ABSTRACT
Adult tongue epithelium is continuously renewed from epithelial progenitor cells, a process that requires hedgehog (HH) signaling. In mice, pharmacological inhibition of the HH pathway causes taste bud loss within a few weeks. Previously, we demonstrated that sonic hedgehog (SHH) overexpression in lingual progenitors induces ectopic taste buds with locally increased SOX2 expression, suggesting that taste bud differentiation depends on SOX2 downstream of HH. To test this, we inhibited HH signaling in mice and observed a rapid decline in Sox2 and SOX2-GFP expression in taste epithelium. Upon conditional deletion of Sox2, differentiation of both taste and non-taste epithelial cells was blocked, and progenitor cell number increased. In contrast to basally restricted proliferation in controls, dividing cells were overabundant and spread to suprabasal epithelial layers in mutants. SOX2 loss in progenitors also led non-cell-autonomously to taste cell apoptosis, dramatically shortening taste cell lifespans. Finally, in tongues with conditional Sox2 deletion and SHH overexpression, ectopic and endogenous taste buds were not detectable; instead, progenitor hyperproliferation expanded throughout the lingual epithelium. In summary, we show that SOX2 functions downstream of HH signaling to regulate lingual epithelium homeostasis.
KEY WORDS: Cell differentiation, Mouse, Progenitor proliferation, Taste bud, Smoothened antagonist
Summary: A combination of genetic and pharmacological manipulation shows that SOX2 is required downstream of Hedgehog signaling for differentiation of taste bud cells, as well as for tongue epithelium renewal.
INTRODUCTION
In mammals, the adult lingual epithelium can be categorized into non-taste and taste components. The majority of the tongue surface is covered by keratinized, non-taste epithelium made up of mechanosensory filiform papillae, which are curved, spinous-shaped structures with small mesenchymal cores (Hume and Potten, 1976). The more complex taste epithelium consists of collections of neuroepithelial taste cells organized within taste buds, which in turn lie in specialized taste papillae on the tongue surface. In rodents, fungiform papillae (FFP) are arrayed on the anterior two-thirds of the tongue, interspersed among filiform papillae of the non-taste epithelium. Each rodent FFP houses a single apical taste bud surrounded by keratinocytes that make up the papilla walls, which in turn surround a mesenchymal core. Murine taste buds comprise ∼60-100 fusiform taste receptor cells responsible for detecting, transducing and transmitting to the brain the five basic tastes, i.e. salty, sweet, bitter, umami/savory and sour (Chaudhari and Roper, 2010).
Both non-taste and taste epithelium are continually renewed from basally located progenitor cells. Previous tritiated thymidine studies in mice show that the non-taste epithelium has a turnover rate of 5-7 days; it is one of the fastest renewing tissues in mammals, only slightly slower than the pace of renewal of the intestinal epithelium (3-5 days) (Barker, 2014; Hume and Potten, 1976; Liu et al., 2012). By contrast, similar approaches to define taste cell lifespan concluded that these cells are longer lived, with a median lifespan of 10-14 days (Beidler and Smallman, 1965; Delay et al., 1986; Farbman, 1980), although some taste bud cells persist up to 44 days (Hamamichi et al., 2006; Perea-Martinez et al., 2013).
More recent studies using inducible genetic systems to lineage label keratin (K) 5+ and K14+ (also known as KRT5 and KRT14, respectively – Mouse Genome Informatics) basal epithelial cells in the tongue have demonstrated that this population comprises bipotential progenitors (Gaillard et al., 2015; Okubo et al., 2009). K5/14+ cells located basally in the non-taste epithelium are progenitor cells that give rise to differentiated K13+ (KRT13) keratinocytes. K13+ cells contribute to suprabasal epithelial layers of filiform papillae and FFP, and are ultimately shed at the tongue surface (Iwasaki et al., 2006; Okubo et al., 2009); this lineage progression resembles that of the interfollicular epidermis of the skin (Winter et al., 1990). In taste epithelium, K5/14+ cells situated immediately adjacent to each bud are referred to as perigemmal (PG) cells. These mitotically active progenitors generate cells that exit the cell cycle, enter taste buds, and become immediate taste cell precursors (also known as type IV cells) located basally in each bud. Ultimately, these intragemmal (IG) cells, i.e. inside taste buds, differentiate into mature taste cells within 2.5-3 days of their last division (Barlow, 2015; Barlow and Klein, 2015; Cho et al., 1998; Hamamichi et al., 2006; Miura et al., 2006, 2014; Nguyen and Barlow, 2010; Perea-Martinez et al., 2013). Besides contributing to taste buds, K5/K14+ progenitor cells within FFP replenish themselves and provide keratinocytes to the region of the taste pore and adjacent K13+ non-taste epithelium of the taste papilla (Okubo et al., 2009). The intrinsic differences between non-taste and taste epithelial cell lineages suggest differential molecular regulation of cell fates in these two tissue compartments.
One molecular regulator of cell renewal in many epithelia is sonic hedgehog (SHH) signaling. SHH is expressed by postmitotic taste precursor cells (Miura et al., 2014), whereas mitotically active K5/K14+ progenitors surrounding each bud express the SHH target genes Ptch1 and Gli1, suggesting that SHH signals from within the bud to adjacent progenitors, to regulate proliferation and/or taste cell differentiation (Miura, 2003; Miura and Barlow, 2010; Miura et al., 2001, 2014). HH signaling-dependent cancers, such as basal cell carcinomas, are frequently treated with hedgehog pathway inhibitors (HPIs) to inhibit constitutive HH pathway activation (Ng and Curran, 2011; Rubin and de Sauvage, 2006; Wong and Dlugosz, 2014). Although these chemotherapeutics efficiently target tumors, patients experience disturbingly altered taste sensation (LoRusso et al., 2011; Rodon et al., 2014; Tang et al., 2012). Moreover, in mice, HPIs lead to taste bud loss, as well as loss of taste nerve responses (Kumari et al., 2015, 2017; Yang et al., 2015), indicating that HH signaling is required for taste cell renewal.
Another important regulator of taste bud differentiation is SOX2, which belongs to the family of SRY-related HMG box transcription factors that are critical for cell fate determination during development and stem cell maintenance/differentiation in many adult tissues (Arnold et al., 2011; Liu et al., 2012). The importance of SOX2 during embryonic taste development was first demonstrated by failure of Sox2 hypomorphic mutants to generate FF taste buds (Okubo et al., 2006). In the adult tongue, SOX2 is expressed at low levels by K14+ cells, and Sox2 genetic lineage tracing confirms that SOX2+ basal keratinocytes also function as bipotential stem cells for taste and non-taste lingual epithelium (Ohmoto et al., 2017). Furthermore, SOX2 is also expressed in PG K14+ taste bud progenitors, in basal cells within taste buds and in a subset of mature taste receptor cells (Ohmoto et al., 2017; Okubo et al., 2006; Suzuki, 2008), suggesting that SOX2 may be key to proper taste cell differentiation from progenitors. Interestingly, overexpression of SHH in K14+ progenitors leads to formation of ectopic taste buds, which are associated with increased SOX2 expression in epithelial cells surrounding and within ectopic buds (Castillo et al., 2014). These results suggested the testable hypothesis that renewal of adult lingual epithelium is positively regulated by HH signaling, which in turn requires downstream SOX2 function.
Here, we test this idea by assessing the impact of HH pathway inhibition on SOX2 expression using HhAntag, an HPI that blocks the HH effector smoothened (SMO) (Yauch et al., 2008). Additionally, we genetically delete Sox2 (SOX2cKO) (Shaham et al., 2009) or pair SOX2cKO with SHH overexpression (SHH-YFPcKI) (Castillo et al., 2014) in K14+ progenitors to explicitly test whether SOX2 is required for taste cell differentiation. Using SOX2-GFP mice, we find that pharmacological inhibition of the HH pathway, which blocks the differentiation program of taste buds (Castillo-Azofeifa et al., 2017), also leads to downregulation of SOX2-GFP in taste bud progenitors and taste buds. Furthermore, we show that SOX2 function in lingual progenitors is required broadly for lingual epithelial cell maintenance; in SOX2cKO mice, K14+ progenitors fail to differentiate and instead proliferate. Unexpectedly, we find that SOX2 function in progenitors is required non-cell-autonomously for survival of differentiated taste bud cells, as taste cells rapidly undergo apoptosis when Sox2 is deleted from progenitors only. Finally, loss of SOX2 abrogates the ability of SHH to induce ectopic taste buds; instead, SHH overexpression in SOX2cKO epithelium results in hyperproliferation of basal epithelial cells, suggesting that in the absence of SOX2, SHH switches from a pro-taste differentiation signal to a robust mitogen.
RESULTS
Adult taste buds are mildly but significantly affected within 1 week of HhAntag treatment
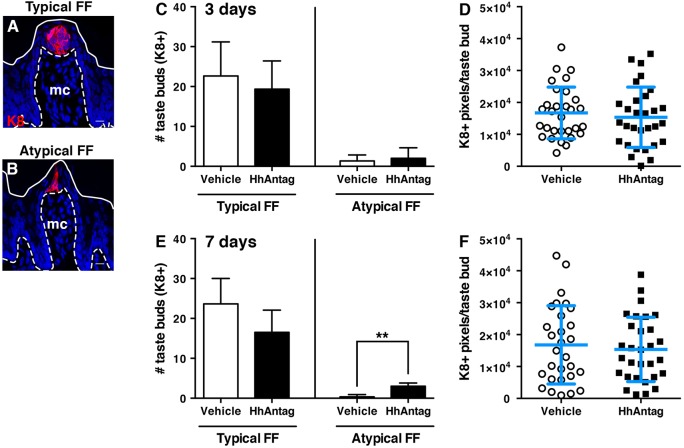
We and others have found that taste buds are significantly reduced after 21 days of HPI treatment (Castillo-Azofeifa et al., 2017; Kumari et al., 2015, 2017; Yang et al., 2015); during 2-4 weeks of drug exposure, FFP of typical appearance and their taste buds (Fig. 1A) are gradually lost as the number of atypical, i.e. degenerating, FFP taste buds (Fig. 1B) (Nagato et al., 1995; Oakley et al., 1990) increases (Castillo-Azofeifa et al., 2017; Kumari et al., 2015). However, because differentiation of taste progenitors into new taste cells takes ∼3 days from their last division, we hypothesized that HPIs would affect taste bud renewal well in advance of taste bud loss. Using K8 (KRT8) immunostaining to mark mature taste buds (Fig. 1A) (Knapp et al., 1995), we found that neither typical FFP taste bud number and size nor atypical FFP number differed from controls after 3 days of drug (Fig. 1C,D). By 7 days of HhAntag treatment, typical FFP number was slightly, but not significantly, decreased, a trend that was similar for taste bud size (Fig. 1E,F); however, atypical FFP number increased significantly in drug-treated mice. These data suggested to us that taste bud homeostasis might already be affected by short-term inhibition of HH signaling.
