Abstract
The effects of Castanea sativa Mill. have been studied in high fat diet (HFD) overweight rats. Natural Extract of Chestnut bark (Castanea sativa Mill.) (ENC®), rich in ellagitannins, has been studied in 120 male Sprague-Dawley rats, divided in four groups. Two groups were controls: regular (RD) and HDF diet. Two groups received ENC® (20 mg/kg/day): RD + ENC® and HFD + ENC®. At baseline and at 7, 14 and 21 days, weight gain, serum lipids, plasma cytokines, liver histology, microsomial enzymes and oxidation, intestinal oxidative stress and contractility were studied. HFD increased body weight, increased pro-inflammatory cytokines, induced hepatocytes microvescicular steatosis, altered microsomial, increased liver and intestinal oxidative stress, deranged intestinal contractility. In HFD-fed rats, ENC® exerted antiadipose and antioxidative activities and normalized intestinal contractility, suggesting a potential approach to overweight management associated diseases.
Introduction
Unhealthy sedentary lifestyle and excessive eating have influenced the increase of closely related disturbances, such as obesity, insulin resistance, hypertension, and dyslipidemia, and the outstanding example of metabolic syndrome [1]. Metabolic syndrome is characterized by chronic dysregulation of metabolic and immune processes [2], low-grade inflammation [3], antioxidant impairment [4], xenobiotic metabolism homeostasis disruption [5], vascular smooth muscle contractility alterations [6]. Increased oxidative stress (OS) in obese patients [7] appears to be involved in the onset and progression of the pathological alterations of different organs [8–12]. Obesity-induced inflammatory dysfunctions may be, at least in part, reversible by “Comprehensive lifestyle interventions” [13]. Nutraceuticals use in the preclinical phase is a “proactive reverse approach” tool to health conditions in prevention time [14] and studies are being conducted to understand the mechanisms of action of bioactive nutraceutical compounds.
Traditionally, Castanea sativa Mill. leaves or bark has been widely used against various diseases, for its high antioxidant [15], cardioprotective activity [16], antihelmintic, antibacterial and antiviral effects [17], neuroprotective activity [18, 19], antispasmodic action on gallbladder [20] and intestine [15, 21].
High levels of tannins and their antioxidant activity have suggested that Castanea sativa Mill. bark extract associated to diet as a supplement, can help to prevent and treat the pathological processes underlying obesity.
In this study, we show the protective effect of Natural Extract of Chestnut [ENC® (Castanea sativa Mill. bark extract)] on body weight gain, liver inflammation and function, liver and intestinal oxidative state, gastric and intestinal contractility in high-fat diet rats in order to propose its use in the prevention and possibly co-management of obesity.
Materials and methods
Plants materials and chemicals
Bark extract of a Castanea sativa Mill. (ENC®), (supplied by SilvaTeam S.p.a., San Michele Mondovì, Italy) was obtained with a process previously described [21]. The powder (92–95% dry matter) is made by 77% tannins and its chemical composition has been previously characterized by HPLC-DAD-MS analysis. [16]. It is rich in phenolic compounds such as: castalin, vescalin, castalgin, vescalgin, ellagic acid and gallic acid. The amount of ellagic acid and of gallic acid found in ENC® were 10.69 ± 0.28% and 24.01 ± 0.57% respectively. The extract used in the study has been obtained as previously described and the percentage composition of the phenolic compounds has been obtained. Further information about ENC® is available at SILVATEAM website (http://it.silvateam.com/). Detailed information about chemicals are listed in S1 File.
Animals and experimental design
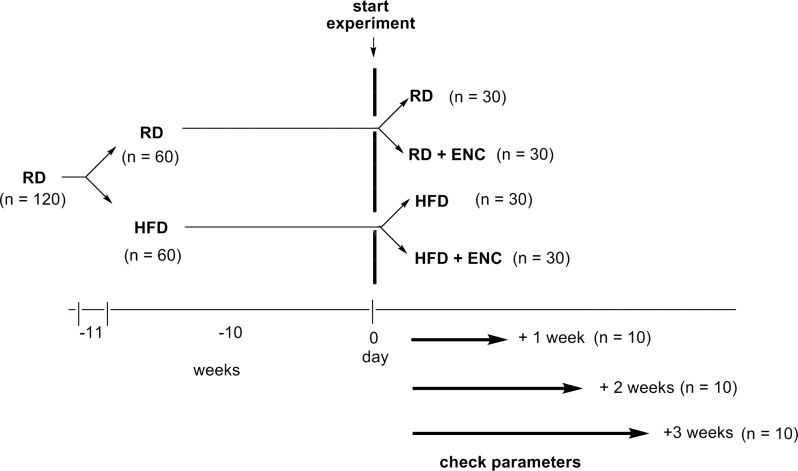
120 male Sprague-Dawley rats were purchased from Envigo RMS S.R.L. San Pietro al Natisone (Udine, Italy) at 9 weeks of age weighing 270–300 g. They were housed over 7 days adaptation under 12h-light/12h-dark cycle, 22°C, 60% humidity, with water and food ad libitum. The rats were split randomly into two groups and fed specific diets as follows: regular diet (RD, n = 60: 18.7% crude protein, 5.6% crude fat, 4.5% crude fibre by Mucedola s.r.l.); high fat diet (HFD, n = 60: 23% crude protein, 34% crude fat, 5% crude fibre, by Mucedola s.r.l.). HFD has been reported in S2 File. Animals maintained the dietary regimens described for 10 weeks, then each group was assigned to the treatment as follows: RD animals with vehicle (tap water) (n = 30), RD animals with ENC® 20 mg/kg administered by gavage (n = 30), HDF group with vehicle (tap water) (n = 30), HFD diet with ENC® 20 mg/kg administered by gavage (n = 30). (Fig1). The dose of ENC® to be administered to rats was extrapolated by using the translational dose calculation [22], which express the human equivalent dose (HED, mg/kg) as:
where Km was 6 and 37 for rat and human, respectively. As Abidov used 300 mg/day in man, a value in agreement with the human dose of 225 mg/day [23], the resulting final dose for rat was thus calculated to be 20 mg/kg. Furthermore, 21 days of treatment was chosen as it is the most common duration period to highlight metabolic changes and possible toxic effects and to better evaluate the time-course of the observed changes. At every end point (7, 14, 21 days) the animals were sacrificed by decapitation (n = 10/ end point) and the parameters reported.
Fig 1. Overview of the experimental design.
After one-week housing period, animals were randomly assigned to RD or HFD groups (10-weeks fattening period). HFD and control rats were further split into 4 groups as depicted. Animals were treated for 7, 14 or 21 days during which they received ENC® (20 mg/kg/day) or vehicle (tap water).
Ethical statements
The work was conducted according to the guidelines set forth to EU Directive 2010/63/EU and to ARRIVE guidelines [24]. The protocol was approved by the Institutional Ethics Committee of the University of Bologna (Protocol 21/79/14) and transmitted to the Ministry of Health. Details of Humane end points.docx are in S3 File.
Body-weight gain and food intake
Body weight and food consumption measurements started the first day of the study and continued for the entire experiment (3 weeks).
Determination of lipids, transaminases and inflammation
Serum lipids and transaminases
Triglyceride, HDL-cholesterol, LDL-cholesterol, total cholesterol and transaminases (ALT and AST) concentrations were measured by using commercially available kits (see S4 File).
Plasma inflammatory response
Biomarkers of inflammation have been quantitatively evaluated in RD- and HFD- rats, in basal conditions and after ENC® addition: IL-1α, IL-1β, IL-2, IL-5, IL-6, IL-7, IL-12p70, IL-17A, IL-18, and TNF-α (pro-inflammatory) and IL-4, IL-10, IL-13 (anti-inflammatory), by the Luminex MAGPIX® system (Millipore Corp., Billerica, MA, USA). For details, see S4 File).
Histology
Liver necro-inflammatory score and architectural changes, fibrosis and cirrhosis were assessed after haematoxylin and eosin tissue staining (See S4 File).
