Abstract
Liver cirrhosis (LC) is a major cause of secondary sarcopenia. Sarcopenia makes the prognosis worse; thus, novel therapeutic options for sarcopenia in patients with LC are urgently required as they are currently limited. In this retrospective study, 158 patients with LC were screened, and 35 of those patients who were treated with L‐carnitine for more than 6 months and for whom skeletal muscle mass changes could be evaluated by computer tomography were enrolled. Of the 158 patients, 79 patients who did not receive L‐carnitine supplementation served as controls. Cases and controls were propensity score matched for age, sex, presence of hepatocellular carcinoma, and branched chain amino acid administration, and changes in skeletal muscle mass and clinical data were compared. The 35 patients who received L‐carnitine supplementation and 35 propensity score‐matched patients who did not receive carnitine supplementation comprised the final enrollment. Compared with control patients, patients who received L‐carnitine had significantly worse liver function, which is associated with rapid progress of skeletal muscle depletion. However, loss of skeletal muscle mass was significantly suppressed in patients receiving L‐carnitine, and a significant effect was observed in patient subgroups stratified by age, sex, presence of hepatocellular carcinoma, and branched chain amino acid administration. The change ratios of most laboratory data, including vitamin D and insulin‐like growth factor 1 levels, were similar in the two groups, but ammonia levels were significantly less in those receiving L‐carnitine. However, even in patients receiving L‐carnitine but not showing an ammonia decrease, loss of skeletal muscle was significantly suppressed. Conclusion: L‐carnitine suppresses loss of skeletal muscle mass and may therefore be a novel therapeutic option for sarcopenia in patients with LC. (Hepatology Communications 2018; 00:000‐000)
Sarcopenia is defined as skeletal muscle mass depletion and a decrease in muscle strength.1, 2 These changes are generally observed with aging and result in a high risk of falls, disturbance of gait, and reduction in the ability to perform activities of daily living. Aging negatively affects protein synthesis,3 and skeletal muscle mass decreases progressively year by year in elderly people. This aging‐related sarcopenia is defined as primary sarcopenia. Several diseases, including inflammatory disease, malignancy, chronic kidney disease, and chronic liver disease, are reported to cause secondary sarcopenia.4 The prevalence of sarcopenia in patients with liver cirrhosis (LC) is high and is estimated to be around 40%.5 Because hepatocytes perform the central function of glucose, lipid, and protein metabolism, dysfunction of the liver causes a glycogen storage dysfunction in the liver that facilitates the utilization of glycogen and branched chain amino acids (BCAAs) from the skeletal muscle, resulting in the progression of proteolysis.6 In addition, hyperammonemia induced by LC causes high concentrations of myostatin, which strongly inhibits skeletal muscle synthesis.7 In patients with LC and poor liver function, loss of skeletal muscle mass progresses more rapidly.8 Importantly, the complications associated with sarcopenia in patients with LC make the prognosis worse.9, 10 Thus, reagents that can suppress development or progression of mass depletion of skeletal muscle or sarcopenia in patients with LC are urgently required; however, therapeutic options remain limited.11
L‐carnitine (L‐b‐hydroxy‐g‐N‐trimethylaminobutyric acid) is an essential nutrient that plays a pivotal role in fatty acid metabolism. Carnitine binds to long‐chain acyl‐coenzyme A and converts it to acylcarnitine, which is transported to the mitochondria. Carnitine is absorbed mainly from food; however, one fourth of carnitine is synthesized in the kidney and liver. Primary carnitine deficiency causes hepatic steatosis, hepatomegaly, hyperammonemia, skeletal myopathy, and cardiomyopathy.12, 13 Secondary carnitine deficiency can cause various disorders, including increased renal tubular loss of carnitine (Fanconi syndrome), hemodialysis, a poor diet, and LC.14
In Japan, L‐carnitine is sometimes prescribed to patients with LC, targeting carnitine deficiency. Several reports have documented that L‐carnitine supplementation resulted in restoration of hyperammonemia and improvements in muscle cramp symptoms in patients with LC.15, 16 In addition, supplementation of carnitine improved liver steatosis and fibrosis in subjects with nonalcoholic steatohepatitis (NASH).17
To the best of our knowledge, it remains unclear whether carnitine supplementation affects sarcopenia and loss of skeletal muscle mass in patients with LC. Therefore, in this retrospective propensity score‐matched study, we investigated the effects of carnitine supplementation on the loss of skeletal muscle mass in patients with LC.
Patients and Methods
STUDY DESIGN AND PATIENTS
In this retrospective study, we screened a total of 158 patients with LC between June 2012 and September 2017 at Hokkaido University Hospital. Of those, a total of 35 patients were treated with L‐carnitine supplementation and were followed for more than 6 months. These 35 patients met the following inclusion criteria: (i) initial computed tomography (CT) conducted in the 100 days before initiation of L‐carnitine supplementation and (□A) subsequent CT scans conducted at least 6 months later that enabled evaluation of the change in skeletal muscle mass and clinical parameters. CT or magnetic resonance imaging examinations were typically conducted in patients with LC every 6 to 12 months according to the guidelines of the Japan Society of Hepatology.18 Patients were excluded if they did not have adequate clinical information or they discontinued L‐carnitine supplementation during the observational period. In addition, of the 158 patients with LC who were screened, 79 patients who did not receive L‐carnitine supplementation and underwent a paired CT scan during the study period and a second CT scan more than 6 months after the initial CT scan served as controls. Cases (patients with L‐carnitine supplementation) and controls were matched for age, sex, presence of hepatocellular carcinoma (HCC), and BCAA administration, using propensity score matching (Fig. 1). Changes in skeletal muscle mass and clinical data were compared to clarify the effect of carnitine supplementation on patients with LC.
Figure 1.
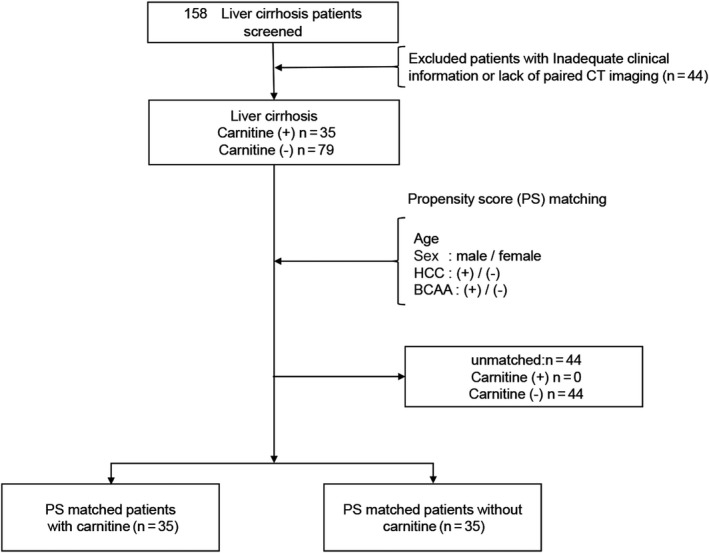
Patient flow chart. Of 158 patients with LC who were screened, 44 were excluded due to inadequate clinical information or lack of paired CT imaging. Thus, 35 patients who were administered L‐carnitine and 79 control patients who were not administered L‐carnitine were enrolled. After propensity score matching for age, sex, presence of HCC, and supplementation of BCAAs, 35 patients who received L‐carnitine supplementation were selected as cases and 35 patients who did not receive carnitine supplementation were selected as matched controls.