Fig. 1.
Adult taste buds are mildly but significantly affected by 1 week of HhAntag treatment. (A,B) Although the morphology of typical FF (A) and atypical FF (B) papillae differ, both house K8+ taste buds (red). Images are confocal compressed z-stacks. Nuclei are counterstained with Draq5 (blue); dashed lines delimit the basement membrane; solid lines delimit the epithelial surface; mc, mesenchymal core. Scale bars: 10 μm. (C) After 3 days of HhAntag treatment, typical FF and atypical FF taste bud numbers do not differ from vehicle-treated controls. (D) The number of K8+ pixels, i.e. taste bud size, is comparable between vehicle and HhAntag-treated mice at 3 days. (E) After 7 days, HhAntag-treated mice tend to have fewer typical FF taste buds, whereas atypical FF taste buds are significantly increased. (F) At 7 days, taste bud size does not differ between HhAntag-treated and control mice. N=3 mice for 3 days; N=3-4 mice for 7 days; n=30 taste buds for vehicle or 30-40 for HhAntag (ten taste buds randomly selected per mouse). Data are represented as mean±s.d.; **P<0.01 (Student's t-test).
SOX2-GFP expression in FFP epithelium and taste buds is significantly decreased by inhibition of HH signaling
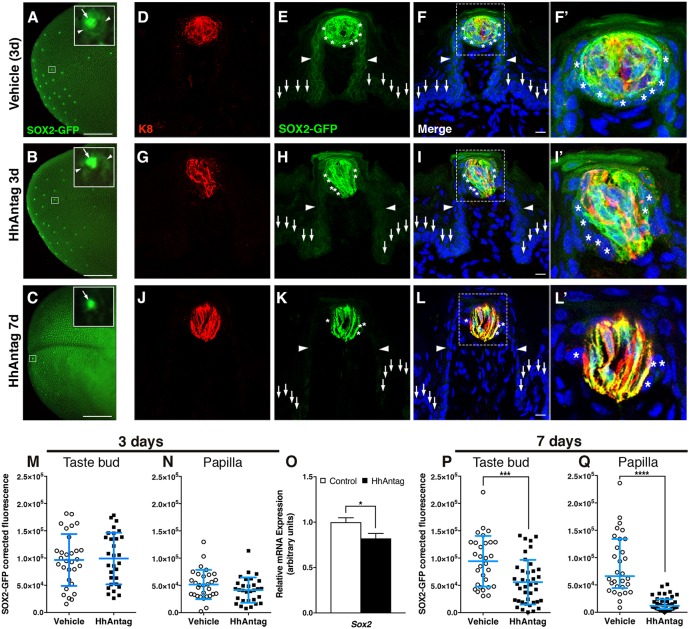
As taste buds were impacted, albeit minimally, at 7 days, we reasoned that if SOX2 plays a role in taste cell renewal downstream of SHH, then SOX2 expression would be affected by short-term drug treatment. Hence, we examined GFP expression in SOX2-GFP mice treated with HhAntag or vehicle for 3 or 7 days. In intact control tongues, SOX2-GFP expression is readily detectable in FFP at low magnification (Fig. 2A). At higher magnification, dimmer GFP expression is evident in FFP epithelial cells surrounding SOX2-GFP taste buds (Fig. 2A, inset, arrowheads and arrow, respectively); this pattern of SOX2-GFP expression was comparable in tongues of mice treated with drug for 3 days (Fig. 2B and inset). By contrast, after HhAntag treatment for 7 days, GFP expression was substantially reduced (Fig. 2C). Upon closer examination, GFP expression appeared to be limited to apical FF taste buds (Fig. 2C, inset and arrow), with little or no GFP signal in FFP epithelium surrounding buds.
Fig. 2.
SOX2-GFP expression is reduced in taste buds and papillae in mice treated with HhAntag. (A) In whole-mount preparations of vehicle-treated tongues at 3 days, taste buds appear to have high SOX2-GFP expression (inset, arrow), whereas adjacent FF papilla walls express lower SOX2-GFP (inset, arrowheads). (B) After 3 days of HhAntag treatment, a similar pattern of bright GFP expression in taste buds (inset, arrow) with dimmer GFP expression in papilla epithelium (inset, arrowheads) is evident. (C) After 7 days of HhAntag treatment, fewer GFP+ taste buds are detectable (low power view and inset, arrow), and FFP SOX2-GFP expression is absent. (D-L′) (D-F′) In immunostained tissue sections of control tongues, bright GFP+ K8+ cells are present in taste buds and GFP+/K8− PG cells surround taste buds (asterisks); GFP+ cells populate FF papilla walls (arrowheads), whereas nearby non-taste epithelium is dimly GFP+ (vertical arrows). (G-I′) After 3 days of HhAntag treatment, a similar pattern to that of controls is observed; GFP+/K8+ taste bud cells and K8− PG cells (asterisks), GFP+ FFP epithelium (arrowheads) and dimly GFP+ non-taste epithelium (vertical arrows). (J-L′) After 7 days of HhAntag treatment, there are fewer GFP+/K8+ taste cells, GFP+ PG cells are lacking (asterisks) and GFP in FFP (arrowheads) and non-taste epithelium (vertical arrows) is absent. F′, I′ and L′ show high magnification views of the boxed areas in F, I and L, respectively. (M,N) At 3 days, SOX2-GFP corrected fluorescence intensity in taste buds does not differ between vehicle- and HhAntag-treated mice; there is a small but non-significant decrease in GFP+ fluorescence in FFP epithelium and the PG cell compartment (see Materials and Methods). (O) Expression of Sox2 is significantly reduced following 3 days of HhAntag treatment. (P,Q) After 7 days of HhAntag treatment, SOX2-GFP intensity is significantly reduced both in taste buds, and in PG cells plus FFP walls. Nuclei are counterstained with Draq5 (blue). All images are compressed confocal z-stacks. N=3 mice 3 days; N=3-4 mice 7 days; n=30 taste buds and papillae for vehicle or 30-40 for HhAntag; N=5 mice 3 days qPCR. Data are represented as mean±s.d. (except P, in which data are represented as median with interquartile range); *P<0.05, ***P<0.001, ****P<0.0001 (Student's t-test or Mann–Whitney U-test). Scale bars: 1 mm (A-C); 10 μm (D-L).
We examined SOX2-GFP expression more closely in tissue sections of anterior tongues. Notably, SOX2-GFP reporter expression recapitulated SOX2 protein expression in adult tongue (Fig. 3A; Ohmoto et al., 2017; Okubo et al., 2009; Suzuki, 2008). Specifically, SOX2-GFP is highest in a subset of cells within K8+ taste buds (Fig. 2D-F′, red), which have been proposed to represent immature and/or type I taste cells (Suzuki, 2008). SOX2-GFP is also more moderately expressed in PG cells immediately surrounding each bud (Fig. 2F,F′, asterisks) and epithelial walls of FFP (Fig. 2E,F, arrowheads), as well as at low levels in adjacent non-taste basal epithelial cells (Fig. 2E,F, arrows). Broadly, SOX2+ basal keratinocytes outside of taste buds are considered taste bud stem cells (Ohmoto et al., 2017), but it is not known whether specific subsets of PG and/or FFP wall SOX2+ cells function in this role.
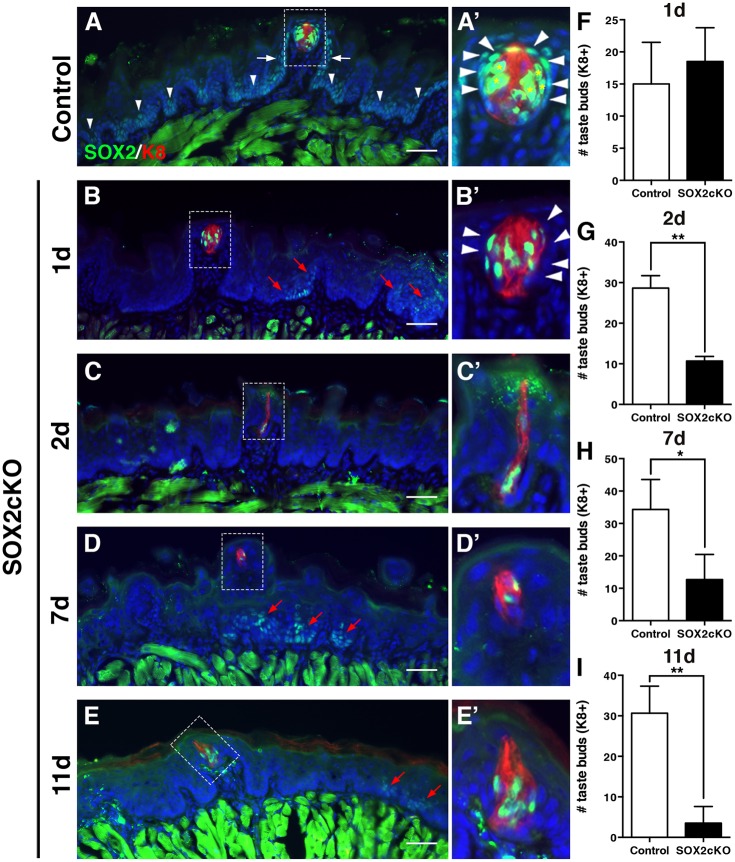
Fig. 3.
Genetic ablation of Sox2 in K14+ progenitors disrupts taste bud renewal. (A,A′) In control mice (K14+/+;Sox2flox/flox), SOX2 immunoreactivity (green) is high in taste bud cells (K8+, red, asterisks) and PG cells (A′, arrowheads). SOX2 is expressed at low levels by basal cells in FFP walls (white arrows) and non-taste epithelium (arrowheads in A). (B,B′) One day after Sox2 deletion (K14CreERT2/+;Sox2flox/flox), SOX2+ cells are found within most taste buds, PG cells lack SOX2 expression (B′, arrowheads) and SOX2+ epithelial cells outside of buds are limited to sparse, scattered clusters (B, red arrows). (C-E′) A similar pattern of SOX2 immunoreactivity is observed at 2 (C,C′), 7 (D,D′) and 11 (E,E′) days after Cre induction; SOX2+ cells are observed in occasional taste buds and scattered small clusters of more dimly SOX2+ cells are evident in non-taste epithelium (red arrows). A′-E′ show high magnification views of the boxed areas in A-E, respectively. (F) Taste bud number in mutant mice does not differ from controls 1 day after Sox2 deletion. (G) Deletion of Sox2 results in significant loss of K8+ taste buds after 2 days. (H) A similar reduction in taste bud number is evident at 7 days post-SOX2cKO, and by 11 days only 15% of taste buds remain in mutant tongues (I). The morphology of most remaining FF taste buds is disrupted in mutant mice; taste buds have fewer cells and/or more elongate morphology compared with controls (A′-E′). Nuclei are counterstained with Draq5 (blue). Scale bars: 50 μm. All are fluorescence images. N=3-5 mice per condition. Data are represented as mean+s.d.; *P<0.05, **P<0.01 (Student's t-test).
