Abstract
Aims
Clinical trial patients are highly motivated but may encounter difficulty in taking study medication regularly when treatment burden is substantial. We assessed a brief behavioural intervention, given in addition to a standard trial protocol.
Methods
We performed a two-arm adherence sub-study, within a twelve-month randomised controlled drug trial evaluating the impact of statin and/or omega-3 EE90 treatment in 800 patients with type 2 diabetes, in 59 United Kingdom general practices cluster-randomised to action-planning or control groups. The former delivered an initial written exercise prompting participants to formulate action-plans to take study medication regularly, with brief nurse encouragement to use action-plans at later visits, whilst the latter followed the standard trial protocol. The primary outcome was proportion of days on which study medication were taken as intended measured by electronic medication containers.
Results
Adjusted mean (95% CI) proportion of days with medication taken as intended was 79.3% (76.3% to 82.3%) for the 30 action-planning practices (321 participants), compared with 78.5% (75.8% to 81.1%) for the 29 control group practices (479 participants, with a mean intervention effect of 0.9% (95%CI -3.1% to +4.9%, p=0.67). Adjusted odds ratios for ≥80% trial medication adherence for action-planning compared with control practices were 1.29 (0.90 to 1.84) and 1.38 (0.96 to 1.99) respectively.
Conclusions
Low-intensity action-planning interventions used alone are unlikely to have a clinically important impact on medication adherence. These findings, do not exclude their contribution, as part of a multifactorial intervention, to improving treatment adherence. ISRCTN number 76737502.
Keywords: type 2 diabetes, statin, omega-3 EE90, adherence, behavioural intervention
Introduction
Diabetes is a major public health problem. The prevalence is projected to reach 642 million by 2040,[1] with a high clinical and economic burden as people with diabetes have a two-to four fold increased risk for cardiovascular disease compared to the general population, and an increased incidence of retinopathy, peripheral nerve damage and renal problems.[2] Treatment with statins (HMG-CoA reductase inhibitors) reduces the risk of a first cardiovascular event in people with diabetes, even in those without high baseline LDL cholesterol values.[3] However, up to half of medication for diabetes may not be taken as prescribed, including statin therapy. Medication non-adherence reduces treatment efficacy and wastes healthcare resources.
Interventions to promote adherence need to be sufficiently effective to justify their cost, with the components straightforward to deliver, particularly when implemented at scale. Non-adherence falls into two broad categories: (i) that arising from the patient’s decision to take less medication than prescribed or to miss a dose or day of medication (intentional non-adherence); (ii) forgetting to take medication (non-intentional non-adherence).[4] One approach for people who forget to take medicines is to help them define specific action plans that will increase the chances of carrying out the desired behaviour and establishing a regular habit.[5] Previous work using this approach includes increasing consumption of vitamin C pills,[5] attendance for cervical cytology screening [6] and uptake of breast self-examination.[7]
Clinical trial participants are often highly motivated to take the study medication, but may still encounter difficulty in taking it regularly. This is particularly so when there is a high burden of medication and when the trial period extends beyond a few weeks. The Atorvastatin in Factorial with Omega fatty acids for Risk Reduction in type 2 Diabetes (AFORRD) trial, a one-year, primary care based factorial design clinical trial investigating the impact of statin and/or omega-3 EE90 treatment on estimated cardiovascular risk with statins provided an opportunity to test the effectiveness of an embedded action-planning intervention.
This sub-study aimed to assess the degree to which an action planning intervention could improve adherence to trial medication when added to the standard trial procedures.[8]
Methods
Study setting and population
We performed a cluster-randomised sub-study, embedded within the AFORRD trial run by the University of Oxford Diabetes Trials Unit in an academic collaboration with Pfizer Ltd, in 59 United Kingdom general practices. The protocol was approved by local and national ethics committees and carried out in accordance with the Declaration of Helsinki and good clinical practice guidelines. The study design, inclusion criteria, and primary results have been published.[8] AFORRD participants were recruited between November 2004 and July 2005.
