Abstract
Noninvasive serum biomarkers (nonalcoholic fatty liver disease fibrosis score [NFS], fibrosis 4 score [FIB‐4], or enhanced liver fibrosis [ELF] test) are recommended as first‐line tools to determine the risk of advanced fibrosis in nonalcoholic fatty liver disease. We aimed to assess the utility of a pragmatic approach to screening for clinically significant fibrosis in primary care and diabetes clinics. We recruited 252 patients from an endocrine clinic or primary care facility. Anthropometric measurements, ELF test, ultrasound, and liver stiffness measurements (LSMs) were performed. Clinically significant fibrosis was defined as LSM ≥8.2 kPa or ELF ≥9.8. A subgroup of patients underwent liver biopsy (n = 48) or had imaging diagnostic of cirrhosis (n = 14). Patients were 57.3 ± 12.3 years old with a high prevalence of metabolic syndrome (84.5%), type 2 diabetes (82.5%), and body mass index (BMI) ≥40 kg/m2 (21.8%). LSM met quality criteria in 230 (91.3%) patients. NFS and FIB‐4 combined had a high negative predictive value (90.0%) for excluding LSM ≥8.2 kPa. However, 84.1% of patients had indeterminate or high NFS or FIB‐4 scores requiring further assessment. LSM ≥8.2 kPa and ELF ≥9.8 were present in 31.3% and 28.6% of patients, respectively. Following adjustment for age, BMI, sex, and presence of advanced fibrosis, older age was independently associated with ELF ≥9.8 (adjusted odds ratio, 1.14; 95% confidence interval, 1.06‐1.24), whereas increasing BMI was independently associated with LSM ≥8.2 kPa (adjusted odds ratio, 1.15; 95% confidence interval, 1.01‐1.30). Concordant LSM <8.2 kPa and ELF <9.8 and concordant LSM ≥8.2 kPa and ELF ≥9.8 had a high negative predictive value (91.7%) and positive predictive value (95.8%) for excluding and identifying clinically significant fibrosis, respectively. Conclusion: Simple scoring tools alone lack accuracy. LSM accuracy is influenced by severe obesity, whereas age impacts the ELF test. Further studies are required to confirm whether combining LSM and ELF may enhance accuracy and confidence in identifying clinically significant fibrosis. (Hepatology Communications 2018; 00:000‐000)
Nonalcoholic fatty liver disease (NAFLD), estimated to affect 25% of the adult population globally,2016 is the most common chronic liver disorder seen in primary care.2012 The most important predictor of mortality in NAFLD is the extent of liver fibrosis.2017 In particular, the presence of advanced fibrosis (Brunt stage 3‐4) is associated with increased risks of overall and liver‐related mortality.2015, 2011 These patients may benefit from specialist care and surveillance for liver cancer and liver decompensation. Reports from transplant registries in the United States show that NAFLD is now the second leading cause of liver disease2015 and the second most common cause of primary liver cancer among adults awaiting liver transplantation.2014 These data highlight the need for early identification of patients with NAFLD at increased risk of progressive liver disease and cirrhosis. Patients with type 2 diabetes mellitus (T2DM) and NAFLD are more likely to develop nonalcoholic steatohepatitis (NASH), advanced fibrosis/cirrhosis, and hepatocellular carcinoma (HCC),2013 and diabetic patients have a 2‐fold to 3‐fold increased risk of mortality from chronic liver disease.2014 Consequently, there is increasing interest in the development and implementation of a NAFLD screening strategy for the diabetic population that can be included in clinical practice guidelines.2016
Although NAFLD screening in the community is not currently recommended, European clinical practice guidelines advise that “… the progressive form of NAFLD (non‐alcoholic steatohepatitis (NASH)), particularly when associated with advanced fibrosis, should be identified in patients at risk (age >50 years, T2DM or metabolic syndrome (MetS)), because of its prognostic implications.”2016 Primary care clinicians and hospital specialists other than gastroenterologists and hepatologists have a key role in identifying patients with NAFLD at risk of significant liver disease who may require specialist referral for further evaluation or who need closer management of metabolic comorbidities and lifestyle interventions. However, many primary care clinicians and hospital specialists underestimate the prevalence and do not fully recognize the clinical spectrum of NAFLD and how this is assessed.2018, 2013
The European associations for the study of the liver, diabetes, and obesity advocate the use of noninvasive serum biomarkers (NAFLD fibrosis score [NFS], fibrosis 4 [FIB‐4], or enhanced liver fibrosis [ELF] test) for first‐line risk stratification, identifying NAFLD cases at low risk of advanced fibrosis/cirrhosis.2016 The simple scoring systems (NFS and FIB‐4) combine routine biochemical tests with clinical risk factors for fibrosis, such as age or diabetes, and have high negative predictive values for excluding advanced fibrosis.2010 The ELF test is a commercial panel of markers (tissue inhibitor of matrix metalloproteinase, hyaluronic acid, and the aminoterminal peptide of procollagen III) focusing on matrix turnover that has good diagnostic accuracy for advanced fibrosis in NAFLD.2008 In contrast to the European associations, the United Kingdom National Institute for Health and Care Excellence (NICE) guidelines on the assessment and management of NAFLD recommend using the ELF test as the first‐line test for advanced fibrosis in people who have been diagnosed with NAFLD.2016 Both guidelines recommend that if significant fibrosis cannot be ruled out, patients should be referred to a liver clinic for further evaluation, including liver stiffness measurements (LSMs). In patients with NAFLD, the LSM has a high negative predictive value and a modest positive predictive value for detecting advanced fibrosis.2016 This approach, however, requires a dedicated complex instrument with an XL probe for overweight subjects, an experienced operator, and competent interpretation of the results.
The optimal strategy to screen for advanced fibrosis in NAFLD remains under discussion, and in Australia, there are currently no guidelines for NAFLD management. The purpose of this study was to assess the prevalence of NAFLD with clinically significant fibrosis in T2DM clinics and at‐risk populations in primary care. In addition, we aimed to assess the utility of a pragmatic approach to screening for clinically significant fibrosis in these populations using noninvasive serum biomarkers and LSMs and to determine the extent of agreement between these biomarkers.
Patients and Methods
This was a cross‐sectional analysis of a prospective study involving patients identified with NAFLD between October 2015 and August 2017.
CASE ASCERTAINMENT/STUDY ELIGIBILITY
The source population included (i) consecutive patients scheduled for review in the diabetes clinic at Princess Alexandra Hospital, Brisbane, and (ii) all patients referred to secondary care from primary care due to a history of fatty liver, T2DM, or metabolic syndrome during the study period. All eligible patients were invited to attend the liver clinic at Princess Alexandra Hospital for further clinical assessment.
STUDY POPULATION
Patients were eligible to be included in the study if they had attended the liver clinic and had a diagnosis of NAFLD defined by demonstration of hepatic steatosis by liver ultrasound in the presence of metabolic risk factors and the exclusion of significant alcohol consumption (≥20 g/day) or other chronic liver diseases (including a prior history of alcohol‐related liver disease).2016 Patients were excluded if they had stage 5 chronic kidney disease (estimated glomerular filtration rate <15 mL/minute), renal replacement therapy, history of organ transplant, or if other causes of hepatic steatosis were suspected. The derivation of the study population is detailed in Supporting Fig.S1. We recruited 35% of the entire patient cohort (59.5% of the primary care cohort) from a single general practice (Inala Primary Care) following screening of their at‐risk patient population. This general practice has expertise in chronic disease and complex diabetes management and delivers an integrated primary–secondary care diabetes service. The study clinicians (P.P. and E.P.) worked closely with this general practice to raise awareness about NAFLD in their at‐risk patient populations.
