point of this study is that we maintained the dose constraint to the urethra (Dmax <217 Gy) despite increasing the treatment dose from 145 to 160 Gy.
In conclusion, the use of real-time, intraoperative planning with dose escalation to 160 Gy results in better disease control without an increase in toxicity when the dose constraints to the urethra are maintained.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
For this type of study formal consent is not required.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Forman D., Bray F., Brewster D.H., editors. Cancer incidence in five continents, vol. X. IARC Scientific Publication N. 164. 2017. [Google Scholar]
- 2.Registro Nacional de Prostate cancer-Asociación Española de Urología, www.aeu.es/userfiles/presentacion_datos_nacionales_registro_nacional_cap.pdf.
- 3.National Comprehensive Cancer Network Guidelines. 2016. www.nccn.org Available at: [accessed May 2016] [Google Scholar]
- 4.Steven H., Stokes M.D. Comparison of biochemical disease-free survival of patients with localized carcinoma of the prostate undergoing radical prostatectomy, transperineal ultrasound-guided radioactive seed implantation, or definitive external beam irradiation. Int J Radiat Oncol Biol Phys. 2000;47:129–136. doi: 10.1016/s0360-3016(99)00526-x. [DOI] [PubMed] [Google Scholar]
- 5.Davis B., Horwitz E., Lee W.R. American Brachytherapy Society consensus guidelines for transrectal ultrasound-guided permanent prostate brachytherapy. Brachytherapy. 2012;11:6–19. doi: 10.1016/j.brachy.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Ash D., Flynn A., Battermann J. ESTRO/EAU/EORTC recommendations on permanent seed implantation for localized prostate cancer. Radiother Oncol. 2000;57(3):315–321. doi: 10.1016/s0167-8140(00)00306-6. [DOI] [PubMed] [Google Scholar]
- 7.Salembier C., Lavagnini P., Nickers P. Tumour and target volumes in permanent prostate brachytherapy: a supplement to the ESTRO/EAU/EORTC recommendations on prostate brachytherapy. Radiother Oncol. 2007;83:3–10. doi: 10.1016/j.radonc.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Roach M., 3rd, Hanks G., Thames H., Jr. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO, Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 9.RTOG/EORTC Radiation Morbidity Scoring Schema. Available at www.rtog.org.
- 10.Common Terminology Criteria for Adverse Events (CTCAE). Available at www.evs.nci.nih.Gov.
- 11.The R Project for Statistical Computing. R version 3.2.3 (Wooden Christmas-tree).
- 12.Zelelefsky M., Kuban D., Levy L. Multi-institutional analysis of long-term outcome for stages T1–T2 prostate cancer treated with permanent seed implantation. Int J Radiat Oncol Biol Phys. 2007;67:327–333. doi: 10.1016/j.ijrobp.2006.08.056. [DOI] [PubMed] [Google Scholar]
- 13.Dickinson P., Malik J., Mandall P. Five years outcomes following 125I seed brachytherapy for low risk prostate cancer at three United Kingdom cancer centers. BJU Int. 2014;113:748–753. doi: 10.1111/bju.12358. [DOI] [PubMed] [Google Scholar]
- 14.Martínez E., Daidone A., Gutierrez C. Permanent seed brachytherapy for clinically localized prostate cancer: long-term outcomes in a 700 patient cohort. Brachytheapy. 2015;14:166–172. doi: 10.1016/j.brachy.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Prada J., Juan G., González-Suárez H. Prostate-specific antigen relapse-free survival and side-effects in 734 patients with up to 10 years of follow-up with localized prostate cancer treated by permanent 125Iodine implants. BJU Int. 2010;106:32–36. doi: 10.1111/j.1464-410X.2009.09096.x. [DOI] [PubMed] [Google Scholar]
- 16.Silvester J., Grimm P., Wong J. Fifteen-year biochemical relapse-free survival, cause-specific survival, and overall survival following 125Iodine prostate brachytherapy in clinically localized prostate cancer: Seattle experience. Int J Radiat Oncol Biol Phys. 2011;81:376–381. doi: 10.1016/j.ijrobp.2010.05.042. [DOI] [PubMed] [Google Scholar]
