Abstract
Background
Increasing evidence shows cancer/testis antigens (CTAs) play a key role in oncogenesis. Our pre-study finds that MAGEA1, MAGEA10, MAGEB2, KK-LC-1, and CTAG1A/B have high expression frequencies at the protein level. We aim to explore their prognostic role and correlations with clinical characteristics in resected lung cancer at the mRNA level.
Methods
Thirty-eight surgical lung cancer samples were included. Validation study was performed based on The Cancer Genome Atlas database. The prognostic roles of CTAs were evaluated by Kaplan–Meier and multivariate analysis.
Results
High expression of MAGEA1 (16.7% vs 65.0%, P=0.004), MAGEA10 (61.1% vs 95.0%, P=0.016), MAGEB2 (55.6% vs 95.0%, P=0.007), and KK-LC-1 (16.7% vs 55.0%, P=0.020) was closely correlated with lymph node metastasis at diagnosis. Patients with TNM stage II or III had a higher expression of MAGEA10 (57.1% vs 91.7%, P=0.034) and KK-LC-1 (14.3% vs 50.0%, P=0.039) compared with patients in TNM stage I. High CTAG1A/B expression showed unfavorable prognosis in all cases (P<0.05). Subgroup analysis showed high CTAG1A/B expression was a negative prognostic factor of survival (P=0.031) in patients with TNM stage II or III. Although no statistical significance was reached, high CTAG1A/B also showed a similar prognostic trend in lung adenocarcinoma (ADC) and squamous cell carcinoma. The Cancer Genome Atlas database showed the negative prognostic role of CTAG1A/B was mainly induced by CTAG1B (NY-ESO-1, P=0.047) and high CTAG1B expression (hazard ratio =2.733, 95% CI: 1.348–5.541, P=0.005) was an independent negative prognostic factor of lung ADC.
Conclusion
CTAs represent potential candidate targets for immunotherapy and their expression was closely correlated with tumor stage. High CTAG1B expression was an independent negative prognostic factor of lung ADC.
Keywords: lung cancer, cancer/testis antigens, immunotherapy, prognosis
Introduction
With the aging and growth of population as well as the prevalence of established risk factors, cancer incidence and mortality have been increasing.1 GLOBOCAN 2012 data shows lung cancer remains the first leading cause of cancer-related death in male patients worldwide. In female patients of more developed countries, it has even surpassed breast cancer to be the leading cause of cancer death.1 There is no doubt that cancer will create a giant burden on societies in the following decades.
In a recent study, it is found that adjuvant gefitinib can significantly prolong the disease-free survival (28.7 vs 18.0 months, P=0.0054) of resected stage II–III EGFR-mutant non-small-cell lung cancer compared with traditional chemotherapy. Its impact on overall survival (OS) need to be further studied because the OS data are not yet mature.2 Studies involving Nivolumab (PD-1 inhibitor) have achieved great success and the results show that Nivolumab can significantly improve the OS of lung cancer patients with a favorable safety profile.3–5 Lung cancer treatment has become more and more standardized and patients may have more chances of being treated with variable treatment strategies. However, the prognosis of lung cancer remains unsatisfactory, with a 5-year survival rate of <10%.6 Treatment strategies with high efficiency and low toxicity are urgently needed.
Immunotherapy has been an active area in cancer research in recent years. However, the identification of ideal targets is the main point restricting the study of immunotherapy. Cancer/testis antigens (CTAs) are considered ideal targets for cancer treatment due to their high immunogenic, restricted expression in germ cells and malignancies.7–9 Generally, germ cells do not express human leukocyte antigen (HLA) class I molecules, leading them escape from the attack of T cells. Though somatic tissues, such as thyroid, breast, and retina may have the expression of CTAs, their expression is far below that in testis tissue and their function remains to be discovered. CTA-induced immunotherapy can specifically identify and kill cancer cells expressing corresponding CTAs, while they have limited toxicities on patients at the same time.10–12
One important point needing to be considered when selecting ideal targets for immunotherapy is the expression frequency of CTAs. Our previous study (unpublished data) shows that MAGEA1, MAGEA10, MAGEB2, KK-LC-1, and CTAG1A/B have a high expression rate in lung cancer samples. Increasing evidence has demonstrated that CTAs play important roles in oncogenesis, including sustained growth, angiogenesis, evading apoptosis, invasion, metastasis, and so on. MAGEA, MAGEB, and MAGEC families have been demonstrated to promote cancer cells survival and inhibit apoptosis through the binding and regulation of tumor suppressor p53.13 CTAs, including CTAG1B (NY-ESO-1), SSX, and so on also play a key role in tumor invasion and metastasis.14 MAGEA1 and CTAG1B show higher expression frequencies in metastatic melanoma compared with primary tumor.15,16 KK-LC-1 is a novel CTA that was first identified in human lung cancer, and is also expressed in a wide range of malignancies.17 CTAs expression is heterogeneous and their expression has significant regional and racial distinctions.9,18–23 In this study, we aim to analyze the expression rate of the aforementioned 5 CTAs and explore their relationship with clinical features in resected lung cancer patients of China.