Informed written consent was obtained from each eligible patient, and the protocol was approved by the Metro South Health and University of Queensland human research ethics committees (HREC/15/QPAH/301; UQ2015001047).
CLINICAL DATA
Data were collected prospectively by the study clinician (P.P.). Basic demographic and limited clinical information were available for the source population, including the NAFLD fibrosis score and/or FIB‐4 tests that were calculated using available clinical and laboratory data. Overall risk stratification was performed using the results of the NFS and the FIB‐4 score. Patients were deemed to be at “high risk” of advanced fibrosis if either score was high but at “low risk” if both their NFS and FIB‐4 scores were low. In the absence of a high score, “indeterminate risk” was assigned if one or both scores were indeterminate.
For patients included in the study, medical history was obtained during the initial consultation in the liver clinic using a structured questionnaire. Question items included self‐reported sociodemographic characteristics, history of tobacco use, recreational drug use, previously diagnosed liver disease, medical conditions, and use of medications. Alcohol intake was assessed using a standardized questionnaire and the Alcohol Use Disorders Identification Test,1993 as described by Patel etal.2017
Patients underwent a clinical assessment that included anthropometric measurements, laboratory tests (routine biochemical, hematologic, and serologic assays and ELF test), transient elastography, and liver ultrasound. In Australia, the ELF test is marketed with a recommended cutoff of ≥9.8 for “severe fibrosis”2011 (Brunt fibrosis stage ≥F2), and we have previously shown that this score reliably identified advanced liver fibrosis in patients with chronic liver disease.2015 Body mass index (BMI) was adjusted according to ethnicity for patients from South Asia (overweight, BMI ≥23 to <27.5; class 1 obese, BMI ≥27.5 to 30.0).2004 Metabolic syndrome was defined per the International Diabetes Federaton guidelines.2006
Transient elastography was performed after a 3‐hour fast using FibroScan technology (Echosens, Paris, France) with the standard M or XL probes in line with the manufacturer's instructions. Examinations were performed by a trained clinical nurse (with experience in performing more than 400 LSMs) and reviewed by a hepatologist (K.A.S.) with extensive FibroScan experience (more than 2,000 LSMs performed). Recommended standard FibroScan operating procedures were followed along with adherence to criteria for definition of reliable LSMs as follows: minimum of 10 valid measurements with a success rate of ≥60% and interquartile range (IQR) ≤30% of the final (median) result. The XL probe was used when the skin‐capsule depth was ≥2.5 cm. Although optimal liver stiffness cut‐off values in NAFLD remain under discussion,2018 for the purposes of this study, we used cut‐off values of 8.2 kPa for clinically significant fibrosis,2016 ≥9.5 kPa for advanced fibrosis,2017 and >13 kPa to indicate cirrhosis2017, 2016; the same cut‐off value was used for both probes.2017, 2016
Demonstration of steatosis by liver ultrasound was determined by the presence of increased hepatic echogenicity and beam attenuation, resulting in the renal cortex appearing relatively hypoechoic to the liver parenchyma, absence of the normal echogenic walls of the portal veins, and poor visualization of the diaphragm and deep portions of the liver.2002 Evidence of cirrhosis or portal hypertension on liver imaging was determined by liver surface nodularity or signs of portal hypertension, including portal vein dilatation, splenomegaly (spleen length >13 cm), portosystemic collaterals, and ascites.
LIVER HISTOLOGY
Patients with clinically significant fibrosis based on the FibroScan examination, discordant results of investigations for cirrhosis, or interest in participating in a clinical therapy trial were invited to undergo liver biopsy. Percutaneous liver biopsy was performed under ultrasound guidance by an experienced radiologist using the Tru‐Cut biopsy needle. Liver histology was assessed by a single experienced pathologist (A.D.C.) who was blinded to the clinical data. Histologic scoring was performed according to the system of Kleiner etal.2005 Fibrosis was staged from 0 to 4 as follows: stage 1, zone 3 perisinusoidal only or portal/periportal only; stage 2, zone 3 perisinusoidal and portal fibrosis; stage 3, bridging fibrosis; and stage 4, cirrhosis. Steatosis was estimated as a percentage of the parenchyma containing fat droplets and graded (0‐3),2005 and necroinflammatory activity was also graded according to severity (0‐3). NASH was defined by the presence of hepatic steatosis with both inflammation and hepatocyte ballooning, with or without fibrosis.
DATA ANALYSIS
Participant sociodemographic and clinical characteristics were described using frequency and percentage for categorical variables, mean and SD for continuous data normally distributed, and median and IQR for non‐normally distributed data. Correlation between continuous variables was assessed using Spearman's rho correlation (nonparametric case). The relationship between two categorical variables was assessed using Pearson's chi‐squared test or Fisher's exact test, as appropriate. The comparison of a continuous variable between two groups was tested using independent t tests or Mann‐Whitney U tests when the normality assumption was not met and one‐way analysis of variance or Kruskal‐Wallis test (nonparametric case) when comparing more than two groups. Tukey's post‐hoc tests or Dunn's test (when appropriate) were performed when overall significance was demonstrated between groups. Univariate logistic regression was performed to identify potential predictors of LSM ≥8.2 kPa or ELF ≥9.8. All variables with P < 0.2 were included in a multiple logistic regression with stepwise selection to identify factors influencing the outcome. Odds ratios (ORs), adjusted ORs (aORs), and 95% confidence intervals (CIs) were reported. All P values were two sided, and statistical significance was set at α = 0.05. Data analysis was conducted using SPSS Inc. version 24.0 (StataCorp L.P., College Station, TX; 2013).
Results
SOURCE POPULATION
A total of 504 patients from a hospital diabetes clinic and 571 primary care patients were assessed with a NAFLD fibrosis and/or FIB‐4 test using available laboratory/clinical data. Compared to the diabetes cohort, patients from primary care were less likely to be male or have T2DM and had a lower BMI and higher serum liver enzyme levels (Supporting Table S1). Using published cutoffs,2010 indeterminate or high NAFLD fibrosis scores were present in 82.9% of the hospital diabetes cohort and 76.9% of the primary care cohort. Indeterminate or high FIB‐4 scores were present in 34.3% and 34.8% of the hospital diabetes cohort and primary care cohort, respectively (Supporting Table S1). Overall, 77.6% of patients had indeterminate or high scores in at least one test, requiring further evaluation to establish the presence or absence of clinically significant fibrosis.
STUDY POPULATION
A total of 283 patients accepted the invitation to have further clinical assessment in the hepatology clinic. Following initial clinical review, 19 patients did not meet inclusion criteria (absence of steatosis on liver ultrasound examination with no evidence of clinically significant liver disease [LSM <8.2], n = 11; excess alcohol intake, n = 3; drug‐induced steatosis, n = 2; other, n = 3) and were excluded from the study and 12 patients withdrew. The demographic and clinical characteristics of the final cohort (n = 252) are summarized in Table 1. Differences between the final cohort and source population are detailed in Supporting Tables S1and S2. In particular, diabetes was less prevalent in the study cohort (P = 0.005), and those included had higher BMI (P = 0.001), alanine aminotransferase (ALT) (P < 0.001), and aspartate aminotransferase (AST) (P < 0.001) values, and fewer patients were stratified as low risk overall (P = 0.017). Overall, the mean age of subjects included in the study was 57.3 ± 12.3 years and 54.4% were male patients. The majority of subjects (78.6%) were Caucasian, with a mean BMI of 35.1 ± 8.3 kg/m2 and mean girth of 117.2 ± 19.0 cm. Patients had a high prevalence of metabolic syndrome (84.5%) and T2DM (82.5%), and more than one fifth of the cohort had class 3 obesity or higher (BMI ≥40 kg/m2).
