Abstract
Nonalcoholic fatty liver disease (NAFLD) is becoming common in the United States and throughout the world and can progress to cirrhosis, hepatocellular carcinoma, and death. There is a strong association between coronary artery disease and NAFLD due to common risk factors, such as metabolic syndrome, obesity, and diabetes mellitus. Subclinical atherosclerosis, defined as coronary artery calcification in asymptomatic patients, has been shown to have a higher incidence in patients with NAFLD. We performed a meta‐analysis to examine the association of NAFLD with subclinical atherosclerosis measured by coronary artery calcium (CAC) scoring. Data were extracted from 12 studies selected using a predefined search strategy. NAFLD was diagnosed by abdominal ultrasound or computed tomography scans. The rate of coronary artery calcification was analyzed using random effects models, and publication bias was assessed using Egger's regression test. A total of 42,410 subjects were assessed, including 16,883 patients with NAFLD. Mean CAC score was significantly higher in subjects with NAFLD compared to those without NAFLD (odds ratio with random effects model, 1.64; 95% confidence inteval, 1.42‐1.89). This association remained significant through subgroup analyses for studies with >1,000 subjects and a higher CAC score cutoff of >100. Higher aspartate aminotransferase levels were also associated with increased subclinical atherosclerosis (mean difference 1.77; 95% confidence interval, 1.19‐2.34). Conclusion: There is an increased prevalence of subclinical atherosclerosis in patients with NAFLD, where subclinical atherosclerosis is defined using a “real world” clinical biomarker, namely the CAC score. Prospective studies are needed to establish a causative link between NAFLD and coronary artery disease. (Hepatology Communications 2018; 00:000‐000)
Nonalcoholic fatty liver disease (NAFLD) is now the most common chronic liver disease in the United States1 with a prevalence of 10%‐30% using different screening tests.2, 3 NAFLD encompasses a wide spectrum of hepatic conditions ranging from simple steatosis (nonalcoholic fatty liver [NAFL]) to nonalcoholic steatohepatitis (NASH), which can progress to cirrhosis and end‐stage liver disease.4 NAFLD has become a major cause of cirrhosis in Western countries and is predicted to overtake hepatitis C as the leading cause for liver transplantation within the next 10‐20 years.5
Several lines of evidence suggest a strong association between NAFLD and coronary artery disease (CAD). First, prevalence of NAFLD is significantly higher in patients having co‐existing obesity, diabetes mellitus, or metabolic syndrome, all of which are established risk factors for CAD.2 Second, NAFLD and CAD share several common environmental and genetic factors.6, 7, 8, 9, 10, 11 Third, studies have demonstrated that NAFLD is associated with more severe symptomatic coronary atherosclerosis and is even considered an independent risk factor for CAD.12, 13 Finally, cardiovascular disease has been identified as the most common cause of death in NAFLD patients with advanced liver fibrosis.4, 14, 15
Similar to the progression of NAFL to NASH in a percentage of patients, subclinical atherosclerosis, defined as the presence of coronary arterial atherosclerosis in asymptomatic individuals, is a prodrome that can progress to clinically evident CAD.16, 17 Improved cardiovascular diagnostic modalities, such as carotid intima‐media thickness (CIMT),18, 19 carotid‐femoral pulse velocity,20 and coronary calcium scoring,18, 21, 22, 23 have resulted in an improved ability to detect subclinical atherosclerosis, understand its natural history, and have a meaningful impact on clinical care. A consistent methodology for measuring brachial artery flow‐mediated dilation has not yet been adopted, and CIMT, although useful to assess atherosclerosis, cannot quantify the degree of calcification in atherosclerotic plaque. Therefore, of these various modalities, coronary artery calcium (CAC) scoring has emerged as an economical, sensitive, and specific method to screen for CAD, with an increased risk for prevalent coronary heart disease observed at all levels of a CAC score >0.6, 7, 24, 25 A large registry analysis demonstrated that higher CAC scores increase all‐cause mortality after adjusting for traditional risk factors, with risk‐adjusted relative risk ratios for a CAC score ranging between 2.2‐fold and 12.5‐fold for scores 11 to >1,000, respectively.26 This provides strength to the growing evidence that progressive coronary atherosclerosis, albeit subclinical, is an important predictor of mortality and that CAC scoring is an effective tool to assess cardiovascular risk.
Recently, several studies have evaluated the association of subclinical atherosclerosis with NAFLD.27, 28 Although these studies uniformly demonstrate an association of subclinical atherosclerosis with NAFLD, they lack a unified method of detecting subclinical atherosclerosis or use modalities not readily available in clinical practice. A recent study by Jaruvongvanich and colleagues29 demonstrated an association between atherosclerosis measured by CAC scores and NAFLD in populations that included known CAD. However, the question remains whether subclinical atherosclerosis is actually associated with NAFLD. There has not been a meta‐analysis evaluating the association of subclinical atherosclerosis alone, as defined by the CAC score, with NAFLD.