Patients and methods
Patients and specimens
This study used 38 lung cancer samples for real-time polymerase chain reaction (RT-PCR). All patients had pathologically confirmed lung cancer and the tissues were surgically obtained at our hospital between May 2013 and August 2013. Our study was approved by the Ethics Committee of Harbin Medical University Cancer Hospital and written informed consent was obtained from each patient. The tumor stage was classified based on the eighth edition of lung cancer stage classification.24
Quantitative real time-PCR
Five relative high expressed CTAs (MAGEA1, MAGEA10, MAGEB2, KK-LC-1, and CTAG1A/B) in our pre-study were selected to examine their expression at mRNA level. Total RNA from lung cancer frozen tissue specimens was extracted using TRIZOL reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Complementary DNA was synthesized using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) in a final reaction volume of 20 μL. RT-PCR was performed using SYBR Green Master Mix (Roche Applied Science, Mannheim, Germany) and QuantStudio® 3 Thermo Fisher (Applied Biosystems). β-actin was used as the internal control. The primers were synthesized by Oligo-fectamine (Invitrogen, San Diego, CA, USA). There were no nonspecific amplification products observed in any of the reactions based on the analysis of the dissociation curves. Each RT-PCR experiment was repeated 3 times. Ct represents the threshold cycle. The data were normalized to the β-actin expression levels, which was named ∆Ct value. The sample with the largest MAGEA1, MAGEA10, MAGEB2, KK-LC-1, and CTAG1A/B ∆Ct was used as control and the relative gene expression levels were calculated using the comparative Ct (∆∆Ct) method. The relative expression was calculated as 2−∆∆Ct. High or low CTAs expression was determined by a receiver operating characteristic (ROC) curve analysis according to the lymph node metastasis status at diagnosis (patients with lymph node metastasis at diagnosis were registered as 1, otherwise 0). Youden index was calculated as (sensitivity + specificity −1). The optimal cut-off value was determined as CTA expression with the largest Youden index. High expression was determined as CTA expression larger than the optimal cut-off value, and low expression as CTA expression lower than the optimal cut-off value. The primer sets are seen in Table 1.
Table 1.
Polymerase chain reaction amplification programs
| Gene | Direction | Primers from 5′ to 3′ |
|---|---|---|
| MAGEA1 | Forward | ATCAACTTCACTCGACAGAGGC |
| Reverse | CGGAACAAGGACTCCAGGATAC | |
| MAGEA10 | Forward | GTCCAAGCACCCTACAGGTC |
| Reverse | TTCTGCCTTTGTGATCGGCT | |
| MAGEB2 | Forward | GAGCCAGAGTTGTAGCCAGG |
| Reverse | AGACCTCATTGGGGGTGAGA | |
| KKLC1 | Forward | ACTGCTTCCCAACTACCAGC |
| Reverse | ACACAGAATGCTGCTCGCTA | |
| CTAG1A/B | Forward | TGTCCGGCAACATACTGACT |
| Reverse | ACTGCGTGATCCACATCAAC | |
| β-actin | Forward | CAGAAGGATTCCTATGTGG |
| Reverse | CATGATCTGGGTCATCTTC |
The Cancer Genome Atlas (TCGA) data
RNA-seq data of these CTAs for lung cancer and corresponding clinical information were downloaded from the TCGA database. Patients with missing clinicopathological data, including age, gender, tumor stage, OS, and survival status were excluded. High and low CTAs (MAGEA1, MAGEA10, MAGEB2, and KK-LC-1) expression was also determined by ROC curve analysis used previously. However, in total cases, only few patients in TCGA database expressed CTAG1A (n=106) and CTAG1B (n=43), and the relative expression levels were low, especially in lung squamous cell carcinoma (SCC) patients. Patients with CTAG1A and CTAG1B relative expression levels >0 were registered as high expression, whilst those with levels ≤0 were registered as low expression.
Statistical analysis
The OS time was defined from diagnosis to death or the last follow-up if the patients were still alive. The survival curves and 95% CIs were obtained using the Kaplan–Meier method, and the survival in different groups was compared by log-rank tests. The relationships between CTAs and clinicopathological features were assessed by the chi-squared test. The univariate analysis was used to evaluate the potential prognostic role of variables. The prognostic factors with P-values <0.05 in the univariate analysis were examined in the multivariate analysis. SPSS 16.0 statistical software was used for statistical analysis. All P-values <0.05 were considered statistically significant.
Results
Patient characteristics
The patient characteristics are listed in Table 2. The mean age for all cases was 56.53±7.76 years (range 32–71). Most patients were male (68.4%, n=26) with an age of <60 years at diagnosis (63.2%, n=24). Over half of the patients (68.4%, n=26) had a smoking history. Twenty patients (52.6%) had lymph node metastasis at diagnosis. The TNM stage for all patients was listed as stage I (36.8%, n=14), II (31.6%, n=12), and III (31.6%, n=12). The main pathological types were adenocarcinoma (ADC, 50%, n=19), SCC (34.2%, n=13), and others (15.8%, n=6). There were 16 (42.1%) and 3 (7.9%) patients receiving the treatment of postoperative adjuvant chemotherapy and radiotherapy, respectively.