Table 1.
Clinical and Demographic Data for the Final Study Cohort
|
Diabetic Clinic n = 104 (%) |
Primary Care n = 148 (%) |
P Value | ||
|---|---|---|---|---|
| Age* | 59.5 (52.0‐66.0) | 58.0 (45.5‐68.0) | 0.358 | |
| Male sex,† n (%) | 68 (65.4) | 69 (46.6) | 0.003 | |
| Caucasian ethnicity,† n (%) | 86 (82.7) | 112 (75.7) | 0.181 | |
| Metabolic syndrome,† n (%) | 99 (95.2) | 114 (77.0) | <0.001 | |
| Type 2 diabetes,‡ n (%) | 104 (100) | 104 (70.3) | <0.001 | |
| Body mass index* (kg/m2) | 36.0 (30.3‐41.7) | 32.3 (28.8‐37.5) | 0.004 | |
| Waist* (cm) | 123.0 (108.0‐134.0) | 111.0 (100.3‐126.0) | <0.001 | |
| BMI‡ categorized, n (%) | normal weight | 2 (2.0) | 9 (6.1) | 0.049 |
| overweight | 19 (18.3) | 40 (27.0) | ||
| class I obesity | 27 (26.0) | 45 (30.4) | ||
| class II obesity | 26 (25.0) | 29 (19.6) | ||
| ≥class III obesity | 30 (28.8) | 25 (16.9) | ||
| Serum liver enzymes* | ALT (IU/mL) | 30 (21‐42) | 34 (22‐62) | 0.015 |
| AST (IU/mL) | 21 (15‐31) | 25 (17‐40) | 0.009 | |
| GGT (IU/mL) | 28 (20‐50) | 36 (20‐68) | 0.061 | |
| Platelet count§ (×109) | 244 ± 62 | 249 ± 65 | 0.599 | |
| Serum albumin* (g/L) | 40 (38‐42) | 42 (40‐44) | 0.001 | |
| LSM range*,‖ | 2.6‐51.4 | 2.5‐63.9 | 0.300 | |
| LSM ≥8.2 kPa,†,‖ n (%) | 28 (31.1) | 44 (31.4) | 0.960 | |
| ELF test range* | 7.3‐13.0 | 6.6‐12.6 | 0.947 | |
| ELF ≥9.8,† n (%) | 27 (26.0) | 45 (30.4) | 0.442 | |
| NFS,‡ n (%) | low | 4 (3) | 44 (29.7) | <0.001 |
| indeterminate | 66 (63.5) | 74 (50.0) | ||
| high | 34 (32.7) | 30 (20.3) | ||
| FIB‐4 score,† n (%) | low | 61 (58.7) | 89 (60.1) | 0.972 |
| indeterminate | 38 (36.5) | 52 (35.1) | ||
| high | 5 (4.8) | 7 (4.7) | ||
*Continuous data presented (median [IQR]) analyzed using Mann‐Whitney U test; †categorical data presented analyzed using Pearson's chi‐squared test; ‡categorical data presented analyzed using Fisher's exact test; §continuous data presented (mean ± SD) analyzed using an independent t test; ‖LSM presented for the 230 patients with reliable LSM.
A total of 41.9%, 37.2%, and 47.3% of patients from primary care had gamma‐glutamyltransferase (GGT), AST, and ALT levels above laboratory reference ranges (GGT, males ≥55 U/L, females ≥38 U/L; AST, males ≥35 U/L, females ≥31 U/L; ALT, males ≥45 U/L, females ≥34 U/L) compared with 28.8%, 20.4%, and 26.9% of patients from the diabetes clinic (P = 0.034, P = 0.004, and P = 0.001, respectively). Overall, 6% of patients had a platelet count <150 × 109/L.
LSM met quality criteria in 230 (91.3%) patients. Significantly, almost one quarter (23.6%) of patients with severe obesity (BMI ≥40 kg/m2) compared to 4.6% of patients with BMI <40 kg/m2 had a FibroScan that did not meet validity criteria (P < 0.001). Patients without a valid LSM were older (P = 0.02), had a greater girth (P < 0.001), and had a higher ELF score (P = 0.023). Median LSM was 6.1 kPa with a range from 2.5 to 63.9 kPa and required use of the XL probe in 76.5% of subjects. LSM ≥8.2 kPa, consistent with clinically significant fibrosis, was present in 31.1% and 31.4% of patients from the diabetic clinic and primary care cohorts, respectively; 25.6% and 22.9% had an LSM ≥9.5 kPa (consistent with advanced fibrosis); and 18.9% and 14.3% had an LSM >13 kPa (concerning for cirrhosis), in the diabetic and primary care cohorts, respectively. Fourteen patients (LSM 10.1‐63.9 kPa) had liver imaging consistent with cirrhosis.
Overall, the mean ELF score was 9.3 ± 1.0, with a range from 6.6‐13.0. An ELF score ≥9.8, consistent with severe fibrosis, was present in 26.0% and 30.4% of patients from the diabetic clinic and primary care cohorts, respectively. There was a moderate positive correlation between LSM and ELF test results (Spearman's rho = 0.40, P < 0.001; Supporting Fig. S2) with concordance between the tests in 76.5% of patients with a valid LSM result. The United Kingdom NICE guidelines2016 advocate using ELF ≥10.51 to diagnose advanced fibrosis. In our study population, 13.1% had an ELF score ≥10.51.
The LSM results according to low, indeterminate, and high NFS and FIB‐4 risk stratification scores are illustrated in Fig. 1. In contrast to low NFS scores, low FIB‐4 scores did not “rule out” subjects with elevated LSM ≥8.2 kPa. The combination of a low NFS and FIB‐4 provided a better negative predictive value (90%) than FIB‐4 alone for excluding clinically significant fibrosis (defined by LSM ≥8.2 kPa). Characteristics of the low‐risk study population (low score on the FIB‐4 and NFS; n = 40) are summarized in Supporting Table S3 In comparison with the indeterminate/high‐risk cohort, subjects with combined low‐risk FIB‐4 and NFS were younger, had lower prevalence of metabolic syndrome and T2DM, had smaller girth (all P < 0.001), and lower BMI (P = 0.002). In contrast, serum ALT levels were higher in the low‐risk patients compared to the indeterminate/high‐risk cohort.
Figure 1.
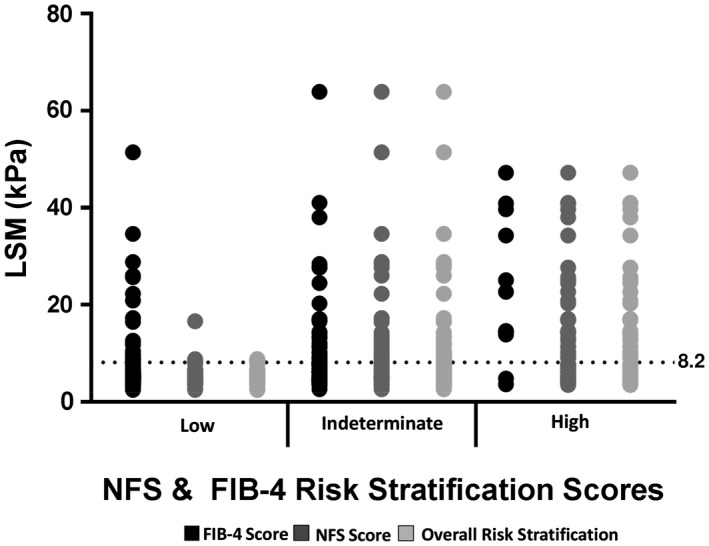
LSM results according to low, indeterminate, and high NFS and FIB‐4 risk stratification scores.