Randomisation
Following recruitment of their first AFORRD participant, participating practices were matched in pairs for size and location and randomly allocated by a statistician with no involvement in the sub-study to receive either the additional action-planning intervention (action-planning group) or the standard trial protocol (control group).
Intervention
Action-planning group participants were asked to complete an extra task when completing other self-reported trial measures in a questionnaire sent by post two weeks after the first medication-dispensing visit. This task was a written exercise, presented on a single sheet of paper as two additional questions, asking the participant to formulate a written plan for taking their study medication. The two questions (“When do you plan to take your study medication?” and “Where do you plan to take your study medication?”) were intended to help the participant specify a series of contingent circumstances that would help prompt them to take their medication, e.g. “When I brush my teeth in the morning in the bathroom, I will take my trial medicines”. At the two subsequent general practice visits (18 and 32 weeks) these action-planning sheets were again completed by trial participants attending practices allocated to deliver the action planning intervention. At these two visits, the practice based research nurse also asked whether participants had concerns about the medication and encouraged the participants to complete action-planning sheets at 18 and 32 weeks by giving information about the benefits of making action plans for taking medication as prescribed. These discussions were intended to be brief, e.g. to last no more than one minute.
Control group practice participants were asked to complete the self-reported trial measures as specified in the study protocol at the same time points as action planning group participants completed their action planning sheets. Research nurses in the control group practices were asked to check whether participants had concerns about taking the study medicines, but were not trained in the use of action-planning or prompting participants to use action plans to take their medication.
Research nurses in practices allocated to the action-planning and control groups received separate documentation and training to ensure fidelity to the sub-study procedures. Intervention scripts were piloted before the trial. Trial operational manuals had sections that were customised and included prompts for the intervention, and one hour of training on sub-study procedures was delivered to the practice-based research nurses with group-specific training videos. The control group video focussed on demonstrating standard trial procedures including advising about side-effects of medication. The action planning video demonstrated, in addition, the principle of the action-planning intervention so that the nurses could practice it themselves and then guide trial participants in completing further action-planning sheets at their 18 and 32 week visits after the medication dispensing visit. The study research nurse followed up practice nurses by telephone.
Procedures
Two weeks after initial assessment and consent procedures for the main trial and sub-study, participants returned to the practice and were given a 16-week supply of both trial medications, dispensed in an electronic medication-monitoring device (eMems V®, Aardex, Switzerland). AFORRD trial participants were allocated in a two-by-two factorial design to a tablet (atorvastatin 20 mg or matched placebo) and to a capsule (omega-3 EE90 2g or a matched placebo). Practice research nurses were trained to show trial participants how to use the eMems device, which maintained a record of the clock time and date whenever the container cap was removed. Two weeks after the first medication-dispensing visit, all participants received a two-page self-report questionnaire about perceived risks of future heart disease, with a medication action-planning sheet for those in the action-planning group.
At 16 weeks, participants attended a brief assessment visit at which they received a further two-week supply of study medication. They were seen again at 18 weeks and given a further 14 weeks study medication supply. This included an additional tablet (atorvastatin or placebo) to intensify lipid-lowering therapy in those with an estimated 10-year cardiovascular risk ≥20%. Action-plan practice participants completed a further action-planning sheet, with additional explanation if needed from the nurse. At 32-weeks, study medication was provided for the final 20-week study period and participants in action-planning group practices completed a further action-planning sheet.
Additional measurements were collected at the 52-week final follow-up visit and the eMems devices for each of the three study medications (statin, omega-3 EE90, statin intensification) returned to the coordinating centre for the data to be downloaded.
Outcomes
The primary outcome for this sub-study was the proportion of days on which all three study medications were taken as intended. Secondary outcomes were the proportion of study medications taken in each of the four study periods (0-16, 16 to 18, 18-32 and 32-52 weeks) overall, and for each of the three trial treatments separately. eMems devices were read and data uploaded to the clinical database masked to group allocation.
The trial was intended to allow identification of a difference in study medication adherence of five percent between action planning and control groups in the proportion of days in which medication was taken as required by the AFORRD trial protocol. We calculated a target sample size of 1000 participants in 70 general practices based on a standard deviation of 17 for the number of days on which medication is taken per 100 days, alpha 0.05, beta 0.8, interclass correlation coefficient (ICC) of 0.05 and 11% inflation to account for drop out following randomisation.