Table 2.
Patient characteristics
| Variables | n | % |
|---|---|---|
| Gender | ||
| Male | 26 | 68.4 |
| Female | 12 | 31.6 |
| Age (years) | ||
| <60 | 24 | 63.2 |
| ≥60 | 14 | 36.8 |
| Smoking | ||
| Never | 12 | 31.6 |
| Ever | 26 | 68.4 |
| T stage | ||
| T1 or 2 | 32 | 84.2 |
| T3 or 4 | 6 | 15.8 |
| N stage | ||
| N0 | 18 | 47.4 |
| N1 or 2 | 20 | 52.6 |
| TNM | ||
| I | 14 | 36.8 |
| II or III | 24 | 63.2 |
| Pathology | ||
| ADC | 19 | 50.0 |
| SCC | 13 | 34.2 |
| Others | 6 | 15.8 |
| Chemotherapy | ||
| Yes | 16 | 42.1 |
| No | 22 | 57.9 |
| Radiotherapy | ||
| Yes | 3 | 7.9 |
| No | 35 | 92.1 |
Abbreviations: ADC, adenocarcinoma; SCC, squamous cell carcinoma.
CTAs expression
The optimal cut-off value for 5 CTAs based on ROC analysis result was as follows: 2,391.75 for MAGEA1 (AUC=0.739; 95% CI: 0.580–0.897; P=0.012), 27.64 for MAGEA10 (AUC=0.667; 95% CI: 0.492–0.841; P=0.079), 16.11 for MAGEB2 (AUC=0.694; 95% CI: 0.524–0.895; P=0.041), 6,199.82 for KK-LC-1 (AUC=0.667; 95% CI: 0.492–0.841; P=0.079), and 178.85 for CTAG1A/B (AUC=0.594; 95% CI: 0.409–0.780; P=0.320), respectively. High expression of MAGEA1, MAGEA10, MAGEB2, KK-LC-1, and CTAG1A/B was found in 16 (42.1%), 30 (79.0%), 29 (76.3%), 14 (36.8%), and 26 (68.4%) cases, respectively.
The relationships between CTAs expression and patient characteristics
Clinicopathological features, including gender, age, smoking history, pathological T stage, pathological N stage, TNM stage, and pathological types were chosen to compare their relationship with CTAs expression. High expression of MAGEA1 (N0 vs N1–2, 16.7% vs 65.0%, P=0.004), MAGEA10 (N0 vs N1–2, 61.1% vs 95.0%, P=0.016), MAGEB2 (N0 vs N1–2, 55.6% vs 95.0%, P=0.007), and KK-LC-1 (N0 vs N1–2, 16.7% vs 55.0%, P=0.020) was closely correlated with lymph node metastasis at diagnosis. Although the difference was not statistically significant, CTAG1A/B expression also had a similar trend (N0 vs N1–2, 55.6% vs 80.0%, P=0.164). High CTAs expression was seen in patients with pathological N1–2 stage. Additionally, high MAGEA10 (I vs II + III, 57.1% vs 91.7%, P=0.034) and KK-LC-1 (I vs II + III, 14.3% vs 50.0%, P=0.039) expression were associated with advanced TNM stage (Table 3). Although no statistical significance was found in the rest 3 CTAs, they all had a similar trend that CTAs had a higher expression rate in patients with advanced stage. In addition, patients with age ≥60 years had a higher CTAG1A/B expression rate compared with patients with age <60 years (54.2% vs 92.9%, P=0.027). There was no significant difference found between CTAs expression and gender, smoking history, pathological T stage, and pathological types.
Table 3.