FACTORS ASSOCIATED WITH LSM ≥8.2 kPa AND/OR ELF ≥9.8
Patients with LSM ≥8.2 kPa or ELF ≥9.8 had an increased prevalence of T2DM, lower platelet counts, and higher BMI, AST, and GGT compared to patients with LSM <8.2 kPa or ELF <9.8 (Table 2). Patients with LSM ≥8.2 kPa had a greater waist circumference (P < 0.001), poorer diabetic control (P = 0.031), and higher ALT (P < 0.001), whereas patients with ELF ≥9.8 were older (P < 0.001). Further assessment comparing ELF ≥9.8 and the United Kingdom NICE Guideline threshold of ELF ≥10.51 is summarized in Supporting Table S4. Patients with a higher ELF score were older (P < 0.001), had raised AST and GGT levels (both P < 0.001), lower platelet counts (P = 0.006), and higher LSM scores (P < 0.001). A post‐hoc analysis did not identify any significant differences between ELF ≥9.8 and <10.51 and ELF ≥10.51.
Table 2.
Patient Characteristics According to LSM and ELF Scores
|
LSM* <8.2 n = 158 |
LSM* ≥8.2 n = 72 |
P Value |
ELF Score <9.8 n = 180 |
ELF Score ≥9.8 n = 72 |
P Value | |
|---|---|---|---|---|---|---|
| Age† (years) | 57.0 (49.0‐66.0) | 59.5 (52.3‐66.0) | 0.505 | 55.0 (45.0‐63.0) | 65.0 (59.0‐72.0) | <0.001 |
| BMI† (kg/m2) | 31.3 (28.1‐35.9) | 37.5 (32.9‐42.8) | <0.001 | 32.5 (29.3‐38.3) | 36.0 (30.5‐41.4) | 0.031 |
| Girth‡ (cm) | 109.7 ± 15.3 | 127.8 ± 17.4 | <0.001 | 117.7 ± 17.5 | 122.3 ± 18.3 | 0.055 |
| T2DM,§ n (%) | 120 (75.9) | 67 (93.1) | 0.002 | 142 (78.9) | 66 (91.7) | 0.016 |
| HbA1C ≥7%,‖ n (%) | 80 (50.6) | 48 (66.7) | 0.031 | 102 (56.7) | 40 (55.6) | 0.872 |
| ALT† (IU/mL) | 29 (20‐48) | 43 (31‐64) | <0.001 | 32 (21‐52) | 33 (22‐57) | 0.658 |
| AST† (IU/mL) | 21 (16‐29) | 34 (22‐47) | <0.001 | 21 (15‐32) | 31 (18‐48) | <0.001 |
| GGT† (IU/mL) | 27 (19‐45) | 59 (38‐107) | <0.001 | 29 (20‐50) | 50 (25‐97) | <0.001 |
| Platelets‡ (× 109) | 254 ± 61 | 232 ± 69 | 0.016 | 254 ± 61 | 229 ± 68 | 0.004 |
| Albumin† (g/L) | 42 (40‐44) | 41 (38‐43) | 0.016 | 41 (39‐44) | 41 (36‐43) | 0.155 |
| ELF Score† | 8.9 (8.4‐9.5) | 9.9 (9.3‐10.5) | <0.001 | ‐ | ‐ | ‐ |
| ELF ≥ 9.8,‖ n (%) | 21 (13.3) | 39 (54.2) | <0.001 | ‐ | ‐ | ‐ |
| LSM† (kPa) | ‐ | ‐ | ‐ | 5.5 (4.6‐7.6) | 11.8 (4.2‐19.5) | <0.001 |
| LSM ≥ 8.2,‖ n (%) | ‐ | ‐ | ‐ | 33 (19.4) | 39 (65.0) | <0.001 |
*LSM data presented for 230 patients meeting quality criteria; †continuous data (median [IQR]) analyzed using Mann‐Whitney U Test; ‡continuous data (mean ± SD) analyzed using an independent t test; §categorical data analyzed using Fisher's exact test; ‖categorical data analyzed using Pearson's chi‐squared test.
Factors associated with LSM ≥8.2 kPa or ELF ≥9.8 were similar between the primary care and diabetic cohorts (Supporting Table S5A,B); however, within the primary care cohort, the mean platelet level was lower in patients with elevated LSM (P = 0.013) and ELF scores (P = 0.004) compared to patients with LSM <8.2 kPa or ELF <9.8. This relationship was not seen in the diabetic cohort (P = 0.495 and P= 0.318, respectively).
Multivariable logistic regression analyses were used to identify factors associated with having LSM ≥8.2 kPa compared to LSM <8.2 kPa, and with having ELF ≥9.8 compared to ELF <9.8 (Table 3). All factors identified as potentially associated with LSM ≥8.2 kPa or ELF ≥9.8 in the univariate analysis (P < 0.2) were included in multiple logistic regression analyses with stepwise selection. Higher BMI, presence of metabolic syndrome, higher AST, higher GGT, and lower platelet count were identified as significant determinants of LSM ≥8.2 kPa. Further assessment considering BMI as categorical (normal plus overweight [<30 kg/m2], category 1‐2 obesity [31‐39.9 kg/m2], and category 3 obesity and above [≥40 kg/m2]) identified that BMI ≥40 kg/m2 (aOR, 15.05; 95% CI, 5.08‐44.58) but not BMI 30‐39 kg/m2 (aOR, 1.79; 95% CI, 0.71‐4.43) when compared to BMI <30 kg/m2 was associated with LSM ≥8.2 kPa. Older age, higher BMI, higher AST, higher GGT, and lower platelet count were identified as factors significantly associated with ELF ≥9.8 compared to ELF <9.8 (Table 3). In this model, when age <50 years was compared to age categories 50 to 59, 60 to 64, and ≥65 years, there was a marked increase in the aOR for an association with ELF ≥9.8 (aOR, 19.42; 95% CI, 3.52‐107.19; aOR, 40.02; 95% CI, 6.70‐239.08; and aOR, 113.29, 95% CI, 18.96‐676.80, respectively).
Table 3.
Multivariable Stepwise Logistic Regression for LSM and ELF Scores
| LSM <8.2 kPa or ≥8.2 kPa | ELF <9.8 or ≥9.8 | |||||
|---|---|---|---|---|---|---|
| aOR | 95% CI | Adjusted P Value | aOR | 95% CI | Adjusted P Value | |
| Age (years) | ‐ | ‐ | ‐ | 1.15 | 1.10‐1.20 | <0.001 |
| BMI (kg/m2) | 1.13 | 1.07‐1.19 | <0.001 | 1.08 | 1.04‐1.13 | <0.001 |
| Metabolic syndrome | 5.08 | 1.28‐20.18 | 0.021 | ‐ | ‐ | ‐ |
| AST (IU/mL) | 1.03 | 1.00‐1.05 | 0.020 | 1.04 | 1.02‐1.07 | 0.001 |
| GGT (IU/mL) | 1.01 | 1.00‐1.02 | 0.007 | 1.01 | 1.00‐1.01 | 0.056 |
| Platelet count (× 109) | 0.99 | 0.99‐1.00 | 0.013 | 0.99 | 0.99‐1.00 | 0.041 |
FACTORS ASSOCIATED WITH CONCORDANT OR DISCORDANT LSM AND ELF SCORES
Patient characteristics according to concordant/discordant scores are summarized in Table 4. Patients with concordant elevated LSM and ELF test were more likely to be diabetic (P = 0.005) than patients in the other groups. They also had higher AST and GGT levels than those patients with concordant low LSM and ELF test (P < 0.001). Discordant LSMs and ELF scores were present in 54 (23.5%) patients, with no difference in the rate of discordance between the primary care and diabetic cohorts (P = 0.782). Patients with ELF ≥9.8 despite LSM <8.2 kPa were significantly older when compared to patients with concordant low ELF test and LSM as well as patients with low ELF test and high LSM (P < 0.001). Patients with ELF ≥9.8 despite LSM <8.2 kPa were less likely to have elevated liver enzymes than patients with concordantly raised LSM and ELF test (P < 0.001). In contrast, patients with high LSM and low ELF test had a higher BMI and waist circumference than those patients with both low LSM and ELF test (P = 0.002 and P < 0.001, respectively) and those with discordantly raised ELF test and low LSM (P < 0.001). Patients with discordant raised LSM and low ELF test were more likely to have mildly raised ALT and GGT levels when compared to patients with discordant raised ELF test and low LSM (P = 0.004 and P = 0.008, respectively).