In this study, LC was diagnosed by liver biopsy, Fibroscan data, or radiologic findings, such as CT or magnetic resonance imaging, and laboratory data. The study protocol conformed to the ethical guidelines of the Declaration of Helsinki and was approved by the ethics committee of Hokkaido University Hospital. Informed consent was obtained from all participating patients. This study was registered at the University Hospital Medical Information Network Clinical Trials Registry as UMIN000030755.
CALCULATION METHOD FOR PSOAS MUSCLE MASS INDEX
Skeletal muscle mass was calculated using the psoas muscle mass index (PMI) on CT images. We obtained CT images using a multidetector CT scanner (Aquilion 64; Toshiba Medical Systems, Tochigi, Japan). The PMI was calculated as follows: the sum of the L3 level cross‐sectional area of the right and left psoas muscle mass was measured by manual tracing, and this value was divided by height squared (cm2/m2).19 To evaluate the monthly change in PMI, Δ PMI/month (%) was calculated as follows: Δ PMI/month (%) = ([psoas muscle area on the second CT scan – psoas muscle area on the initial CT scan]/psoas muscle area on the initial CT scan) × 100/interval between CT scans (m).8
CLINICAL AND LABORATORY ASSESSMENT
Patients underwent physical examinations and blood tests at least every 3 months. Treatment safety and tolerability were monitored at every assessment point. Clinical characteristics and laboratory data were collected at the start of carnitine supplementation in patients receiving L‐carnitine, at the initial CT image conduction point in the control group, and at the second CT image conduction point in both groups. Clinical data included body mass index (kg/m2), etiology of LC, Child‐Pugh grade, presence of HCC, stage of HCC, and blood test results (white blood cells, hemoglobin, platelet counts, prothrombin time, serum albumin, ammonia, total bilirubin, aspartate aminotransferase, alanine aminotransferase, cholinesterase, and alpha‐fetoprotein). Serum insulin‐like growth factor 1 (IGF1) and 25‐hydroxyvitamin D (25(OH) vitamin D) levels were analyzed by immunoradiometric assay (SRL, Tokyo, Japan).20
In addition, because the IGF1 level is affected by age and sex, we analyzed it using sex‐ and age‐adjusted normalizing z scores according to Lee etal.21 The severity of liver disease was evaluated by the Child‐Pugh score. We monitored the change of concomitant drugs during the observational period; this included BCAAs, which were administered to patients with lower serum albumin levels (<3.5 g/dL) based on the judgement of the attending doctor.
Changes in PMI and Δ PMI/month (%) were evaluated and compared between patients with L‐carnitine supplementation and controls. In addition, the changes in clinical data, including Child‐Pugh score, were compared between patients with L‐carnitine supplementation and controls.
STATISTICAL ANALYSIS
Continuous variables were analyzed using the paired Mann‐Whitney U test, and categorical variables were analyzed using the Fisher's exact test. We applied 1:1 propensity score matching to balance the assignment of patients with L‐carnitine supplementation; the variables were age, sex, presence of HCC, and administration of BCAAs.22 We selected the variables that affect skeletal muscle mass and sarcopenia.23, 24 The model's reliability was measured with the Hosmer–Lemeshow test. All P values were two tailed, and the level of significance was set at P < 0.05. All statistical data were generated using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphic user interface for R25 or Prism 7.03 (GraphPad Software, Inc., La Jolla, CA).
Results
PATIENT CHARACTERISTICS
Of the 158 patients with LC who were screened, 35 patients taking L‐carnitine and 79 control patients were enrolled. After propensity score matching for age,sex, the presence of HCC, and administration of BCAAs, 35 patients who were not taking L‐carnitine were selected as matched controls. We administered L‐carnitine to 27, 6, and 2 patients with LC for hyperammonemia, muscle cramp, and for prevention of carnitine deficiency due to LC, respectively. After propensity score matching, the two groups with LC were well matched (Table 1); the propensity score derivation model was confirmed by the Hosmer–Lemeshow test, and the value was not significant (P = 0.65). The baseline characteristics in patients taking L‐carnitine and controls are shown in Table 1. Compared with the controls, patients with L‐carnitine supplementation had significantly worse liver function parameters (higher Child‐Pugh scores and ammonia levels and lower platelet counts, white blood cell counts, prothrombin times, serum albumin levels, hemoglobin levels, and cholinesterase levels), which were associated with rapid progression of skeletal muscle mass depletion.8 The distribution of LC etiologies was similar in the two groups. The average L‐carnitine dose during the observational period was 1,018 mg/day.
Table 1.
Comparison of Baseline Clinical and Biochemical Characteristics in Patients With L‐Carnitine Supplementation and Controls
| Variables |
Overall (n = 70) |
L‐Carnitine Oral Administration Group (n = 35) |
L‐Carnitine Nonoral Administration Group (n = 35) |
P Value |
|---|---|---|---|---|
| Age, years | 67 (20‐87) | 67 (20‐87) | 67 (31‐83) | 0.38 |
| Sex, male/female | 53/17 | 27/8 | 26/9 | >0.99 |
| Etiology, HBV/HCV/NBNC | 19/18/33 | 8/12/15 | 11/6/18 | 0.31 |
| Hepatocellular carcinoma, +/− | 35/35 | 17/18 | 18/17 | >0.99 |
| Stage, I/II/III/IV | 11/8/7/8 | 5/3/3/6 | 6/5/4/2 | 0.51 |
| Child‐Pugh grade, A/B/C | 30/33/7 | 10/19/6 | 20/14/1 | 0.03 |
| Child‐Pugh score | 7 (5‐13) | 7 (5‐13) | 6 (5‐10) | 0.02 |
| Follow‐up period, month | 11 (6‐20) | 11 (7‐20) | 11 (6‐14) | 0.34 |
| BCAA at initial point, +/− | 45/25 | 23/12 | 22/13 | >0.99 |
| Addition of BCAA during the observational period, +/− | 8/17 | 5/7 | 3/10 | 0.41 |
| Total BCAA | 53/17 | 28/7 | 25/10 | 0.58 |
| Psoas muscle mass, cm2/m2 |
M 3.37 (1.20‐6.37) F 2.07 (0.54‐4.25) |
M 3.15 (1.76‐6.37) F 1.73 (0.54‐3.74) |
M 3.49 (1.20‐5.13) F 2.15 (0.97‐4.25) |
M 0.45 F 0.32 |
| Body mass index, kg/m2 | 24.6 (17.8‐35.0) | 24.7 (18.3‐35.0) | 24.6 (17.8‐31.2) | 0.81 |
| White blood cell,/μL | 3,900 (1,200‐9,700) | 3,600 (2,200‐7,000) | 4,700 (1,200‐9,700) | 0.04 |
| NLR | 2.18 (0.67‐9.00) | 2.04 (0.83‐9.00) | 2.20 (0.67‐5.13) | 0.77 |
| Hemoglobin, mg/dL | 12.5 (6.4‐17.40) | 11.3 (6.4‐17.0) | 13.8 (8.5‐17.4) | 0.01 |
| Platelet counts, ×104 /mm3 | 8.70 (1.90‐26.1) | 7.9 (3‐18) | 11.8 (1.9‐26.1) | < 0.01 |
| Prothrombin time, % | 71.3 (19.5‐109.5) | 63.7 (23.4‐107.8) | 78.8 (19.5‐98) | < 0.01 |
| Serum albumin, g/dL | 3.6 (2.0‐4.8) | 3.4 (2.0‐4.4) | 3.7 (2.5‐4.8) | < 0.01 |
| Total bilirubin, mg/dL | 1.1 (0.4‐5.1) | 1.3 (0.4‐5.1) | 1.0 (0.4‐3.2) | 0.09 |
| Aspartate aminotransferase, IU/L | 35 (14‐88) | 41.5 (16‐88) | 32 (14‐76) | 0.09 |
| Alanine aminotransferase, IU/L | 26 (7‐67) | 27 (8‐61) | 24 (7‐67) | 0.46 |
| γ‐glutamyltransferase, IU/L | 41 (10‐350) | 36.5 (10‐142) | 76 (12‐350) | < 0.01 |
| Ammonia, mg/dL | 72 (11‐280) | 113 (16‐280) | 46 (11‐192) | < 0.01 |
| Cholinesterase, IU/L | 177 (68‐439) | 164 (68‐305) | 229 (95‐439) | < 0.01 |
| eGFR, mL/minute/1.73 m2 | 76.3 (38.2‐172.1) | 75.5 (44.1‐143.6) | 79.8 (38.2‐172.1) | 0.89 |
| C reactive protein, mg/dL | 0.10 (0.02‐2.22) | 0.10 (0.02‐2.22) | 0.08 (0.02‐1.91) | 0.83 |
| HbA1c, % | 5.7 (3.6‐9.6) | 5.5 (3.6‐9.6) | 5.8 (4.4‐8.9) | 0.20 |
| Alpha‐fetoprotein, ng/mL | 5.7 (1.0‐200,115.9) | 7.55 (1.0‐1,512.7) | 4.95 (1.2‐200,115.9) | 0.14 |
| FIB4‐index | 5.70 (1.11‐26.67) | 6.64 (1.11‐18.59) | 3.27 (1.54‐26.67) | < 0.01 |
Data are presented as number of patients or median (range) values.