Hepatic Phase I, Phase II and antioxidant enzyme activities
Tissue collection and preparation of hepatic subcellular fractions, Phase I and II, antioxidant enzymes and their activities, listed below, were assessed as reported in S4 File).
Phase I
NADPH-(CYP)-c-reductase (CYP-red), Aminopyrine N-demethylase (APND)- CYP3A1/2, p-Nitrophenol hydroxylase (p-NFH)-CYP2E1, Pentoxyresorufin O-dealkylase (PROD)-CYP2B1/2, Ethoxyresorufin O-deethylase, (EROD)-CYP1A1, Methoxyresorufin O-demethylase (MROD)-CYP1A2, Ethoxycoumarin O-deethylase (ECOD)-CYP1A1/2.
Phase II
Glutathione S-transferase (GST) activity, UDP-glucuronosyl transferase (UDPGT).
Antioxidant enzymes
Catalase (CAT) activity, NAD(P)H:quinone reductase (NQO1), Oxidised glutathione reductase activity (GSSG-red), Superoxide dismutase activity (SOD).
Oxidative stress evaluation in ileal and colonic tissues
OS biomarkers were evaluated in colon and ileum by assay: Details are reported in S4 File).
Spontaneous and induced contractility studies
Gastric fundus basal contractility was investigated. Ileum and proximal colon induced contractility were studied against cholinergic receptors and calcium channels (S4 File).
Data presentation and statistical analysis
Results are presented as mean ± standard deviation (S.D.) or error standard (S.E.M.) from n rats. The experimental design was subjected to ‘a priori power analysis’ to ensure that the size of treated- and control- groups was adequate (G*Power v. 3.1.9.2). To improve clarity, since the content of serum lipids and transaminase, plasma pro- anti-inflammatory cytokines, MDA in ileum and colon as well as hepatic Phase I, II and antioxidant enzyme activities did not change significantly between day 0 and day 21 in both RD- and HFD-rats, these have been pooled and reported as RD 0–21 or HFD 0–21. Plasma cytokine content was reported as percent changes of the baseline value [100 (%)] of RD-rats. Data were compared by ANOVA followed by Bonferroni’s post hoc tests and in all comparisons, P value less than 0.05 was considered significant. Before performing the analysis, ANOVA assumption were always tested using the method of Bartlett (data sampled from populations with identical SDs) and Kolmogorov and Smirnov (data sampled from populations that follow Gaussian distributions).
Changes in spontaneous contractions of gastric fundus were considered significant when they were higher than 10% variations in each range [25].
Functional induced contractility of agonist Carbacol (CCh) was expressed as pEC50 values. The antagonism activity of atropine against CCh was expressed as pA2 values determined from Schild plots [26] constrained to slope –1.0, as required by theory [27]. Differences between mean values were performed by using a two-tailed unpaired Student’s t-test for continuous data. In all comparisons, P value less than 0.05 was considered significant.
Results
Body weight gain and food intake
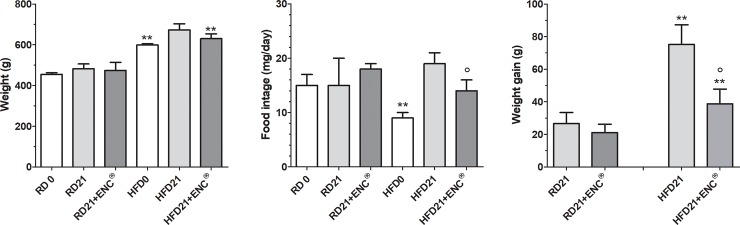
Three weeks of HFD increased significantly rat body weight, with respect to RD (598.1 ± 41.4 g vs 455.8 ± 20.0 g, P < 0.01), despite a general lower food intake of the former group (Fig 2). In RD-rats, weight, weight gain and food intake did not show significant differences with ENC®, while in HFD-rats ENC® caused a significant decrease in food intake and weight gain with respect to HFD-rats (Fig 2).
Fig 2. Body weight, food intake and weight gain of RD-rats, HFD-rats with or without ENC® (20 mg/kg/day) for 21 days.
RD 0 or HFD 0: data measured at day 0; RD 21 or HFD 21: data measured at day 21. Results are reported as mean ± S.D. (n = 10/group) and analyzed by using ANOVA followed by Bonferroni post test. **P < 0.01 vs RD-fed group, same time. °P < 0.05 vs HFD21.
It is worth outlining that 21 days of ENC® treatment did not cause any death in either RD- or HFD-rats. Moreover, physical observations of treated animals showed no signs of changes in the skin, fur, eyes mucous membrane, behavior patterns, tremors, salivation, suggesting an apparent safe profile of ENC® treatment.
Liver gross appearance and histology
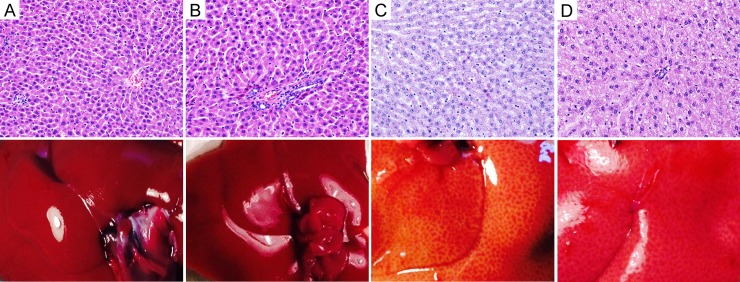
RD-rats presented normal liver histology, while HFD induced a moderate hepatocytes microvesicular steatosis; after ENC® microvesicular steatosis was only in scattered hepatocytes (Fig 3), with a regression of liver steatosis. The livers did not present any sign of necroinflammatory score (arbitrary score of 0 on a scale range from 0 to 18). Similarly, the gross appearance of the liver showed an enlargement and lightening in color that decreased after ENC® assumption. Histological parameters are detailed in S1 Table.
Fig 3.
Histology of liver tissues (upper panels) and gross appearance of the liver (lower panels) of RD-rats, HFD-rats with or without ENC® (20 mg/kg/day) for three weeks. A: RD control rat: normal rat liver showing central vein and a regular parenchyma. B: RD rat after ENC®: the liver shows a normal portal tract and mild hepatocyte pleomorphism. C: HFD rat: the hepatocytes show a moderate microvescicular steatosis. D: HFD rat after ENC®: microvescicular steatosis is evident only in scattered hepatocytes. (H&E) 10x.
Lipids, transaminases and inflammation
Serum lipids and transaminases
As reported in Table 1, HFD-rats serum cholesterol levels were higher than those of RD-rats (P < 0.01 vs RD 0–21). Cholesterol was significantly lowered by ENC® administration in HFD-rats (-10%, P < 0.05). LDL Cholesterol was higher in HFD rats than in controls (P < 0.05 vs RD 0–21); ENC® decreased LDL levels in HFD-rats (P < 0.05 vs HFD 0–21), while it left them unchanged in RD-rats. At the beginning of the study, HDL Cholesterol was lower in HFD- than in RD-rats (P < 0.05 vs RD 0–21). ENC® increased HDL in the HFD group (P < 0.05 vs HFD0-21), whereas it did not affect that of RD-rats. Triglycerides were increased by about 63% in HFD with respect to RD- rats (P < 0.01 vs RD 0–21); interestingly, in the former group ENC® decreased triglycerides levels (P < 0.01 vs HFD 0–21). Finally, aspartate transaminases and alanine transaminases were not affected by the treatment in both RD- and HFD-rats. RD-rats and HFD-rats with or without ENC® (21 days).