Table 4.
Patient Characteristics According to Concordant or Discordant LSM and ELFScores
|
Low LSM Low ELF n = 137 |
Low LSM High ELF n = 21 |
High LSM Low ELF n = 33 |
High LSM High ELF n = 39 |
P Value | |
|---|---|---|---|---|---|
| Age* (years) | 55.0 ± 12.3 | 66.0 ± 8.0 | 50.9 ± 13.2 | 62.6 ± 8.1 | <0.001 |
| Male sex,† n (%) | 74 (54.0) | 11 (52.4) | 19 (57.6) | 22 (56.4) | 0.973 |
| Caucasian ethnicity,† n (%) | 104 (75.9) | 13 (61.9) | 27 (81.8) | 33 (84.6) | 0.213 |
| BMI‡ (kg/m2) | 31.3 (28.2‐35.6) | 30.5 (27.1‐37.3) | 40.5 (34.6‐45.3) | 36.3 (31.6‐42.2) | <0.001 |
| Waist circumference* (cm) | 110.1 ± 14.6 | 107.3 ± 19.3 | 132.2 ± 15.9 | 124.0 ± 17.8 | <0.001 |
| T2DM,§ n (%) | 104 (75.9) | 16 (76.2) | 29 (87.9) | 38 (97.4) | 0.006 |
| Metabolic syndrome,§ n (%) | 107 (78.1) | 17 (81.0) | 30 (90.9) | 38 (97.4) | 0.011 |
| eGFR‡ (mL/minute) | 90 (78‐90) | 81 (63‐90) | 90 (86‐90) | 80 (65‐90) | 0.003 |
| ALT‡ (IU/mL) | 30 (21‐51) | 22 (18‐34) | 46 (31‐61) | 38 (33‐66) | <0.001 |
| AST‡ (IU/mL) | 21 (16‐30) | 24 (15‐29) | 30 (16‐37) | 40 (27‐66) | <0.001 |
| GGT‡ (IU/mL) | 26 (20‐44) | 33 (17‐65) | 50 (23‐79) | 72 (47‐128) | <0.001 |
| Platelet count‡ (× 109) | 247 (208‐293) | 245 (216‐303) | 243 (210‐291) | 209 (162‐263) | 0.019 |
| Albumin‡ (g/L) | 42 (40‐44) | 41 (40‐42) | 40 (38‐43) | 41 (39‐43) | 0.066 |
| HbA1c‡ (%) | 7.3 (5.9‐8.6) | 6.7 (5.8‐7.7) | 7.7 (6.8‐9.0) | 7.7 (6.7‐9.1) | 0.029 |
Data only presented for the 230 patients for whom a valid LSM reading was calculated. *Continuous data (mean ± SD) analyzed using a one‐way analysis of variance; †categorical data analyzed using Pearson's chi‐squared test; ‡continuous data (median [IQR]) analyzed using the Kruskal‐Wallis test; §categorical data analyzed using Fisher's exact test.
Abbreviations: eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c.
FACTORS ASSOCIATED WITH LSM ≥8.2 kPa AND/OR ELF ≥9.8 IN PATIENTS WITH ADVANCED FIBROSIS CONFIRMED BY HISTOLOGY OR IMAGING
A total of 48 patients underwent liver biopsy (Supporting Table S6), and an additional 14 patients had liver imaging diagnostic of cirrhosis. The mean liver biopsy length was 17.4 ± 3.2 mm, and mean number of portal tracts was 15.5 ± 6.1. All patients had NASH, and 25 patients (52.1%) had advanced fibrosis (Brunt fibrosis stage 3 or 4). Within this subgroup of 62 patients, histology or imaging consistent with advanced fibrosis was present in 78.8% of patients with ELF ≥9.8 and 85.4% with LSM ≥8.2 kPa.
Univariate logistic regression within this subgroup identified similar factors to be associated with both LSM ≥8.2 kPa and ELF ≥9.8 as in the complete study population. The presence of advanced fibrosis (diagnosed by histology and/or imaging), BMI, age, and sex as variables of clinical relevance were included in a multivariable logistic regression with stepwise selection. Advanced fibrosis (aOR, 51.40; 95% CI, 6.32‐418.17) and BMI (aOR, 1.15; 95% CI, 1.01‐1.30) were found to be significantly associated with LSM ≥8.2 kPa. In contrast, age but not BMI was independently associated with ELF ≥9.8 (aOR, 1.14; 95% CI, 1.06‐1.24). The aOR for the association between advanced fibrosis and ELF ≥9.8 was 3.06 (95% CI, 0.74‐12.73), but this did not reach statistical significance.
Because BMI did not affect the ELF test result, we examined whether combined classification by ELF (<9.8 or ≥9.8) and LSM (<8.2 kPa or ≥8.2 kPa) increased the accuracy of identifying advanced fibrosis. Combining ELF ≥9.8 and LSM ≥8.2 kPa had a positive predictive value of 95.8% with a specificity of 95.5% for identifying advanced fibrosis (Fig. 2). In contrast, when both ELF <9.8 and LSM <8.2 kPa were present, there was a negative predictive value of 91.7% with a sensitivity of 97.3% for excluding advanced fibrosis.
Figure 2.
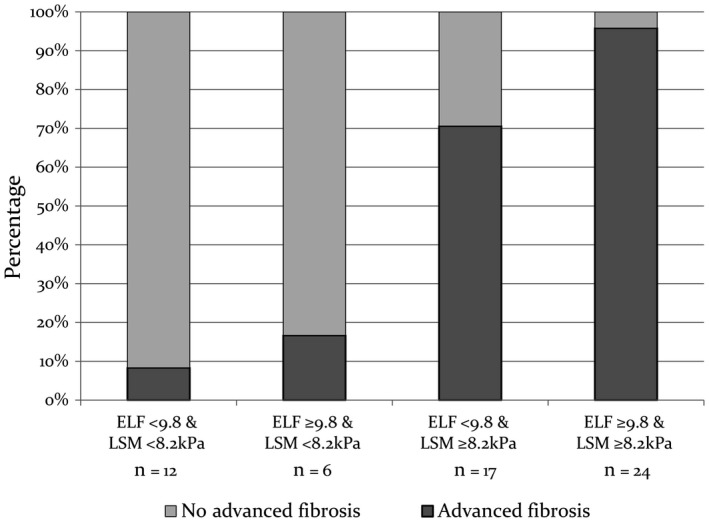
Prevalence of advanced fibrosis (diagnosed by histology and/or imaging) according to LSM and ELF concordance.