Abbreviations: eGFR, estimated glomerular filtration ratio; FIB4, fibrosis‐4; HbA1c, hemoglobin A1c; HBV, hepatitis B virus; NBNC, non‐HBV non‐HCV; NLR, neutrophil–lymphocyte ratio.
During the observational period, a total of 8 patients were started on BCAAs (5 patients received L‐carnitine and 3 controls); however, the total number of patients administered BCAAs was still similar in the two groups (P = 0.59).
COMPARISON OF CHANGE IN Δ PMI IN PATIENTS TAKING L‐CARNITINE AND CONTROLS
We evaluated the change between the initial and second CT scans in the cross‐sectional areas of the right and left psoas muscles at the L3 level using manual tracing.19 The initial CT scan showed a similar overall median value of PMI for the two groups (3.07 [range, 0.54‐6.37] cm2/m2 and 3.36 [0.97‐5.13] cm2/m2; P = 0.37). Median values of PMI in male and female patients were similar in the L‐carnitine and control groups (P = 0.45 and P = 0.32, respectively) (Fig. 2A). At the second CT scan imaging point, the overall median values of PMI were similar in the two groups (2.97 [0.74‐5.77] cm2/m2 and 3.02 [0.88‐4.84] cm2/m2; P = 0.54), and the median values of PMI in male and female patients were similar in the two groups (P = 0.96 and P = 0.49, respectively) (Fig. 2B). However, the median Δ PMI/month value was 0.27% (range, −6.72%‐4.07%) in the L‐carnitine group and −1.24% (range, −6.26%‐4.07%) in the control group, and this difference was significant (P < 0.01) (Fig. 2C). Likewise, this significant difference was observed in male and female subgroups (P = 0.01 and P < 0.01, respectively) (Fig. 2C).
Figure 2.
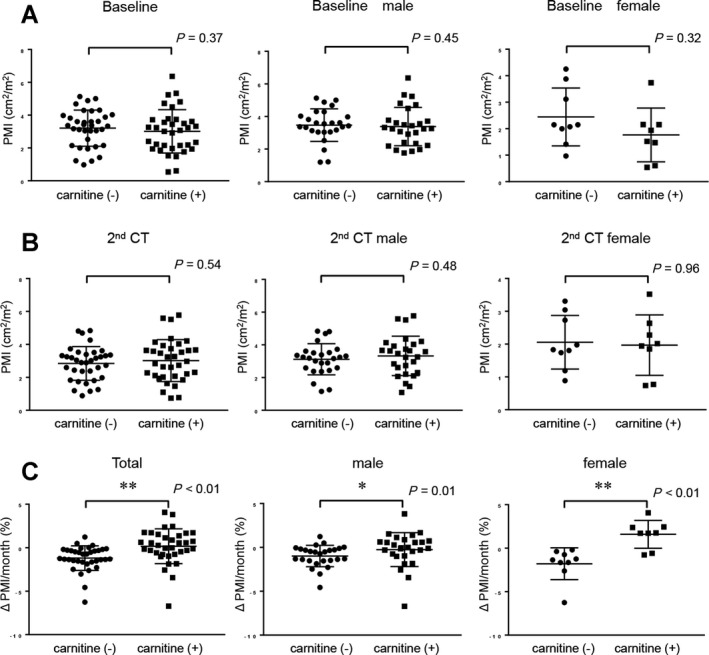
Comparison of PMI and Δ PMI/month in patients with L‐carnitine supplementation and controls. (A) Comparison of baseline PMI in patients with L‐carnitine supplementation and controls (overall cohort, males, and females). (B) Comparison of PMI at the second CT examination point in patients with L‐carnitine supplementation and controls (overall cohort, males, and females). (C) Comparison of Δ PMI/month in patients with L‐carnitine supplementation and controls (overall cohort, males, and females). Data were analyzed with the Mann‐Whitney U test. Asterisk indicates a statistically significant difference (*P < 0.05, **P < 0.01).
STRATIFIED ANALYSIS OF CHANGES IN PMI
We next conducted stratified analyses to clarify the effect of L‐carnitine supplementation on PMI reduction in patients less than 65 years and patients 65 years and older. L‐carnitine supplementation significantly suppressed loss of skeletal muscle regardless of age (P= 0.01 in patients <65 years old and P = 0.01 in patients ≥65 years old) (Fig. 3A,B).
Figure 3.
