Abstract
Zygomycete fungi were classified as a single phylum, Zygomycota, based on sexual reproduction by zygospores, frequent asexual reproduction by sporangia, absence of multicellular sporocarps, and production of coenocytic hyphae, all with some exceptions. Molecular phylogenies based on one or a few genes did not support the monophyly of the phylum, however, and the phylum was subsequently abandoned. Here we present phylogenetic analyses of a genome-scale data set for 46 taxa, including 25 zygomycetes and 192 proteins, and we demonstrate that zygomycetes comprise two major clades that form a paraphyletic grade. A formal phylogenetic classification is proposed herein and includes two phyla, six subphyla, four classes and 16 orders. On the basis of these results, the phyla Mucoromycota and Zoopagomycota are circumscribed. Zoopagomycota comprises Entomophtoromycotina, Kickxellomycotina and Zoopagomycotina; it constitutes the earliest diverging lineage of zygomycetes and contains species that are primarily parasites and pathogens of small animals (e.g. amoeba, insects, etc.) and other fungi, i.e. mycoparasites. Mucoromycota comprises Glomeromycotina, Mortierellomycotina, and Mucoromycotina and is sister to Dikarya. It is the more derived clade of zygomycetes and mainly consists of mycorrhizal fungi, root endophytes, and decomposers of plant material. Evolution of trophic modes, morphology, and analysis of genome-scale data are discussed.
Keywords: Entomophthoromycotina, fungi, Glomeromycotina, Kickellomycotina, Mortierellomycotina, Mucoromycota, Mucoromycotina, paraphyly, systematics, Zoopagomycota Zoopagomycotina
INTRODUCTION
Despite advances in our understanding of evolutionary relationships within Kingdom Fungi, the earliest diverging events are still poorly understood. Included among these unresolved events are the evolutionary transitions that ultimately culminated in modern diversity and in the emergence of terrestrial fungi, including subkingdom Dikarya, which comprises the phyla Ascomycota and Basidiomycota. Resolving the earliest branches in the fungal genealogy is essential to identify characteristics of the ancestral fungi, to determine what traits emerged with the dawn of terrestrial ecosystems, and to obtain an accurate assessment of the morphological and genetic homologies associated with fungal lifestyles. Central to this transition are the fungi that were once classified in the phylum Zygomycota Moreau (1954). However, because the monophyly of Zygomycota was not supported in recent phylogenetic analyses (e.g. James et al. 2006, Liu et al. 2009, Chang et al. 2015), these fungi are informally referred to herein as zygomycetes.
Zygomycetes are filamentous, nonflagellated fungi that mark the major transition away from the earliest diverging zoosporic fungi in Cryptomycota, Chytridiomycota, and Blastocladiomycota toward the rise of the nonflagellated, filamentous, multicellular Dikarya. The zygomycetes include: (i) Phycomyces blakesleeanus and other important model organisms; (ii) species such as Rhizopus stolonifer that cause economically significant pre- and postharvest diseases of fruits; (iii) members of Glomeromycota that colonize roots and form endomycorrhizal symbioses with more than 80% of land plants; and (iv) diverse and important pathogens or commensals of insects, nematodes, and other soil invertebrates (Benny et al. 2014, Redecker and Schüßler 2014). Some zygomycetes significantly benefit humans by the production of compounds such as lycopene, fatty acids, and biodiesel, but they can also cause rare and deadly human diseases such as zygomycosis (Papanikolaou and Panayotou 2007, Wang et al. 2011, Doggett and Wong 2014).