Statistical analysis
We included data for the primary analysis from all randomised participants, excluding those withdrawn because of site-specific protocol violations where the wrong questionnaire was handed out to participants at two sites.
Data are presented as means (1 SD), with estimated intra-cluster correlation coefficients (95% confidence intervals). The primary study analysis of overall adherence used a significance level of 5%. Other pre-specified analyses included the measures of adherence for each of the three trial treatments and changes in adherence over the course of the trial. A predefined subgroup analysis was carried out to test for interaction between participant characteristics and adherence.
Two measures of medication adherence were derived from the electronic monitoring data: (i) the primary outcome - mean percentage of days on which the correct dose of medication was taken; (ii) the proportion of participants taking ≥80% of their medication. Mean measures of adherence were compared between participants registered to practices allocated to the action-planning and control groups.
Medication was considered taken ‘as prescribed’ if there was one recorded opening of the eMems device on a given day. We defined a day as starting at 03.00 am and ending at 03.00 am the following day.[9] Summary measures of individual adherence were calculated as the number of days in which medication was taken as prescribed, divided by the total number of days observed. We calculated mean adherence in the action-planning and control groups overall and for each of the four study time periods.
Generalised estimating equation linear models were used to account for clustering within practice and to present mean adherence (95% confidence intervals) based on robust standard errors. Summary measures were derived using R (R - Foundation, Vienna, Austria) and models fitted using Stata version 11.2.
Between group trends for the proportion of participants taking medication on each day were examined by fitting regression lines for the action-planning and control groups over the whole trial, assuming that an intercept not different to zero would indicate no difference in treatment adherence.
Neither the sponsor nor the funder had any role in trial design, interpretation or reporting of the trial.
Results
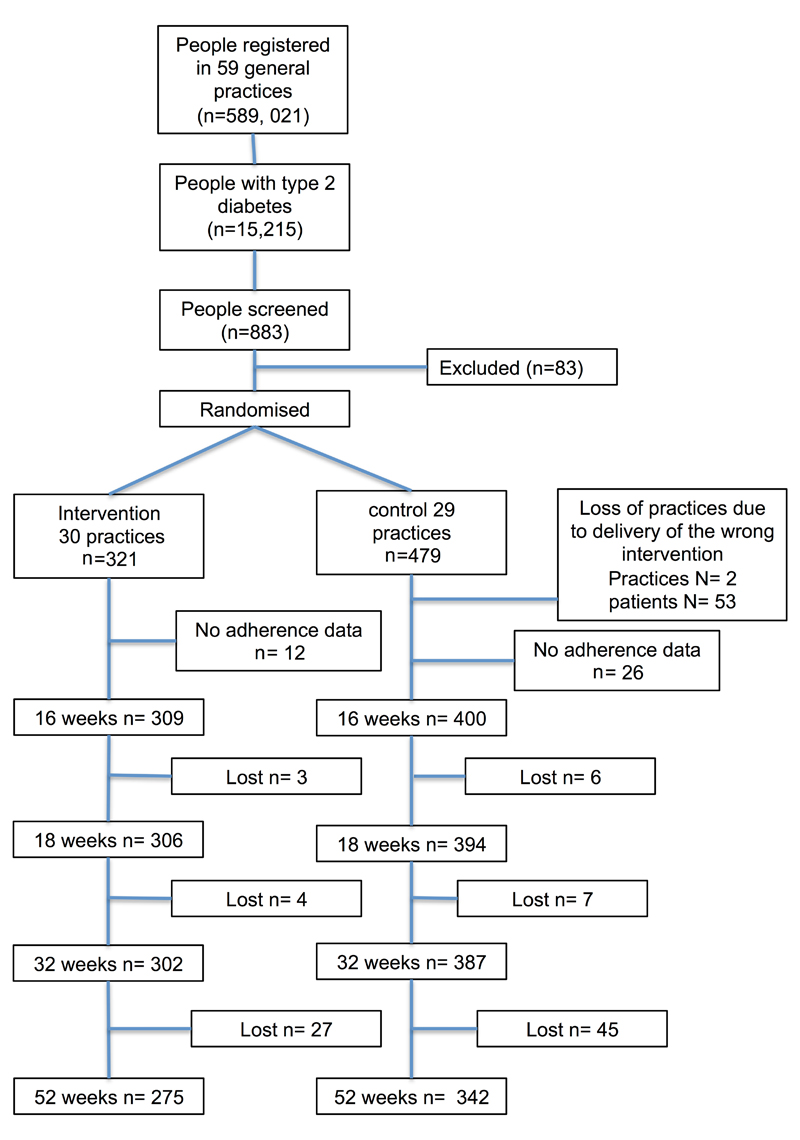
A participant flow diagram is shown in Figure 1. 800 participants were recruited in 59 practices, 30 computer-allocated at random to action-planning (321 participants), and 29 (479 participants) to the control group. A total of 53 patients registered with two control group practices were excluded from the analysis as they were found to have incorrectly received action-planning intervention self-report measures during the study. In total 321 participants in 30 practices received the additional action-planning intervention and 426 participants in 27 practices received the standard trial protocol.