Correlation between CTAs expression at mRNA level and clinicopathological features
| Variables |
MAGEA1
|
MAGEA10
|
MAGEB2
|
KK-LC-1
|
CTAG1A/B
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High
|
Low
|
P-valuea | High
|
Low
|
P-value | High
|
Low
|
P-value | High
|
Low
|
P-value | High
|
Low
|
P-value | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||||||
| Gender | 0.289 | 0.689 | 1.000 | 0.296 | 1.000 | ||||||||||
| Male | 9 (34.6) | 17 (65.4) | 21 (80.8) | 5 (19.2) | 20 (76.9) | 6 (23.1) | 8 (30.8) | 18 (69.2) | 18 (69.2) | 8 (30.8) | |||||
| Female | 7 (58.3) | 5 (41.7) | 9 (75.0) | 3 (25.0) | 9 (75.0) | 3 (25.0) | 6 (50.0) | 6 (50.0) | 8 (66.7) | 4 (33.3) | |||||
| Age (years) | 0.187 | 0.216 | 0.115 | 0.298 | 0.027 | ||||||||||
| <60 | 8 (33.3) | 16 (66.7) | 17 (70.8) | 7 (29.2) | 16 (66.7) | 8 (33.3) | 7 (29.20) | 17 (70.8) | 13 (54.2) | 11 (45.8) | |||||
| ≥60 | 8 (57.1) | 6 (42.9) | 13 (92.9) | 1 (7.1) | 13 (92.9) | 1 (7.1) | 7 (50.0) | 7 (50.0) | 13 (92.9) | 1 (7.1) | |||||
| Smoking | 0.713 | 0.411 | 0.673 | 1.000 | 0.694 | ||||||||||
| Never | 5 (50.0) | 5 (50.0) | 7 (70.0) | 3 (30.0) | 7 (70.0) | 3 (30.0) | 4 (40.0) | 6 (60.0) | 6 (60.0) | 4 (40.0) | |||||
| Ever | 11 (39.3) | 17 (60.7) | 23 (82.1) | 5 (17.9) | 22 (78.6) | 6 (21.4) | 10 (35.7) | 18 (64.3) | 20 (71.4) | 8 (28.6) | |||||
| T stage | 1.000 | 1.000 | 0.613 | 0.383 | 0.357 | ||||||||||
| T1–2 | 14 (43.8) | 18 (56.2) | 25 (78.1) | 7 (21.9) | 25 (78.1) | 7 (21.9) | 13 (40.6) | 19 (59.4) | 23 (71.9) | 9 (28.1) | |||||
| T3–4 | 2 (33.3) | 4 (66.7) | 5 (83.3) | 1 (16.7) | 4 (66.7) | 2 (33.3) | 1 (16.7) | 5 (83.3) | 3 (50.0) | 3 (50.0) | |||||
| N stage | 0.004 | 0.016 | 0.007 | 0.020 | 0.164 | ||||||||||
| N0 | 3 (16.7) | 15 (83.3) | 11 (61.1) | 7 (38.9) | 10 (55.6) | 8 (44.4) | 3 (16.7) | 15 (83.3) | 10 (55.6) | 8 (44.4) | |||||
| N1–2 | 13 (65.0) | 7 (35.0) | 19 (95.0) | 1 (5.0) | 19 (95.0) | 1 (5.0) | 11 (55.0) | 9 (45.0) | 16 (80.0) | 4 (20.0) | |||||
| TNM | 0.088 | 0.034 | 0.052 | 0.039 | 0.296 | ||||||||||
| I | 3 (21.4) | 11 (78.6) | 8 (57.1) | 6 (42.9) | 8 (57.1) | 6 (42.9) | 2 (14.3) | 12 (85.7) | 8 (57.1) | 6 (42.9) | |||||
| II + III | 13 (45.8) | 11 (54.2) | 22 (91.7) | 2 (8.3) | 21 (87.5) | 3 (12.5) | 12 (50.0) | 12 (50.0) | 18 (75.0) | 6 (25.0) | |||||
| Pathology | 0.743 | 1.000 | 1.000 | 1.000 | 0.728 | ||||||||||
| ADC | 7 (40.0) | 12 (60.0) | 15 (78.9) | 4 (21.1) | 14 (73.7) | 5 (26.3) | 7 (36.8) | 12 (63.2) | 12 (63.2) | 7 (36.8) | |||||
| None | 9 (44.4) | 10 (55.6) | 15 (78.9) | 4 (21.1) | 15 (78.9) | 4 (21.1) | 7 (36.8) | 12 (63.2) | 14 (73.7) | 5 (26.3) | |||||
| ADC | |||||||||||||||
Note:
P-value was calculated by Fisher’s exact test.
Abbreviations: ADC, adenocarcinoma; CTAs, cancer/testis antigens.
CTAs expression and OS
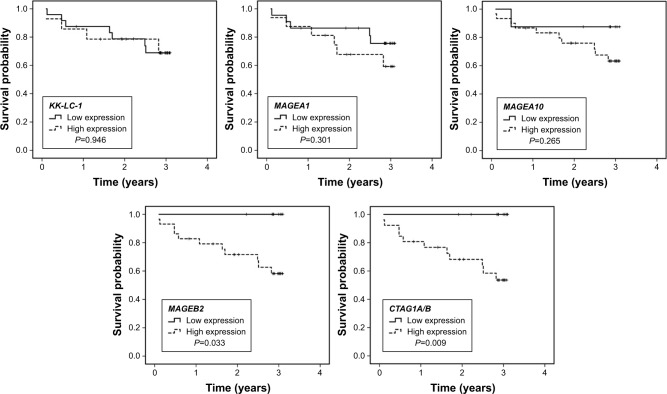
The prognostic roles of all variables in Table 2 and 5 CTAs expression were tested to evaluate their potential prognostic values. The 3 years survival rate for patients with high and low MAGEB2 expression was 58.2% and 100.0% (P=0.033), respectively. The 3 years survival rate for patients with high and low CTAG1A/B expression was 53.6% and 100.0% (P=0.009), respectively. High expression of MAGEB2 and CTAG1A/B were correlated with unfavorable prognosis (Figure 1). The 3 years survival rates of patients with high MAGEA1, MAGEA10, and KK-LC-1 expressions were 59.2%, 63.3%, and 68.8%, compared with 75.6%, 87.5%, and 68.9% in patients with low corresponding CTAs expression. The expression of MAGEA1, MAGEA10, and KK-LC-1 did not have significant association with the prognosis (P>0.05). In addition, N stage (P=0.023) and TNM stage (P=0.026) were also closely correlated with OS on univariate analysis. No significant survival difference was found in age, gender, smoking history, T stage, pathological types, chemotherapy, and radiotherapy. No variables held statistical significance on multivariate analysis (P>0.05). Our results showed that MAGEB2 and CTAG1A/B expression was associated with the prognosis of resected lung cancer patients, but they were not independent prognostic factors.