In this study, we focus on the association of NAFLD with subclinical atherosclerosis by CAC scoring and determine the risk conferred by NAFLD on CAD.
Materials and Methods
This systematic review followed a developed protocol (http://onlinelibrary.wiley.com/doi/10.1002/hep4.1199/full) and was reported according to the meta‐analyses of observational studies in epidemiology guidelines.30
ELIGIBILITY CRITERIA
Both cross‐sectional and cohort studies reporting hepatic steatosis by imaging and evidence of subclinical atherosclerosis by CAC scoring were included in this meta‐analysis. CAC scores were reported using the scoring system proposed by Agatston.31 Hepatic steatosis could be identified either by computed tomography (CT) or by ultrasonography (US). Inclusion and exclusion criteria were determined a priori to the literature search (http://onlinelibrary.wiley.com/doi/10.1002/hep4.1199/full). Studies that included patients who presented with symptoms of CAD, such as acute chest pain, or subjects who had a history of prior CAD were excluded to maintain the integrity of the definition of subclinical atherosclerosis.
SEARCH STRATEGY
MEDLINE, EMBASE, and OVID searches were conducted to identify studies published between 1980 and 2017 that associated NAFLD with subclinical atherosclerosis. The search terms and their variation for NAFLD used included “fatty liver”, “Non‐alcoholic fatty liver disease”, “NASH”, “NAFLD”, “non‐alcoholic fatty liver disease”, and “non‐alcoholic”. The search terms and their variation for subclinical atherosclerosis and the CAC score included “coronary calcium, subclinical atherosclerosis”, “preclinical atherosclerosis”, “spiral CT”, “mdct”, “coronary artery disease”, “cardiac imaging techniques”, “multi‐slice ct”, “cardiac ct”, and “multidetector ct”. The search strategy for MEDLINE is provided in http://onlinelibrary.wiley.com/doi/10.1002/hep4.1199/full. Studies were not limited by language and standard citation, and related article chasing was used. Study authors were contacted if additional data were needed. Abstracts and unpublished studies were not considered for review or data extraction.
STUDY SELECTION AND DATA EXTRACTION
Two review authors (V.T. and D.K.) independently screened studies and abstracts of the studies identified by electronic searches using the Covidence platform (https://www.covidence.org/home). Study quality was assessed using the Newcastle‐Ottawa Scale32 modified for cross‐sectional studies, with a score more than 5 indicating higher quality.33 The study by Sinn et al.34 is a cohort study; therefore, quality assessment was performed using the original Newcastle‐Ottawa Scale. The same review authors performed data extraction. A third investigator (C.K.) resolved any disagreements in study selection, quality assessment, and data extraction.
The data abstracted included demographics (including age, sex, and body mass index [BMI]), baseline risk factors for metabolic syndrome (hypertension, diabetes mellitus, dyslipidemia, smoking, exercise levels), serum aminotransferases (alanine aminotransferase [ALT], aspartate aminotransferase [AST]), number of subjects with and without NAFLD, number of subjects divided into groups based on CAC scores as well as with and without subclinical atherosclerosis, and number of subjects with and without calcified plaques. Additional information collected included country of origin of the study, type of study (cohort, cross‐sectional), and outcomes assessed.
DATA SYNTHESIS AND STATISTICAL ANALYSIS
The primary summary measure was level of CAC in subjects with NAFLD by imaging. Other data pooled for analysis included ALT, AST, and BMI, if available, in subjects with or without coronary artery calcifications.
The rate of coronary artery calcification in subjects with NAFLD was analyzed using both fixed and random effects models using the DerSimonian–Laird estimator and Hartung–Knapp–Sidik–Jonkman method to estimate pooled odds ratio (OR) and 95% confidence intervals (95% CI). Similarly, pooled analysis of continuous variables, including aminotransferase and BMI, were assessed to estimate mean difference (MD) and 95% CI. Using the chi2 test, statistically significant heterogeneity was defined as P < 0.10. Further, I 2 was assessed to evaluate for true variability between the two arms, and a result >50% was identified as significant.
Subgroup analyses, for studies with more than >1,000 subjects, CAC score cutoffs of >0 versus >100, study location (Western versus Asian), and method of assessment of hepatic steatosis (US versus CT) were also performed to explore sources of heterogeneity (http://onlinelibrary.wiley.com/doi/10.1002/hep4.1199/full).