Figure 1.
Participant Flow Diagram
Baseline participant characteristics are shown in Table 1. The action-planning group were slightly younger than the control group, and had fewer male participants. Table 2 shows the impact of the action-planning intervention compared with control, with no difference between groups in the primary outcome for all medication streams over one year, with a between group difference of 0.9% (-3.1% to +4.9%). There was a small, non-significant but consistent higher adherence in the action-planning group over the course of the study of between 2% and 4% after adjustment for the intra-cluster effects, most apparent between the start of the trial and 32 weeks across each of the reported outcomes for adherence to individual medications and their combinations.
Table 1.
Baseline participant characteristics by randomised allocation
| Action-planning | Standard practice | ||
|---|---|---|---|
| Number of practices | 30 | 29 | |
| Number of participants | 321 | 479 | |
| Gender (male)1 | 174 (54%) | 285 (59%) | 0.14 |
| Age (years)2 | 61.5 (11.1) | 63.9 (12.0) | 0.004 |
| Ethnicity1 | |||
| White | 308 (96.0%) | 415 (86.6%) | |
| Asian/Asian British | 8 (2.5%) | 31 (6.5%) | |
| Black/Black British | 4 (1.2%) | 29 (6.1%) | |
| Other | 1 (0.3%) | 4 (0.8%) | 0.001 |
| Diabetes duration (years)3 | 4.7 (1, 7) | 5.1 (1, 7) | 0.28 |
| Weight (kg) 2 | 89.4 (19.9) | 86.8 (18.9) | 0.07 |
| HbA1c (mmol/mol) 2 | 52.1 (11.6) | 52.4(11.7) | 0.75 |
| HbA1c (%) 2 | 6.9 (1.1) | 6.9 (11.1) | 0.75 |
| Total cholesterol (mmol/l)2 | 5.1 (0.8) | 4.9 (0.9) | 0.005 |
| Self-reported adherence3 | 24 (23 to 25) | 24 (23, 25) | 0.86 |
Data are N (%)1, mean (SD)2, median (Q1, Q2)3
P values are taken from chi-squared or Kruskal Wallace
Table 2.