Figure 1.
Overall survival curves of all patients according to CTAs expression.
Note: High MAGEB2 and CTAG1A/B expression showed poorer prognosis than low expression (P<0.05), while the expression of the other 3 CTAs showed no significant correlations with survival (P>0.05).
Abbreviation: CTAs, cancer/testis antigens.
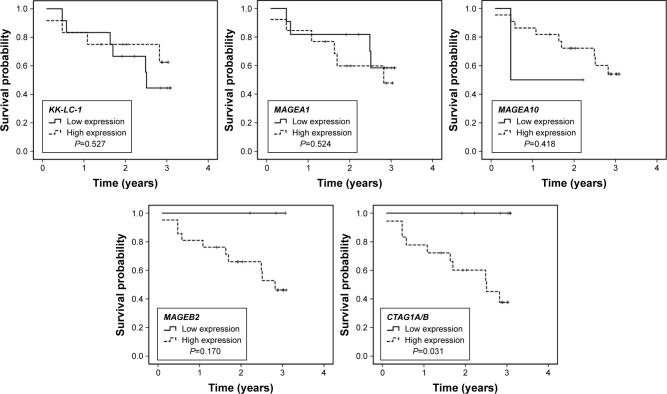
Subgroup analysis of CTAs expression and OS
Given CTAs showed a significant higher expression in patients with stage II and III, subgroup analysis based on TNM stage was performed to assess the potential prognostic roles of CTAs expression on selected patients. The variables evaluated were just the same with the analysis for all cases. When the analysis was limited to patients with stage II and III, only high expression of CTAG1A/B also had a negative impact on survival (Figure 2, P=0.031).
Figure 2.
Overall survival curve of patients with pathological stage II and III according to CTAs expression.
Note: In patients with stage II and III, high expression of CTAG1A/B showed poorer prognosis than low expression (P=0.031).
Abbreviation: CTAs, cancer/testis antigens.
Subgroup analysis based on variable pathological types showed that only pathological N stage (P=0.019) and TNM stage (P=0.038) were closely associated with the prognosis of ADC patients, while no clinicopathological characteristics were associated with the prognosis of non-ADC patients (P>0.05). Although no statistical significance was found between CTAs expression and OS of lung ADC and non-ADC patients, high CTAG1A/B expression also had a similar prognostic trend in ADC (P=0.052) and non-ADC cases (P=0.100).
Validation of CTAs expression in TCGA database
A total of 962 cases (479 ADC and 483 SCC) in TCGA database were enrolled to validate the aforementioned results in our study. The potential prognostic parameters included pathological types (ADC vs SCC), age (<60 vs ≥60 years), gender (male vs female), tumor stage (I + II vs III + IV), T stage (T1–2 vs T3–4), N stage (N0 vs N1–3) and CTAs expression. For total cases, only tumor stage (P<0.001) T (P<0.001) and N (P<0.001) were closely associated with OS on univariate analysis, while no CTAs expression was correlated with OS (P>0.05). When all parameters were evaluated in multivariate analysis, only T stage (hazard ratio [HR]=1.525, 95% CI: 1.131–2.057, P=0.006) and N stage (HR=1.438, 95% CI: 1.131–1.828, P=0.003) held statistical significance.
Subgroup analysis was also performed based on variable pathological types. In ADC patients, tumor stage (P<0.001), T stage (P<0.001), N stage (P<0.001), and CTAG1B (P=0.047) were closely associated with the prognosis of patients on univariate analysis (Figure 3). When all parameters were evaluated in multivariate analysis, MAGEA1 (HR=1.487, 95% CI: 1.006–2.197, P=0.046), CTAG1B (HR=2.733, 95% CI: 1.348–5.541, P=0.005), T stage (HR=1.820, 95% CI: 1.170–2.829, P=0.008), and N stage (HR=2.246, 95% CI: 1.564–3.224, P<0.001) were all found to be independent prognostic factors of lung ADC patients. In SCC patients, only tumor stage (P=0.012) and T stage (P=0.004) were closely associated with the prognosis of patients, and no parameters were found to have statistical significance on multivariate analysis.