Publication bias was assessed through Egger's regression test for funnel plot asymmetry. Lastly, visual evaluation of the funnel plot asymmetry was performed. Primary analyses were done using RStudio (version 0.99.484); subgroup analyses were performed using Review Manager (version 5.3.5).35
Results
STUDY LEVEL DATA
The initial search generated a total of 1,620 studies, using (((“Non‐alcoholic Fatty Liver Disease”[Mesh]) OR fatty liver)) AND atherosclerosis, with 208 potential relevant papers retrieved for detailed assessment using Covidence software (Fig. 1). Among these, 29 studies were eligible for full text screening; 12 studies were included in the final analysis.22, 36, 37, 38, 39, 40, 41, 42 The characteristics of each study and the Newcastle‐Ottawa Scale for quality assessment are given in Table 1. A total of 42,410 subjects were assessed in these studies, comprising 16,883 patients with NAFL and 25,527 without. The mean age was 52.9 years, with most studies having a predominance of male subjects. Hepatic steatosis was assessed by CT scan‐based liver to spleen attenuation ratios in five studies and by US (diagnosed by increased liver echogenicity) in seven studies. We note that four studies used a higher CAC score of >100 compared to a CAC score >0 in the rest. Common risk factors adjusted for in most of the studies were age, sex, and components of the metabolic syndrome. Some studies also adjusted for ethnicity,22, 30 physical activity,22, 42 socioeconomic level,22, 36 and alcohol intake.22, 23 One study recruited only male subjects42 and another only female subjects.
Figure 1.
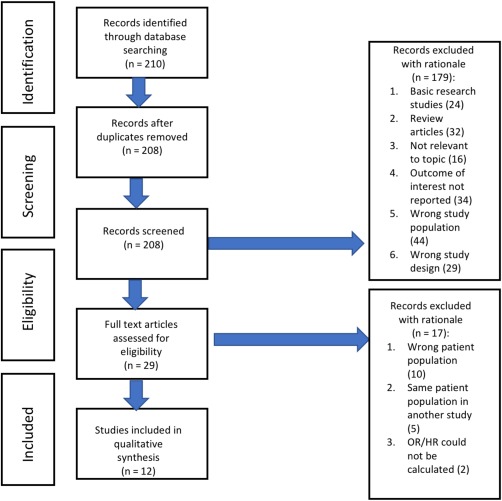
Study flowsheet. Abbreviation: OR, odds ratio; HR, hazard ratio.
Table 1.
Details of Studies Included in the Meta‐Analysis
| Design | Number of Subjects | Age | Sex (% Males) | NAFLD Determination | CAC Definition | Plaques (Calcified) | Risk Factors Adjustment | Study Quality | |
|---|---|---|---|---|---|---|---|---|---|
| Chen et al.37 | Cross‐sectional | 295 | 52.6 ± 11 | 65% | CT (L:S ratio) | CAC >100 | smoking, hypertension, diabetes, dyslipidemia | **********‡ | |
| Chhabra et al.38 | Cross‐sectional | 377 | 62.3 ± 8.5 vs 55.9 ± 9.5† | 51.90% | CT (L:S ratio) | CAC >100 | age, sex, smoking, dyslipidemia, hypertension, diabetes | ********* | |
| Juarez‐Rojas et al.39 | Cross‐sectional | 765 | 55 ± 9 vs 54.3 ± 10a | 47% | CT (L:S ratio) | CAC >0 | age, smoking, BMI, total cholesterol, CRP | ********* | |
| Al Rifai et al.36 | Cross‐sectional | 3,976 | 61.2 ± 9.6 vs 63.3 ± 10.5a | 45.10% | CT (L:S ratio) | CAC >0 | age, gender, ethnicity, smoking, LDL, statin, education | ********** | |
| Jung et al.40 | Cross‐sectional | 1,218 | 52.5 ± 8 vs 51 ± 10a | 49.50% | US | CAC >100 | age, gender, BMI, waist to hip ratio, uric acid, BP, TGs, HDL, DM, statin | ******* | |
| Kang et al.28 | Cross‐sectional | 772 | 55 ± 9 vs 45.8 ± 8.4† | 68% | US | CAC >100 | 59% | age, smoking, HTN, DM2, LDL, HDL, metabolic syndrome | ********* |
| Kim et al.23 | Cross‐sectional | 4,023 | 57.5 ± 9 vs 56.4 ± 9.6a | 60.70% | US | CAC >0 | age, sex, BMI, waist circumference, alcohol, smoking, cholesterol, HTN, DM2, HDL, CRP, TGs | ********** | |
| Kim et al.43 | Cross‐sectional study | 919 | 59.5 ± 6 vs 57 ± 7a | 0% | US | CAC >0 | age, BMI, hypertension, diabetes, hyperlipidemia, insulin resistance | ****** | |
| Kim et al.41 | Cross‐sectional analysis of longitudinal cohort data | 1,575 | 40.0 ± 5.3 vs 39.8 ± 5.5 | 89.6% | US | CAC >0 | age, sex, diabetes, cholesterol, hypertension, smoking, BMI | ******* | |
| Lee et al.42 | Cross‐sectional | 21,335 | 40.85 ± 6.5 vs 40.15 ± 7a | 100% | US | CAC >0 | age, diabetes, HTN, smoking, physical inactivity | ********** | |
| Sinn et al.34 | Cohort study | 4,731 | 52.1 ± 7.2 vs 52.3 ± 7.1 | 91% | US | CAC >0 | age, sex, smoking, alcohol consumption, hypertension, hyperlipidemia, diabetes. | ******* | |
| Van Wagner et al.22 | Cross‐sectional analysis of longitudinal cohort data | 2,424 | 50.5 ± 3.7 vs 49.9 ± 3.6a | 42.70% | CT (L:S ratio) | CAC >0 | age, sex, race, socioeconomic level, alcohol intake, physical activity score | ********* |
NAFLD vs non‐NAFLD; †CAC vs non‐CAC; ‡ stands for study qualtiy.