Proportion of days of medication taken (95% confidence interval) for each trial treatment by four study visit periods, adjusted for cluster effect
| Medication monitored | Group | n = 709 | Timeframe (weeks) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0-16 | n | 16-18 | n | 18-32 | n | 32-52 | n | Average (across all non-zero scores) | |||
| Mean (95 % C.I) | |||||||||||
| Intervention | 298 | 83.6 (80.2 to 87.0) | 280 | 82.5 (78.1 to 86.8) | 289 | 81.1 (77.6 to 84.6) | 271 | 77.2 (73.6 to 80.7) | 298 | 78.4 (75.2 to 81.7) | |
| Tablets | Control | 388 | 80.8 (77.7 to 83.9) | 352 | 80.6 (76.4 to 84.9) | 362 | 79.7 (76.6 to 82.9) | 342 | 77.0 (73.8 to 80.1) | 388 | 76.2 (73.2 to 79.1) |
| Difference (Interv | 686 | 2.8 (-1.8 to 7.5) | 632 | 1.9 (-4.2 to 7.9) | 651 | 1.4 (-3.3 to 6.1) | 613 | 0.2 (-4.5 to 4.9) | 686 | 2.2 (-2.1 to 6.6) | |
| p value | 0.24 | 0.55 | 0.55 | 0.93 | 0.32 | ||||||
| Intervention | 302 | 82.5 (79.1 to 85.8) | 280 | 82.1 (77.7 to 86.5) | 289 | 80.4 (76.8 to 83.9) | 273 | 77.0 (73.6 to 80.5) | 302 | 77.4 (74.1 to 80.8) | |
| Capsules | Control | 388 | 79.9 (76.8 to 83.0) | 350 | 80.1 (75.8 to 84.4) | 362 | 79.0 (75.8 to 82.2) | 343 | 75.9 (72.9 to 79.0) | 388 | 75.4 (72.4 to 78.5) |
| Difference | 690 | 2.5 (-2.1 to 7.1) | 630 | 2.0 (-4.2 to 8.1) | 651 | 1.3 (-3.5 to 6.2) | 616 | 1.1 (-3.5 to 5.7) | 690 | 1.9 (-2.6 to 6.5) | |
| p value | 0.28 | 0.53 | 0.58 | 0.64 | 0.40 | ||||||
| Intervention | 265 | 86.8 (84.3 to 89.3) | 260 | 78.3 (73.9 to 82.8) | 265 | 83.0 (80.3 to 85.7) | |||||
| Additional | Control | 338 | 83.9 (81.7 to 86.1) | 325 | 78.3 (74.1 to 82.4) | 338 | 79.6 (77.2 to 82.0) | ||||
| Difference | 603 | 2.9 (-0.5 to 6.2) | 585 | -0.07 (-6.0 to 6.2) | 603 | 3.4 (-0.2 to 7.0) | |||||
| p value | 0.09 | 0.98 | 0.07 | ||||||||
| Intervention | 291 | 81.5 (77.8 to 85.2) | 268 | 82.4 (77.9 to 86.9) | 263 | 84.5 (82.1 to 87.0) | 251 | 77.0 (73.0 to 81.1) | 268 | 81.6 (78.6 to 84.6) | |
| Tablets and Capsules | Control | 376 | 79.1 (75.7 to 82.5) | 333 | 81.1 (76.6 to 85.5) | 330 | 82.6 (80.3 to 84.8) | 314 | 79.9 (76.1 to 83.7) | 333 | 81.7 (78.9 to 84.5) |
| Difference | 667 | 2.4 (-2.6 to 7.4) | 601 | 1.3 (-5.0 to 7.6) | 593 | 1.9 (-1.4 to 5.3) | 565 | -2.9 (-8.4 to 2.7) | 601 | -0.13 (-4.2 to 3.9) | |
| p value | 0.35 | 0.69 | 0.25 | 0.31 | 0.95 | ||||||
| Intervention | 265 | 263 | 83.4 (80.8 to 86.0) | 251 | 77.1 (73.1 to 81.0) | 263 | 79.3 (76.3 to 82.3) | ||||
| All medications | Control | 378 | 331 | 81.1 (78.8 to 83.4) | 313 | 79.4 (75.8 to 83.1) | 331 | 78.5 (75.8 to 81.1) | |||
| Difference | 643 | 594 | 2.3 (-1.2 to 5.8) | 564 | -2.4 (-7.8 to 3.0) | 594 | 0.9 (-3.1 to 4.9) | ||||
| p value | 0.19 | 0.39 | 0.67 | ||||||||
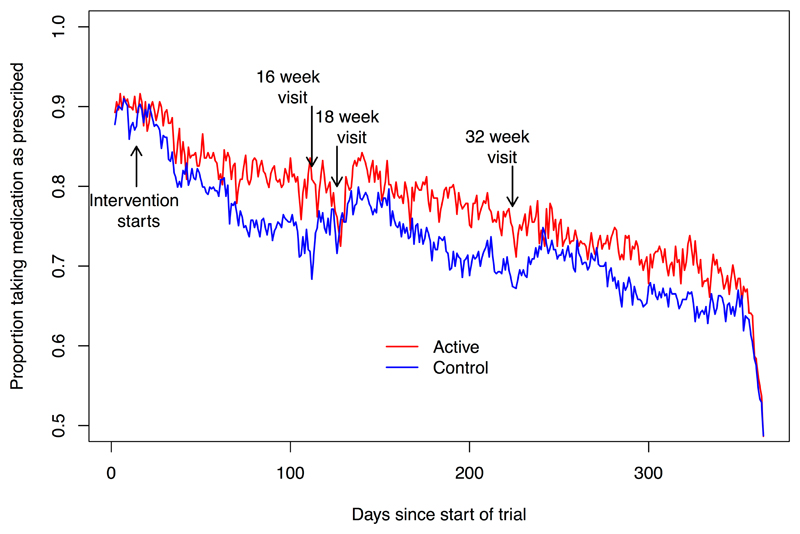
Figure 2 provides a descriptive analysis of persistence with study medication at the group level in the extent to which the tablet and capsule streams were taken over the course of the study. Trends for the proportion of participants taking medication on each day of the trial between groups were significantly different (p < 0.001). The estimate (95% confidence intervals) of difference over-time was 1.9% (1.3% to 2.4%), corresponding to the estimates of mean differences in adherence derived from individual data.
Figure 2.
Proportion of trial participants in the action-planning and control groups taking their tablet and capsule streams of study medication. Reported by day of study.