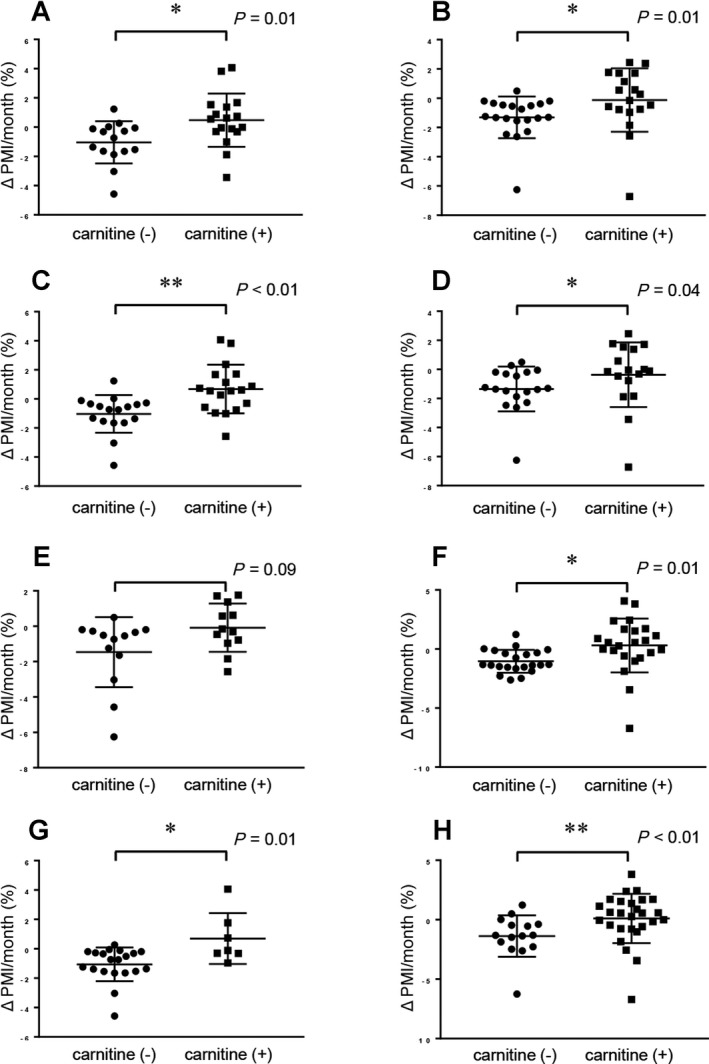
Comparison of Δ PMI/month in patients with L‐carnitine supplementation and controls in subgroups stratified according to age, presence of HCC, and BCAA supplementation. (A) Comparison of Δ PMI/month in patients with L‐carnitine supplementation and controls (patients with LC and less than 65 years old). (B) Comparison of Δ PMI/month in patients with L‐carnitine supplementation and controls (patients with LC and 65 years and older). (C) Comparison of Δ PMI/month in patients with L‐carnitine supplementation and controls (patients with LC without HCC). (D) Comparison of Δ PMI/month in patients with L‐carnitine supplementation and controls (patients with LC with HCC). (E) Comparison of Δ PMI/month in patients with L‐carnitine supplementation and controls (patients with LC not taking BCAA supplementation at baseline). (F) Comparison of Δ PMI/month in patients with L‐carnitine supplementation and controls (patients with LC taking BCAA supplementation at baseline). (G) Comparison of Δ PMI/month in patients with L‐carnitine supplementation and controls (patients with LC who did not experience an ammonia decrease). (H) Comparison of Δ PMI/month in patients with L‐carnitine supplementation and controls (patients with LC who experienced an ammonia decrease). Data were analyzed with the Mann‐Whitney U test. Asterisk indicates a statistically significant difference (*P < 0.05, **P < 0.01).
We also compared patients with and without HCC and patients who received BCAA supplementation and those who did not. L‐carnitine supplementation significantly suppressed the loss of skeletal muscle in patients with and without HCC (Fig. 3C,D) and in patients who received BCAA administration and those who did not (P = 0.01) (Fig. 3F). In patients who were not receiving BCAA supplementation at baseline, carnitine supplementation tended to suppress the loss of skeletal muscle (P = 0.08), but the difference was not significant (Fig. 3E).
Regardless of whether or not a patient had decreased ammonia levels at the second CT scan point (compared with the initial level) (Fig. 3G,H), L‐carnitine supplementation significantly suppressed progression of skeletal muscle loss. Additionally, as shown in http://onlinelibrary.wiley.com/doi/10.1002/hep4.1207/full, patients with L‐carnitine supplementation showed significantly less loss of skeletal muscle compared with control group patients, regardless of changes in ammonia levels between the initial and second CT points.
COMPARISON OF CHANGES IN LABORATORY DATA AND CLINICAL FACTORS IN PATIENTS TAKING L‐CARNITINE AND CONTROLS
Clinical factors at the second CT evaluation point in patients with L‐carnitine supplementation and controls are shown in Table 2. Compared to control patients, patients with L‐carnitine supplementation had significantly lower platelet counts, white blood cell counts, prothrombin times, serum albumin and hemoglobin levels, and cholinesterase levels. Ammonia levels were similar (67 [9‐349] mg/dL in the L‐carnitine group and 53 [15‐190] mg/dL in the control group; P= 0.55) at the second CT evaluation point. Next, we compared the changes in laboratory data between the initial and second CT points in patients with L‐carnitine supplementation and controls. Ammonia levels were significantly decreased in patients with L‐carnitine supplementation compared to controls (−3.37% and 1.28% in the L‐carnitine and control groups, respectively; P < 0.01) (Table 3). Oher measurements were not significantly different between the two groups.
Table 2.
Comparison of Clinical and Biochemical Characteristics in Patients With LC With or Without Carnitine Supplementation at the Second CT Imaging Point
| Variables |
Overall (n = 70) |
L‐Carnitine Oral Administration Group (n = 35) |
L‐Carnitine Nonoral Administration Group (n = 35) |
P Value |
|---|---|---|---|---|
| Psoas muscle mass (at 2nd CT) |
M 3.25 (1.10‐5.77) F 1.94 (0.74‐3.52) |
M 3.47 (1.10‐5.77) F 1.97 (0.74‐3.52 |
M 3.22 (1.15‐4.84) F 1.83 (0.88‐3.31) |
M 0.49 F 0.96 |
| White blood cell count,/μL | 4,100 (1,200‐12,200) | 3,800 (1,900‐6,300) | 4,400 (1,200‐12,200) | <0.01 |
| NLR | 2.85 (0.59‐9.86) | 3.10 (0.88‐9.86) | 2.70 (0.59‐8.70) | 0.60 |
| Hemoglobin, mg/dL | 11.8 (6.7‐18.2) | 11.2 (6.7‐17.3) | 12.8 (7.3‐18.2) | 0.04 |
| Platelet counts, ×104/mm3 | 9.3 (2.1‐31.2) | 7.7 (3.2‐31.2) | 11.1 (2.1‐25) | <0.01 |
| Prothrombin time, % | 70.8 (29.5‐106.2) | 63 (30‐97.6) | 74.2 (29.5‐106.2) | 0.03 |
| Serum albumin, g/dL | 3.7 (1.8‐4.7) | 3.2 (1.8‐4.5) | 3.8 (2.2‐4.7) | <0.01 |
| Total bilirubin, mg/dL | 1.1 (0.3‐4.6) | 1.4 (0.3‐3.5) | 1.1 (0.4‐4.6) | 0.17 |
| Aspartate aminotransferase, IU/L | 34 (12‐157) | 37 (23‐140) | 31 (12‐157) | 0.13 |
| Alanine aminotransferase, IU/L | 25 (7‐89) | 27 (11‐65) | 22 (7‐89) | 0.11 |
| γ‐glutamyltransferase, IU/L | 44 (10‐607) | 32 (10‐588) | 80 (12‐607) | <0.01 |
| Ammonia, mg/dL | 53 (9‐349) | 67 (9‐349) | 53 (15‐190) | 0.55 |
| Cholinesterase, IU/L | 185 (26‐394) | 153 (26‐281) | 204 (99‐394) | <0.01 |
| eGFR, mL/minute/1.73 m2 | 72.3 (27.4‐176.7) | 65.6 (37.4‐176.7) | 78.3 (27.4‐140.9) | 0.37 |
| C reactive protein, mg/dL | 0.13 (0.02‐4.11) | 0.22 (0.02‐9.58) | 0.115 (0.02‐2.26) | 0.51 |
| HbA1c, % | 5.8 (3.4‐9.2) | 5.8 (3.4‐9.2) | 5.85 (4.6‐9.0) | 0.39 |
| Alpha‐fetoprotein, ng/mL | 4.7 (1.0‐413503) | 7.8 (1.0‐38,967.0) | 4.0 (1.2‐413,503) | 0.30 |
| FIB4‐index | 5.65 (0.73‐27.17) | 6.69 (0.73‐18.77) | 3.91 (1.24‐27.17) | 0.01 |
Data are presented as number of patients or median (range) values.