Abbreviations: BP, blood pressure; CRP, C‐reative protein; DM, diabetes mellitus; HDL, high‐density lipoprotein; HTN, hypertension; LDL, low‐density lipoprotein; L:S, liver to spleen ratio; TG, triglyceride.
RISK OF CAC IN SUBJECTS WITH NAFLD
Data from the 12 studies were synthesized to evaluate the risk of CAC score between subjects with and without NAFLD. Mean CAC score was significantly higher in subjects with NAFLD compared to those without, with a pooled OR with random effects model of 1.64 and 95% CI of 1.42‐1.89 (Fig. 2). All the evaluated studies demonstrated a similar increase in odds of a high CAC score in patients with NAFLD, with an OR as high as 2.84 in one study.38 There was significant heterogeneity between the studies (I 2, 72%; P < 0.01). Subgroup analyses were subsequently performed based on the number of subjects in the study (>1,000 versus <1,000), origin of population of study (Asian versus non‐Asian), and method of detection of liver steatosis (CT versus US) (http://onlinelibrary.wiley.com/doi/10.1002/hep4.1199/full). Five out of 12 studies had less than 1,000 subjects) with a total of 3,128 subjects. Subgroup analysis performed for these studies demonstrated a similar trend of a higher CAC score in subjects with NAFLD (OR, 1.96; 95% CI, 1.59‐2.40; I 2 0% for studies with <1,000 subjects and OR, 1.54; 95% CI, 1.34‐1.77; I 2 = 80% for studies with >1,000 subjects). Studies with >1,000 subjects showed significant heterogeneity. Test for subgroup differences yielded chi2 = 3.54, P = 0.06, I 2 = 71.8% (http://onlinelibrary.wiley.com/doi/10.1002/hep4.1199/full). A similar analysis was performed in studies with higher CAC score cut‐off values of >100 (four studies with 2,622 subjects) and lower CAC score cut‐off values (eight studies with a total of 39,748 subjects) (Fig. 3). NAFLD continued to be a risk factor for subclinical atherosclerosis even in the subgroup with a high CAC cut‐off value (OR, 2.11; 95% CI, 1.61‐2.76; I 2 = 0%). Test for subgroup differences showed heterogeneity between the two subgroups (chi2 = 3.89, P = 0.05, I 2 = 74.3%).
Figure 2.
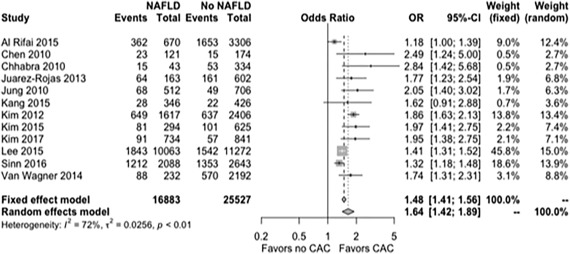
Forest plot showing relationship of NAFLD with subclinical atherosclerosis.
Figure 3.
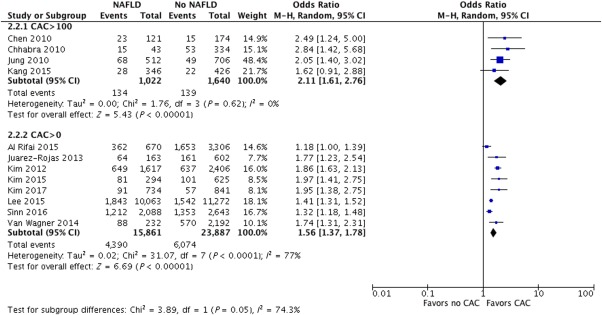
Forest plot showing subgroup analysis of NAFLD in studies with CAC >100 and CAC>0. Abbreviation: M‐H, Mantel‐Haenszel method.
When studies were grouped into the origin of the study population, eight studies with a total number of 24,742 subjects were performed in Asian populations, with a prevalence of subclinical atherosclerosis in subjects with NAFLD (OR, 1.67; 95% CI, 1.45‐1.93). There were four studies performed in non‐Asian populations (7,542 subjects), with similar results noted (OR, 1.64; 95% CI, 1.19‐2.26). Although both subgroups were heterogeneous, test for subgroup differences showed no heterogeneity (chi2 = 0.01, P = 0.92, I 2 = 0%).