- 17.Ferrer M., Guedea F., Suárez J.F. Quality of life impact of treatments for localized prostate cancer: Cohort study with a 5 year follow-up. Radiother Oncol. 2013;108:306–313. doi: 10.1016/j.radonc.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 18.Takahisa E., Atsunori Y., Nobuko K. Predictive factors for urinary toxicity after iodine-125 prostate brachytherapy with or without supplemental external beam radiotherapy. Brachytherapy. 2016;15:218–295. doi: 10.1016/j.brachy.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Keyes M., Miller S., Pickles T. Late urinary side effects 10 years after low-dose-rate brachytherapy: population-based results from a multiphysician practice treating with a standardized protocol and uniform dosimetric goals. Int J Radiat Oncol Biol Phys. 2014;90:570–578. doi: 10.1016/j.ijrobp.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 20.Matzkin H., Chen J., German L., Mabjeesh N. Comparison between preoperative and real-time intraoperative planning 125I permanent prostate brachytherapy: long-term clinical biochemical outcome. Radiat Oncol. 2013;8(288) doi: 10.1186/1748-717X-8-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guinot J.L., Ricós J.V., Tortajada M.I. Comparison of permanent I125 seeds implants with two different techniques in 500 cases of prostate cancer. J Contemp Brachyther. 2015;7(4):258–264. doi: 10.5114/jcb.2015.53525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishiyama H., Satoh T., Sekiguchi A. Comparison of three different techniques of low-dose-rate seed implantation for prostate cancer. J Contemp Brachyther. 2015;7(1):3–9. doi: 10.5114/jcb.2015.48603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X.A., Wang J., Stewart R.D. Dose escalation in permanent brachytherapy for prostate cancer: dosimetric and biological considerations. Phys Med Biol. 2003;48:2753–2765. doi: 10.1088/0031-9155/48/17/302. [DOI] [PubMed] [Google Scholar]
- 24.Matzkin H., Kaver I., Stenger A., Agai R., Esna N., Chen J. Iodine-125 brachytherapy for localized prostate cancer and urinary morbidity: a prospective comparison of two seed implant methods-preplanning and intraoperative planning. Urology. 2003;62(3):497–502. doi: 10.1016/s0090-4295(03)00407-2. [DOI] [PubMed] [Google Scholar]
- 25.Waterman F.M., Yue N., Corn B.W., Dicker A.P. Edema associated with I-125 or Pd-103 prostate brachytherapy and its impact on post-implant dosimetry: an analysis based on serial CT acquisition. Int J Radiat Oncol Biol Phys. 1998;41:1069–1077. doi: 10.1016/s0360-3016(98)00152-7. [DOI] [PubMed] [Google Scholar]
- 26.Gómez-Iturriaga A., Crook J., Borg J., Ma C. Biochemical disease-free rate and toxicity for men treated with Iodine-125 prostate cancer with D90 > 180 Gy. Int J Radiat Oncol Biol Phys. 2010;78(2):422–427. doi: 10.1016/j.ijrobp.2009.07.1723. [DOI] [PubMed] [Google Scholar]
- 27.Crook J., McLean M., Yeung I., Williams T., Lockwood G. MRI-CT fusion to assess postbrachytherapy prostate volume and the effects of prolonged edema on dosimetry following transperineal interstitial permanent prostate brachytherapy. Brachytherapy. 2004;3:55–60. doi: 10.1016/j.brachy.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Su Y., Davis B.J., Furutani K.M., Hermn M.G., Robb R.A. Dosimetry accuracy as a function of seed localization uncertainty in permanent prostate brachytherapy: increased seed number correlates with less variability in prostate dosimetry. Phys Med Biol. 2007;52:3105–3119. doi: 10.1088/0031-9155/52/11/012. [DOI] [PubMed] [Google Scholar]
- 29.Kao J., Stone N., Lavaf A., Dumane V., Cesaretti J.A., Stock R.G. 125I monotherapy using D90 implant doses of 180 Gy or greater. Int J Radiat Oncol Biol Phys. 2008;70(1):96–101. doi: 10.1016/j.ijrobp.2007.06.067. [DOI] [PubMed] [Google Scholar]