Table 1. Serum parameters of RD-rats and HFD-rats supplemented with or without ENC® for 21 days.
| RD | RD+ ENC®a | HFD | HFD+ ENC®a | |
|---|---|---|---|---|
| Day | 0–21 | 21 | 0–21 | 21 |
| ASTb | 242 ± 60 | 236 ± 70 | 237 ± 60 | 238 ± 60 |
| ALTc | 67 ± 23 | 64 ± 25 | 60 ± 23 | 60 ± 21 |
| Cholesterold | 3.16 ± 0.23 | 3.10 ± 0.19 | 3.68 ± 0.17** | 3.28 ± 0.16° |
| LDL Cholesterold | 0.90 ± 0.03 | 0.88 ± 0.09 | 1.02 ± 0.02* | 0.92 ± 0.07° |
| HDL Cholesterold | 2.34 ± 0.08 | 2.36 ± 0.03 | 2.18 ± 0.04* | 2.26 ± 0.03° |
| Triglycerided | 1.24 ± 0.14 | 1.22 ± 0.16 | 2.03 ± 0.10** | 1.46 ± 0.07°° |
a) ENC® 20 mg/kg/day.
b) AST, Aspartate transaminases (Units/ml).
c) ALT, Alanine transaminases (Units/ml).
d) Expressed as mmol/l.
To improve clarity, since values between RD 0 day and RD 21 days did not change significantly, they have been pooled; the same was for HFD 0 day and HFD 21 days. Results are reported as mean ± S.D. (n = 10/group) and analyzed by using ANOVA followed by Bonferroni post test.
*P < 0.05
**P < 0.01, vs RD-rats, same time.
°P < 0.05
°°P < 0.01 vs HFD 0–21.
Inflammatory response
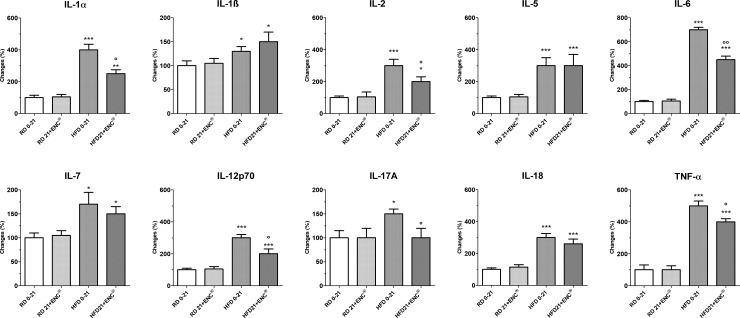
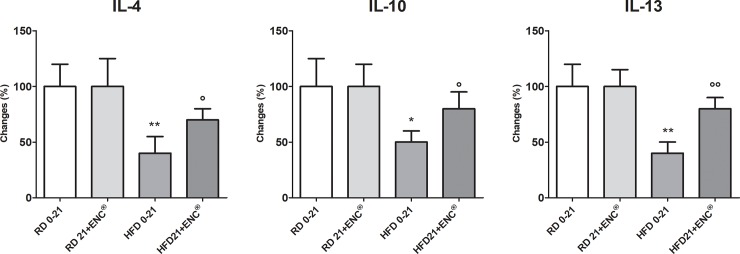
HFD-rats had higher pro-inflammatory cytokines plasma levels than RD-rats (Fig 4), changing from + ~ 30% (IL-1ß) to +~ 600% (IL-6). In the former group, however, 21 days ENC® decreased significantly IL1α, IL-2, IL-6, IL-12p70, IL17A and TNF-α plasma levels, while it left unchanged those of IL-1ß, IL-5 and IL-18. Interestingly, the anti-inflammatory cytokines IL-4, IL-10 and IL-13 were significantly lower in overweight animals (- 50–60%, P<0.05 or 0.01 vs RD 0–21) (Fig 5). ENC® reverted this effect, restoring anti-inflammatory cytokines plasma content to values very close to RD-rats. Finally, ENC® did not affect cytokines plasma contents in RD-rats.
Fig 4. Plasma pro-inflammatory cytokines in RD-rats, and HFD-rats with or without ENC® (20 mg/kg/day) for 21 days.
RD0-21 or HFD0-21: pooled data between days 0–21. Data are reported as mean ± S. D. and expressed as a percent changes of the baseline value [100 (%)] of RD-fed rats. *P < 0.05, **P < 0.01, ***P < 0.001 vs RD same time. °P < 0.05, °°P < 0.01 vs HFD 0–21 (ANOVA followed by Bonferroni post test).
Fig 5. Plasma anti-inflammatory cytokines in RD-rats, and HFD-rats with or without ENC® (20 mg/kg/day) for 21 days.
RD0-21 or HFD0-21: pooled data between days 0–21. Data are reported as mean ± S.D. and expressed as percent changes of the baseline value [100 (%)] of RD-rats. *P < 0.05, **P < 0.01, ***P < 0.01 vs RD same time. °P < 0.05, °°P < 0.01 vs HFD 0–21 (ANOVA followed by Bonferroni post test).
Hepatic Phase I, Phase II and antioxidant enzyme activities
Hepatic Phase-I enzymes
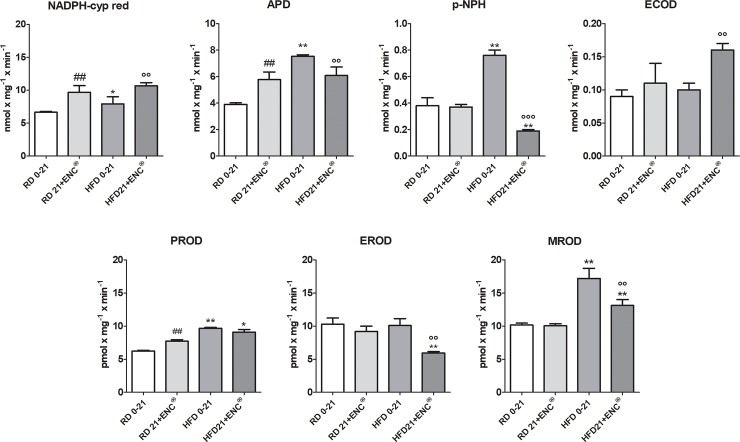
In RD-rats, ENC increased the activity NADPH cyp red, APD and PROD, leaving unaffected that of pNPH, ECOD, EROD and MROD (Fig 6). Also HFD-rats reported a widespread increment of microsomal hepatic mono-oxygenases respect to RD-rats. In particular, CYP3A1/2, CYP2E1 and CYP1A2 isoform-linked activities, here measured as APD, p-NPH and MROD, respectively as well as NADPH cyp-red were significantly raised (P < 0.01 vs RD0-21) (Fig 6), while ECOD and EROS were not changed. ENC® was effective in counteracting the changes caused by HFD, reducing almost all enzymes increased activities (P < 0.01 vs HFD 0–21). Hepatic phase-I enzymes full changes are detailed in S2 Table.
Fig 6. Changes in liver phase-I enzymes in RD-rats, and HFD-rats with or without ENC® (20 mg/kg/day) for 21 days.
RD0-21 or HFD0-21: pooled data between days 0–21. Bars represent the means ± S.D. *P < 0.05, **P < 0.01, vs RD same time; °P < 0.05, °°P < 0.01, °°°P < 0.001 vs HFD0-21; ## P <001 vs RD0-21.
Hepatic phase-II enzymes
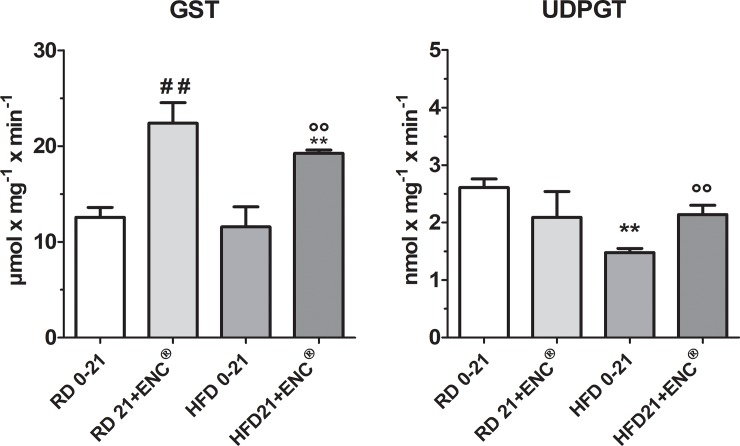
ENC® supplementation induced a significant up-regulation of GST activity in both RD- (≈ 78%, P < 0.01) and HFD-rats (≈66%, P < 0.01) (Fig 7). In HFD-rats, UDPGT was decreased with respect to RD-rats and ENC® restored the values toward those observed in RD-rats. Detailed information is in S2 Table.