Finally, subgroup analysis was performed based on the method of detection of liver steatosis (CT versus US). Seven studies (34,573 subjects) used US to detect hepatic steatosos, with an increased prevalence of CAC in NAFLD (OR, 1.64; 95% CI, 1.42‐1.90). The other five studies with 7,837 subjects used CT as a tool to detect hepatic steatosis, and a similar relationship between NAFLD and CAC was noted (OR, 1.73; 95% CI, 1.27‐2.36). Test for subgroup differences showed minimal heterogeneity (chi2 = 0.09, P = 0.77, I 2 = 0%).
SERUM AMINOTRANSFERASE LEVELS IN SUBJECTS WITH CORONARY ARTERY CALCIFICATION
Only four studies reported serum aminotransferase levels for a total of 5,467 subjects. Almost all studies individually reported an association of elevated ALT with CAC with the exception of one study.38 Higher AST levels were associated with increased subclinical atherosclerosis (MD, 1.77; 95% CI, 1.19‐2.34). A trend with ALT levels was observed, with elevated ALT levels in patients with increased subclinical atherosclerosis (MD, 1.89; 95% CI, –3.44 to 7.21); however, this was not significant (Fig. 4).
Figure 4.

Forest plot showing association of ALT (top) and AST (bottom) with CAC.
Sensitivity analyses were performed by excluding the cohort study from the pooled analysis, with similar results. Further, studies with only male,42 only female,43 and then both subjects were excluded from the pooled analysis; this yielded similar results, albeit with a slightly increased heterogeneity. Additionally, analysis was repeated after excluding the cohort study (Sinn et al.34) from the analysis, with similar results (http://onlinelibrary.wiley.com/doi/10.1002/hep4.1199/full).
EVALUATION FOR PUBLICATION BIAS
The funnel plot did show asymmetry; this was confirmed with a nonsignificant Egger's test. The studies were heterogeneous (http://onlinelibrary.wiley.com/doi/10.1002/hep4.1199/full).
Discussion
In this pooled analysis evaluating 12 published studies with an aggregate of 42,410 patients, we evaluated the association of NAFLD with subclinical atherosclerosis. By examining “real‐world” clinical biomarkers, namely CAC scoring for subclinical atherosclerosis and US or CT for NAFLD, we demonstrated an increased prevalence of subclinical atherosclerosis in subjects with NAFLD compared to subjects without hepatic steatosis, after adjusting for risk factors predisposing to CAD. This is the first meta‐analysis that evaluates the association of subclinical atherosclerosis as defined by CAC scoring with the presence of NAFLD in subjects without known CAD. In general, the results from this study are in concordance with the individually reported studies and also are in line with other studies that use more complicated measurement modalities, namely the measurement of CIMT and carotid plaques44 and brachial artery flow‐mediated dilations.45
Although a recent meta‐analysis29 suggests an association between subclinical atherosclerosis and NAFLD, our study provides more definitive evidence as it was performed in patient populations without existing CAD; also, the availability of two additional studies after the previous meta‐analysis was published further strengthens this association. Moreover, we report similar results in subgroup analyses that differentiated larger studies from studies with small sample sizes.
In our study, a significantly higher mean AST was observed in the cohort with coronary artery calcification; ALT levels trended higher in patients with coronary artery calcification; however, when only non‐U.S. populations (i.e., more homogeneous) were studied, this was a significant association. Further study needs to be performed to evaluate the role of an elevated AST value in patients at a high risk of developing coronary atherosclerosis.
Despite the general concordance of our results with previous studies, the results from this study seemingly differ from two other studies. First, Van Wagner and colleagues22 observed a weakening in the relationship between NAFLD and subclinical atherosclerosis after adjusting for abdominal or general obesity. Second, a subgroup analysis evaluating the association of NAFLD and CAC score in 398 black and white individuals from the Multi‐Ethnic Study of Atherosclerosis (MESA) cohort at a single center in North Carolina did not find an association between NAFLD and CAC score.46 However, in the larger cross‐sectional analysis of the entire MESA cohort, a significant relationship was identified between NAFLD and subclinical atherosclerosis even after adjusting for obesity and metabolic syndrome. These contrasting observations can be explained on the basis of the population characteristics of each individual study; the patients in Van Wagner's study were younger, potentially explaining the weak association between NAFLD and CAC score, and the patients in the MESA subgroup were small in number and from a uniform group of individuals. In contrast, larger studies including ethnically diverse populations have demonstrated that Hispanics, followed by Asians, have the strongest association of NAFLD with subclinical atherosclerosis.36
There has also been an emerging association of NAFLD with “nontraditional” cardiovascular risk factors, such as elevated uric acid levels, decreased vitamin D, and adiponectin levels.47, 48, 49 Experimental data have demonstrated an increased transcription of several candidate genes responsible for atherogenesis in NASH but not NAFLD, which suggests a putative association of severity of NASH with the development of atherosclerosis.9, 41 A similar incremental rise from NAFLD to NASH in inflammatory markers C‐reactive protein, interleukin‐6, tumor necrosis factor, and transforming growth factor β has been described in a few studies.49, 50 Early NAFLD models have described messenger RNA up‐regulation of the inflammasome components nucleotide‐binding domain, leucine‐rich‐containing family, pyrin domain‐containing‐3, pyrin domain and apoptosis‐associated speck‐like protein containing a CARD, and caspase. Although significant, these markers only result in incomplete inflammasome activation, but there is an increase in expression of inflammasome components that would lead to more complete activation in NASH.21
Unlike NAFLD, there is a paucity of prospective studies on the natural history of subclinical atherosclerosis progression. Despite a single conflicting study,19 most of the published literature has demonstrated progression of subclinical atherosclerosis over relatively short periods of time.48, 49 Notably, one interesting finding is that this progression occurred over months rather than years, indicating that there may be other unknown factors apart from simple aging involved that could contribute to this rapid progression. With recent research suggesting that the presence of NAFLD is associated with increased progression of coronary atherosclerosis, further study in this area may yield novel results along with potential changes in the way both diseases are managed.