Footnote for Figure 2. Difference in adherence between the active and control groups, (P (trend) < 0.001)
Secondary analyses of the proportion of participants taking ≥80% of each medication stream over the full treatment period gave an adjusted odds ratio of 1.29 (0.90 to 1.84, p=0.16) for statin tablets and 1.38 (0.96 to 1.99, p=0.08) for omega-3 EE90 capsules, with 1.24 (0.81 to 1.89, p=0.32) for the statin intensification tablets. Pre-planned sub-group analyses showed no interactions between adherence and pre-defined participant subgroups.
Discussion
Summary
Although this trial provides no evidence for a clinically-relevant action-planning intervention impact, it does not exclude a small effect of such an approach on the extent to which people take their trial medication. The results suggest a consistent, trend with an estimated 2% and 3% increase in the proportion of study medication taken when comparing the action-planning with the control group. Additional analyses provide a consistent picture, with a significant difference in the proportion of participants taking >80% of their trial capsule medicines, and an overall analysis of the proportion of participants taking their initial statin tablet as the trial progresses showing a significant difference in trends as this proportion falls over time. This is a population likely to be more adherent to medication having been self-selected for participation in a clinical trial. Not withstanding the characteristics of the trial participants, this study raises the possibility of a small effect on adherence.
Strengths and limitations
This was a challenging intervention to implement within the framework of a randomised controlled trial where motivation to take study medication was likely to be high. Despite this, the average proportion of study medication taken was around 80% of that intended. Procedures were designed to be practical and consistent, with use of a standardised action-planning sheet to help ensure uniform intervention delivery, and the use of videos sent to practice nurses after participants had been recruited to provide training. However, objective evidence that this training translated into fidelity to use of the intervention by participants is not available. Electronic medication monitors were used to provide an objective measurement of adherence, which had additional advantages in carrying out subsequent exploratory analyses to establish response to medication.[10]
There were a number of practical difficulties encountered in embedding the cluster study within a randomised trial. For example, the total number of participants recruited (and determined by recruitment to the main AFORRD trial) was less than originally envisaged, so the trial did not have the planned power to identify differences of the size observed. Although electronic medication monitoring has the potential to affect adherence, other studies have not demonstrated that it has a clinically significant impact.[11] In addition, differing rates of recruitment to each practice after randomisation and in the eighteen weeks after recruitment of the first participant in each practice, led to an unintended unbalanced allocation of participants. As with any cluster-randomised study, it is not possible to fully exclude systematic differences between populations. However, the initial similarities in adherence observed over the first two weeks of the study before implementation of the intervention would suggest that groups were similar in their adherence behaviour.