SOX2-GFP expression in tongues of mice treated for 3 days with HhAntag was comparable to controls: a subset of K8+ taste bud cells (red), and PG cells adjacent to taste buds, as well as basal cells of the FFP walls and non-taste epithelium outside of FFP were all GFP+ (Fig. 2G-I′). However, SOX2-GFP expression was significantly altered by 7 days of HhAntag treatment. GFP was virtually absent in FFP walls and PG cells (Fig. 2K-L′, arrowheads and asterisks, respectively); only elongate IG K8+ taste cells within taste buds remained GFP+ (Fig. 2J-L′, red). These qualitative observations were confirmed by quantification of corrected SOX2-GFP fluorescence in: (1) the tissue compartment comprising the putative taste bud stem cell population, i.e. FFP walls plus PG cells (Fig. 2E,H,K, arrowheads and asterisks, respectively); and (2) K8+ taste buds (Fig. 2D,G,J) (see Materials and Methods). HhAntag given for 3 days did not alter IG SOX2-GFP expression (Fig. 2M), whereas in FFP walls and PG cells, fluorescence was slightly, but not significantly, reduced (Fig. 2N). Using qPCR, we found that Sox2 mRNA was reduced by 3 days of drug treatment (Fig. 2O), which correlates with the trend in reduced SOX2-GFP fluorescence outside of buds and indicates that HhAntag treatment leads to bona fide reduced expression of Sox2 in tongue epithelium. By 7 days of HhAntag treatment, SOX2-GFP fluorescence was significantly reduced in taste buds (Fig. 2P) and to an even greater extent in the FFP walls and PG compartment (Fig. 2Q). These data indicate that HH signaling regulates SOX2 expression and suggest that HH signaling and SOX2 together are required to regulate taste bud cell renewal.
Genetic ablation of Sox2 in K14+ progenitors disrupts taste bud renewal
Differentiation of taste buds is perturbed in Sox2 hypomorphic mouse embryos, indicating that SOX2 is required for early development of the taste epithelium (Okubo et al., 2006). To determine whether SOX2 is required for adult taste cell renewal, we conditionally deleted Sox2 in lingual epithelial progenitor cells by dosing K14+/CreER;Sox2flox/flox mice once with tamoxifen (SOX2cKO). Previously, we reported that K14-CreER activation results in broad but mosaic reporter gene expression within K14+ lingual progenitor cells and their daughter cells (Castillo et al., 2014). Taste cells within buds do not express K14 (Gaillard et al., 2015) and, thus, K14-CreER activation does not directly and immediately turn on reporter expression in differentiated taste cells; rather, new taste cells generated from labeled progenitors inherit reporter expression and thus are only evident in buds several days after CreER induction (Okubo et al., 2009). As expected, SOX2, as evidenced by immunostaining, was deleted in the majority of basal progenitors 1 day post-tamoxifen (Fig. 3B), although small clusters of SOX2+ cells were detected in the non-taste epithelium, due to K14CreER mosaicism (Fig. 3B, red arrows). At 1 day post-induction, SOX2 was also deleted from PG cells of the majority of taste buds (Fig. 3A′,B′, arrowheads; Fig. S1A, gray versus cyan bars). By contrast, Sox2 was not deleted in K8+ taste buds of Sox2cKO mice; SOX2+ cells were detected in almost all taste buds of mutants after 1 day (Fig. 3A′,B′; Fig. S1A, gray versus cyan bars), consistent with the lack of K14 expression (Gaillard et al., 2015) and, therefore, of CreER activity in differentiated taste cells.
Despite broad deletion of Sox2 in FFP and PG cells at 1 day post-tamoxifen, taste buds were detected in normal numbers and with normal morphology (Fig. 3B′,F). Two days post-induction, however, the number of K8+ taste buds was dramatically reduced in mutants compared with controls (Fig. 3G). Most remaining taste buds had perturbed morphology, ranging from narrow clusters of elongated cells, to small round collections of two or three K8+ cells (Fig. S1B-E). Remarkably, despite their abnormal morphologies, almost all possessed SOX2+ IG cells, i.e. located inside taste buds, but only a quarter were still surrounded by some SOX2+ PG cells (Fig. S1F,G), and none exhibited SOX2+ cells in FFP walls (Fig. S1B-G, Fig. S1A, green bars). Following this initial drastic loss, taste bud number decreased more slowly as assessed at days 7 and 11 post-induction (Fig. 3H,I). Most remaining taste buds at 7 days had an elongated morphology or were very small, although some normally appearing buds were also present. In these mutants, almost all buds, regardless of morphology, housed IG SOX2+ cells (Fig. 3D,D′; Fig. S1B-I, Fig. S1A, blue bars); however, some taste buds with standard morphology also had SOX2+ cells in FFP and large numbers of SOX2+ PG cells (Fig. S1H,I) suggesting that in these normally appearing buds Sox2 simply had not been deleted from the K14+ progenitors due to mosaicism of K14CreER. In Sox2cKO tongues at day 11, taste buds were only detected in two of four mutants. Some of these had a normal appearance and persisted because of a failure of local Sox2 deletion, whereas the remainder comprised aggregates of a few K8+ cells and mostly lacked SOX2+ IG, PG and FFP cells (Fig. S1A, magenta bars).
SOX2 in lingual epithelium is required to maintain adult taste buds and indicate that taste cell expression of SOX2 is not sufficient to maintain taste buds
Intriguingly, taste buds disappeared very rapidly in SOX2cKO mice (within 2 days), considering that rodent taste cells have an average lifespan of 14-21 days (Beidler and Smallman, 1965; Farbman, 1980). This swift disappearance suggested that taste bud loss was not simply due to a failure of SOX2-depleted progenitors to fulfill normal replacement of taste bud cells, but rather that taste bud cells en masse were actively lost in the absence of SOX2 in progenitors. In control tongues, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)+ cells are evident in the most superficial layer of the lingual epithelium, as these cells undergo cell death and form the barrier layer of the tongue surface (Fig. 4A, arrows). By contrast, apoptotic cells are rarely found within taste buds or basal layers of the epithelium (Fig. 4A) (Ichimori et al., 2009; Zeng and Oakley, 1999). One day after Sox2 deletion, however, we detected many TUNEL+/K8+ taste cells in mutants compared with controls as well as increased TUNEL+ cells in the FFP wall and PG region (Fig. 4B-D). By 2 days of SOX2cKO, however, TUNEL+ cells were again only seen in superficial layers (data not shown), as in controls. Importantly, genetic deletion of Sox2 1 day after tamoxifen induction is restricted to basal keratinoctyes outside of taste buds, whereas SOX2 expression is maintained within taste buds (see Fig. 3B,B′, Fig. S1A). Thus, precipitous taste bud loss, preceded by a dramatic increase in taste cell apoptosis, cannot be cell-autonomous, i.e. due to SOX2 loss in differentiated taste cells. Rather, we hypothesize that SOX2 acts in local progenitors to indirectly support taste cell survival.
Fig. 4.
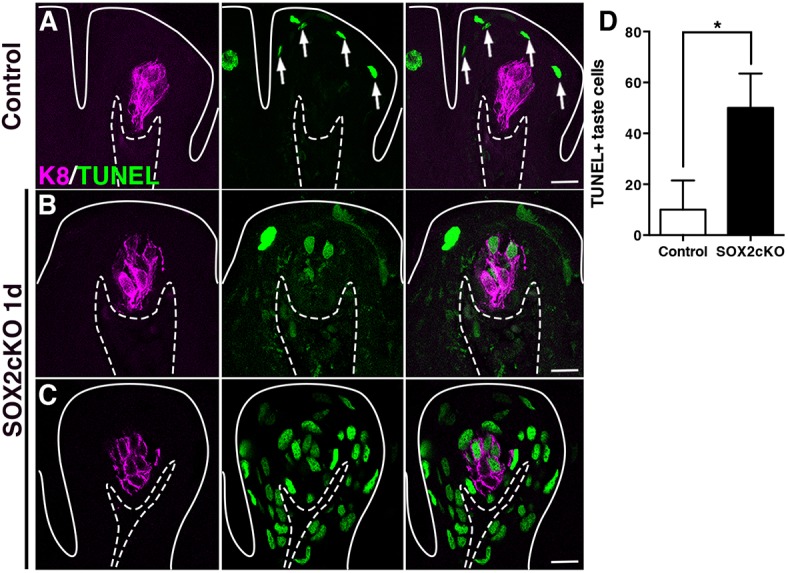
Deletion of Sox2 in progenitor cells induces taste cell apoptosis non-cell-autonomously. (A) In control FFP, TUNEL+ nuclei (green) are typically detected in superficial keratinocytes as they enucleate to form the acellular surface layer of the tongue epithelium (white arrows). (B,C) One day after SOX2cKO, TUNEL+/K8+ taste cells (magenta) are detected in numerous mutant FFP (B), and some FFP have extensive TUNEL+ cells (C). Dashed lines delimit the basement membrane; solid lines delimit the epithelial surface. Scale bars: 20 µm. (D) One day after SOX2cKO, mutant mice have significantly more TUNEL+/K8+ taste cells than do controls. N=3 mice per condition. Data are represented as mean+s.d.; *P<0.05 (Student's t-test).
Loss of SOX2 in K14+ progenitors blocks differentiation of taste and non-taste epithelial cells
In addition to increased taste cell death, we reasoned that rapid loss of taste buds in the absence of SOX2 might have direct effects on the progenitor population. Hence, we next investigated whether SOX2cKO affected differentiation of K14+ progenitors. In control taste papillae, K14+ basal progenitors comprise basal cells of the FFP walls and PG cells (Fig. 5A, green). The pattern of K14 expression is similar 1 day after SOX2cKO (data not shown). By contrast, at 2 days post-induction, in SOX2cKO tongues K14+ cells expand above the basal epithelial layer and encroach upon FF taste buds (Fig. 5B, arrowheads). By 7 and 11 days, many more K14+ cells occupy the suprabasal epithelium, including regions formerly occupied by taste buds (Fig. 5C,D). Additionally, at later time points, many suprabasal K14+ cells were abnormally elongated and flattened, in contrast to basally located, ovoid K14+ cells (Fig. 5C,D, red arrows).