Fig 7. Changes in liver phase-II enzymes in RD-rats, and HFD-rats with or without ENC® (20 mg/kg/day) for 21 days.
RD0-21 or HFD0-21: pooled data between days 0–21. Bars represent the means ± S.D. ##P < 0.01 vs RD0-21; **P < 0.01 vs RD same time; °°P < 0.01 vs HFD0-21.
Hepatic antioxidant enzymes
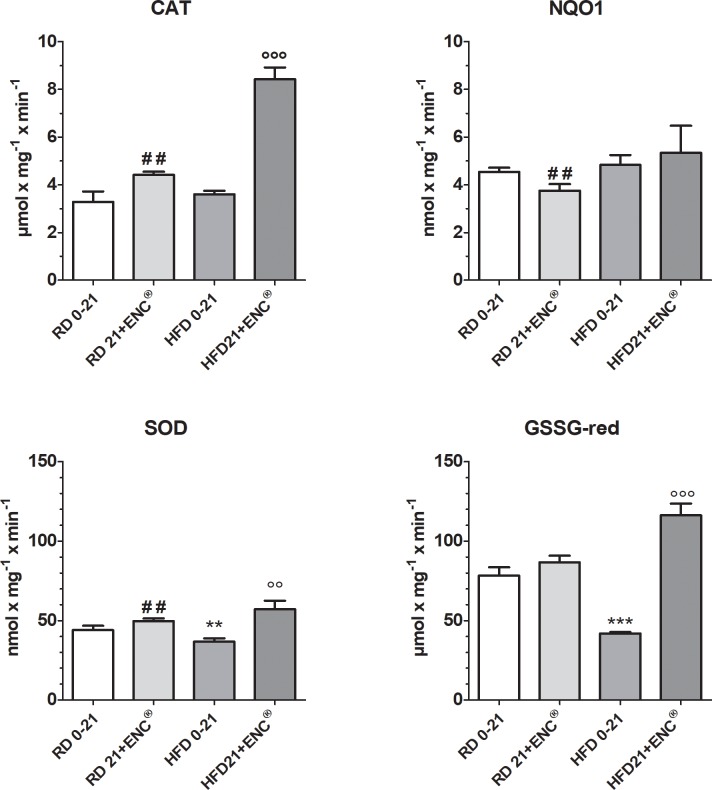
Hepatic antioxidant enzymes CAT and NQ01 were similar in RD and HFD rats. On the contrary, as shown in Fig 8, HFD decreased SOD and GSSG-red with respect to RD. In RD feed rats, the treatment with ENC® increased CAT and SOD and decreased NQ01 activities, while in HFD animals, it caused an increment of CAT, SOD and GSSG-red, completely restoring these enzyme antioxidant potential which exceeded the values recorded in RD group (Fig 8). For detailed information about the changes in the time course of antioxidant enzymes activities, see S2 Table.
Fig 8. Changes in hepatic antioxidant enzymes after 21 days ENC® supplementation (20 mg/kg/day) in RD-fed and HFD-fed rats.
RD0-21 or HFD0-21: pooled data between days 0–21. Bars represent the means ± S.D. of ten measurements performed on ten rats. ** P< 0.01 vs RD-fed group, same time. °°P < 0.01, °°° P <0.001 vs HFD0-21, ##P < 0.01 vs RD0-21.
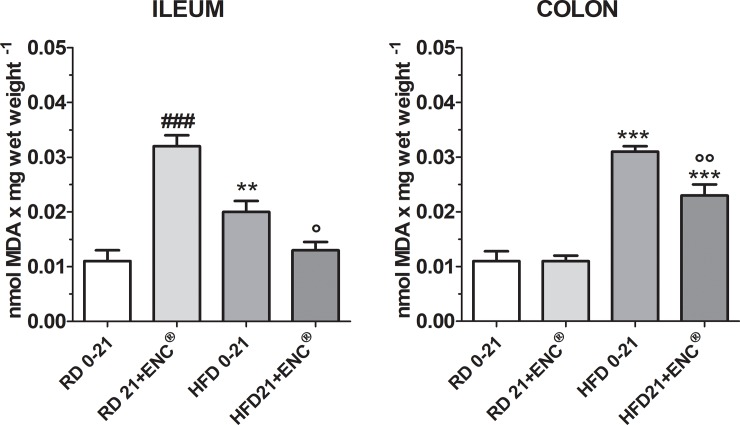
Oxidative stress in ileal and colonic tissues
Malondialdehyde (MDA) assay, a product generated from lipid oxidation, was used to measure the level of OS in the different tissues. HFD-rats showed a significant increase in the MDA values respect to RD-rats (P < 0.01 in the ileum and P<0.001 in the colon) confirming that a HFD induces OS (Fig 9). Interestingly, the RD-rats, treated with ENC® for 21 days had a high MDA value in the ileum suggesting that the phenolic compounds present in the ENC® may have a pro-oxidant effect on this tissue at the dose of extract used. Nevertheless, 21 days of ENC® administration was able to counteract HFD-induced OS in the ileum (Fig 9). The pro-oxidant effect of ENC® in RD-rats was not evident in colon tissue. On the other hand, OS induced by the HFD on this tissue was 2.5 times higher than that of the RD-rats (P < 0.001); after 21 days ENC®treatment MDA was significantly reduced (P < 0.01 vs HFD 0–21) confirming the antioxidant properties of ENC® (Fig 9).
Fig 9. MDA equivalents in ileum and colon homogenates in RD-rats and HFD-rats supplemented with or without ENC® (20 mg/kg/day) for 21 days.
Data are expressed as mean ± SD. **P < 0.01, ***P < .001 vs RD same time. °P < 0.05, °°P < 0.01 vs HFD0-21 (ANOVA followed by Bonferroni post test).
Spontaneous and induced contractility studies
Spontaneous contractility
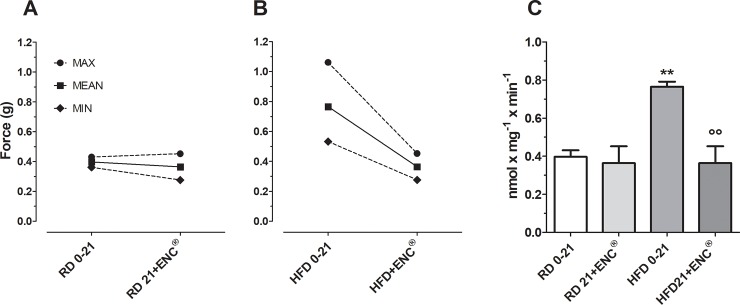
The gastric spontaneous contractility in HFD-rats was characterized by a higher basal tone than in RD-rats (P < 0.01). After ENC® the basal tone was unchanged in RD-rats while in HFD-rats the contraction force was reduced to values within the range of RD-rats, suggesting that 21 days ENC® treatment restored the gastric tonic state to values within the normal range (Fig 10).
Fig 10.
Basal gastric spontaneous contractility in RD- (panel A) and HFD-rats (panel B) before and after 21 days supplementation with ENC® (20 mg/kg/day). Panel C: columns comparing the specific values for RD- and HFD-rats. Data are expressed as mean ± SD. **P < 0.01, vs RD same time; °°P < 0.01 vs HFD0-21 (ANOVA followed by Bonferroni post test).