Relationship to previous studies
This study was carried out in a population similar to those of motivated individuals receiving care for type 2 diabetes where adherence rates of 80% to 90% have been observed. [12] Previous intervention studies have often used imprecise measurement of adherence. Systematic reviews show mixed results to a wide range of interventions, targeted at both patients and health systems, to support adherence.[13] Multi-component and intensive interventions appear to be more effective, but the extent to which the success of interventions is dependent on specific characteristics of the setting is often unclear.
Interpretation and significance
Summary statistics that are estimated over an aggregate period of time provide only a limited account of trends in the implementation of the dosing regimen over time.[14] The effects of the action planning intervention on adherence noted here might not be sufficient to lead to changes in drug levels that would affect lipid levels achieved. Statins, for example, have a long duration of effect. However, interventions in this population that improve adherence are likely to be possible, but will be multi-component, utilising a number of strategies, including addressing motivation, providing feedback on medication use and supporting medication taking as a habitual behaviour. Action planning is a low-cost intervention that could be delivered at scale and thus might form a component of innovative approaches to supporting medication adherence. Modelling to establish the conditions under which these small effects on adherence might lead to a cost-effective intervention before carrying out further research would be helpful.
Conclusion
Interventions using low-intensity action-planning only are unlikely to have a clinically important impact on medication adherence. However, these findings do not exclude the possibility that self-completed action plans, supported by brief advice from clinical staff, could improve adherence to treatment as a component of a multifactorial intervention, particularly if delivered at scale and low cost.
Novelty Statement.
Clinical trial participants are highly motivated to take study medication, but may have problems in doing so if the medication burden is high.
Action plans are a promising approach for people who forget to take their medicines.
There have been no large-scale evaluations of this approach.
We did a large, cluster-randomised trial to evaluate the impact of an action planning based intervention on study medication adherence compared with a standard trial protocol.
We exclude a clinically important effect of action-planning intervention alone, but not a smaller effect that might contribute as part of a multi-factorial intervention.
Table 3.
Proportion of days covered (week 2-52) for all medication (or tablets and capsules separately) for pre-defined subgroups of the trial population adjusted for cluster effect.
| n | Intervention | Control | Diff (Int-Cont) | p value for interaction | ||
|---|---|---|---|---|---|---|
| Gender | Male | 384 | 79.7 (75.9 to 83.5) | 74.2 (70.4 to 77.9) | 3.0 (-1.9 to 8.0) | 0.98 |
| Female | 302 | 78.0 (73.1 to 82.9) | 75.5 (70.9 to 80.0) | 2.5 (-4.2 to 9.2) | ||
| Age | <= 63 | 341 | 78.8 (74.5 to 83.2) | 75.6 (71.5 to 79.8) | 3.2 (-2.8 to 9.2) | 0.66 |
| > 63 | 345 | 78.0 (73.3 to 81.0) | 77.0 (73.1 to 81.0) | 1.0 (-5.1 to 7.1) | ||
| HbA1c | <= 7 % | 422 | 78.9 (75.0 to 82.8) | 76.1 (72.8 to 79.4) | 1.1 (-4.2 to 6.1) | 0.29 |
| > 7 % | 264 | 78.4 (73.3 to 83.5) | 73.1 (68.1 to 78.1) | 5.3 (-1.8 to 12.4) | ||
| Duration | <= 3 years | 393 | 80.3 (76.5 to 84.1) | 76.4 (71.1 to 81.7) | 2.9 (-2.2 to 8.1) | 0.81 |
| > 3 years | 293 | 76.4 (71.1 to 81.7) | 74.6 (70.1 to 79.2) | 1.8 (-5.2 to 8.7) | ||
| BMI | <= 30 | 347 | 78.8 (74.8 to 82.8) | 75.4 (70.8 to 80.1) | 2.0 (-3.2 to 7.1) | 0.55 |
| > 30 | 339 | 78.8 (74.1 to 83.4) | 74.7 (70.3 to 79.1) | 4.1 (-2.4 to 10.6) | ||
| Education | No qualification | 361 | 76.7 (72.1 to 81.4) | 77.7 (73.4 to 81.8) | -0.9 (-7.2 to 5.3) | 0.28 |
| Any qualification | 325 | 80.3 (75.4 to 85.1) | 74.9 (70.4 to 79.5) | 5.3 (-1.3 to 11.9) |
Acknowledgements
This trial, sponsored by the University of Oxford, was funded by Pfizer Ltd. in partnership with the University of Oxford Diabetes Trials Unit. Aardex Ltd supplied the eMEMs devices. Pfizer provided atorvastatin study medication and matching placebo and Pronova Biopharma Norge AS provided omega-3 EE90 study medication and matching olive oil capsules. Steering Committee members, coordinating centre staff and clinical investigators are listed elsewhere.[8] RRH and AF are NIHR Senior Investigators.