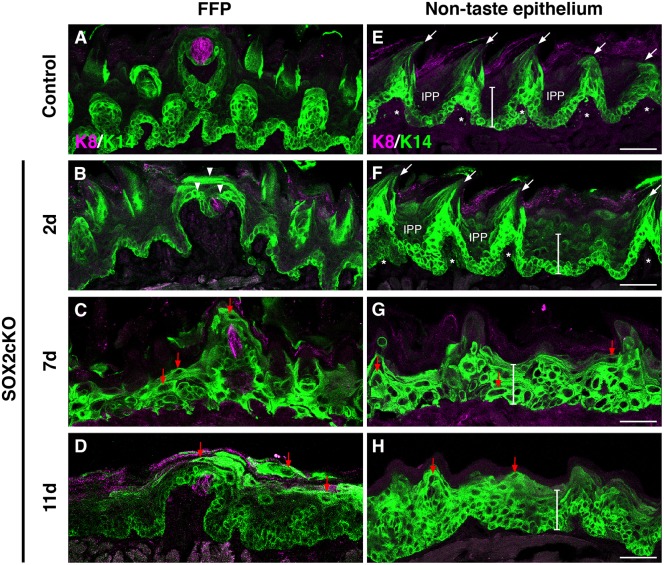
Fig. 5.
Loss of SOX2 in K14+ progenitors blocks fate acquisition of taste and non-taste epithelial cells. (A) In control FFP, K14+ progenitors (green) are limited to the basal epithelial layer, and are adjacent to taste buds (K8, magenta). (B) Two days after Sox2 deletion in K14CreERT2/+;Sox2flox/flox (SOX2cKO) mice, K14+ cells are found in suprabasal epithelial layers and have expanded around K8+ taste buds (arrowheads). (C,D) By 7 and 11 days post-SOX2cKO, K14+ cells comprise most of the FF epithelium, and many of these cells have enlarged cell somata with elongated processes (red arrows). (E) Control non-taste epithelium is characterized by basally located K14+ progenitors and filiform papillae (arrows; asterisks mark each filiform papilla core), interspersed with K14− interpapillary (IPP) regions. The white bar spans the epithelium from the basement membrane to the superficial cellular layers (73 μm). (F) Filiform papillae are initially evident two days after SOX2cKO, but K14+ cells are uncharacteristically detected in suprabasal layers. (G,H) At 7 (G) and 11 (H) days after SOX2cKO, filiform papillae are no longer evident, K14+ cells span the entire thickness of the epithelium (white bars), and many K14+ cells have atypical morphologies (red arrows). Scale bars: 50 µm.
As SOX2 is expressed in non-taste epithelial progenitors, we reasoned that in the absence of SOX2 keratinocyte differentiation might also be disturbed throughout the tongue. Mechanosensory filiform papillae with small, short pits between them make up the majority of the non-taste epithelium. K14+ progenitors in control tongues surround the mesenchymal core of each filiform papilla (Fig. 5E, asterisks), reside in filiform papilla per se (Fig. 5E, arrows), and lie at the basement membrane of the interpapillary pits (IPPs) (Fig. 5E); this pattern did not differ 1 day after SOX2cKO (data not shown). At 2 days following Sox2 deletion, spinous-shaped filiform papillae with mesenchymal cores were present (Fig. 5F, arrows and asterisks); however, IPPs now comprised mostly K14+ progenitors instead of K14− differentiated keratinocytes, and layers of K14+ cells within the IPPs extended to the tongue surface (Fig. 5F, IPP and white vertical bar). At 7 and at 11 days post-induction, filiform papillae were lost altogether and K14+ progenitor cells expanded throughout the entire depth of the non-taste epithelium (Fig. 5G,H, white vertical bars).
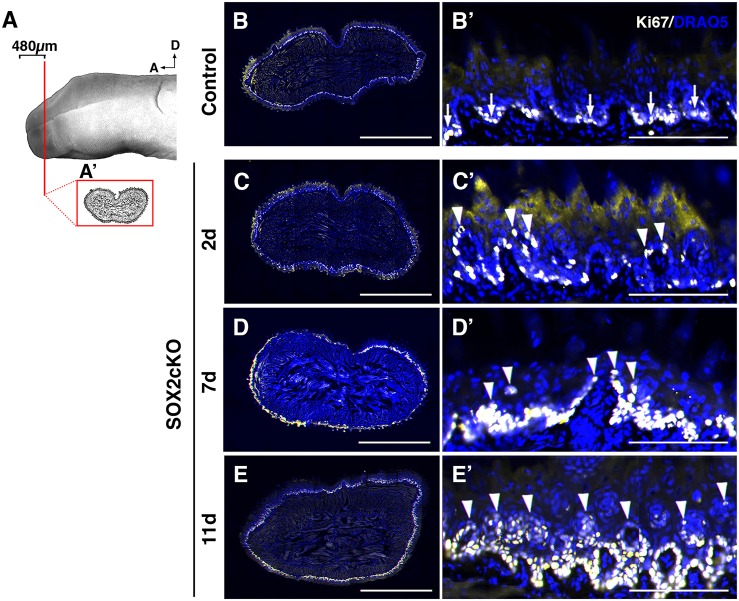
To determine whether K14+ progenitors expand in the lingual epithelium because of sustained proliferation, we used Ki67 (MKI67) as a marker to detect actively cycling cells (Fig. 6A,A′). In control tongues, the majority of progenitor cells are Ki67+ and are restricted to the basal epithelial layer (Fig. 6B,B′). At 2 days, proliferating cells had a disorganized distribution, with numerous Ki67+ cells in suprabasal layers of the epithelium (Fig. 6C,C′, arrowheads). With prolonged SOX2 loss, progressively more proliferating cells were detected both basally and suprabasally (Fig. 6D-E′, arrowheads indicate suprabasal cells).
Fig. 6.
Proliferation is disorganized in SOX2cKO mice. (A) Proliferating cells (Ki67+) were assessed in representative transverse sections through the anterior tongue (A′) (first 480 μm from the tip). (B,B′) In controls, Ki67+ (light yellow) cells are restricted to the basal layer of the lingual epithelium. (C-E′) In SOX2cKO mice, Ki67+ cells also reside basally, but progressively more Ki67+ cells are found in suprabasal layers at later times post SOX2cKO (C′-E′, arrowheads). B′-E′ show high magnification views of the dorsal lingual epithelium in B-E, respectively. Nuclei are counterstained with Draq5 (blue). All images are scanned best focus sections. A, anterior; D, dorsal. Scale bars: 1 mm (B-E); 125 μm (B′-E′).
Thus, our data indicate that genetic deletion of Sox2 in lingual progenitor cells quickly leads to impaired taste and non-taste cell differentiation, overexpansion of progenitor cells via aberrant proliferation, and taste bud cell death. The combination of these cellular events likely underlies the swift decrease in FF taste buds and loss of filiform papillae upon deletion of Sox2.
Overexpression of SHH in SOX2cKO lingual progenitors transforms SHH from a pro-taste differentiation factor to an epithelial mitogen
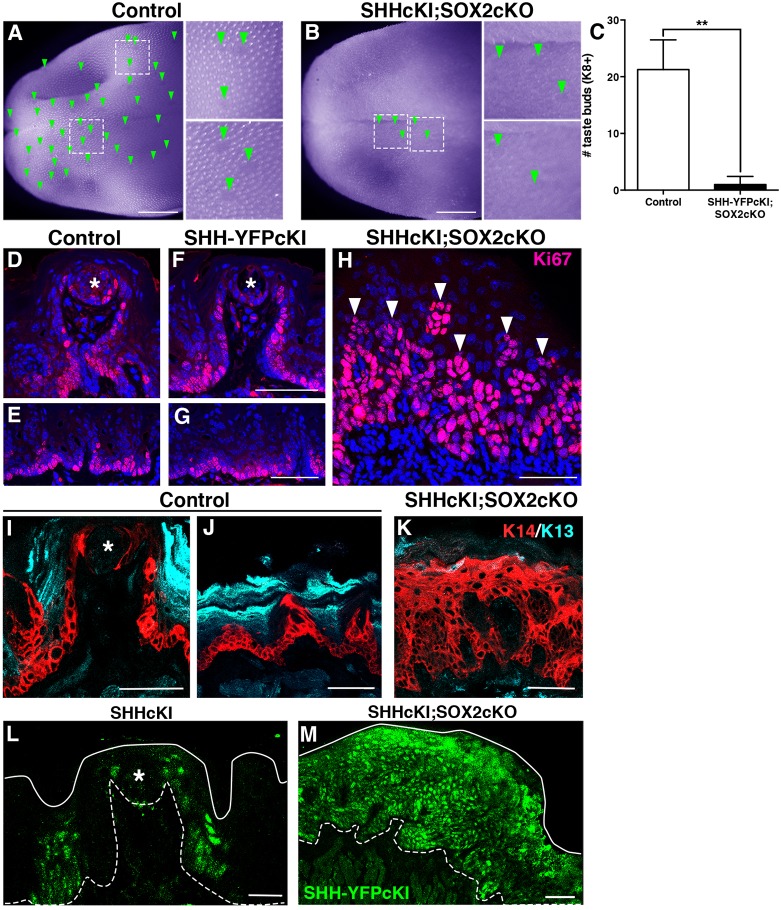
SHH overexpression in K14+ progenitors cells induces de novo differentiation of taste buds in regions of the lingual epithelium formerly thought to be incapable of sustaining taste cell differentiation (Castillo et al., 2014). Hence, we next investigated whether SHH overexpression in K14+ progenitors can drive taste cell differentiation in the absence of SOX2. We took advantage of a Shh conditional knock-in allele, SHH-IRES-YFPcKI (SHHcKI), together with SOX2cKO to drive SHH expression and Sox2 deletion in K14+ progenitors of adult K14+/CreER;RosaShh-IRES-YFPcKI;Sox2flox/flox mice (SHHcKI-SOX2cKO). Control and mutant mice were given a single tamoxifen dose and tongues were analyzed at 11 days. In genetic controls (RosaShh-IRES-YFPcKI;Sox2flox/flox treated with tamoxifen), FF taste buds are readily visible on the tongue surface as translucent ovoids (Fig. 7A, arrowheads) interspersed among spinous filiform papillae. In SHHcKI-SOX2cKO mice, neither FF nor filiform papillae were abundant on the tongue surface; those FFP that remained likely reflected mosaic Cre activation (Fig. 7B, arrowheads). The loss of taste buds was confirmed in K8-immunostained tissue sections; SHHcKI-SOX2cKO mice exhibited on average one K8+ FF taste bud in the anteriormost 1.5 mm of the tongue per mouse compared with ∼20 buds evident in the same region of each tongue in controls (Fig. 7C). Thus, deletion of Sox2 prevents SHH-dependent ectopic taste bud formation, a result consistent with our previous observation that ectopically induced taste buds expressed high levels of SOX2 (Castillo et al., 2014).