Abbreviations: eGFR, estimated glomerular filtration ratio; FIB4, fibrosis‐4; HbA1c, hemoglobin A1c; NLR, neutrophil–lymphocyte ratio.
Table 3.
Comparison of Changes in Biochemical Profiles and Clinical Factors in Patients With LC With or Without L‐Carnitine Supplementation
| Variables |
Overall (n = 70) |
L‐Carnitine Oral Administration Group (n = 35) |
L‐Carnitine Nonoral Administration Group (n = 35) |
P Value |
|---|---|---|---|---|
| White blood cell count, /μL | 0.00 (−3,400‐7,400) | −1,000 (−3,400‐2,100) | 1,000 (−1,900‐7,400) | 0.16 |
| NLR | 0.06 (−7.19‐7.24) | 0.06 (−7.19‐7.24) | 0.11 (−0.80‐5.26) | 0.47 |
| Hemoglobin, mg/dL | −0.40 (−4.70‐5.80) | 0.00 (−4.70‐5.80) | −0.70 (−4.10‐3.90) | 0.20 |
| Platelet counts, ×104/mm3 | 0.10 (−15.00‐7.80) | 0.20 (−4.90‐6.10) | 0.10 (−15.00‐7.80) | 0.44 |
| Prothrombin time, % | −1.45 (−35.3‐27.1) | −2.7 (−27.0‐39.0) | 0.70 (−35.3‐27.1) | 0.54 |
| Serum albumin, g/dL | 0.00 (−1.30‐1.40) | −0.05 (−1.30‐1.40) | 0.00 (−1.10‐1.00) | 0.94 |
| Total bilirubin, mg/dL | −0.1 (−3.8‐1.8) | −0.1 (−3.8‐1.8) | −0.1 (−0.5‐1.4) | 0.83 |
| Aspartate aminotransferase, IU/L | 1 (−55‐98) | 1 (−55‐93) | 1 (−27‐98) | 0.70 |
| Alanine aminotransferase, IU/L | 0 (−27‐55) | 0.50 (−27‐39) | −1.0 (−24‐55) | 0.49 |
| γ‐glutamyltransferase, IU/L | −1 (−222‐546) | −1 (−115‐546) | −1 (−222‐430) | 0.82 |
| Ammonia, mg/dL | −9 (−146‐146) | −31 (−146‐146) | 6 (−88‐115) | <0.01 |
| Cholinesterase, IU/L | −5.5 (−130‐68) | −2 (−66‐51) | −8 (−130‐68) | 0.78 |
| eGFR, mL/minute/1.73 m2 | −1.3 (−65.4‐33.1) | −2.3 (−65.4‐33.1) | −0.4 (−31.2‐23.0) | 0.35 |
| C reactive protein, mg/dL | 0.00 (−1.65‐4.05) | 0.00 (−1.65‐4.05) | 0.00 (−1.63‐1.07) | 0.15 |
| HbA1c, % | 0.0 (−2.1‐2.9) | 0.2 (−2.1‐1.7) | 0.0 (−2.1‐2.9) | 0.29 |
| Alpha‐fetoprotein, ng/mL | −0.25 (−1,434.1‐213,387.1) | −0.35 (−1,434.1‐37,454.6) | −0.05 (−11.0‐213,387.1) | 0.48 |
| FIB4‐index | 0.08 (−5.53‐16.18) | −0.11 (−5.53‐6.81) | 0.12 (−2.80‐16.18) | 0.56 |
Data are presented as number of patients or median (range) values.
Abbreviations: eGFR, estimated glomerular filtration ratio; FIB4, fibrosis‐4; HbA1c, hemoglobin A1c; NLR, neutrophil–lymphocyte ratio.
COMPARISON OF CHANGES IN VITAMIN D AND IGF1 IN PATIENTS TAKING L‐CARNITINE AND CONTROLS
We subsequently analyzed the change in serum concentration of 25(OH) vitamin D and IGF1,both of which have been reported to be associated with loss of skeletal muscle.26, 27 Baseline IGF1 levels and IGF1 z scores were lower in patients with L‐carnitine supplementation compared to controls (P = 0.08 for IGF1 levels; P = 0.06 for IGF1 z scores) (Fig. 4). The Δ IGF1 and Δ IGF1 z scores were not significantly different between patients with L‐carnitine supplementation and controls (P = 0.61 and P = 0.80, respectively). Baseline levels of 25(OH) vitamin D were significantly lower (P < 0.01) in patients with L‐carnitine supplementation compared to controls, but Δ 25(OH) vitamin D did not differ significantly between the two groups (P = 0.15).
Figure 4.
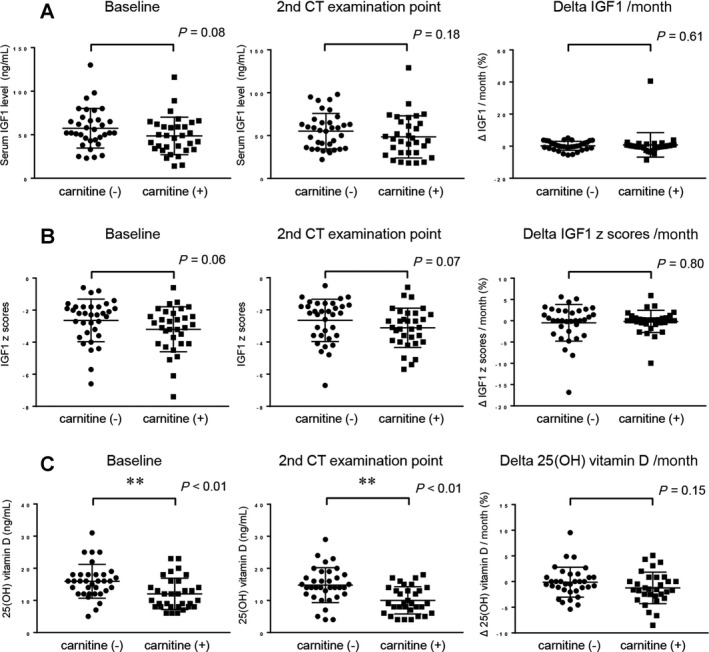
Comparison of serum IGF1 and 25(OH) vitamin D levels at baseline and at the second CT examination point and the change in IGF1 in patients with L‐carnitine supplementation and controls. (A) Comparison of serum IGF1 levels at baseline, at the second CT examination point, and Δ IGF1 in patients with L‐carnitine supplementation and controls. (B) Comparison of IGF1 z scores at baseline, at the second CT examination point, and Δ IGF1 z scores in patients with L‐carnitine supplementation and controls. (C) Comparison of serum 25(OH) vitamin D 1 levels at baseline, at the second CT examination point, and Δ 25(OH) vitamin D in patients with L‐carnitine supplementation and controls. Data were analyzed with the Mann‐Whitney U test. Asterisk indicates a statistically significant difference (*P < 0.05, **P < 0.01).