IMPACT ON PATIENT MANAGEMENT
Following review by a hepatologist (E.E.P.), 70 patients (27.8% of the total cohort) were considered to have NAFLD with clinically significant fibrosis based on liver histology, imaging, or a combination of noninvasive markers and clinical assessment. In 63 of these 70 patients (90%), this was a new diagnosis of clinically significant fibrosis and led to a change in clinical management, with a recommendation for ongoing review in the liver clinic and HCC surveillance.
Discussion
To our knowledge, this is the first prospective Australian study to assess a pragmatic approach to screening for NAFLD with clinically significant fibrosis in T2DM clinics and at‐risk populations in primary care, using noninvasive serum biomarkers and LSM. Overall in our cohort of patients with NAFLD, we found a prevalence of clinically significant fibrosis of 27.8%; this was a new diagnosis in 90% of these cases. This is of concern because the extent of liver fibrosis is the most important predictor of all‐cause mortality in NAFLD. The new diagnosis of clinically significant fibrosis led to a change in clinical management, with a recommendation for ongoing review in the liver clinic and HCC surveillance.
In our cohort of “at‐risk” subjects, 31.3% had an elevated LSM ≥8.2 kPa, suggesting significant liver fibrosis. We believe the high rate of clinically significant fibrosis in our patient population reflects the high prevalence of risk factors for more advanced liver disease in this cohort, specifically complex T2DM, metabolic syndrome, and severe obesity. Among patients with diabetes in Hong Kong seen in a hospital setting (n = 1,770) with a median BMI of 26.6 kg/m2, 17.1% had LSM ≥9.6 kPa using the M probe, whereas patients requiring use of the XL probe (n = 114) had higher BMI, waist circumference, systolic blood pressure, and hemoglobin A1c and 27.2% had LSM ≥9.3 kPa.2016 These findings differ from two community‐based studies of patients with T2DM, where the prevalence of significant fibrosis was lower.2017, 2016 In a French community‐based diabetic population (n = 669), the proportion of patients with LSM ≥8 kPa, 9.6 kPa, and 13 kPa was 12.7%, 7.3%, and 2.1%, respectively.2017 In that study, the mean BMI was 29.6 kg/m2, metabolic syndrome was present in 56.2% of patients (compared to 84.5% of our patients), and the XL probe was required in only 35% of patients. Similarly, among 216 subjects from the community with T2DM and NAFLD (a subset of a larger population‐based study), the overall predicted probability of LSM ≥8.0 kPa was 17.2%.2016
Even in our considerably obese cohort, FibroScan was feasible in 91.3% of patients, similar to the reported 85% success rate in a mixed overweight and obese diabetic cohort.2014 Although obesity is associated with LSM failure and unreliability using the M probe, there is a higher success rate with the XL probe, which uses a lower frequency, a more sensitive ultrasonic transducer, a deeper focal length, a larger vibration amplitude, and a greater depth of measurement below the skin surface.2010 In previous NAFLD studies, LSM cut‐off values for advanced fibrosis using the M probe ranged from 8‐12 kPa, with 84%‐100% sensitivity and 83%‐97% specificity.2016 Relatively few published data are available using the XL probe, in particular the appropriate cut‐off values for fibrosis and whether severe obesity and steatosis influence the reliability and accuracy of these measurements.2012, 2012, 2013, 2012, 2016, 2013 Importantly, we found that, among 41 patients with LSM ≥8.2 kPa (40 required the XL probe), 85.4% had advanced fibrosis or cirrhosis on histology or imaging. In contrast, only 2 of 18 patients with LSM <8.2 kPa who underwent liver biopsy had Brunt fibrosis stage F3 and none had cirrhosis. Therefore, our data support studies2016, 2018 demonstrating that, in this high‐risk NAFLD cohort, LSM has a valuable role in the assessment of advanced fibrosis.
Although the discordance between LSM and histology was low in our liver biopsy group, high BMI (>35 kg/m2) has been associated with overestimation of fibrosis by LSM with the XL probe compared to histologic assessment.2012, 2012 In addition, in almost one quarter of our patients with BMI ≥40 kg/m2, FibroScan did not meet validity criteria, and in our total cohort, BMI, particularly ≥40 kg/m2, was independently associated with LSM ≥8.2. In view of this, we propose that the accuracy and confidence in noninvasive liver fibrosis assessment may be improved by combining LSM with a serum biomarker of fibrosis that is independent of BMI. Among our at‐risk patients, 28.6% had a serum ELF ≥9.8, the manufacturer's cutoff for severe fibrosis. Overall, there was a moderate positive correlation between LSM and ELF test results (P < 0.001, Spearman's rho = 0.40), with concordance between the two noninvasive biomarkers in 76.5% of cases. Among 24 patients with both LSM ≥8.2 kPa and ELF ≥9.8 who proceeded to liver biopsy, 23 had advanced fibrosis or cirrhosis on histologic examination and the remaining subject had stage 2 fibrosis. In contrast, only 1 of 12 patients with both LSM <8.2 kPa and ELF <9.8 who underwent liver biopsy had Brunt fibrosis stage F3. Our data support a study in chronic hepatitis B2014 and a mixed liver disease etiology cohort2012 that showed combining LSM and the ELF test may enhance accuracy and confidence in identifying advanced fibrosis. However, the small number of patients with histologic data in our study limits our ability to draw firm conclusions, and further studies are required to clarify the benefit of combining these tests.
In the cohort of subjects with a liver biopsy, age (but not BMI) was independently associated with ELF ≥9.8, with a marked increase in the aOR for older age categories. The original European liver fibrosis test incorporated age, but the algorithm was simplified by dropping the age variable, without apparent loss of diagnostic performance.2011 The median age of the original ELF cohort was 43 years (range, 19‐75 years), and the independent patient cohorts used to validate simplification of the ELF test had similar median ages,2011 whereas the mean age of subjects included in the current NAFLD study was 57.3 ± 12.3 years. This difference in cohort age may have impacted diagnostic test performance. Similarly, in a study of 400 healthy volunteers, age was found to be the most relevant factor influencing the ELF score, with a significant increase in ELF score across decades from <20 to >60 years.2013 Clearly, further analysis of the impact of age on ELF score discrimination is required.
Our study demonstrates that simple scoring systems alone lack accuracy and that a sequential algorithm using complementary biomarkers is required to appropriately stratify patients according to fibrosis risk. Following screening with the NFS, only 20.2% of patients had a low risk of advanced fibrosis. In our population with T2DM, the utility of the NFS is limited by the inclusion of age, BMI, and diabetes, leading to a high prevalence (>50%) of indeterminate results. In contrast, screening with the FIB‐4 led to a low prevalence (<5%) of subjects at high risk of advanced fibrosis, likely reflecting the well‐preserved liver function in patients with NAFLD. However, if both the NFS and FIB‐4 scores are low, clinically significant fibrosis (defined by LSM ≥8.2 kPa) appears to be largely excluded (negative predictive value 90.0%). Although assessment with FibroScan and ELF was performed in the current study in a tertiary hospital center, we suggest that these tests may be undertaken by primary care clinicians or other specialists to identify patients with NAFLD and clinically significant fibrosis who require hepatology referral for further evaluation. Our proposed algorithm for sequential use of these complementary biomarkers is summarized in Fig. 3.
Figure 3.