Figure 3.
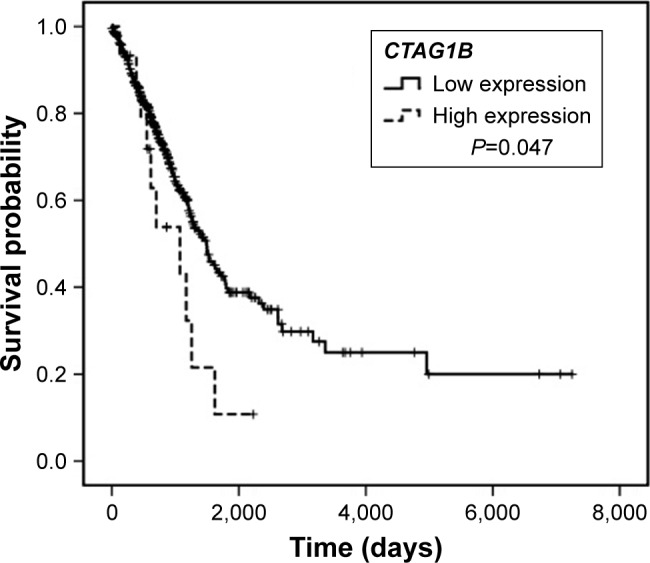
Negative prognostic role of CTAG1B in lung adenocarcinoma demonstrated by The Cancer Genome Atlas database.
Discussion
In our study, we found that high expression rate of these 5 CTAs ranged 36.8%–79.0%. CTAs expression at mRNA level was closely associated with pathological N and TNM stages. Patients with stage II–III and lymph node metastasis had a higher CTAs expression rate compared with patients with stage I and no lymph node metastasis. In addition, CTAG1A/B expression had a negative impact on OS of resected lung cancer patients. TCGA database analysis showed that the negative prognostic role of CTAG1A/B was mainly induced by CTAG1B, and high CTAG1B expression was an independent prognostic factor of lung ADC cases.
More and more attention has been paid to the study of immunotherapy and targeted therapy due to the limited benefits of conventional chemotherapy and the associated toxicities.2 CTAs represent potential candidates for the management of cancers due to their restricted expression in cancer and immune-privileged tissues, such as testis and placenta. These immune-privileged tissues cannot present CTAs because of the lacking expression of HLA class I molecules, and this leads to the escape of effector T cell attack. Previous studies have demonstrated the anti-tumor effect of immunotherapy.10–12,25 Immunotherapy has 2 significant benefits. On the one hand, if immunotherapy shows great advantages over conventional therapies, patients can benefit more from treatment with immunotherapy. On the other hand, if immunotherapy has as much effect as conventional therapies, that is also good for patients because the toxicities of immunotherapy are usually less. Both the conditions have significant effect on cancer patients.
One notable characteristic of CTAs is their heterogeneity in variable malignancies. Lung cancer belongs to CTA-rich cancers due to its high frequency of CTAs expression. The frequency of 5 tested CTAs in our study varies largely, ranging 36.8%–79.0%. Regional and racial difference may influence CTAs expression. The KK-LC-1 (36.8% vs 33.0%) and MAGEA1 (42.1% vs 31.0%) expression is similar with previous studies, while the MAGEA10 (79.0% vs 28.0%) expression varies largely with previous studies.22,26 In our study, MAGEB2 and CTAG1A/B expression frequency represents 76.3% and 68.4%, which is higher than that in TCGA database. The majority of patients involved in TCGA database were White (n=722), Black and African American (n=79), American Indian (n=1), and unknown origins (n=145), while only 15 cases were of Asian origin. The TCGA database shows that CTAG1A (13.3% vs 11.0%) and CTAG1B (6.7% vs 4.4%) expression in Asians was higher than non-Asian race. All patients enrolled in our study were of Asian origin, and CTAs expression heterogeneity was significant in variable race.
In our study, CTAs expression was closely associated with pathological lymph node metastasis and advanced tumor stage. Patients with stage II and III show a higher CTAs expression compared with patients with stage I, and this is similar with previous studies.22,27 We also find that patients with lymph node metastasis have a higher CTAs expression frequency compared with patients without lymph node metastasis. A previous study demonstrates that MAGEC1 and MAGEC2 expression can independently predict lymph node metastasis.28 More lymph node metastases are found in patients with positive expression of MAGEC1 and MAGEC2, which is in favor of our results.
Cancer stem cells (CSCs) play an important role in cancer invasion and metastasis.29 Previous studies have demonstrated that several CTAs involving MAGEB2 and NY-ESO-1 (CTAG1B) show preferential expression in CSCs compared with non-CSCs.30 They can be regarded as cancer/testis/stem genes, which are closely associated with the progress of tumor migration, invasion, and the formation of secondary metastatic lesions.14,31,32 In our study, the results show that MAGEB2 and CTAG1A/B are closely correlated with the prognosis of resected lung cancer patients. In subgroup analysis, CTAG1A/B expression still shows unfavorable prognosis in patients with stage II and III. This can be partly interpreted by the theory mentioned previously. The TCGA database analysis shows that the negative prognostic role of CTAG1A/B is mainly induced by CTAG1B, and high CTAG1B expression is an independent negative prognostic factor of lung ADC patients. Unfortunately, the role of MAGEB2 is not confirmed by TCGA database analysis.