Funding: Pfizer Ltd.
Sponsor: University of Oxford
Footnotes
Declarations of duality of interest.
RRH declares research funding from Bayer, Astra Zeneca, Merck and honoraria from Amgen, Bayer, Elcelyx, Jannsen, Intarcia, Merck, Novartis, and Novo Nordisk WH has done consultancy work for AbbVie Ltd. All other authors confirm that they have no dualities of interest.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas. 7th Edition. Brussels, Belgium: 2015. [Google Scholar]
- 2.Yach D, Stuckler D, Brownell K. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat Med. 2006;12:62–6. doi: 10.1038/nm0106-62. [DOI] [PubMed] [Google Scholar]
- 3.Colhoun H, Betteridge D, Durrington P, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–96. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 4.Barber N. Should we consider non-compliance a medical error? Qual Saf Health Care. 2002;11:81–4. doi: 10.1136/qhc.11.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheeran P, Orbell S. Implementation intentions and repeated behaviour: augmenting the predictive validity of the theory of planned behaviour. Eur J Soc Psychol. 1999;29:349–69. [Google Scholar]
- 6.Sheeran P, Orbell S. Using implementation intentions to increase attendance for cervical cancer screening. Health Psychol. 2000;19:283–9. doi: 10.1037//0278-6133.19.3.283. [DOI] [PubMed] [Google Scholar]
- 7.Rutter DR, Steadman L, Quine L. An implementation intentions intervention to increase uptake of mammography. Ann Behav Med. 2006;32:127–34. doi: 10.1207/s15324796abm3202_10. [DOI] [PubMed] [Google Scholar]
- 8.Holman RR, Paul S, Farmer A, et al. Atorvastatin in Factorial with Omega-3 EE90 Risk Reduction in Diabetes (AFORRD): a randomised controlled trial. Diabetologia. 2009;52:50–9. doi: 10.1007/s00125-008-1179-5. [DOI] [PubMed] [Google Scholar]
- 9.Vrijens B, Vincze G, Kristanto P, et al. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ. 2008;336:1114–7. doi: 10.1136/bmj.39553.670231.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neil HAWH, Ceglarek UU, Thiery JJ, et al. Impact of atorvastatin and omega-3 ethyl esters 90 on plasma plant sterol concentrations and cholesterol synthesis in type 2 diabetes: A randomised placebo controlled factorial trial. Atherosclerosis. 2010;213:6–6. doi: 10.1016/j.atherosclerosis.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Sutton S, Kinmonth AL, Hardeman W, et al. Does Electronic Monitoring Influence Adherence to Medication? Randomized Controlled Trial of Measurement Reactivity. Ann Behav Med. doi: 10.1007/s12160-014-9595-x. Published Online First: 27 February 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farmer AJ, Rodgers LR, Lonergan M, et al. Adherence to Oral Glucose–Lowering Therapies and Associations With 1-Year HbA 1c: A Retrospective Cohort Analysis in a Large Primary Care Database. Diabetes Care. 2015:dc151194–6. doi: 10.2337/dc15-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database SystRev. 2014;11:CD000011. doi: 10.1002/14651858.CD000011.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmac. 2012;73:691–705. doi: 10.1111/j.1365-2125.2012.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]