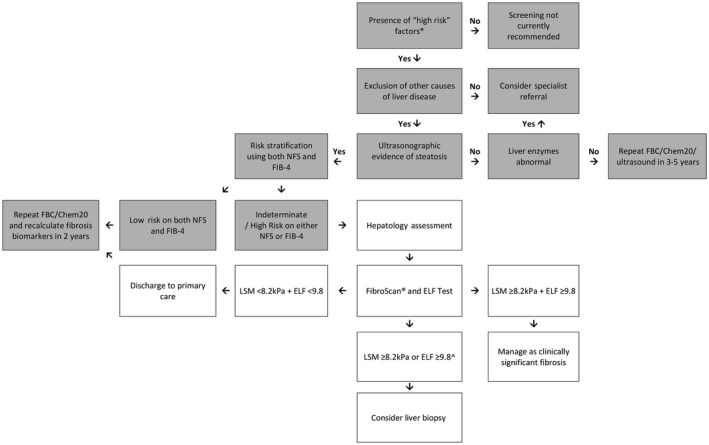
Proposed algorithm to assess for NAFLD and clinically significant fibrosis in at‐risk subjects. Boxes shaded in gray represent care undertaken prior to hepatology assessment. For patients with evidence of advanced fibrosis/cirrhosis on imaging or biochemical abnormalities consistent with advanced fibrosis (e.g., low platelet count, low serum albumin level, AST:ALT reversal) specialist referral should be considered. 1Metabolic high‐risk factors include age >50 years, presence of diabetes or metabolic syndrome. ∧If LSM <8.2 kPa, consider an alternate cause for ELF ≥9.8 (e.g., extrahepatic fibrosis, increased age). Abbreviation: FBC, full blood count.
Strengths of our study include the prospective recruitment of unselected “real‐world” subjects from both diabetes clinics and primary care facilities and the pragmatic use of a combination of readily available biomarkers that apply published cut‐off values for severe fibrosis. Our study has a number of limitations. In particular, liver biopsy was only performed in a subset of patients who were selected based on increased likelihood of advanced disease or patient interest in participating in clinical therapy trials. In addition to diabetes, a high proportion of patients had metabolic syndrome and severe obesity, likely contributing to the high prevalence of clinically significant fibrosis. As a consequence, the study results reflect our source populations (hospital diabetes clinics and primary care facilities with a high prevalence of complex T2DM and metabolic comorbidity), and fibrosis prevalence may be different in other populations.
The most concerning result of our study is the high prevalence of previously unrecognized severe liver fibrosis in these patient populations. The development of advanced fibrosis is the major determinant of liver‐related outcomes as well as for predicting overall mortality in patients with NAFLD.2015, 2015 According to European and American practice guidelines,2016, 2018 patients with advanced fibrosis or cirrhosis should be considered for HCC screening as cirrhosis is an important risk factor for HCC and early diagnosis improves the applicability and cost effectiveness of therapies. However, several studies have identified that patients with cirrhosis due to NAFLD are less likely to receive surveillance for HCC,2017, 2016 and this may be partly due to failure to recognize cirrhosis in clinical practice.2016 Clearly, our data demonstrate that diagnosis and risk stratification of patients with NAFLD in these high‐risk cohorts are important unmet clinical needs and warrant proactive strategies to ensure recognition. We believe that the use of simple noninvasive scores followed by use of the ELF test and FibroScan may help to resolve the diagnosis of advanced fibrosis in the vast majority of patients with NAFLD referred from the community or diabetes clinics.
Abbreviations
- aOR
adjusted odds ratio
- BMI
body mass index
- CI
confidence interval
- ELF
enhanced liver fibrosis
- FIB‐4
fibrosis 4
- HCC
hepatocellular carcinoma
- IQR
interquartile range
- LSM
liver stiffness measurement
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NFS
nonalcoholic fatty liver disease fibrosis score
- NICE
National Institute for Health and Care Excellence
- OR
odds ratio
- T2DM
type 2 diabetes mellitus
Supporting information
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep4.1208/full
Supporting Information
Potential conflict of interest: Professor Rosenberg is on the speakers' bureau and received grants from Siemens; he is among the inventors and patent holders for the enhanced liver fibrosis test. The other authors have nothing to report.
Supported by the Pathology Queensland‐Study, Education, and Research Trust Fund; the Australian National Health and Medical Research Council (Career Development Fellowship #1083090 to P.C.V.); and Siemens Healthineers (unrestricted educational grant and enhanced liver fibrosis test).
References
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease‐meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73‐84. [DOI] [PubMed] [Google Scholar]
- Armstrong MJ, Houlihan DD, Bentham L, Shaw JC, Cramb R, Olliff S, etal. Presence and severity of non‐alcoholic fatty liver disease in a large prospective primary care cohort. JHepatol 2012;56:234‐240. [DOI] [PubMed] [Google Scholar]
- Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, etal. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta‐analysis. Hepatology 2017;65:1557‐1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, etal. Fibrosis stage is the strongest predictor for disease‐specific mortality in NAFLD after up to 33 years of follow‐up. Hepatology 2015;61:1547‐1554. [DOI] [PubMed] [Google Scholar]
- Younossi ZM, Stepanova M, Rafiq N, Makhlouf H, Younoszai Z, Agrawal R, etal. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver‐related mortality. Hepatology 2011;53:1874‐1882. [DOI] [PubMed] [Google Scholar]
- Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, etal. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547‐555. [DOI] [PubMed] [Google Scholar]
- Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014;59:2188‐2195. [DOI] [PubMed] [Google Scholar]
- Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 2013;10:330‐344. [DOI] [PubMed] [Google Scholar]
- Zoppini G, Fedeli U, Gennaro N, Saugo M, Targher G, Bonora E. Mortality from chronic liver diseases in diabetes. Am J Gastroenterol 2014;109:1020‐1025. [DOI] [PubMed] [Google Scholar]
- Cusi K. Treatment of patients with type 2 diabetes and non‐alcoholic fatty liver disease: current approaches and future directions. Diabetologia 2016;59:1112‐1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) . EASL‐EASD‐EASO clinical practice guidelines for the management of non‐alcoholic fatty liver disease. Diabetologia 2016;59:1121‐1140. [DOI] [PubMed] [Google Scholar]
- Patel PJ, Banh X, Horsfall LU, Hayward KL, Hossain F, Johnson T, etal. Underappreciation of non‐alcoholic fatty liver disease by primary care clinicians: limited awareness of surrogate markers of fibrosis. Intern Med J 2018;2:144‐151. [DOI] [PubMed] [Google Scholar]
- Bergqvist CJ, Skoien R, Horsfall L, Clouston AD, Jonsson JR, Powell EE. Awareness and opinions of non‐alcoholic fatty liver disease by hospital specialists. Intern Med J 2013;43:247‐253. [DOI] [PubMed] [Google Scholar]
- McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non‐invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non‐alcoholic fatty liver disease. Gut 2010;59:1265‐1269. [DOI] [PubMed] [Google Scholar]
- Guha IN, Parkes J, Roderick P, Chattopadhyay D, Cross R, Harris S, etal. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology 2008;47:455‐460. [DOI] [PubMed] [Google Scholar]
- Glen J, Floros L, Day C, Pryke R; Guideline Development Group . Non‐alcoholic fatty liver disease (NAFLD): summary of NICE guidance. BMJ 2016;354:i4428. [DOI] [PubMed] [Google Scholar]
- Cassinotto C, Boursier J, de Ledinghen V, Lebigot J, Lapuyade B, Cales P, etal. Liver stiffness in nonalcoholic fatty liver disease: a comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology 2016;63:1817‐1827. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption‐‐II. Addiction 1993;88:791‐804. [DOI] [PubMed] [Google Scholar]