Fig. 7.
Overexpression of SHH in SOX2cKO lingual progenitors transforms SHH from a pro-taste differentiation factor to an epithelial mitogen. (A) In a control tongue (RosaShh-IRES-YFPcKI;Sox2flox/flox) viewed in whole mount (pseudocolored purple to enhance contrast), FFP are evident as clear ovals (green arrowheads, insets) embedded within the spinous filiform papillae that cover the tongue surface. (B) In double mutant tongues (SHHcKI;SOX2cKO in K14+ progenitors) at 11 days, FFP (green arrowheads, insets) and filiform papillae are mostly absent in tongues. (C) Tallies of taste buds in immunostained tissue sections from the first 1.5 mm of the tongue show that K8+ taste buds are drastically diminished in SHHcKI;SOX2cKO mice compared with the same region in controls. (D-H) In control and SHHcKI tongues, cycling cells (Ki67, magenta) have the same basal distribution as K14+ progenitors in taste (D,F; taste bud, asterisks) and non-taste (E,G) epithelia. (H) In the absence of SOX2, SHHcKI massively increases epithelial proliferation (Ki67+, magenta, arrowheads). (I) In control mice, K14+ progenitors (red) are adjacent to taste buds (asterisk) and in FFP walls, and K13+ (cyan) differentiated keratinocytes make up the surface of the FFP. (J) In non-taste epithelium, K14+ progenitors reside basally, whereas differentiated K13+ keratinocytes are found suprabasally. (K) The lingual epithelium of SHHcKI;SOX2cKO mice is populated almost exclusively by K14+ cells at the expense of differentiated K13+ cells. (L) SHH-YFPcKI+ patches (green) 14 days post-Cre induction are limited to discrete patches in the presence of SOX2, as reported previously (Castillo et al., 2014). Asterisk indicates FF taste bud. (M) In the absence of SOX2 11 days post-induction, SHH-YFPcKI+ patches (green) are greatly expanded. (D-H) Nuclei are counterstained with Draq5 (blue). In L,M, dashed lines delimit the basement membrane; solid lines delimit the epithelial surface. D-M are compressed confocal z-stacks. Scale bars: 1 mm (A,B); 50 μm (D-K,M); 20 μm (L). N=4 mice per condition. Data are represented as mean+s.d.; **P<0.01 (Student's t-test).
We also assessed the gross morphology of the dorsal, lateral and ventral epithelia in tongue cryosections from mutants and controls. In general, despite induction of ectopic taste buds, lingual epithelium has a normal morphology in SHHcKI mutants. In control and SHHcKI mice, FFP, readily identifiable by nuclear counterstain, are found predominantly on the dorsal surface of the tongue (Fig. S2A,B, arrowheads), whereas in SHHcKI-SOX2cKO mutants, FF taste buds were mostly absent (Fig. S2C). Dorsal, lateral and ventral epithelia in control and SHHcKI tongues have well-organized FF and filiform papillae with discrete mesenchymal cores (Fig. S2D,E,G,H, asterisks), and there is a clear distinction between the basal epithelial layer and the lamina propria, i.e. connective tissue, below (Fig. S2D,E,G,H). In SHHcKI-SOX2cKO mice, however, papillary epithelial invaginations with mesenchymal cores virtually disappear, the epithelium appears to contain more cells, and the boundary between epithelium and lamina propria is indistinct (Fig. S2C,F,I). This disorganized phenotype is more severe in the lateral and ventral aspects of the tongue, where, in controls, taste buds do not typically reside.
We next examined the extent of epithelial proliferation in control, SHHcKI and SHHcKI-SOX2cKO tongues, reasoning that an increase in proliferation may underlie the aberrant morphology seen in SHHcKI-SOX2cKO lingual epithelia. In both controls and SHHcKI mice, Ki67+ cells were restricted to the basal epithelial layer of both FFP and non-taste epithelium, and the proportion of proliferating epithelial cells appeared to be comparable in control and SHHcKI tongues (Fig. 7D-G); these observations are consistent with the minimal impact of loss of SHH signaling on lingual epithelial proliferation (Castillo-Azofeifa et al., 2017; Kumari et al., 2017). By contrast, but similar to the impact of SOX2cKO alone (see Fig. 6E′), Ki67+ cells were dramatically increased and found throughout a greatly expanded epithelium in SHHcKI-SOX2cKO tongues (Fig. 7H). The expanded domains in double mutant epithelium comprised primarily K14+ progenitors, lacked the differentiated K13+ keratinocytes that occupy suprabasal layers in controls (Fig. 7I-K), and coincided with large areas of SHH-overexpressing cells (Fig. 7L,M).
DISCUSSION
Little is known about the functional role of SOX2 in adult lingual epithelium, or about the connection, if any, between HH signaling and SOX2. Specifically, we wanted to investigate whether the interaction of these two factors is crucial for taste bud homeostasis. Previously, we showed that SHH overexpression in K14+ progenitors of the adult non-taste epithelium results in elevated SOX2 levels, and these ectopic patches of high SHH/SOX2 expression coincide with the development of ectopic taste buds (Castillo et al., 2014). These findings suggested that the mechanism by which SHH regulates taste bud homeostasis is by increasing SOX2 expression levels.
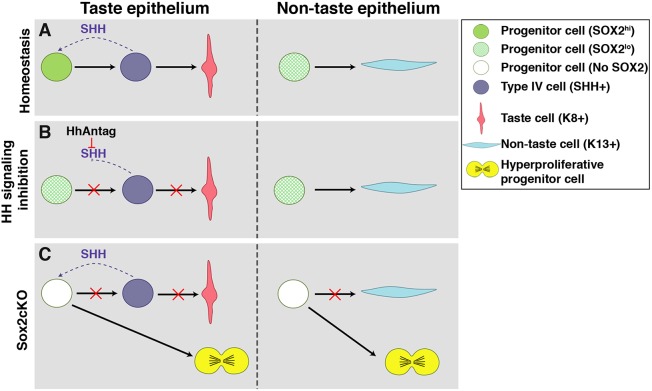
In the present study, we demonstrate that SOX2 is downstream of the HH signaling cascade and that SOX2 is essential for translating HH activity into the appropriate cellular output (Fig. 8A). We show that HH signaling inhibition results in rapid downregulation of SOX2 in both taste progenitors and IG taste bud cells, disrupting taste cell fate (Fig. 8B). Additionally, Sox2 conditional deletion in lingual progenitor cells promotes taste cell death, prevents differentiation of taste cells and lingual keratinocytes, and expands the undifferentiated progenitor cell population. Finally, we demonstrate that SHH functions as a mitogen rather than a pro-taste bud differentiation factor in the absence of SOX2 (Fig. 8C).
Fig. 8.
Summary of SHH regulation of SOX2 in tongue epithelium. (A) In taste epithelium, progenitor cells have increased SOX2 expression in response to SHH signaling, which promotes replenishment of SHH+ postmitotic precursor cells that differentiate into K8+ taste cells. In non-taste epithelium, progenitor cells are distant from any SHH source and thus maintain low SOX2 expression, essential for K13+ keratinocyte differentiation. (B) Inhibition of HH signaling reduces SOX2 expression preventing differentiation of taste epithelium, without affecting non-taste keratinocyte differentiation. (C) Genetic deletion of SOX2 in K14+ progenitors prevents differentiation of taste and non-taste cells, instead promoting progenitor proliferation and overall epithelial hyperplasia.
We first sought to elucidate whether acute use of an HPI impacts taste bud maintenance, as chronic administration of HPIs in humans and mice affects the taste system (Kumari et al., 2015, 2017; LoRusso et al., 2011; Rodon et al., 2014; Tang et al., 2012; Yang et al., 2015). After 1 week of HhAntag treatment, taste bud number and size were slightly, but not significantly, decreased, and the incidence of degenerating FFP was significantly increased. Even though these differences were small or not statistically significant, they nonetheless suggested that HH-dependent molecular mechanisms regulating taste cell renewal were already affected by 7 days but had not yet been translated into a robust cellular phenotype.
In the adult tongue, SOX2 is moderately expressed in PG cells and FFP walls and more highly expressed in a subset of IG taste bud cells, whereas SOX2 expression is low in the basal keratinocytes of the non-taste epithelium (Ohmoto et al., 2017; Okubo et al., 2006, 2009; Suzuki, 2008). In adult mice, after transection of the glossopharyngeal nerve innervating the posterior circumvallate taste papilla, taste buds disappear, as does SOX2 expression in taste progenitors and taste buds. Nerve regeneration is required for taste bud regeneration (Cheal and Oakley, 1977) and, similarly, nerve regeneration leads to reappearance of high levels of SOX2 expression in PG cells followed by expression within the regenerating taste buds (Suzuki, 2008), suggesting that SOX2 is involved in adult taste bud regeneration. Monitoring SOX2 activity via SOX2-GFP expression in HhAntag-treated mice, we found that SOX2-GFP was significantly decreased in FFP taste epithelium after 7 days of drug treatment. Interestingly, with HPI treatment, we detected a greater effect on SOX2-GFP expression in the PG progenitors compared with SOX2-GFP inside taste buds, which aligns with the PG pattern of recovery of SOX2 expression prior to taste bud regeneration after denervation (Suzuki, 2008). In summary, these data support the model that blocking HH signaling: (1) triggers downregulation of SOX2 expression in HH-responding PG progenitors, followed by (2) reduced SOX2 expression in mature taste cells, and finally (3) taste bud regression (Fig. 8A,B).
Prior studies have suggested that SOX2 is required but not sufficient for embryonic taste bud formation. Sox2 hypomorphic embryos, which express ∼20% of normal SOX2 levels, fail to form differentiated taste buds at birth, but Sox2 gain of function does not induce taste bud differentiation (Okubo et al., 2006). Our findings extend a requirement for SOX2 to adult taste bud renewal, as loss of SOX2 in adult tongue progenitors causes taste bud deterioration. Compared with the effect of HH pathway inhibition, which primarily impacts taste buds, however, Sox2 deletion prompts a much more dramatic lingual phenotype that includes perturbation of taste buds and non-taste epithelium. This result is not unexpected, as the SOX2 transcription factor functions in a host of processes, and is regulated by or itself regulates a multitude of signaling pathways (Liu et al., 2013b), including the Wnt pathway, which is a key regulator of taste and lingual epithelial cell renewal. Although the role of SOX2, if any, in mediating Wnt signaling in tongue is unknown, loss of Sox2 likely disrupts many molecular regulators.