Induced contractility
Ileum- and distal colon-induced contractility by Carbachol (CCh) and KCl (80 mM) was studied. In RD-fed rats, CCh caused a rapid phasic response, while KCl induced a tonic contraction of rat ileum and proximal colon, as previously reported [25]. Atropine antagonized in concentration dependent manner the response to CCh both in ileum and colon (Table 2 and S3 Table).
Table 2. Agonist [carbachol (CCh)] and antagonist (atropine) activities expressed as pEC50 or pA2, respectively, in ileum and proximal colon of RD- and HFD-rats supplemented with or without ENC® for 21 days.
| RD | RD + ENC®a | HFD | HFD+ ENC®a | ||
|---|---|---|---|---|---|
| Day | 0–21 | 21 | 0–21 | 21 | |
| Ileum | CCh b | 6.27 ± 0.03 | 6.34 ± 0.05 | 5.89 ± 0.02** | 5.90 ± 0.04* |
| Atropine c | 8.95 ± 0.03 | 8.90 ± 0.05 | 8.68 ± 0.04** | 8.91 ± 0.02 | |
| Proximal Colon | CCh b | 5.92 ± 0.02 | 5.80 ± 0.02## | 5.83 ± 0.03* | 5.43 ± 0.02**°° |
| Atropinec | 8.77 ± 0.03 | 8.57 ± 0.04## | 7.73 ± 0.07*** | 8.73 ± 0.02**°° |
a) RD-and HFD-rats have been given 21 days ENC® (20 mg/kg/day b.w).
b) Data are expressed as pEC50. pEC50 = –log EC50. EC50 values are the means ± S.E.M. of at least four independent experiments and were calculated by a non-linear regression curve-fitting computer program [28] [50]
c) Data are expressed as pA2. pA2 values ± S.E.M. were calculated from Schild plots [26], constrained to slope –1.0 [27] (pA2 is the positive value of the intercept of the line derived by plotting log (DR– 1) vs log [antagonist]. The log (DR– 1) was calculated from three different antagonist concentrations, and each concentration was tested from four to six times. Dose-ratio (DR) values represent the ratio of the potency of the agonist carbachol (EC50) in the presence of the antagonist and in its absence. Parallelism of concentration–response curves was checked by linear regression, and slopes were tested for significance (P < 0.05).
*P <0.05
**P < 0.01
***P <0.001 vs RD, same time.
°°P < 0.01, vs HFD0-21.
## P< 0.01 vs RD0-21 (ANOVA followed by Bonferroni post test).
In ileum, HFD significantly reduced CCh and atropine pA2 (P < 0.01) with respect to RD-rats; ENC® decreased CCh pD2 difference (P<0.05). In ileum ENC® did not change CCh pD2 after days 21 treatment in HFD-rats, while atropine pA2 values were not different from RD-rats. In RD-rats proximal colon, ENC® slightly reduced CCh potency, less than in the ileum, and Atropine potency. In HFD-rats colon, both CCh and Atropine were less potent than in the same organs of RD-rats. In RD-rat ileum and proximal colon, Nifedipine inhibited tonic contraction induced by 80 mM KCl (Fig 11) (for detailed data, see S3 Table).
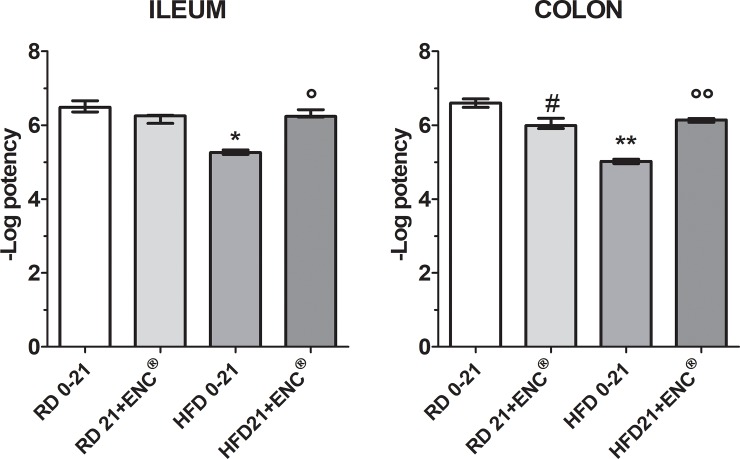
Fig 11. Potency of Nifedipine (pEC50) on ileum and distal colon in RD-and HFD-rats with or without ENC® (20 mg/kg/day) for 21 days.
The spasmolytic effect induced by Nifedipine was used as reference. Data are expressed as mean ± S.E.M.. *P<0.05, P<0.01 vs RD0-21; °P<0.05, °°P<0.01 vs HFD 0–21 (ANOVA followed by Bonferroni post test).
After ENC® administration, no significant changes in Nifedipine intrinsic activity and potency were observed in RD-fed rats ileum. In HFD-fed rats ileum, Nifedipine showed the same intrinsic activity, but at concentrations which differ by one order of magnitude (Fig 11) (For detailed data, see S3 Table). Potency on the HFD-fed rats ileum was about 16.39 times lower than on RD-fed rats. After 21 days ENC® treatment, Nifedipine potency was not quite different from that observed in RD-fed rats ileum. In RD-fed rats colon, Nifedipine potency decrease was related to the days of treatment and became lower by 4.04 at the end of the experiment (21 days of treatment). In HFD-fed rats colon, Nifedipine presented intrinsic activity and potency significantly different from RD-fed rats colon. Potency was reduced by 37.72 times. 21 days ENC® treatment of HFD-fed rats increased Nifedipine potency on the colon, which attained values very close to those observed in RD-fed rats colon (Fig 11) (for detailed data, see S3 Table).
Discussion
Polyphenols have been shown to exert several beneficial effects in different obesity experimental models [16, 20, 29–31], by scavenging reactive oxygen and nitrogen species and modulating genes associated with metabolism, drug metabolizing enzymes, detoxification proteins, protecting from chronic diseases [32].
A down-regulation of liver oxidative defenses has been reported in obesity experimental models [33]. Consistently, in the present work, ENC® restored the antioxidant enzymatic ability in HFD-rats and suppressed pro-inflammatory / anti-inflammatory cytokines imbalance in HFD rat liver. A crucial point is the decrease of TNF-α, a key factor in the development of Non Alcoholic Fatty Liver Disease (NAFLD) and Non Alcoholic Steato Hepatitis (NASH) [34], of IL-6, hepatoprotector in liver steatosis by reducing oxidative stress [35], of IL-10 [36] and of IL-17A, implicated in both regulation of obesity and NAFLD [37].
NAFLD is characterized by a general dysregulation of drug metabolism enzymes. Several studies reported that GSTM1, GSTM2, GSTM4 and GSTM5 mRNA levels are suppressed in patients with steatosis and NASH [38]. Moreover, GST activity decreases with disease progression with a reduced pool of glutathione, highlighting the reduced ability to counteract OS in these patients [39].
Finally, GSTM1-null genotype has been associated to NAFLD [40] and UDPGT levels were decreased in HFD-fed rats [41]. In HFD-rats ENC® increased enzymatic activity of GST and UDPGT and it can reduce the dysregulation of drug metabolism enzymes. Moreover, these finding could be clinically relevant for obese patients who have additional comorbidities and are receiving multiple medications.
These outcomes are extremely important in a therapeutic schedule of obese patients at possible risk of NAFLD and NASH. ENC® slightly reduced liver steatosis induced by HFD. Since flavonoids induce a cytoprotective effect through Nrf2-dependent gene expression [42] we think that up-regulation of antioxidant and detoxifying enzymes is obtained of Nrf2-ARE signalling pathway. Intracellular accumulation of xeno- and endobiotics is regulated at several levels by drug metabolizing enzymes.