- Patel PJ, Smith D, Connor JP, Horsfall LU, Hayward KL, Hossain F, etal. Alcohol consumption in diabetic patients with nonalcoholic fatty liver disease. Can J Gastroenterol Hepatol 2017;2017:7927685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADVIA Centaur Enhanced Liver Fibrosis (ELF) Test Specifications. Tarrytown, NY:Siemens Healthcare Diagnostics Inc.; 2011. [Google Scholar]
- Fagan KJ, Pretorius CJ, Horsfall LU, Irvine KM, Wilgen U, Choi K, etal. ELF score >/ = 9.8 indicates advanced hepatic fibrosis and is influenced by age, steatosis and histological activity. Liver Int 2015;35:1673‐1681. [DOI] [PubMed] [Google Scholar]
- WHO Expert Consultation . Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157‐163. Erratum in: Lancet 2004;363:902. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Zimmet P, Shaw J. Metabolic syndrome‐‐a new world‐wide definition. A consensus statement from the International Diabetes Federation. Diabet Med 2006;23:469‐480. [DOI] [PubMed] [Google Scholar]
- Wong VW, Chan WK, Chitturi S, Chawla Y, Dan YY, Duseja A, etal. Asia‐Pacific Working Party on non‐alcoholic fatty liver disease guidelines 2017‐Part 1: definition, risk factors and assessment. JGastroenterol Hepatol 2018;33:70‐85. [DOI] [PubMed] [Google Scholar]
- Roulot D, Roudot‐Thoraval F, NKontchou G, Kouacou N, Costes JL, Elourimi G, etal. Concomitant screening for liver fibrosis and steatosis in French type 2 diabetic patients using Fibroscan. Liver Int 2017;37:1897‐1906. [DOI] [PubMed] [Google Scholar]
- Koehler EM, Plompen EP, Schouten JN, Hansen BE, Darwish Murad S, Taimr P, etal. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: The Rotterdam study. Hepatology 2016;63:138‐147. [DOI] [PubMed] [Google Scholar]
- Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, etal. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002;123:745‐750. [DOI] [PubMed] [Google Scholar]
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, etal.; Nonalcoholic Steatohepatitis Clinical Research Network . Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313‐1321. [DOI] [PubMed] [Google Scholar]
- Kwok R, Choi KC, Wong GL, Zhang Y, Chan HL, Luk AO, etal. Screening diabetic patients for non‐alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut 2016;65:1359‐1368. [DOI] [PubMed] [Google Scholar]
- Morling JR, Fallowfield JA, Guha IN, Nee LD, Glancy S, Williamson RM, etal.; Edinburgh Type 2 Diabetes Study investigators . Using non‐invasive biomarkers to identify hepatic fibrosis in people with type 2 diabetes mellitus: the Edinburgh type 2 diabetes study. JHepatol 2014;60:384‐391. [DOI] [PubMed] [Google Scholar]
- de Ledinghen V, Vergniol J. Transient elastography for the diagnosis of liver fibrosis. Expert Rev Med Devices 2010;7:811‐823. [DOI] [PubMed] [Google Scholar]
- Mikolasevic I, Orlic L, Franjic N, Hauser G, Stimac D, Milic S. Transient elastography (FibroScan((R))) with controlled attenuation parameter in the assessment of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease ‐ where do we stand? World J Gastroenterol 2016;22:7236‐7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ledinghen V, Wong VW, Vergniol J, Wong GL, Foucher J, Chu SH, etal. Diagnosis of liver fibrosis and cirrhosis using liver stiffness measurement: comparison between M and XL probe of FibroScan(R). JHepatol 2012;56:833‐839. [DOI] [PubMed] [Google Scholar]
- Wong VW, Vergniol J, Wong GL, Foucher J, Chan AW, Chermak F, etal. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol 2012;107:1862‐1871. [DOI] [PubMed] [Google Scholar]
- Wong GL, Vergniol J, Lo P, Wai‐Sun Wong V, Foucher J, Le Bail B, etal. Non‐invasive assessment of liver fibrosis with transient elastography (FibroScan(R)): applying the cut‐offs of M probe to XL probe. Ann Hepatol 2013;12:570‐580. [PubMed] [Google Scholar]
- Myers RP, Pomier‐Layrargues G, Kirsch R, Pollett A, Duarte‐Rojo A, Wong D, etal. Feasibility and diagnostic performance of the FibroScan XL probe for liver stiffness measurement in overweight and obese patients. Hepatology 2012;55:199‐208. [DOI] [PubMed] [Google Scholar]
- Weiss J, Rau M, Meertens J, Hering I, Reichert L, Kudlich T, etal. Feasibility of liver stiffness measurement in morbidly obese patients undergoing bariatric surgery using XL probe. Scand J Gastroenterol 2016;51:1263‐1268. [DOI] [PubMed] [Google Scholar]
- Durango E, Dietrich C, Seitz HK, Kunz CU, Pomier‐Layrargues GT, Duarte‐Rojo A, etal. Direct comparison of the FibroScan XL and M probes for assessment of liver fibrosis in obese and nonobese patients. Hepat Med 2013;5:43‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuppalanchi R, Siddiqui MS, Van Natta ML, Hallinan E, Brandman D, Kowdley K, etal.; NASH Clinical Research Network . Performance characteristics of vibration‐controlled transient elastography for evaluation of non‐alcoholic fatty liver disease. Hepatology 2018;67:134‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RP, Pomier‐Layrargues G, Kirsch R, Pollett A, Beaton M, Levstik M, etal. Discordance in fibrosis staging between liver biopsy and transient elastography using the FibroScan XL probe. JHepatol 2012;56:564‐570. [DOI] [PubMed] [Google Scholar]
- Wong GL, Chan HL, Choi PC, Chan AW, Yu Z, Lai JW, etal. Non‐invasive algorithm of enhanced liver fibrosis and liver stiffness measurement with transient elastography for advanced liver fibrosis in chronic hepatitis B. Aliment Pharmacol Ther 2014;39:197‐208. [DOI] [PubMed] [Google Scholar]
- Crespo G, Fernandez‐Varo G, Marino Z, Casals G, Miquel R, Martinez SM, etal. ARFI, FibroScan, ELF, and their combinations in the assessment of liver fibrosis: a prospective study. JHepatol 2012;57:281‐287. [DOI] [PubMed] [Google Scholar]
- Parkes J, Guha IN, Roderick P, Harris S, Cross R, Manos MM, etal. Enhanced Liver Fibrosis (ELF) test accurately identifies liver fibrosis in patients with chronic hepatitis C. JViral Hepat 2011;18:23‐31. [DOI] [PubMed] [Google Scholar]
- Lichtinghagen R, Pietsch D, Bantel H, Manns MP, Brand K, Bahr MJ. The Enhanced Liver Fibrosis (ELF) score: normal values, influence factors and proposed cut‐off values. JHepatol 2013;59:236‐242. [DOI] [PubMed] [Google Scholar]
- Angulo P, Kleiner DE, Dam‐Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, etal. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389‐397.e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, etal. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328‐357. [DOI] [PubMed] [Google Scholar]
- Tavakoli H, Robinson A, Liu B, Bhuket T, Younossi Z, Saab S, etal. Cirrhosis patients with nonalcoholic steatohepatitis are significantly less likely to receive surveillance for hepatocellular carcinoma. Dig Dis Sci 2017;62:2174‐2181. [DOI] [PubMed] [Google Scholar]
- Piscaglia F, Svegliati‐Baroni G, Barchetti A, Pecorelli A, Marinelli S, Tiribelli C, etal.; HCC‐NAFLD Italian Study Group . Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: a multicenter prospective study. Hepatology 2016;63:827‐838. [DOI] [PubMed] [Google Scholar]
- Walker M, El‐Serag HB, Sada Y, Mittal S, Ying J, Duan Z, etal. Cirrhosis is under‐recognised in patients subsequently diagnosed with hepatocellular cancer. Aliment Pharmacol Ther 2016;43:621‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep4.1208/full
Supporting Information