A comprehensive study showed that SOX2 is expressed in many adult mouse epithelial tissues (e.g. tongue, lungs, lens, glandular stomach, esophagus, forestomach and anus), where it marks basally situated progenitor cells (Arnold et al., 2011). This group showed that ablation of SOX2+ basal cells in the tongue and oral mucosa resulted in inflammation, ulcers and edema in the oral cavity (Arnold et al., 2011). Our data complement this study, as loss of SOX2 in tongue progenitors alters adult lingual epithelium homeostasis by promoting K14+ progenitor proliferation and impeding taste and non-taste cell fates (Fig. 8C). However, our results contrast with reports that hypomorphic SOX2 expression did not affect differentiation of filiform papillae and progenitor proliferation in embryos (Okubo et al., 2006), as well as that stratified epithelial layers in the tongue are unaltered by ablation of SOX2+ progenitors in adults (Arnold et al., 2011). The discrepancies with the developmental study may be due to a more limited function of SOX2 in embryonic lingual epithelium, as opposed to the broader role for SOX2 in adult tongue, where we show it is required for both taste and non-taste epithelium. Alternatively, hypomorphic SOX2 expression may be sufficient for filiform papillae development, where in adults SOX2 levels are lowest. Our findings, however, parallel those from studies of SOX2+ dental epithelial cells that contribute to all epithelial cell lineages of the mouse incisor (Juuri et al., 2012).
Our data support a model in which HH controls tongue epithelium homeostasis by regulating SOX2 levels in lingual progenitors (Fig. 8). In support of this hypothesis, in adult FFP, the PG cells that highly express SOX2 are also GLI1+, and are directly affected by LDE225, an HPI (Kumari et al., 2015; Liu et al., 2013a). Furthermore, a SOX2-SHH link has been reported in embryonic tongues, in which both genes are co-expressed in placodes of the developing taste papillae, and thus SOX2 and SHH may also interact in taste bud development. Imbalance between several signaling circuits involving SHH and SOX2 causes elevated levels of SOX2 in cells that normally differentiate into keratinocytes, and in some cases these cells appear to form taste buds (Beites et al., 2009). A direct relationship between SHH and Sox2 has been documented in telencephalic neuroepithelial cells, in which SOX2 expression is under the control of the transcription factor GLI2 (Takanaga et al., 2009). Furthermore, in non-small-cell lung cancer stem cells, GLI1 was found to bind to the promoter region and regulate Sox2 transcription (Bora-Singhal et al., 2015).
To test the requirement of Sox2 downstream of HH in inducing taste bud differentiation, we overexpressed SHH in lingual epithelial progenitors and simultaneously deleted Sox2. We found that instead of inducing ectopic taste buds, SHH activated hyperproliferation of K14+ basal progenitors and blocked taste and non-taste epithelial differentiation. The tongue epithelial architecture was profoundly changed, developing into a hyperplastic basal cell carcinoma-like phenotype (Kasper et al., 2012; Oro et al., 1997; Wong and Dlugosz, 2014). SHH functions as a mitogen in a variety of tissues under homeostasis. In mouse lung development, SHH regulates cell proliferation of the epithelium and mesenchyme (Bellusci et al., 1997). In the anagen hair follicle, transit-amplifying progeny signal via SHH to the quiescent stem cell pool to proliferate for continual hair regeneration (Hsu et al., 2014), and conditional SHH signaling activation in the adult brain results in expansion of neural stem cells at the expense of their progeny (Ferent et al., 2014). Moreover, several types of cancers are associated with mutations of the HH pathway, including basal cell carcinomas (Jiang and Hui, 2008; Ng and Curran, 2011; Petrova and Joyner, 2014; Rubin and de Sauvage, 2006), and, in a mouse model, SHH overexpression in K14+ cells in the skin causes formation of basal cell carcinoma (Oro et al., 1997). Interestingly, in the tongue epithelium, SHH overexpression alone induces taste bud differentiation; only in the absence of SOX2 does SHH expression cause massive hyperproliferation and basal cell expansion, characteristic of basal cell carcinomas. Furthermore, although SOX2 expression is amplified in many cancers (e.g. Boumahdi et al., 2014), SOX2 expression can be protective in gastric tumors (Sarkar et al., 2016) and oral squamous cell carcinoma (Fu et al., 2016), suggesting that SOX2 loss in the tongue may be pro-oncogenic.
In summary, our results demonstrate that in adult tongue HH signaling functions through SOX2, and SOX2 is required for maintenance and renewal of both taste buds and non-taste lingual epithelium. Overall, our findings suggest that SOX2 is a molecular gatekeeper of HH signaling and possibly other signaling pathways in the adult tongue. Going forward, determining whether Sox2 is a direct or indirect downstream target of HH signaling will aid the development of therapies for mitigating taste disruption due to the use of HPIs as chemotherapy and will advance our understanding of the molecular mechanisms of lingual epithelium homeostasis.
MATERIALS AND METHODS
Animals
Male and female mice were all on a mixed background. Mouse lines used include combinations of the following alleles or transgenes: Sox2GFP (Jax 017592) (Arnold et al., 2011), K14CreER (Li et al., 2000), Sox2flox (Jax 013093) (Shaham et al., 2009), R26RShh-IRES-YFPcKI (Castillo et al., 2014). Mice were 6-12 weeks of age at the start of each experiment and data for this study were gathered from at least three mice per time point. Mice were genotyped as previously described (Arnold et al., 2011; Castillo et al., 2014; Shaham et al., 2009) and rodent work was carried out in accordance to approved protocols by the Institutional Animal Care and Use Committee at the University of Colorado Anschutz Medical Campus and University of California San Francisco.
HhAntag administration
HhAntag was prepared as described by Yauch et al. (2008) and was administered to Sox2GFP mice by oral gavage twice daily at a dose of 100 mg/kg for 3 or 7 days.
RNA extraction and qPCR
RNA from lingual epithelium after 3 days of vehicle versus HhAntag treatment was obtained for four mice for each condition and reverse transcribed following methods detailed by Castillo-Azofeifa et al. (2017). SYBR-Green-based qPCR was used as described with Sox2-specific primers (forward: CCAGCGCATGGACAGCTA; reverse: GCTGCTCCTGCATCATGCT).
Tamoxifen induction of Cre
To delete Sox2 in lingual epithelial cells by Cre activation, K14CreER;Sox2flox/flox mice were gavaged once with a dose of 5 mg tamoxifen (T5648, Sigma) dissolved in corn oil; mice were sacrificed 1, 2, 7 or 11 days later. To misexpress SHH and delete Sox2 in lingual epithelial cells, K14CreER;R26RShh-IRES-YFPcKI;Sox2flox/flox mice received a single tamoxifen dose of 5 mg and tissue was collected 11 days post-tamoxifen induction.
Tissue preparation
Harvested tongues were fixed by immersion or perfusion. For immersion fixation, animals were euthanized by CO2 inhalation followed by cervical dislocation. Tongues were dissected, rinsed in sterile ice-cold PBS, and immersed in 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB) overnight at 4°C. Tissue was embedded in Tissue-Tek O.C.T. compound (4583, Sakura), frozen, and stored at −80°C. For perfusion fixation, animals were anesthetized by intraperitoneal injection of 250 mg/kg Avertin (2,2,2-tribromoethanol) and transcardially perfused with periodate-lysine-paraformaldehyde (PLP) (Pieri et al., 2002). Dissected tongues were post-fixed in PLP for 3 h at 4°C and then cryoprotected in 20% sucrose in PB overnight at 4°C. Tissue was embedded in Tissue-Tek O.C.T. compound, frozen, and stored at −80°C. Processing of immersion- or perfusion-fixed tongues was restricted to the anterior 1.5 mm of the tongue with a high density of FFP. Eight sets of serial cryosections (12 μm) per tongue were collected on Superfrost Plus Slides (12-550-15, Fisher Scientific).
Immunofluorescence
Immunofluorescence was performed on immersion- or perfusion-fixed 12 μm cryosections as described (Nguyen and Barlow, 2010). Primary antisera were: rat anti-K8 (Troma) (1:250; Developmental Studies Hybridoma Bank, University of Iowa), chicken anti-GFP (used to detect GFP or YFP; 1:1000; GFP-1020, Aves Labs), goat anti-SOX2 (1:500; sc-17320, Santa Cruz), rabbit anti-K14 (1:3500; PRB-155P, Covance), rabbit anti-Ki67 (1:200; RM-9106-S, Thermo Fisher Scientific) and guinea pig anti-K13 (1:500; BP5076, Acris Antibodies). Appropriate secondary antisera from Thermo Fisher Scientific (A11006, A11081, A21247, A21208, A11039, A11055, A11008, A11010, A21245, A21206, A31573, S11225, A11073), Jackson ImmunoResearch (712-165-153, 712-605-150) and Vector Laboratories (PK-6101) were used at 1:1000 (host: goat), 1:800 (host: donkey) or 1:500 (rabbit IgG biotinylated). Sections were counterstained with Draq5 (1:8000; 108410, AbCam), Sytox green nucleic acid stain (1:50,000; S7020, Thermo Fisher Scientific) or DAPI (1:10,000; D3571, Thermo Fisher Scientific), and coverslipped with Fluormount G (0100-01, SouthernBiotech) or ProLong Gold Antifade (P36930, Thermo Fisher Scientific). All antibodies have been previously validated (Castillo et al., 2014; Castillo-Azofeifa et al., 2017).
TUNEL assay was performed on immersion-fixed 12 μm cryosections as described (Gaillard et al., 2015) using In Situ Cell Death Detection Kit, TMR red (12156792910, Roche).
Image acquisition and analysis
All image acquisition and analyses were performed blind to condition. Fluorescence and bright-field images were acquired using a Zeiss Axioplan II microscope, an Olympus SZX12 stereo microscope or Leica DM5000 B, a Retiga 4000R camera with Q-Capture Pro-7 software, an Axiocam CCD camera with Axiovision software or Leica DFC 500 with LAS V4.9 software. Confocal images were obtained as a z-stack of 0.76 μm optical sections acquired sequentially using a Leica TCS SP5 II confocal microscope with LASAF software or Zeiss Observer Z1 with ZEN blue software. Whole-tissue section scans were acquired sequentially using a Leica DFC 365FX camera on a Leica DM6000B microscope with the imaging software Surveyor by Objective Imaging. A series of 20× images was obtained for each fluorophore (Texas Red, FITC, Cy5), aligned and stitched together using the Best Focus option in the Surveyor software. The final rendering is a mosaic RGB image of each section.