Our study aims at exploring CTAs expression frequency at mRNA level in resected lung cancer and analyzes their relationship with clinicopathological characteristics. There are some limitations of our study. First, the surgical samples in our study are only 38 and the results need to be demonstrated in larger samples. Second, we do not assess the CTAs expression at protein level due to the lack of enough samples. In addition, there may be some limitations present due to the retrospective features of our study.
Conclusion
In summary, high MAGEA1, MAGEA10, MAGEB2, KK-LC-1, and CTAG1A/B expression at mRNA level range is 36.8%–79.0%. CTAs represent potential candidates for cancer immunotherapy and their expression is closely associated with pathological lymph node metastasis and tumor stage. High CTAG1A/B expression is a negative prognostic factor in resected lung cancer patients, and its negative role is mainly induced by CTAG1B based on TCGA database analysis. High CTAG1B expression is an independent negative prognostic factor of lung ADC patients.
Acknowledgments
We thank Ke Pan and Cassian Yee (Departments of Melanoma Medical Oncology and Immunology, MD Anderson Cancer Center, Houston, Texas) for providing direction to this project.
This work was supported by the National Natural Scientific Foundation of China (numbers 81673007, 81572824 and 81773133) and the Postdoctoral Scientific Research Developmental Fund of Heilongjiang Province (LBH-Q16155).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II–IIIA (N1–N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol. 2018;19(1):139–148. doi: 10.1016/S1470-2045(17)30729-5. [DOI] [PubMed] [Google Scholar]
- 3.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horn L, Spigel DR, Vokes EE. Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057) J Clin Oncol. 2017;35(35):3924–3933. doi: 10.1200/JCO.2017.74.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 7.Nakagawa K, Noguchi Y, Uenaka A, et al. XAGE-1 expression in non-small cell lung cancer and antibody response in patients. Clin Cancer Res. 2005;11(15):5496–5503. doi: 10.1158/1078-0432.CCR-05-0216. [DOI] [PubMed] [Google Scholar]
- 8.Curigliano G, Viale G, Ghioni M, et al. Cancer-testis antigen expression in triple-negative breast cancer. Ann Oncol. 2011;22(1):98–103. doi: 10.1093/annonc/mdq325. [DOI] [PubMed] [Google Scholar]
- 9.Kim J, Reber HA, Hines OJ, et al. The clinical significance of MAGEA3 expression in pancreatic cancer. Int J Cancer. 2006;118(9):2269–2275. doi: 10.1002/ijc.21656. [DOI] [PubMed] [Google Scholar]
- 10.Yee C, Thompson JA, Byrd D, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99(25):16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapuis AG, Thompson JA, Margolin KA, et al. Transferred melanoma-specific CD8+ T cells persist, mediate tumor regression, and acquire central memory phenotype. Proc Natl Acad Sci U S A. 2012;109(12):4592–4597. doi: 10.1073/pnas.1113748109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapuis AG, Ragnarsson GB, Nguyen HN, et al. Transferred WT1-reactive CD8+ T cells can mediate antileukemic activity and persist in post-transplant patients. Sci Transl Med. 2013;5(174):174ra27. doi: 10.1126/scitranslmed.3004916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang B, O’Herrin SM, Wu J, et al. MAGE-A, mMage-b, and MAGE-C proteins form complexes with KAP1 and suppress p53-dependent apoptosis in MAGE-positive cell lines. Cancer Res. 2007;67(20):9954–9962. doi: 10.1158/0008-5472.CAN-07-1478. [DOI] [PubMed] [Google Scholar]
- 14.Whitehurst AW. Cause and consequence of cancer/testis antigen activation in cancer. Annu Rev Pharmacol Toxicol. 2014;54:251–272. doi: 10.1146/annurev-pharmtox-011112-140326. [DOI] [PubMed] [Google Scholar]
- 15.Barrow C, Browning J, Macgregor D, et al. Tumor antigen expression in melanoma varies according to antigen and stage. Clin Cancer Res. 2006;12(3 Pt 1):764–771. doi: 10.1158/1078-0432.CCR-05-1544. [DOI] [PubMed] [Google Scholar]