This study, while strong for its numbers and evaluation of clinically available tests for subclinical atherosclerosis and NAFLD, is limited by the inherent limitations of US or CT in determining hepatic steatosis and estimations of hepatic fat as well by the inability of these modalities to differentiate between simple steatosis and NASH. While US remains the most widely used imaging method to detect steatosis, its accuracy is diminished considerably in mild hepatic steatosis. Similary, while the CT scan has a better diagnostic yield for focal steatosis, using it as a tool for detecting hepatic steatosis has several limitations, including unnecessary radiation exposure. Additionally, the CAC score cut‐off values for subclinical atherosclerosis in asymptomatic subjects varied between studies with a range from <0 to <100. While a CAC score >100 is clearly linked to adverse cardiovascular outcomes, the association between low CAC (>0‐10) and adverse cardiovascular events is less defined. Next, the analyzed studies were performed in heterogeneous populations with varying levels of normal cutoffs for transaminases, and this could affect the association of transaminases with increased coronary calcification. We attempted to contact several authors of studies where dichotomization of study subjects based on CAC score was not presented; however, we received only one response.40 The studies included in the meta‐analysis had a high level of heterogeneity. On the basis of subgroup analyses, this heterogeneity can probably be explained by the use of different cutoffs for CAC and studies with large versus small numbers of study subjects. We also excluded non‐English language citations. Finally, given that our meta‐analysis included smaller studies, there remains a risk for selection and ascertainment bias.
In conclusion, NAFLD appears to be associated with subclinical atherosclerosis by CAC scoring. Further prospective studies are needed to establish whether a causative relationship exists between NAFLD and coronary atherosclerosis and whether management of subclinical atherosclerosis will impact coronary or hepatic outcomes.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- CAC
coronary artery calcium
- CACS
coronary artery calcium score
- CAD
coronary artery disease
- CI
confidence interval
- CIMT
carotid intima‐media thickness
- CT
computed tomography
- MD
mean difference
- MESA
Multi‐Ethnic Study of Atherosclerosis
- MOOSE
Meta‐analyses Of Observational Studies in Epidemiology
- NAFL
nonalcoholic fatty liver
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- OR
odds ratio
- US
ultrasonography
Supporting information
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep4.1199/full.
Supporting Information
Potential conflict of interest: Dr. Etzion advises and is on the speakers' bureau for Gilead and AbbVie; he consults for Eiger and advises HepQuant.The other authors have nothing to report.
REFERENCES
- 1. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non‐alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55:2005‐2023. [DOI] [PubMed] [Google Scholar]
- 2. Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34:274‐285. [DOI] [PubMed] [Google Scholar]
- 3. Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle‐aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011;140:124‐131. [DOI] [PubMed] [Google Scholar]
- 4. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 2002;346:1221‐1231. [DOI] [PubMed] [Google Scholar]
- 5. Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547‐555. [DOI] [PubMed] [Google Scholar]
- 6. Hamirani YS, Pandey S, Rivera JJ, Ndumele C, Budoff MJ, Blumenthal RS, et al. Markers of inflammation and coronary artery calcification: a systematic review. Atherosclerosis 2008;201:1‐7. [DOI] [PubMed] [Google Scholar]
- 7. Kim BJ, Kim NH, Kim BS, Kang JH. The association between nonalcoholic fatty liver disease, metabolic syndrome and arterial stiffness in nondiabetic, nonhypertensive individuals. Cardiology 2012;123:54‐61. [DOI] [PubMed] [Google Scholar]
- 8. Gómez M, Vila J, Elosua R, Molina L, Bruguera J, Sala J, et al. Relationship of lipid oxidation with subclinical atherosclerosis and 10‐year coronary events in general population. Atherosclerosis 2014;232:134‐140. [DOI] [PubMed] [Google Scholar]
- 9. Sookoian S, Gianotti TF, Rosselli MS, Burgueño AL, Castaño GO, Pirola CJ. Liver transcriptional profile of atherosclerosis‐related genes in human nonalcoholic fatty liver disease. Atherosclerosis 2011;218:378‐385. [DOI] [PubMed] [Google Scholar]