Many tannins possess anti-obesity properties [43]. Pomegranate leaf extract containing abundant tannins, for example, can hamper the development of obesity and hyperlipidemia in high-fat diet induced obese mice by inhibiting the pancreatic lipase activity and suppressing energy intake [44]. This effect was much less evident when the animals were treated with its main isolated compounds (ellagic acid and tannic acid), suggesting a possible synergic effects among the component of the extract. Furthermore, growing number of studies demonstrate the potential role of ellagic acid in the regulation of lipid metabolism and in attenuating obesity and metabolic syndrome in rodents, mainly because of its antioxidant and anti-inflammatory properties (for a review see [45]). Thus it is possible that all the properties reported for ellagic acid are shared also by ENC® or its gut metabolites. Is is important to outline, however, that the amount of ellagic- and gallic-acid contained in ENC® is ~ 1–2% of the injected dose, suggesting that other components may contribute to the observed effects. It is possible that other polyphenols found in ENC® might act synergistically by increasing, for example, the potency of ellagic and gallic acids at its specific molecular target(s) or by increasing the bioavailability of the main active components. In this sense, the literature already reports the existence of synergistic effects among several phytochemicals [46].
Obesity has been correlated with an induction of some cytochrome P450 isoforms (CYPs) [5, 47, 48]. The NADPH-dependent overproduction of reactive oxygen species (ROS) is linked to CYP induction [49].
In HFD-rats an up-regulation of mainly CYP2E1, CYP3A1 and CYP2B1/2 CYPs has been found. CYP2E1 values were reduced by four times by ENC®. CYP2E1 up-regulation has been associated to obesity, fatty liver, and NASH in both humans and rodents and correlates with NAFLD severity [50] and progression of liver steatosis to NASH: CYP2E1 up-regulation leads to enhanced ROS production, with an increase of lipid peroxidation and inflammation [51].
HFD led to a general impairment of cellular redox homeostasis responsible for the observed alterations of phase I and II antioxidant enzymes. We focused on the peroxidation products in intestinal tissues from all experimental groups. In RD-rats ileum, ENC® increased MDA levels, likely due to the high amount of ellagitannins, that are slowly hydrolysed in the digestive tract, to release ellagic acid. In the colon OS was increased, as observed in the mouse distal colon of HFD mice [52].
The compound inhibited HFD-induced oxidative stress increase, in agreement with published data showing that polyphenols rich extracts provide benefits in gut inflammation, for their antioxidant activity, due to a prebiotic [53] action that may help reduce OS in obese patients.
Since obesity seems associated to increased gastric emptying, we evaluated the basal gastric tone of HFD- and RD-rats. ENC® restored the gastric tone in HFD-fed rats. HFD alters the composition of cells membranes, resulting in an impaired response to endogenous agonists such as Ach and histamine; also intracellular calcium release may be disturbed. In a previous work, we demonstrated that ENC® exerts a non-competitive and concentration-dependent antagonist effect towards muscarinic and histaminergic receptors and inhibits BaCl2-induced contractions: these effects may be, at least in part, responsible for ENC® beneficial effects towards gastric smooth muscle contractility alterations related to obesity [21]. To evaluate the intestinal cell membrane functionality, we used CCh and atropine as a muscarinic receptor agonist and antagonist respectively, and nifedipine as calcium channel blocker. ENC® spasmolytic effect depends on a combination of effects towards different receptors and channels. KCl induced tonic contraction on rat ileum and colon while CCh caused a rapid phasic contraction. Nifedipine completely inhibited K+ induced contraction. In RD-rats, ENC® (21 days) reduced nifedipine potency by 4.04 times in the colon, with a minimal decrease in the ileum (by 1.69 times). Hypercholesterolemic diet has been reported to decrease calcium channel pump activity in pigs coronary macrocirculation [54] and cholesterol enriched cyclodextrin inhibited calcium currents in guinea pig gallbladder smooth muscle [55]. In HFD-rats, nifedipine potency was 16.39 and 37.72 times less potent than in RD-rats ileum and colon, respectively. Importantly, ENC® decreased the activity of L-type calcium channels in RD-rats mainly in colon, but increased their function mostly in the HFD-rats colon. ENC® decreases nifedipine potency in ileum and more strongly in colon. Therefore, in HFD-rats calcium channels function progressively recovers after ENC® administration.
The activity and responsiveness of calcium channels in HFD-fed rats may be worthwhile considering. The induced increase in calcium channels activity may influence the basal tone, affect contractility and alter the response to receptor agonists and antagonists. ENC® showed a not competitive and reversible muscarinic effect on isolated ileal and colonic muscular strips and ENC® restored receptors function in HFD-rats. Also in the case of membrane coupled receptors, the observed increase of the activity showed the restoration of membrane function, that supported the ENC® effects on basal gastric contractility.
Conclusions
In conclusion, our study shows that ENC® may be a promising nutraceutical tool, decreasing spontaneous food assumption, up-regulating hepatic antioxidant enzymes causing the impairment in liver oxidative defenses, restoring liver phase I and II enzymes activity, reducing intestinal oxidative stress, and restoring the intestinal motor function. These ameliorative effects on metabolic situation in obesity might be related to the presence of ellagic and gallic acid. As recently reported on hypertriglyceridemia induced by High-Fructose Diet in rats, ENC® contains such gallic acid equivalents as to decrease hypercholesterolemia induced by HFD [56]. All together these results show that ENC® has the potential to be used in clinical medicine as a dietary supplement in the treatment of obesity complications.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Effects of 21 days ENC® supplementation (20 mg/kg/day) for 21 days on liver phase I (A), phase II (B) and antioxidant (C) enzymes activities in RD and HFD rats.
(DOCX)
Agonist [carbachol (CCh)] and antagonist (atropine) activities expressed as pEC50 or pA2, respectively, (A) and Calcium channels antagonist Nifedipine activity (B) in the isolated rat ileum and distal colon of RD- and HFD-rats supplemented with or without ENC® (20 mg/kg/day) for 21 days.