- 16.Velazquez EF, Jungbluth AA, Yancovitz M, et al. Expression of the cancer/testis antigen NY-ESO-1 in primary and metastatic malignant melanoma (MM)-correlation with prognostic factors. Cancer Immun. 2007;7:11. [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuyama T, Hanagiri T, Takenoyama M, et al. Identification of a new cancer/germline gene, KK-LC-1, encoding an antigen recognized by autologous CTL induced on human lung adenocarcinoma. Cancer Res. 2006;66(9):4922–4928. doi: 10.1158/0008-5472.CAN-05-3840. [DOI] [PubMed] [Google Scholar]
- 18.Ohue Y, Kurose K, Mizote Y, et al. Prolongation of overall survival in advanced lung adenocarcinoma patients with the XAGE1 (GAGED2a) antibody. Clin Cancer Res. 2014;20(19):5052–5063. doi: 10.1158/1078-0432.CCR-14-0742. [DOI] [PubMed] [Google Scholar]
- 19.Ayyoub M, Scarlata CM, Hamaï A, Pignon P, Valmori D. Expression of MAGE-A3/6 in primary breast cancer is associated with hormone receptor negative status, high histologic grade, and poor survival. J Immunother. 2014;37(2):73–76. doi: 10.1097/CJI.0000000000000013. [DOI] [PubMed] [Google Scholar]
- 20.Gure AO, Chua R, Williamson B, et al. Cancer-testis genes are coordinately expressed and are markers of poor outcome in non-small cell lung cancer. Clin Cancer Res. 2005;11(22):8055–8062. doi: 10.1158/1078-0432.CCR-05-1203. [DOI] [PubMed] [Google Scholar]
- 21.Laban S, Atanackovic D, Luetkens T, et al. Simultaneous cytoplasmic and nuclear protein expression of melanoma antigen-A family and NY-ESO-1 cancer-testis antigens represents an independent marker for poor survival in head and neck cancer. Int J Cancer. 2014;135(5):1142–1152. doi: 10.1002/ijc.28752. [DOI] [PubMed] [Google Scholar]
- 22.Shigematsu Y, Hanagiri T, Shiota H, et al. Clinical significance of cancer/testis antigens expression in patients with non-small cell lung cancer. Lung Cancer. 2010;68(1):105–110. doi: 10.1016/j.lungcan.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Scanlan MJ, Simpson AJ, Old LJ. The cancer/testis genes: review, standardization, and commentary. Cancer Immun. 2004;4:1. [PubMed] [Google Scholar]
- 24.Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The Eighth Edition Lung Cancer Stage Classification. Chest. 2017;151(1):193–203. doi: 10.1016/j.chest.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Atanackovic D, Arfsten J, Cao Y, et al. Cancer-testis antigens are commonly expressed in multiple myeloma and induce systemic immunity following allogeneic stem cell transplantation. Blood. 2007;109(3):1103–1112. doi: 10.1182/blood-2006-04-014480. [DOI] [PubMed] [Google Scholar]
- 26.Tajima K, Obata Y, Tamaki H, et al. Expression of cancer/testis (CT) antigens in lung cancer. Lung Cancer. 2003;42(1):23–33. doi: 10.1016/s0169-5002(03)00244-7. [DOI] [PubMed] [Google Scholar]
- 27.Salmaninejad A, Zamani MR, Pourvahedi M, Golchehre Z, Hosseini Bereshneh A, Rezaei N. Cancer/Testis Antigens: Expression, Regulation, Tumor Invasion, and Use in Immunotherapy of Cancers. Immunol Invest. 2016;45(7):619–640. doi: 10.1080/08820139.2016.1197241. [DOI] [PubMed] [Google Scholar]
- 28.Curioni-Fontecedro A, Nuber N, Mihic-Probst D, et al. Expression of MAGE-C1/CT7 and MAGE-C2/CT10 predicts lymph node metastasis in melanoma patients. PLoS One. 2011;6(6):e21418. doi: 10.1371/journal.pone.0021418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fazilaty H, Gardaneh M, Bahrami T, Salmaninejad A, Behnam B. Cross-talk between breast cancer stem cells and metastatic niche: emerging molecular metastasis pathway? Tumour Biol. 2013;34(4):2019–2030. doi: 10.1007/s13277-013-0831-y. [DOI] [PubMed] [Google Scholar]
- 30.Cronwright G, Le Blanc K, Götherström C, Darcy P, Ehnman M, Brodin B. Cancer/testis antigen expression in human mesenchymal stem cells: down-regulation of SSX impairs cell migration and matrix metalloproteinase 2 expression. Cancer Res. 2005;65(6):2207–2215. doi: 10.1158/0008-5472.CAN-04-1882. [DOI] [PubMed] [Google Scholar]
- 31.Ghafouri-Fard S. Expression of cancer-testis antigens in stem cells: is it a potential drawback or an advantage in cancer immunotherapy. Asian Pac J Cancer Prev. 2015;16(7):3079–3081. doi: 10.7314/apjcp.2015.16.7.3079. [DOI] [PubMed] [Google Scholar]
- 32.Yamada R, Takahashi A, Torigoe T, et al. Preferential expression of cancer/testis genes in cancer stem-like cells: proposal of a novel sub-category, cancer/testis/stem gene. Tissue Antigens. 2013;81(6):428–434. doi: 10.1111/tan.12113. [DOI] [PubMed] [Google Scholar]