The most anterior 1.5 mm of each tongue was collected as eight serial sets of 16 cryosections and a single series was used for each of the different immunomarkers. Each fungiform taste bud was counted if: (1) it was found within a FF papilla; and (2) it housed at least 1 K8-immunoreactive (K8+) cell with a nuclear profile. Additionally, FF taste buds were categorized as follows (also see Fig. 1A,B). (1) Typical FF papilla and taste bud (typical FFP): papilla with epithelial invagination into the lamina propria mesenchyme, where a mesenchymal core is defined by a basal epithelial layer; the papilla apex has a plateau-like surface housing a single taste bud. Each taste bud has a characteristic onion-like shape and is composed of fusiform cells. (2) Atypical FF papilla and taste bud (atypical FFP): papilla and mesenchymal core are narrow; the papilla apex is filiform and houses a single taste bud. The taste bud is narrow and has fewer taste cells; the remaining taste cells have a stretched appearance (Nagato et al., 1995; Oakley et al., 1990). All taste buds in either type of papillae were tallied and assigned a number. Typical and atypical FF taste buds were analyzed separately. Sets of ten typical FF taste buds per mouse were randomly selected (random.org) for quantification of the total number of K8+ pixels inside taste buds. We analyzed each confocal optical section from every taste bud z-stack using our imstack toolbox developed in MATLAB (Mathworks, Natick, MA, USA) (Castillo-Azofeifa et al., 2017). We loaded each z-stack into the imstack toolbox and established a rectangular region-of-interest (ROI) that completely encompassed the taste bud. The same ROI dimensions were used for all analyzed images. Signal in all three channels was thresholded using Otsu's method (Otsu, 1979). Signal was designated as only those pixels with an intensity above the calculated threshold value within the taste bud ROI.
We measured the corrected integrated density (CID) of SOX2-GFP immunofluorescence from z-stacks using the open-source platform Fiji (Schindelin et al., 2012). We first established a taste bud region-of-interest (ROIa) encompassing each K8+ taste bud (Texas Red channel). SOX2-GFP CID was quantified within the ROIa to obtain SOX2-GFP corrected fluorescence within each taste bud. To quantify SOX2-GFP corrected fluorescence of FFP walls plus PG cells, we set a new ROI (ROIb) delimiting the papilla walls and taste bud. However, in ROIb we masked the area corresponding to the taste bud ROIa, and quantified SOX2-GFP CID within the ROIb−a to obtain SOX2-GFP corrected fluorescence within each papilla. CID was obtained using the following calculation: corrected integrated density=integrated density – (area selected×mean value of background) (Gavet and Pines, 2010).
For whole-section analysis of cell proliferation by Ki67 immunoreactivity, representative sections were selected from a region of the tongue in which FF and filiform papillae are adequately distributed for the analysis. This region of the tongue is located 480 μm from the tip of the tongue.
Statistical analysis
Normally distributed data were analyzed using the parametric two-tailed Student's t-test with Welch's correction or two-way ANOVA with Tukey's multiple comparisons test. The non-parametric Mann–Whitney U-test was used if the data did not fit a normal distribution. Significance was taken as P<0.05 with a confidence interval of 95%. Data are presented as mean±s.d. for parametric data or as median with interquartile range for non-parametric data.
Supplementary Material
Acknowledgements
We thank Kelly Zaccone, Dong-Kha Tran, Sarah Alto, Rebecca d'Urso and Pauline Marangoni for excellent mouse assistance, Adriane Joo and Jimmy Kuang-Hsien Hu for helpful scientific comments, and Fred deSauvage and Genentech for HhAntag.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: D.C.-A., K.S., O.D.K., L.A.B.; Methodology: D.C.-A., K.S., E.J.G.; Formal analysis: D.C.-A., E.J.G., L.A.B.; Investigation: D.C.-A., K.S., L.G., B.J., L.A.B.; Resources: K.S., O.D.K.; Writing - original draft: D.C.-A., L.A.B.; Writing - review & editing: D.C.-A., K.S., L.G., O.D.K., L.A.B.; Visualization: L.A.B.; Supervision: L.A.B.; Project administration: O.D.K., L.A.B.; Funding acquisition: O.D.K., L.A.B.
Funding
This work was supported by the National Institutes of Health (R01 DC012383 to L.A.B.; NIH R35-DE026602 to O.D.K.). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.164889.supplemental
References
- Arnold K., Sarkar A., Yram M. A., Polo J. M., Bronson R., Sengupta S., Seandel M., Geijsen N. and Hochedlinger K. (2011). Sox2+ adult stem and progenitor cells are important for tissue regeneration and survival of mice. Stem Cell 9, 317-329. 10.1016/j.stem.2011.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N. (2014). Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 15, 19-33. 10.1038/nrm3721 [DOI] [PubMed] [Google Scholar]
- Barlow L. A. (2015). Progress and renewal in gustation: new insights into taste bud development. Development 142, 3620-3629. 10.1242/dev.120394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow L. A. and Klein O. D. (2015). Developing and regenerating a sense of taste. Curr. Top. Dev. Biol. 111, 401-419. 10.1016/bs.ctdb.2014.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beidler L. M. and Smallman R. L. (1965). Renewal of cells within taste buds. J. Cell Biol. 27, 263-272. 10.1083/jcb.27.2.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beites C. L., Hollenbeck P. L. W., Kim J., Lovell-Badge R., Lander A. D. and Calof A. L. (2009). Follistatin modulates a BMP autoregulatory loop to control the size and patterning of sensory domains in the developing tongue. Development 136, 2187-2197. 10.1242/dev.030544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellusci S., Furuta Y., Rush M. G., Henderson R., Winnier G. and Hogan B. L. (1997). Involvement of Sonic hedgehog (Shh) in mouse embryonic lung growth and morphogenesis. Development 124, 53-63. [DOI] [PubMed] [Google Scholar]
- Bora-Singhal N., Perumal D., Nguyen J. and Chellappan S. (2015). Gli1-mediated regulation of Sox2 facilitates self-renewal of stem- like cells and confers resistance to EGFR inhibitors in non–small cell lung cancer. Neoplasia 17, 538-551. 10.1016/j.neo.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumahdi S., Driessens G., Lapouge G., Rorive S., Nassar D., Le Mercier M., Delatte B., Caauwe A., Lenglez S., Nkusi E. et al. (2014). SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature 511, 246-250. 10.1038/nature13305 [DOI] [PubMed] [Google Scholar]
- Castillo D., Seidel K., Salcedo E., Ahn C., de Sauvage F. J., Klein O. D. and Barlow L. A. (2014). Induction of ectopic taste buds by SHH reveals the competency and plasticity of adult lingual epithelium. Development 141, 2993-3002. 10.1242/dev.107631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Azofeifa D., Losacco J. T., Salcedo E., Golden E. J., Finger T. E. and Barlow L. A. (2017). Sonic hedgehog from both nerves and epithelium is a key trophic factor for taste bud maintenance. Development 144, 3054-3065. 10.1242/dev.150342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari N. N. and Roper S. D. S. (2010). The cell biology of taste. J. Cell Biol. 190, 285-296. 10.1083/jcb.201003144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheal M. and Oakley B. (1977). Regeneration of fungiform taste buds: temporal and spatial characteristics. J. Comp. Neurol. 172, 609-625. 10.1002/cne.901720405 [DOI] [PubMed] [Google Scholar]
- Cho Y. K., Farbman A. I. and Smith D. V. (1998). The timing of alpha-gustducin expression during cell renewal in rat vallate taste buds. Chem. Senses 23, 735-742. 10.1093/chemse/23.6.735 [DOI] [PubMed] [Google Scholar]
- Delay R. J., Kinnamon J. C. and Roper S. D. (1986). Ultrastructure of mouse vallate taste buds: II. Cell types and cell lineage. J. Comp. Neurol. 253, 242-252. 10.1002/cne.902530210 [DOI] [PubMed] [Google Scholar]
- Farbman A. I. (1980). Renewal of taste bud cells in rat circumvallate papillae. Cell Tissue Kinet 13, 349-357. 10.1111/j.1365-2184.1980.tb00474.x [DOI] [PubMed] [Google Scholar]
- Ferent J., Cochard L., Faure H., Taddei M., Hahn H., Ruat M. and Traiffort E. (2014). Genetic activation of Hedgehog signaling unbalances the rate of neural stem cell renewal by increasing symmetric divisions. Stem Cell Reports 3, 312-323. 10.1016/j.stemcr.2014.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T.-Y., Hsieh I.-C., Cheng J.-T., Tsai M.-H., Hou Y.-Y., Lee J.-H., Liou H.-H., Huang S.-F., Chen H.-C., Yen L.-M. et al. (2016). Association of OCT4, SOX2, and NANOG expression with oral squamous cell carcinoma progression. J. Oral Pathol. Med. 45, 89-95. 10.1111/jop.12335 [DOI] [PubMed] [Google Scholar]
- Gaillard D., Xu M., Liu F., Millar S. E. and Barlow L. A. (2015). β-catenin signaling biases multipotent lingual epithelial progenitors to differentiate and acquire specific taste cell fates. PLoS Genet. 11, e1005208 10.1371/journal.pgen.1005208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavet O. and Pines J. (2010). Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev. Cell 18, 533-543. 10.1016/j.devcel.2010.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamichi R., Asano-Miyoshi M. and Emori Y. (2006). Taste bud contains both short-lived and long-lived cell populations. Neuroscience 141, 2129-2138. 10.1016/j.neuroscience.2006.05.061 [DOI] [PubMed] [Google Scholar]
- Hsu Y.-C., Li L. and Fuchs E. (2014). Transit-amplifying cells orchestrate stem cell activity and tissue regeneration. Cell 157, 935-949. 10.1016/j.cell.2014.02.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume W. J. and Potten C. S. (1976). The ordered columnar structure of mouse filiform papillae. J. Cell Sci. 22, 149-160. [DOI] [PubMed] [Google Scholar]
- Ichimori Y., Ueda K., Okada H., Honma S. and Wakisaka S. (2009). Histochemical changes and apoptosis in degenerating taste buds of the rat circumvallate papilla. Arch. Histol. Cytol. 72, 91-100. 10.1679/aohc.72.91 [DOI] [PubMed] [Google Scholar]