(DOCX)
Acknowledgments
The authors received no specific funding for this work and are grateful to Alma Mater Studiorum-University of Bologna (Italy) by research financial support (RFO-University of Bologna). The authors thank "GVM Care & Research" for cultural contribution and declare that it did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Alma Mater Studiorum-University of Bologna (Italy) in the form of research financial support awarded to RB, as an Associate Professor of the University of Bologna. GVM Care & Research provided support in the form of cultural contributions for AC. The specific role of this author is articulated in the 'author contributions' section. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Alexopoulos N, Katritsis D, Raggi P. Visceral adipose tissue as a source of inflammation and promoter of atherosclerosis. Atherosclerosis. 2014;233(1):104–12. 10.1016/j.atherosclerosis.2013.12.023 . [DOI] [PubMed] [Google Scholar]
- 2.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88 10.1186/1471-2458-9-88 ; PubMed Central PMCID: PMCPMC2667420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sur G, Floca E, Kudor-Szabadi L, Sur ML, Sur D, Samasca G. The relevance of inflammatory markers in metabolic syndrome. Maedica (Buchar). 2014;9(1):15–8. ; PubMed Central PMCID: PMCPMC4268284. [PMC free article] [PubMed] [Google Scholar]
- 4.Calliste CA, Trouillas P, Allais DP, Duroux JL. Castanea sativa Mill. leaves as new sources of natural antioxidant: an electronic spin resonance study. J Agric Food Chem. 2005;53(2):282–8. 10.1021/jf049341c . [DOI] [PubMed] [Google Scholar]
- 5.Melega S, Canistro D, Pagnotta E, Iori R, Sapone A, Paolini M. Effect of sprout extract from Tuscan black cabbage on xenobiotic-metabolizing and antioxidant enzymes in rat liver. Mutat Res. 2013;751(1):45–51. 10.1016/j.mrgentox.2012.10.013 . [DOI] [PubMed] [Google Scholar]
- 6.Christou DD, Pierce GL, Walker AE, Hwang MH, Yoo JK, Luttrell M, et al. Vascular smooth muscle responsiveness to nitric oxide is reduced in healthy adults with increased adiposity. Am J Physiol Heart Circ Physiol. 2012;303(6):H743–50. 10.1152/ajpheart.00394.2012 ; PubMed Central PMCID: PMCPMC3468458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asayama K, Nakane T, Dobashi K, Kodera K, Hayashibe H, Uchida N, et al. Effect of obesity and troglitazone on expression of two glutathione peroxidases: cellular and extracellular types in serum, kidney and adipose tissue. Free Radic Res. 2001;34(4):337–47. . [DOI] [PubMed] [Google Scholar]
- 8.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37(4):917–23. 10.1053/jhep.2003.50161 . [DOI] [PubMed] [Google Scholar]
- 9.Matsuda M, Shimomura I. Roles of adiponectin and oxidative stress in obesity-associated metabolic and cardiovascular diseases. Rev Endocr Metab Disord. 2014;15(1):1–10. 10.1007/s11154-013-9271-7 . [DOI] [PubMed] [Google Scholar]
- 10.Nguyen JC, Killcross AS, Jenkins TA. Obesity and cognitive decline: role of inflammation and vascular changes. Front Neurosci. 2014;8:375 10.3389/fnins.2014.00375 ; PubMed Central PMCID: PMCPMC4237034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma K. Obesity, oxidative stress, and fibrosis in chronic kidney disease. Kidney Int Suppl (2011). 2014;4(1):113–7. 10.1038/kisup.2014.21 ; PubMed Central PMCID: PMCPMC4220515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun X, Veldhuizen MG, Wray AE, de Araujo IE, Sherwin RS, Sinha R, et al. The neural signature of satiation is associated with ghrelin response and triglyceride metabolism. Physiol Behav. 2014;136:63–73. 10.1016/j.physbeh.2014.04.017 ; PubMed Central PMCID: PMCPMC4195817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 Suppl 2):S102–38. 10.1161/01.cir.0000437739.71477.ee . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santini A, Tenore GC, Novellino E. Nutraceuticals: A paradigm of proactive medicine. Eur J Pharm Sci. 2017;96:53–61. 10.1016/j.ejps.2016.09.003 . [DOI] [PubMed] [Google Scholar]
- 15.Frankic T, Salobir J. In vivo antioxidant potential of Sweet chestnut (Castanea sativa Mill.) wood extract in young growing pigs exposed to n-3 PUFA-induced oxidative stress. J Sci Food Agric. 2011;91(8):1432–9. 10.1002/jsfa.4328 . [DOI] [PubMed] [Google Scholar]
- 16.Chiarini A, Micucci M, Malaguti M, Budriesi R, Ioan P, Lenzi M, et al. Sweet chestnut (Castanea sativa Mill.) bark extract: cardiovascular activity and myocyte protection against oxidative damage. Oxid Med Cell Longev. 2013;2013:471790 10.1155/2013/471790 ; PubMed Central PMCID: PMCPMC3600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basile A, Sorbo S, Giordano S, Ricciardi L, Ferrara S, Montesano D, et al. Antibacterial and allelopathic activity of extract from Castanea sativa leaves. Fitoterapia. 2000;71 Suppl 1:S110–6. . [DOI] [PubMed] [Google Scholar]
- 18.Brizi C, Santulli C, Micucci M, Budriesi R, Chiarini A, Aldinucci C, et al. Neuroprotective Effects of Castanea sativa Mill. Bark Extract in Human Neuroblastoma Cells Subjected to Oxidative Stress. J Cell Biochem. 2016;117(2):510–20. 10.1002/jcb.25302 . [DOI] [PubMed] [Google Scholar]
- 19.Santulli C, Brizi C, Micucci M, Del Genio A, De Cristofaro A, Bracco F, et al. Castanea sativa Mill. Bark Extract Protects U-373 MG Cells and Rat Brain Slices Against Ischemia and Reperfusion Injury. J Cell Biochem. 2017;118(4):839–50. 10.1002/jcb.25760 . [DOI] [PubMed] [Google Scholar]
- 20.Micucci M, Ioan P, Aldini R, Cevenini M, Alvisi V, Ruffilli C, et al. Castanea sativa Mill. extract contracts gallbladder and relaxes sphincter of Oddi in guinea pig: a natural approach to biliary tract motility disorders. J Med Food. 2014;17(7):795–803. 10.1089/jmf.2013.0090 . [DOI] [PubMed] [Google Scholar]
- 21.Budriesi R, Ioan P, Micucci M, Micucci E, Limongelli V, Chiarini A. Stop Fitan: antispasmodic effect of natural extract of chestnut wood in guinea pig ileum and proximal colon smooth muscle. J Med Food. 2010;13(5):1104–10. 10.1089/jmf.2009.0210 . [DOI] [PubMed] [Google Scholar]
- 22.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–61. 10.1096/fj.07-9574LSF . [DOI] [PubMed] [Google Scholar]
- 23.Abidov M, Ramazanov Z, Seifulla R, Grachev S. The effects of Xanthigen in the weight management of obese premenopausal women with non-alcoholic fatty liver disease and normal liver fat. Diabetes Obes Metab. 2010;12(1):72–81. 10.1111/j.1463-1326.2009.01132.x . [DOI] [PubMed] [Google Scholar]
- 24.McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160(7):1573–6. 10.1111/j.1476-5381.2010.00873.x ; PubMed Central PMCID: PMCPMC2936829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Micucci M, Gotti R, Corazza I, Tocci G, Chiarini A, De Giorgio M, et al. Newer Insights into the Antidiarrheal Effects of Acacia catechu Willd. Extract in Guinea Pig. J Med Food. 2017;20(6):592–600. 10.1089/jmf.2016.0154 . [DOI] [PubMed] [Google Scholar]
- 26.Arunlakshana O, Schild HO. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959;14(1):48–58. ; PubMed Central PMCID: PMCPMC1481829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tallarida RJ, Murray RB. Manual of pharmacologic calculations with computer programs. 2nd ed. New York: Springer-Verlag; 1987. x, 297 p. p. [Google Scholar]
- 28.Motulsky H, Christopoulos A. Fitting models to biological data using linear and nonlinear regression: a practical guide to curve fitting. Oxford; New York: Oxford University Press; 2004. 351 p. p. [Google Scholar]
- 29.Girish C, Pradhan SC. Drug development for liver diseases: focus on picroliv, ellagic acid and curcumin. Fundam Clin Pharmacol. 2008;22(6):623–32. 10.1111/j.1472-8206.2008.00618.x . [DOI] [PubMed] [Google Scholar]