Discussion
Liver cirrhosis is one of the major causes of secondary sarcopenia. Importantly, complications of sarcopenia make the prognosis worse in patients with LC,9, 10 and therapeutic options are still limited. Thus, novel therapeutic options for these patients are urgently required. In this study, we analyzed the effect of L‐carnitine supplementation in patients with LC, focusing on changes in skeletal muscle mass. Median Δ PMI/month values were 0.27% and −1.24% in patients with L‐carnitine supplementation and propensity score‐matched controls, respectively, and this difference was significant (P < 0.01) (Fig. 2C). Although patients with L‐carnitine supplementation had significantly worse liver dysfunction (Table 1), which is associated with rapid progression of skeletal muscle depletion,8 loss of skeletal muscle mass was significantly suppressed in patients with L‐carnitine supplementation. To the best of our knowledge, this is the first study to show that L‐carnitine supplementation has a preventive effect on skeletal muscle depletion in patients with LC. Thus, L‐carnitine might be a novel therapeutic option for sarcopenia in patients with LC.
Several mechanisms are thought to be associated with the development of secondary sarcopenia and loss of skeletal muscle mass in patients with LC. In patients with LC, both suppression of protein synthesis and progress of protein catabolism are thought to exist, resulting in loss of skeletal muscle mass. IGF1 is produced by the liver and is associated with protein synthesis. Decreased levels of free IGF1 are sometimes observed in patients with LC,28 and this is suspected to be due to a decrease in the IGF1 production ability of the liver and/or growth hormone receptors in the liver.29 In addition, vitamin D deficiency is usually observed in liver disease, especially in patients with LC. Vitamin D signaling by the vitamin D receptor, which exists in muscle cells,30 regulates myoblast proliferation and differentiation31 and thus decreases IGF1 and vitamin D, which is thought to be one of the mechanisms of skeletal muscle depletion in patients with LC. In addition, the liver is the central organ involved in the metabolism of glucose, lipids, and protein; thus, dysfunction of the liver causes protein energy malnutrition.6 With protein energy malnutrition, the liver cannot store enough glycogen, and consequently amino acids, including BCAAs, and glycogen in the muscle mass are used, resulting in skeletal muscle loss and loss of muscle strength.6 BCAAs have strong anabolic effects on protein metabolism by increasing protein synthesis and decreasing protein degradation. BCAA deficiency is usually observed in patients with LC, resulting in dysfunction of protein synthesis. Furthermore, systemic inflammation, including increased radical oxygen species or chronic inflammation, is usually observed in patients with LC.32 Elevated radical oxygen species suppress protein synthesis, and an inflammatory cytokine, like interleukin‐6 or tumor necrosis factor alpha, progress protein catabolism.33 In addition, hyperammonemia is a major problematic complication in patients with LC and could cause hepatic encephalopathy. Increased ammonia affects the skeletal muscle and activates transforming growth factor beta activated kinase 1, resulting in up‐regulated expression of myostatin.11, 34 Myostatin is a cytokine of the transforming growth factor beta family and suppresses synthesis of skeletal muscle,35 resulting in loss of muscle mass. This complex mechanism is thought to be associated with the development of sarcopenia and loss of skeletal muscle mass in patients with LC; thus, various mechanisms of this therapeutic option might be effective.
To date, therapeutic approaches for patients with LC and sarcopenia have focused on nutrient supplementation and physical activity; however, these therapeutic approaches have not always been effective.36 Recent studies revealed that BCAA supplementation prevented the development of sarcopenia in patients with LC.37 However, novel therapeutic options for patients with LC are still required, especially those focusing on novel mechanisms, including antioxidants and mitochondrial protective agents.36 This study is the first to show the possibility that L‐carnitine supplementation can prevent loss of skeletal muscle in patients with LC. Stratified analysis revealed that this effect was also observed in patients who took BCAAs at the initiation point; thus, L‐carnitine might have the additional effect of preventing loss of skeletal muscle even in patients taking BCAAs.
Muscle changes of between −2% and +2% /100 days were considered “maintenance of tissue” in other reports.8, 38 In this study, the median Δ PMI/month was −1.24% (range, −6.26%‐4.07%) in the control group, and these values were nearly equal to −4.1%/100 days. On the other hand, the mean Δ PMI/month value was 0.27% (range, −6.72%‐4.07%) in the carnitine administration group, and these values were nearly equal to 0.9%/100 days. Thus, in the carnitine group, the PMI was stable, which is in contrast to the control group. The mechanism by which carnitine prevents loss of skeletal muscle has not been clarified; however, there are several hypotheses. Carnitine plays a central role in the transport of long‐chain fatty acids from the cytosol to the mitochondrial matrix. Carnitine binds to long‐chain acyl‐coenzyme A and converts it to acylcarnitine, which is transported to the mitochondria and degraded by β‐oxidation.39 Thus, deficiency of carnitine causes lipid metabolism dysfunction, resulting in hepatic steatosis, skeletal myopathy, and cardiomyopathy.12, 13
Liver cirrhosis is a major cause of secondary carnitine deficiency.14 Several reports have revealed that carnitine supplementation has a positive effect on various liver diseases. Carnitine supplementation reportedly improves liver steatosis and fibrosis in patients with NASH17 and decreases alanine aminotransferase levels in patients infected with hepatitis B virus.40 In addition, we revealed that carnitine supplementation suppresses hepatitis C virus (HCV) replication and HCV‐induced oxidant stress.41 Importantly, L‐carnitine supplementation reduces ammonia levels, which is thought to reflect the ability of L‐carnitine to improve hepatic mitochondrial function.42 Similarly, carnitine supplementation significantly improved hyperammonemia, as shown in Table 3. Hyperammonemia causes elevation of myostatin, which strongly suppresses muscle synthesis.35 Thus, ammonia reduction ability might be one of the main mechanisms behind the prevention of skeletal muscle mass loss in patients with LC. However, patients with LC who experienced reduced ammonia levels and those who did not benefitted significantly from carnitine supplementation, which suppressed the loss of skeletal muscle mass (Fig. 3G,H; http://onlinelibrary.wiley.com/doi/10.1002/hep4.1207/full). Thus, carnitine might have another therapeutic effect other than reducing ammonia levels.
Systemic inflammation with an increase in radical oxygen species is a cause of skeletal muscle loss in patients with LC.33 Gulçin43 identified the antioxidant activities of carnitine in an in vitro model, and Ishikawa etal.44 used the NASH mouse model to show that carnitine reduces oxidative stress by up‐regulating mitochondrial β‐oxidation and redox systems in the liver. Moreover, we previously reported that carnitine supplementation reduced oxidative stress using an in vitro HCV infection model. Thus, carnitine had antioxidant effects that might protect against skeletal muscle loss. In patients with cancer cachexia, in which chronic systemic inflammation and increased oxidative stress are major pathogeneses and skeletal muscle loss is a major feature, carnitine supplementation is effective for symptoms of cachexia through anti‐inflammatory and oxidant effects.45 In this study, the inflammatory parameters, such as the neutrophil–lymphocyte ratio and C reactive protein, in patients with L‐carnitine supplementation without a reduction in ammonia tended to decrease compared to controls without reduced ammonia (http://onlinelibrary.wiley.com/doi/10.1002/hep4.1207/full). In addition, most absorbed or produced carnitine exists in the muscle46; thus, skeletal muscle is affected by states of carnitine deficiency, and several reports have indicated that carnitine supplementation may increase skeletal muscle in patients with L‐carnitine deficiency.47 On the other hand, carnitine supplementation did not affect the change in vitamin D and IGF1 levels, as shown in Fig. 4. Thus, combining carnitine with reagents that improve IGF1 and vitamin D levels might also be effective for preventing progression of skeletal muscle mass loss in patients with LC.