- 10. Hsieh J, Koseki M, Molusky MM, Yakushiji E, Ichi I, Westerterp M, et al. TTC39B deficiency stabilizes LXR reducing both atherosclerosis and steatohepatitis. Nature 2016;535:303‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. An B‐Q, Lu L‐L, Yuan C, Xin Y‐N, Xuan S‐Y. Leptin receptor gene polymorphisms and the risk of non‐alcoholic fatty liver disease and coronary atherosclerosis in the Chinese Han population. Hepat Mon 2016;16:e35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lonardo A, Sookoian S, Pirola CJ, Targher G. Non‐alcoholic fatty liver disease and risk of cardiovascular disease. Metabolism 2016;65:1136‐1150. [DOI] [PubMed] [Google Scholar]
- 13. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non‐alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta‐analysis. J Hepatol 2016;65:589‐600. [DOI] [PubMed] [Google Scholar]
- 14. Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 2013;10:330‐344. [DOI] [PubMed] [Google Scholar]
- 15. Agopian VG, Kaldas FM, Hong JC, Whittaker M, Holt C, Rana A, et al. Liver transplantation for nonalcoholic steatohepatitis: the new epidemic. Ann Surg 2012;256:624‐633. [DOI] [PubMed] [Google Scholar]
- 16. Berry JD, Liu K, Folsom AR, Lewis CE, Carr JJ, Polak JF, et al. Prevalence and progression of subclinical atherosclerosis in younger adults with low short‐ term but high lifetime estimated risk for cardiovascular disease: the coronary artery risk development in young adults study and multi‐ethnic study of atherosclerosis. Circulation 2009;119:382‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Toth PP. Subclinical atherosclerosis: what it is, what it means and what we can do about it. Int J Clin Pract 2008;62:1246‐1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O'Leary DH, Bots ML. Imaging of atherosclerosis: Carotid intima‐media thickness. Eur Heart J 2010;31:1682‐1689. [DOI] [PubMed] [Google Scholar]
- 19. Toledo‐Corral CM, Davis JN, Alderete TL, Weigensberg MJ, Ayala CT, Li Y, et al. Subclinical atherosclerosis in Latino youth: progression of carotid intima‐media thickness and its relationship to cardiometabolic risk factors. J Pediatr 2011;158:935‐940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meyer ML, Tanaka H, Palta P, Cheng S, Gouskova N, Aguilar D, et al. Correlates of segmental pulse wave velocity in older adults: the Atherosclerosis Risk in Communities (ARIC) study. Am J Hypertens 2016;29:114‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ganz M, Csak T, Szabo G. High fat diet feeding results in gender specific steatohepatitis and inflammasome activation. World J Gastroenterol 2014;20:8525‐8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. VanWagner LB, Ning H, Lewis CE, Shay CM, Wilkins J, Carr JJ, et al. Associations between nonalcoholic fatty liver disease and subclinical atherosclerosis in middle‐aged adults: the Coronary Artery Risk Development in Young Adults Study. Atherosclerosis 2014;235:599‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim D, Choi S‐Y, Park EH, Lee W, Kang JH, Kim W, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology 2012;56:605‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sangiorgi G, Rumberger JA, Severson A, Edwards WD, Gregoire J, Fitzpatrick LA, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol 1998;31:126‐133. [DOI] [PubMed] [Google Scholar]
- 25. Cheng YJ, Church TS, Kimball TE, Nichaman MZ, Levine BD, McGuire DK, et al. Comparison of coronary artery calcium detected by electron beam tomography in patients with to those without symptomatic coronary heart disease. Am J Cardiol 2003;92:498‐503. [DOI] [PubMed] [Google Scholar]
- 26. Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, et al. Long‐term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol 2007;49:1860‐1870. [DOI] [PubMed] [Google Scholar]
- 27. Kim HC, Kim DJ, Huh KB. Association between nonalcoholic fatty liver disease and carotid intima‐media thickness according to the presence of metabolic syndrome. Atherosclerosis 2009;204:521‐525. [DOI] [PubMed] [Google Scholar]
- 28. Kang MK, Kang BH, Kim JH. Nonalcoholic fatty liver disease is associated with the presence and morphology of subclinical coronary atherosclerosis. Yonsei Med J 2015;56:1288‐1295.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jaruvongvanich V, Wirunsawanya K, Sanguankeo A, Upala S. Nonalcoholic fatty liver disease is associated with coronary artery calcification: a systematic review and meta‐analysis. Dig Liver Dis 2016;48:1410‐1417. [DOI] [PubMed] [Google Scholar]
- 30. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000;283:2008‐2012. http://www.ncbi.nlm.nih.gov/pubmed/10789670. [DOI] [PubMed] [Google Scholar]
- 31. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827‐832. http://www.ncbi.nlm.nih.gov/pubmed/2407762. [DOI] [PubMed] [Google Scholar]