- 30.Micucci M, Aldini R, Cevenini M, Colliva C, Spinozzi S, Roda G, et al. Curcuma longa L. as a therapeutic agent in intestinal motility disorders. 2: Safety profile in mouse. PLoS One. 2013;8(11):e80925 10.1371/journal.pone.0080925 ; PubMed Central PMCID: PMCPMC3832444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okla M, Kang I, Kim DM, Gourineni V, Shay N, Gu L, et al. Ellagic acid modulates lipid accumulation in primary human adipocytes and human hepatoma Huh7 cells via discrete mechanisms. J Nutr Biochem. 2015;26(1):82–90. 10.1016/j.jnutbio.2014.09.010 . [DOI] [PubMed] [Google Scholar]
- 32.Soory M. Relevance of nutritional antioxidants in metabolic syndrome, ageing and cancer: potential for therapeutic targeting. Infect Disord Drug Targets. 2009;9(4):400–14. . [DOI] [PubMed] [Google Scholar]
- 33.Dinkova-Kostova AT, Kostov RV. Glucosinolates and isothiocyanates in health and disease. Trends Mol Med. 2012;18(6):337–47. 10.1016/j.molmed.2012.04.003 . [DOI] [PubMed] [Google Scholar]
- 34.Braunersreuther V, Viviani GL, Mach F, Montecucco F. Role of cytokines and chemokines in non-alcoholic fatty liver disease. World J Gastroenterol. 2012;18(8):727–35. 10.3748/wjg.v18.i8.727 ; PubMed Central PMCID: PMCPMC3286135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Assal O, Hong F, Kim WH, Radaeva S, Gao B. IL-6-deficient mice are susceptible to ethanol-induced hepatic steatosis: IL-6 protects against ethanol-induced oxidative stress and mitochondrial permeability transition in the liver. Cell Mol Immunol. 2004;1(3):205–11. . [PubMed] [Google Scholar]
- 36.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. 10.1146/annurev.immunol.19.1.683 . [DOI] [PubMed] [Google Scholar]
- 37.Lemmers A, Moreno C, Gustot T, Marechal R, Degre D, Demetter P, et al. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology. 2009;49(2):646–57. 10.1002/hep.22680 . [DOI] [PubMed] [Google Scholar]
- 38.Ip E, Farrell GC, Robertson G, Hall P, Kirsch R, Leclercq I. Central role of PPARalpha-dependent hepatic lipid turnover in dietary steatohepatitis in mice. Hepatology. 2003;38(1):123–32. 10.1053/jhep.2003.50307 . [DOI] [PubMed] [Google Scholar]
- 39.Younossi ZM, Baranova A, Ziegler K, Del Giacco L, Schlauch K, Born TL, et al. A genomic and proteomic study of the spectrum of nonalcoholic fatty liver disease. Hepatology. 2005;42(3):665–74. 10.1002/hep.20838 . [DOI] [PubMed] [Google Scholar]
- 40.Stepanova M, Hossain N, Afendy A, Perry K, Goodman ZD, Baranova A, et al. Hepatic gene expression of Caucasian and African-American patients with obesity-related non-alcoholic fatty liver disease. Obes Surg. 2010;20(5):640–50. 10.1007/s11695-010-0078-2 . [DOI] [PubMed] [Google Scholar]
- 41.Osabe M, Sugatani J, Fukuyama T, Ikushiro S, Ikari A, Miwa M. Expression of hepatic UDP-glucuronosyltransferase 1A1 and 1A6 correlated with increased expression of the nuclear constitutive androstane receptor and peroxisome proliferator-activated receptor alpha in male rats fed a high-fat and high-sucrose diet. Drug Metab Dispos. 2008;36(2):294–302. 10.1124/dmd.107.017731 . [DOI] [PubMed] [Google Scholar]
- 42.Cimino F, Speciale A, Anwar S, Canali R, Ricciardi E, Virgili F, et al. Anthocyanins protect human endothelial cells from mild hyperoxia damage through modulation of Nrf2 pathway. Genes Nutr. 2013;8(4):391–9. 10.1007/s12263-012-0324-4 ; PubMed Central PMCID: PMCPMC3689892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yun JW. Possible anti-obesity therapeutics from nature—a review. Phytochemistry. 2010;71(14–15):1625–41. 10.1016/j.phytochem.2010.07.011 . [DOI] [PubMed] [Google Scholar]
- 44.Lei F, Zhang XN, Wang W, Xing DM, Xie WD, Su H, et al. Evidence of anti-obesity effects of the pomegranate leaf extract in high-fat diet induced obese mice. Int J Obes (Lond). 2007;31(6):1023–9. 10.1038/sj.ijo.0803502 . [DOI] [PubMed] [Google Scholar]
- 45.Kang I, Buckner T, Shay NF, Gu L, Chung S. Improvements in Metabolic Health with Consumption of Ellagic Acid and Subsequent Conversion into Urolithins: Evidence and Mechanisms. Adv Nutr. 2016;7(5):961–72. 10.3945/an.116.012575 ; PubMed Central PMCID: PMCPMC5015040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phan MAT, Paterson J, Bucknall M, Arcot J. Interactions between phytochemicals from fruits and vegetables: Effects on bioactivities and bioavailability. Crit Rev Food Sci Nutr. 2016:1–20. 10.1080/10408398.2016.1254595 . [DOI] [PubMed] [Google Scholar]
- 47.Tomankova V, Anzenbacher P, Anzenbacherova E. Effects of obesity on liver cytochromes P450 in various animal models. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2017;161(2):144–51. 10.5507/bp.2017.026 . [DOI] [PubMed] [Google Scholar]
- 48.Vivarelli F, Canistro D, Sapone A, De Nicola GR, Babot Marquillas C, Iori R, et al. Raphanus sativus cv. Sango Sprout Juice Decreases Diet-Induced Obesity in Sprague Dawley Rats and Ameliorates Related Disorders. PLoS One. 2016;11(3):e0150913 10.1371/journal.pone.0150913 ; PubMed Central PMCID: PMCPMC4795736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Canistro D, Vivarelli F, Cirillo S, Babot Marquillas C, Buschini A, Lazzaretti M, et al. E-cigarettes induce toxicological effects that can raise the cancer risk. Sci Rep. 2017;7(1):2028 10.1038/s41598-017-02317-8 ; PubMed Central PMCID: PMCPMC5435699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aubert J, Begriche K, Knockaert L, Robin MA, Fromenty B. Increased expression of cytochrome P450 2E1 in nonalcoholic fatty liver disease: mechanisms and pathophysiological role. Clin Res Hepatol Gastroenterol. 2011;35(10):630–7. 10.1016/j.clinre.2011.04.015 . [DOI] [PubMed] [Google Scholar]
- 51.Abdelmegeed MA, Banerjee A, Yoo SH, Jang S, Gonzalez FJ, Song BJ. Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced non-alcoholic steatohepatitis. J Hepatol. 2012;57(4):860–6. 10.1016/j.jhep.2012.05.019 ; PubMed Central PMCID: PMCPMC3445664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhattarai Y, Fried D, Gulbransen B, Kadrofske M, Fernandes R, Xu H, et al. High-fat diet-induced obesity alters nitric oxide-mediated neuromuscular transmission and smooth muscle excitability in the mouse distal colon. Am J Physiol Gastrointest Liver Physiol. 2016;311(2):G210–20. 10.1152/ajpgi.00085.2016 ; PubMed Central PMCID: PMCPMC5007291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Z, Summanen PH, Komoriya T, Henning SM, Lee RP, Carlson E, et al. Pomegranate ellagitannins stimulate growth of gut bacteria in vitro: Implications for prebiotic and metabolic effects. Anaerobe. 2015;34:164–8. 10.1016/j.anaerobe.2015.05.012 . [DOI] [PubMed] [Google Scholar]
- 54.Bowles DK, Heaps CL, Turk JR, Maddali KK, Price EM. Hypercholesterolemia inhibits L-type calcium current in coronary macro-, not microcirculation. J Appl Physiol (1985). 2004;96(6):2240–8. 10.1152/japplphysiol.01229.2003 . [DOI] [PubMed] [Google Scholar]
- 55.Jennings LJ, Xu QW, Firth TA, Nelson MT, Mawe GM. Cholesterol inhibits spontaneous action potentials and calcium currents in guinea pig gallbladder smooth muscle. Am J Physiol. 1999;277(5 Pt 1):G1017–26. . [DOI] [PubMed] [Google Scholar]
- 56.Huang DW, Chang WC, Yang HJ, Wu JS, Shen SC. Gallic Acid Alleviates Hypertriglyceridemia and Fat Accumulation via Modulating Glycolysis and Lipolysis Pathways in Perirenal Adipose Tissues of Rats Fed a High-Fructose Diet. Int J Mol Sci. 2018;19(1). 10.3390/ijms19010254 ; PubMed Central PMCID: PMCPMC5796201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Effects of 21 days ENC® supplementation (20 mg/kg/day) for 21 days on liver phase I (A), phase II (B) and antioxidant (C) enzymes activities in RD and HFD rats.
(DOCX)
Agonist [carbachol (CCh)] and antagonist (atropine) activities expressed as pEC50 or pA2, respectively, (A) and Calcium channels antagonist Nifedipine activity (B) in the isolated rat ileum and distal colon of RD- and HFD-rats supplemented with or without ENC® (20 mg/kg/day) for 21 days.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.