There are several methods of calculating skeletal muscle mass. The CT‐determined skeletal muscle index (SMI) is a well‐known and established index for calculation of skeletal muscle mass48; however, special software is required.49 In contrast, the measurement of PMI does not require any software and is reported to be strongly correlated with the SMI.19, 50 In addition, we confirmed a significant relationship between SMI and PMI in the limited number of patients included in this study (http://onlinelibrary.wiley.com/doi/10.1002/hep4.1207/full); thus, we used PMI to evaluate skeletal muscle mass.
There were several limitations to this study. First, this is a retrospective single‐center study and the number of patients was relatively small. The timing of the CT examination and indication of L‐carnitine administration were heterogeneous in each case due to the retrospective and uncontrolled nature of the study. In addition, although propensity matched for age, sex, presence of HCC, and BCAA administration, the clinical backgrounds of patients with L‐carnitine supplementation and controls were significantly different. Carnitine patients had more severe liver dysfunction, which promotes loss of skeletal muscle mass.8 Nevertheless, we showed that carnitine supplementation significantly protected against loss of skeletal muscle mass. In addition, the observational period was limited; thus, whether preventing the loss of skeletal muscle mass can improve overall survival is yet to be clarified, and larger prospective studies with longer observational periods are required. In conclusion, this study revealed the potential of carnitine supplementation as a novel therapeutic option for sarcopenia in patients with LC.
Abbreviations
- 25(OH) vitamin D
25‐hydroxyvitamin D
- BCAA
branched chain amino acid
- CT
computed tomography
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- IGF1
insulin‐like growth factor 1
- LC
liver cirrhosis
- NASH
nonalcoholic steatohepatitis
- PMI
psoas muscle mass index
- SMI
skeletal muscle index
Supporting information
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep4.1207/full.
Supporting Information
Potential conflict of interest: Prof. Sakamoto has received lecture fees from Bristol Myers Squibb and Pharmaceutical K.K.; grants and endowments from MSD K.K., Otsuka Pharmaceutical Co., and Chugai Pharmaceutical Co., Ltd.; and a research grant from Gilead Sciences, Inc. Dr. Morikawa has received research grants from Gilead Sciences, Inc., Bristol Myers Squibb, Otsuka Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Co., Ltd. Dr. Suda has received research grants from Bristol Myers Squibb, and MSD K.K. The other authors have nothing to report.
Supported by the Platform Project for Supporting in Drug Discovery and Life Science Research from the Japan Agency for Medical Research and Development (16fk0210102h0001, 17fk0210102h0001, and 17fk0210106h0501) and the Japan Society for the Promotion of Science KAKENHI grant (15K08980 and 16K09334).
REFERENCES
- 1. Cruz‐Jentoft AJ, Landi F, Schneider SM, Zuniga C, Arai H, Boirie Y, etal. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014;43:748‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thompson DD. Aging and sarcopenia. JMusculoskelet Neuronal Interact 2007;7:344‐345. [PubMed] [Google Scholar]
- 3. Blau HM, Cosgrove BD, Ho AT. The central role of muscle stem cells in regenerative failure with aging. Nat Med 2015;21:854‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, etal. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on sarcopenia in older people. Age Ageing 2010;39:412‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dasarathy S. Consilience in sarcopenia of cirrhosis. JCachexia Sarcopenia Muscle 2012;3:225‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moriwaki H, Miwa Y, Tajika M, Kato M, Fukushima H, Shiraki M. Branched‐chain amino acids as a protein‐ and energy‐source in liver cirrhosis. Biochem Biophys Res Commun 2004;313:405‐409. [DOI] [PubMed] [Google Scholar]
- 7. Nishikawa H, Enomoto H, Ishii A, Iwata Y, Miyamoto Y, Ishii N, etal. Elevated serum myostatin level is associated with worse survival in patients with liver cirrhosis. JCachexia Sarcopenia Muscle 2017;8:915‐925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hanai T, Shiraki M, Ohnishi S, Miyazaki T, Ideta T, Kochi T, etal. Rapid skeletal muscle wasting predicts worse survival in patients with liver cirrhosis. Hepatol Res 2016;46:743‐751. [DOI] [PubMed] [Google Scholar]
- 9. Iritani S, Imai K, Takai K, Hanai T, Ideta T, Miyazaki T, etal. Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. JGastroenterol 2015;50:323‐332. [DOI] [PubMed] [Google Scholar]
- 10. Masuda T, Shirabe K, Ikegami T, Harimoto N, Yoshizumi T, Soejima Y, etal. Sarcopenia is a prognostic factor in living donor liver transplantation. Liver Transpl 2014;20:401‐407. [DOI] [PubMed] [Google Scholar]
- 11. Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. JHepatol 2016;65:1232‐1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koizumi T, Nikaido H, Hayakawa J, Nonomura A, Yoneda T. Infantile disease with microvesicular fatty infiltration of viscera spontaneously occurring in the C3H‐H‐2(0) strain of mouse with similarities to Reye's syndrome. Lab Anim 1988;22:83‐87. [DOI] [PubMed] [Google Scholar]
- 13. Magoulas PL, El‐Hattab AW. Systemic primary carnitine deficiency: an overview of clinical manifestations, diagnosis, and management. Orphanet J Rare Dis 2012;7:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rudman D, Ansley JD. Deficiency of carnitine in cachectic cirrhotic patients. JClin Invest 1977;60:716‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shiraki M, Shimizu M, Moriwaki H, Okita K, Koike K. Carnitine dynamics and their effects on hyperammonemia in cirrhotic Japanese patients. Hepatol Res 2017;47:321‐327. [DOI] [PubMed] [Google Scholar]
- 16. Nakanishi H, Kurosaki M, Tsuchiya K, Nakakuki N, Takada H, Matsuda S, etal. L‐carnitine reduces muscle cramps in patients with cirrhosis. Clin Gastroenterol Hepatol 2015;13:1540‐1543. [DOI] [PubMed] [Google Scholar]
- 17. Malaguarnera M, Gargante MP, Russo C, Antic T, Vacante M, Malaguarnera M, etal. L‐carnitine supplementation to diet: a new tool in treatment of nonalcoholic steatohepatitis‐‐a randomized and controlled clinical trial. Am J Gastroenterol 2010;105:1338‐1345. [DOI] [PubMed] [Google Scholar]
- 18.[No authors listed]. Clinical practice guidelines for hepatocellular carcinoma differ between Japan, United States, and Europe. Liver Cancer 2015;4:85‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Hammad A, Tamai Y, etal. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition 2016;32:1200‐1205. [DOI] [PubMed] [Google Scholar]
- 20. Takano H, Morita T, Iida H, Asada K, Kato M, Uno K, etal. Hemodynamic and hormonal responses to a short‐term low‐intensity resistance exercise with the reduction of muscle blood flow. Eur J Appl Physiol 2005;95:65‐73. [DOI] [PubMed] [Google Scholar]