- 32. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta‐analyses. The Ottawa Hospital Research Institute. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. (Access date 2/1/2018)
- 33. Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, et al. ESH Working Group on CV Risk in Low Resource Settings. Panethnic differences in blood pressure in Europe: a systematic review and meta‐analysis. PLoS One 2016;11:e0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sinn DH, Kang D, Chang Y, Ryu S, Gu S, Kim H, et al. Non‐alcoholic fatty liver disease and progression of coronary artery calcium score: a retrospective cohort study. Gut 2017;66:323‐329. [DOI] [PubMed] [Google Scholar]
- 35. Review Manager (RevMan) [computer program]. Version 5.3. Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [Google Scholar]
- 36. Al Rifai M, Silverman MG, Khurram N, Budoff MJ, Blankstein R, Szklo M, et al. The association of nonalcoholic fatty liver disease, obesity, and metabolic syndrome, with systemic inflammation and subclinical atherosclerosis: the multi‐ethnic study of atherosclerosis (MESA). Atherosclerosis 2015;239:629‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen C‐H, Nien C‐K, Yang C‐C, Yeh Y‐H. Association between nonalcoholic fatty liver disease and coronary artery calcification. Dig Dis Sci 2010;55:1752‐1760. [DOI] [PubMed] [Google Scholar]
- 38. Chhabra R, O'Keefe JH, Patil H, O'Keefe E, Thompson RC, Ansari S, et al. Association of coronary artery calcification with hepatic steatosis in asymptomatic individuals. Mayo Clin Proc 2013;88:1259‐1265. [DOI] [PubMed] [Google Scholar]
- 39. Juárez‐Rojas JG, Medina‐Urrutia AX, Jorge‐Galarza E, González‐Salazar C, Kimura‐Hayama E, Cardoso‐Saldaña G, et al. Fatty liver increases the association of metabolic syndrome with diabetes and atherosclerosis. Diabetes Care 2013;36:1726‐1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jung DH, Lee YJ, Ahn HY, Shim JY, Lee HR. Relationship of hepatic steatosis and alanine aminotransferase with coronary calcification. Clin Chem Lab Med 2010;48:1829‐1834. [DOI] [PubMed] [Google Scholar]
- 41. Kim J, Lee DY, Park SE, Park CY, Lee WY, Oh KW, et al. Increased risk for development of coronary artery calcification in subjects with non‐alcoholic fatty liver disease and systemic inflammation. PLoS One 2017;12:e0180118.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee MK, Park HJ, Jeon WS, Park SE, Park CY, Lee WY, et al. Higher association of coronary artery calcification with non‐alcoholic fatty liver disease than with abdominal obesity in middle‐aged Korean men: the Kangbuk Samsung Health Study. Cardiovasc Diabetol 2015;14:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim MK, Ahn CW, Nam JS, Kang S, Park JS, Kim KR. Association between nonalcoholic fatty liver disease and coronary artery calcification in postmenopausal women. Menopause 2015;22:1323‐1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thakur ML, Sharma S, Kumar A, Bhatt SP, Luthra K, Guleria R, et al. Nonalcoholic fatty liver disease is associated with subclinical atherosclerosis independent of obesity and metabolic syndrome in Asian Indians. Atherosclerosis 2012;223:507‐511. [DOI] [PubMed] [Google Scholar]
- 45. Fan Y, Wei F, Zhou Y, Zhang H. Association of non‐alcoholic fatty liver disease with impaired endothelial function by flow‐mediated dilation: a meta‐analysis. Hepatol Res 2016;46:E165‐E173. [DOI] [PubMed] [Google Scholar]
- 46. Ding J, Kritchevsky SB, Hsu FC, Harris TB, Burke GL, Detrano RC, et al. Association between non‐subcutaneous adiposity and calcified coronary plaque: a substudy of the Multi‐Ethnic Study of Atherosclerosis. Am J Clin Nutr 2008;88:645‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bugianesi E, Pagotto U, Manini R, Vanni E, Gastaldelli A, de Iasio R, et al. Plasma adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severity. J Clin Endocrinol Metab 2005;90:3498‐3504. [DOI] [PubMed] [Google Scholar]
- 48. Li Y, Xu C, Yu C, Xu L, Miao M. Association of serum uric acid level with non‐alcoholic fatty liver disease: a cross‐sectional study. J Hepatol 2009;50:1029‐1034. [DOI] [PubMed] [Google Scholar]
- 49. Targher G, Bertolini L, Rodella S, Zoppini G, Scala L, Zenari L, et al. Associations between plasma adiponectin concentrations and liver histology in patients with nonalcoholic fatty liver disease. Clin Endocrinol (Oxf) 2006;64:679‐683 [DOI] [PubMed] [Google Scholar]
- 50. Northup PG, Argo CK, Shah N, Caldwell SH. Hypercoagulation and thrombophilia in nonalcoholic fatty liver disease: mechanisms, human evidence, therapeutic implications, and preventive implications. Semin Liver Dis 2012;32:39‐48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep4.1199/full.
Supporting Information