- Iwasaki S.-I., Yoshizawa H. and Aoyagi H. (2006). Immunohistochemical expression of keratins 13 and 14 in the lingual epithelium of rats during the morphogenesis of filiform papillae. Arch. Oral Biol. 51, 416-426. 10.1016/j.archoralbio.2005.09.009 [DOI] [PubMed] [Google Scholar]
- Jiang J. and Hui C.-C. (2008). Hedgehog signaling in development and cancer. Dev. Cell 15, 801-812. 10.1016/j.devcel.2008.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juuri E., Saito K., Ahtiainen L., Seidel K., Tummers M., Hochedlinger K., Klein O. D., Thesleff I. and Michon F. (2012). Sox2+ stem cells contribute to all epithelial lineages of the tooth via Sfrp5+ progenitors. Dev. Cell 23, 317-328. 10.1016/j.devcel.2012.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper M., Jaks V., Hohl D. and Toftgård R. (2012). Basal cell carcinoma - molecular biology and potential new therapies. J. Clin. Invest. 122, 455-463. 10.1172/JCI58779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp L., Lawton A., Oakley B., Wong L. and Zhang C. (1995). Keratins as markers. Differentiation 58, 341-349. 10.1046/j.1432-0436.1995.5850341.x [DOI] [PubMed] [Google Scholar]
- Kumari A., Ermilov A. N., Allen B. L., Bradley R. M., Dlugosz A. A. and Mistretta C. M. (2015). Hedgehog pathway blockade with the cancer drug LDE225 disrupts taste organs and taste sensation. J. Neurophysiol. 113, 1034-1040. 10.1152/jn.00822.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari A., Ermilov A. N., Grachtchouk M., Dlugosz A. A., Allen B. L., Bradley R. M. and Mistretta C. M. (2017). Recovery of taste organs and sensory function after severe loss from Hedgehog/Smoothened inhibition with cancer drug sonidegib. Proc. Natl Acad. Sci. USA 114, E10369-E10378. 10.1073/pnas.1712881114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Indra A. K., Warot X., Brocard J., Messaddeq N., Kato S., Metzger D. and Chambon P. (2000). Skin abnormalities generated by temporally controlled RXRalpha mutations in mouse epidermis. Nature 407, 633-636. 10.1038/35036595 [DOI] [PubMed] [Google Scholar]
- Liu Z., Walters B. J., Owen T., Brimble M. A., Steigelman K. A., Zhang L., Mellado Lagarde M. M., Valentine M. B., Yu Y., Cox B. C. et al. (2012). Regulation of p27Kip1 by Sox2 maintains quiescence of inner pillar cells in the murine auditory sensory epithelium. J. Neurosci. 32, 10530-10540. 10.1523/JNEUROSCI.0686-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.-X., Ermilov A., Grachtchouk M., Li L., Gumucio D. L., Dlugosz A. A. and Mistretta C. M. (2013a). Multiple Shh signaling centers participate in fungiform papilla and taste bud formation and maintenance. Dev. Biol. 382, 82-97. 10.1016/j.ydbio.2013.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Lin B., Zhao M., Yang X., Chen M., Gao A., Liu F., Que J. and Lan X. (2013b). The multiple roles for Sox2 in stem cell maintenance and tumorigenesis. Cell. Signal. 25, 1264-1271. 10.1016/j.cellsig.2013.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoRusso P. M., Rudin C. M., Reddy J. C., Tibes R., Weiss G. J., Borad M. J., Hann C. L., Brahmer J. R., Chang I., Darbonne W. C. et al. (2011). Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin. Cancer Res. 17, 2502-2511. 10.1158/1078-0432.CCR-10-2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura H. (2003). Co-expression pattern of Shh with Prox1 and that of Nkx2.2 with Mash1 in mouse taste bud. Gene Expr. Patterns 3, 427-430. 10.1016/S1567-133X(03)00081-4 [DOI] [PubMed] [Google Scholar]
- Miura H. and Barlow L. A. (2010). Taste bud regeneration and the search for taste progenitor cells. Arch. Ital. Biol. 148, 107-118. [PMC free article] [PubMed] [Google Scholar]
- Miura H., Kusakabe Y., Sugiyama C., Kawamatsu M., Ninomiya Y., Motoyama J. and Hino A. (2001). Shh and Ptc are associated with taste bud maintenance in the adult mouse. Mech. Dev. 106, 143-145. 10.1016/S0925-4773(01)00414-2 [DOI] [PubMed] [Google Scholar]
- Miura H., Kusakabe Y. and Harada S. (2006). Cell lineage and differentiation in taste buds. Arch. Histol. Cytol. 69, 209-225. 10.1679/aohc.69.209 [DOI] [PubMed] [Google Scholar]
- Miura H., Scott J. K., Harada S. and Barlow L. A. (2014). Sonic hedgehog-expressing basal cells are general post-mitotic precursors of functional taste receptor cells. Dev. Dyn. 243, 1286-1297. 10.1002/dvdy.24121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagato T., Matsumoto K., Tanioka H., Kodama J. and Toh H. (1995). Effect of denervation on morphogenesis of the rat fungiform papilla. Acta Anat (Basel) 153, 301-309. 10.1159/000147739 [DOI] [PubMed] [Google Scholar]
- Ng J. M. Y. and Curran T. (2011). The Hedgehog's tale: developing strategies for targeting cancer. Nat. Rev. Cancer 11, 493-501. 10.1038/nrc3079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H. M. and Barlow L. A. (2010). Differential expression of a BMP4 reporter allele in anterior fungiform versus posterior circumvallate taste buds of mice. BMC Neurosci. 11, 129 10.1186/1471-2202-11-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B., Wu L.-H., Lawton A. and Desibour C. (1990). Neural control of ectopic filiform spines in adult tongue. Neuroscience 36, 831-838. 10.1016/0306-4522(90)90026-Z [DOI] [PubMed] [Google Scholar]
- Ohmoto M., Ren W., Nishiguchi Y., Hirota J., Jiang P. and Matsumoto I. (2017). Genetic lineage tracing in taste tissues using Sox2-CreERT2 strain. Chem. Senses. 10.1093/chemse/bjx032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo T., Pevny L. H. and Hogan B. L. M. (2006). Sox2 is required for development of taste bud sensory cells. Genes Dev. 20, 2654-2659. 10.1101/gad.1457106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo T., Clark C. and Hogan B. L. M. (2009). Cell Lineage mapping of taste bud cells and keratinocytes in the mouse tongue and soft palate. Stem Cells 27, 442-450. 10.1634/stemcells.2008-0611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oro A. E., Higgins K. M., Hu Z., Bonifas J. M., Epstein E. H. and Scott M. P. (1997). Basal cell carcinomas in mice overexpressing sonic hedgehog. Science 276, 817-821. 10.1126/science.276.5313.817 [DOI] [PubMed] [Google Scholar]
- Otsu N. (1979). A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 9, 62-66. 10.1109/TSMC.1979.4310076 [DOI] [Google Scholar]
- Perea-Martinez I., Nagai T. and Chaudhari N. (2013). Functional cell types in taste buds have distinct longevities. PLoS ONE 8, e53399 10.1371/journal.pone.0053399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova R. and Joyner A. L. (2014). Roles for Hedgehog signaling in adult organ homeostasis and repair. Development 141, 3445-3457. 10.1242/dev.083691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieri L., Sassoli C., Romagnoli P. and Domenici L. (2002). Use of periodate-lysine-paraformaldehyde for the fixation of multiple antigens in human skin biopsies. Eur. J. Histochem. 46, 365-375. 10.4081/1749 [DOI] [PubMed] [Google Scholar]
- Rodon J., Tawbi H. A., Thomas A. L., Stoller R. G., Turtschi C. P., Baselga J., Sarantopoulos J., Mahalingam D., Shou Y., Moles M. A. et al. (2014). A phase I, multicenter, open-label, first-in-human, dose-escalation study of the oral smoothened inhibitor sonidegib (LDE225) in patients with advanced solid tumors. Clin. Cancer Res. 20, 1900-1909. 10.1158/1078-0432.CCR-13-1710 [DOI] [PubMed] [Google Scholar]
- Rubin L. L. and de Sauvage F. J. (2006). Targeting the Hedgehog pathway in cancer. Nat. Rev. Drug Discov. 5, 1026-1033. 10.1038/nrd2086 [DOI] [PubMed] [Google Scholar]
- Sarkar A., Huebner A. J., Sulahian R., Anselmo A., Xu X., Flattery K., Desai N., Sebastian C., Yram M. A., Arnold K. et al. (2016). Sox2 suppresses gastric tumorigenesis in mice. Cell Reports 16, 1929-1941. 10.1016/j.celrep.2016.07.034 [DOI] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676-682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham O., Smith A. N., Robinson M. L., Taketo M. M., Lang R. A. and Ashery-Padan R. (2009). Pax6 is essential for lens fiber cell differentiation. Development 136, 2567-2578. 10.1242/dev.032888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y. (2008). Expression of Sox2 in mouse taste buds and its relation to innervation. Cell Tissue Res. 332, 393-401. 10.1007/s00441-008-0600-1 [DOI] [PubMed] [Google Scholar]
- Takanaga H., Tsuchida-Straeten N., Nishide K., Watanabe A., Aburatani H. and Kondo T. (2009). Gli2 is a novel regulator of Sox2 expression in telencephalic neuroepithelial cells. Stem Cells 27, 165-174. 10.1634/stemcells.2008-0580 [DOI] [PubMed] [Google Scholar]
- Tang J. Y., Mackay-Wiggan J. M., Aszterbaum M., Yauch R. L., Lindgren J., Chang K., Coppola C., Chanana A. M., Marji J., Bickers D. R. et al. (2012). Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N. Engl. J. Med. 366, 2180-2188. 10.1056/NEJMoa1113538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H., Rentrop M., Nischt R. and Schweizer J. (1990). Tissue-specific expression of murine keratin K13 in internal stratified squamous epithclia and its aberrant expression during two-stage mouse skin carcinogenesis is associated with the methylation state of a distinct CpG site in the remote 5′-flanking region of the gene. Differentiation 43, 105-114. 10.1111/j.1432-0436.1990.tb00436.x [DOI] [PubMed] [Google Scholar]
- Wong S. Y. and Dlugosz A. A. (2014). Basal cell carcinoma, hedgehog signaling, and targeted therapeutics: the long and winding road. J. Invest. Dermatol. 134, E18-E22. 10.1038/skinbio.2014.4 [DOI] [PubMed] [Google Scholar]
- Yang H., Cong W.-N., Yoon J. S. and Egan J. M. (2015). Vismodegib, an antagonist of hedgehog signaling, directly alters taste molecular signaling in taste buds. Cancer Med. 4, 245-252. 10.1002/cam4.350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch R. L., Gould S. E., Scales S. J., Tang T., Tian H., Ahn C. P., Marshall D., Fu L., Januario T., Kallop D. et al. (2008). A paracrine requirement for hedgehog signalling in cancer. Nature 455, 406-410. 10.1038/nature07275 [DOI] [PubMed] [Google Scholar]
- Zeng Q. and Oakley B. (1999). p53 and Bax: putative death factors in taste cell turnover. J. Comp. Neurol. 413, 168-180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.