- 21. Lee JJ, Mitchell PD, Hood HC, Grand RJ, Cohen LE. Potential role of IGF‐1 z score to predict permanent linear growth impairment in children with IBD. JPediatr Gastroenterol Nutr 2014;58:472‐476. [DOI] [PubMed] [Google Scholar]
- 22. Sasada S, Miyata Y, Mimae T, Mimura T, Okada M. Impact of lepidic component occupancy on effects of adjuvant chemotherapy for lung adenocarcinoma. Ann Thorac Surg 2015;100:2079‐2086. [DOI] [PubMed] [Google Scholar]
- 23. Nishikawa H, Shiraki M, Hiramatsu A, Moriya K, Hino K, Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res 2016;46:951‐963. [DOI] [PubMed] [Google Scholar]
- 24. Imai K, Takai K, Watanabe S, Hanai T, Suetsugu A, Shiraki M, etal. Sarcopenia impairs prognosis of patients with hepatocellular carcinoma: the role of liver functional reserve and tumor‐related factors in loss of skeletal muscle volume. Nutrients 2017;9 pii: E1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013;48:452‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wintermeyer E, Ihle C, Ehnert S, Stockle U, Ochs G, de Zwart P, etal. Crucial role of vitamin D in the musculoskeletal system. Nutrients 2016;8 pii: E319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sharples AP, Hughes DC, Deane CS, Saini A, Selman C, Stewart CE. Longevity and skeletal muscle mass: the role of IGF signalling, the sirtuins, dietary restriction and protein intake. Aging Cell 2015;14:511‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Assy N, Hochberg Z, Amit T, Shen‐Orr Z, Enat R, Baruch Y. Growth hormone‐stimulated insulin‐like growth factor (IGF) I and IGF‐binding protein‐3 in liver cirrhosis. JHepatol 1997;27:796‐802. [DOI] [PubMed] [Google Scholar]
- 29. Donaghy AJ, Delhanty PJ, Ho KK, Williams R, Baxter RC. Regulation of the growth hormone receptor/binding protein, insulin‐like growth factor ternary complex system in human cirrhosis. JHepatol 2002;36:751‐758. [DOI] [PubMed] [Google Scholar]
- 30. Ceglia L, da Silva Morais M, Park LK, Morris E, Harris SS, Bischoff‐Ferrari HA, etal. Multi‐step immunofluorescent analysis of vitamin D receptor loci and myosin heavy chain isoforms in human skeletal muscle. JMol Histol 2010;41:137‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Buitrago CG, Arango NS, Boland RL. 1alpha,25(OH)2D3‐dependent modulation of Akt in proliferating and differentiating C2C12 skeletal muscle cells. JCell Biochem 2012;113:1170‐1181. [DOI] [PubMed] [Google Scholar]
- 32. Tilg H, Wilmer A, Vogel W, Herold M, Nolchen B, Judmaier G, etal. Serum levels of cytokines in chronic liver diseases. Gastroenterology 1992;103:264‐274. [DOI] [PubMed] [Google Scholar]
- 33. Lin SY, Chen WY, Lee FY, Huang CJ, Sheu WH. Activation of ubiquitin‐proteasome pathway is involved in skeletal muscle wasting in a rat model with biliary cirrhosis: potential role of TNF‐alpha. Am J Physiol Endocrinol Metab 2005;288:E493‐E501. [DOI] [PubMed] [Google Scholar]
- 34. Qiu J, Thapaliya S, Runkana A, Yang Y, Tsien C, Mohan ML, etal. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF‐kappaB‐mediated mechanism. Proc Natl Acad Sci U S A 2013;110:18162‐18167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF‐beta superfamily member. Nature 1997;387:83‐90. [DOI] [PubMed] [Google Scholar]
- 36. Sinclair M, Gow PJ, Grossmann M, Angus PW. Review article: sarcopenia in cirrhosis‐‐aetiology, implications and potential therapeutic interventions. Aliment Pharmacol Ther 2016;43:765‐777. [DOI] [PubMed] [Google Scholar]
- 37. Kitajima Y, Takahashi H, Akiyama T, Murayama K, Iwane S, Kuwashiro T, etal. Supplementation with branched‐chain amino acids ameliorates hypoalbuminemia, prevents sarcopenia, and reduces fat accumulation in the skeletal muscles of patients with liver cirrhosis. JGastroenterol 2018;53:427‐437. [DOI] [PubMed] [Google Scholar]
- 38. Rutten IJ, van Dijk DP, Kruitwagen RF, Beets‐Tan RG, Olde Damink SW, van Gorp T. Loss of skeletal muscle during neoadjuvant chemotherapy is related to decreased survival in ovarian cancer patients. JCachexia Sarcopenia Muscle 2016;7:458‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Flanagan JL, Simmons PA, Vehige J, Willcox MD, Garrett Q. Role of carnitine in disease. Nutr Metab (Lond) 2010;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jun DW, Kim BI, Cho YK, Kim HJ, Kwon YO, Park SY, etal. Efficacy and safety of entecavir plus carnitine complex (GODEX(R)) compared to entecavir monotherapy in patient with ALT elevated chronic hepatitis B: randomized, multicenter open‐label trials. The GOAL study. Clin Mol Hepatol 2013;19:165‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tsukuda Y, Suda G, Tsunematsu S, Ito J, Sato F, Terashita K, etal. Anti‐adipogenic and antiviral effects of l‐carnitine on hepatitis C virus infection. JMed Virol 2017;89:857‐866. [DOI] [PubMed] [Google Scholar]
- 42. Malaguarnera M, Vacante M, Giordano M, Pennisi G, Bella R, Rampello L, etal. Oral acetyl‐L‐carnitine therapy reduces fatigue in overt hepatic encephalopathy: a randomized, double‐blind, placebo‐controlled study. Am J Clin Nutr 2011;93:799‐808. [DOI] [PubMed] [Google Scholar]
- 43. Gulcin I. Antioxidant and antiradical activities of L‐carnitine. Life Sci 2006;78:803‐811. [DOI] [PubMed] [Google Scholar]
- 44. Ishikawa H, Takaki A, Tsuzaki R, Yasunaka T, Koike K, Shimomura Y, etal. L‐carnitine prevents progression of non‐alcoholic steatohepatitis in a mouse model with upregulation of mitochondrial pathway. PLoS One 2014;9:e100627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Silverio R, Laviano A, Rossi Fanelli F, Seelaender M. l‐carnitine and cancer cachexia: clinical and experimental aspects. JCachexia Sarcopenia Muscle 2011;2:37‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brass EP. Supplemental carnitine and exercise. Am J Clin Nutr 2000;72(Suppl.):618S‐623S. [DOI] [PubMed] [Google Scholar]
- 47. Giovenali P, Fenocchio D, Montanari G, Cancellotti C, D'Iddio S, Buoncristiani U, etal. Selective trophic effect of L‐carnitine in type I and IIa skeletal muscle fibers. Kidney Int 1994;46:1616‐1619. [DOI] [PubMed] [Google Scholar]
- 48. Carey EJ, Lai JC, Wang CW, Dasarathy S, Lobach I, Montano‐Loza AJ, etal.; Fitness, Life Enhancement, and Exercise in Liver Transplantation Consortium . A multicenter study to define sarcopenia in patients with end‐stage liver disease. Liver Transpl 2017;23:625‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. van Vugt JL, Levolger S, de Bruin RW, van Rosmalen J, Metselaar HJ, IJzermans JN. Systematic review and meta‐analysis of the impact of computed tomography‐assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation. Am J Transplant 2016;16:2277‐2292. [DOI] [PubMed] [Google Scholar]
- 50. Hiraoka A, Aibiki T, Okudaira T, Toshimori A, Kawamura T, Nakahara H, etal. Muscle atrophy as pre‐sarcopenia in Japanese patients with chronic liver disease: computed tomography is useful for evaluation. JGastroenterol 2015;50:1206‐1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep4.1207/full.
Supporting Information