Abandonment of the phylum Zygomycota was formalized in Hibbett et al. (2007), which treated zygomycete fungi as four subphyla incertae sedis, including Entomophthoromycotina, Kickellomycotina, Mucoromycotina, and Zoopagomycotina and the phylum Glomeromycota. Mortierella was classified with the morphologically similar Mucorales until multigene analyses demonstrated that it was phylogenetically distinct from Mucoromycotina, resulting in the description of the subphylum Mortierollomycotina (Hoffmann et al. 2011). Results from rDNA and multigene molecular phylogenetic studies resolved these zygomycete taxa into two larger groups. One of the groups, informally known as “zygomycetes I”, includes Mucoromycotina and Mortierellomycotina and in some studies, Glomeromycota (James et al. 2006, White et al. 2006, Chang et al. 2015). Mucoromycotina includes Mucor, Rhizopus, and the majority of the most common and best known zygomycetes. Many of these are fast growing, early colonizers of carbon-rich substrates, with several species used in industry for organic acid production and fermentation (Jennessen et al. 2008). Mortierellomycotina are common soil fungi that occur as root endophytes of woody plants and also are commonly isolated as saprobes (Summerbell 2005). Glomeromycota includes the arbuscular mycorrhizal fungi, which arguably comprise the most successful plant-fungal symbiosis on Earth. Glomeromycota has been a phylogenetic enigma because it lacks any known form of sexual reproduction. Morphological hypotheses placed Glomeromycota among the zygomycetes (Gerdemann and Trappe 1974, Morton and Benny 1990), whereas rDNA-based phylogenies placed this phylum as sister to Dikarya (Schüßler et al. 2001). Mitochondrial phylogenies (Nadimi et al. 2012, Pelin et al. 2012) placed Glomeromycota as sister to Mortierellomycotina, which is supported by some but not all genome-scale phylogenies (Tisserant et al. 2013, Chang et al. 2015).
The second of the larger groups, “zygomycetes II”, includes Entomophthoromycota, Kickxellomycotina, and Zoopagomycotina (James et al. 2006, White et al. 2006, Sekimoto et al. 2011, Ebersberger et al. 2012, Chang et al. 2015). Zygomycetes II is more difficult of the two groups to study. In phylogenetic analyses, it has been weakly supported (James et al. 2006, Sekimoto et al. 2011) or strongly supported but based only on a couple of taxa (Chang et al. 2015). Entomophthoromycotina, the “insect destroyers”, includes parasites of insects and mites, commensals of reptiles and amphibians, and poorly known parasites of desmid algae. Kickxellomycotina comprises a diverse assemblage of fungi associated with the hindgut of arthropods, saprobic species with broad substrate ranges and mycoparasites. Zoopagomycotina are either obligate mycoparasites or pathogens of invertebrates, including nematodes, rotifers, and amoebae. Members of the zygomycetes II group are almost exclusively characterized by associations with animals and fungi with essentially no associations with living plants, either as pathogens or symbionts (Benny et al. 2014).
Although the applications of multigene analysis has resulted in limited phylogenetic resolution of zygomycetes in kingdom-level analyses, they have led to significant refinement of evolutionary hypotheses for selected groups of zygomycetes, based on a combination of molecular and morphological data. These include a family-level phylogenetic classification of Mucorales (Hoffmann et al. 2013), testing of ordinallevel phylogenetic and taxonomic hypotheses for Kickxellomycotina (Tretter et al. 2014) and characterization of the major clades of Entomophthoromycota and temporal estimates of their origin in the geologic record (Gryganskyi et al. 2012). However, unlike Dikarya for which genome data and phylogenomic analyses have transformed our understanding of phylogenetic relationships and evolutionary processes (e.g. Floudas et al. 2012, Nagy et al. 2014, Kohler et al. 2015), genome data for zygomycetes have been sparse with respect to phylogenetic depth and breadth (Gryganskyi and Muszewska 2014). These gaps in our knowledge of zygomycete evolution have manifested in a poor understanding of the homology of numerous life history traits essential to Fungi. These include characters associated with genomic, metabolic, reproductive, morphological, biochemical, and ecological traits. We attribute the limited amount of environmental data on zygomycetes to their molecular divergence, limited amplicon-based barcoding success, and paucity of well-annotated zygomycete reference data. For example, Zoopagomycotina comprises 19 genera and 228 described species worldwide, but this subphylum is only represented in GenBank by 125 DNA sequences for 17 species, 12 unnamed isolates, and seven environmental samples (NCBI nucleotide database accessed 21 Jan 2016).
Understanding zygomycete relationships from subphyla to species will provide long-awaited insight into transitions in form and function that changed as fungi colonized land, became multicellular, evolved true filamentous growth, and established intimate associations with other clades of life. A robust phylogenetic classification of zygomycetes will improve communication among biologists, ending the current use of confusing alternative names for poorly defined taxa. Here we leverage a phylogenomic approach with kingdom-wide sampling of species and genome-scale sampling of loci to resolve phylum-level relationships and propose a phylogenetic classification of the zygomycetes.
MATERIALS AND METHODS
Taxon and genome sampling.
Assembled and annotated genomes of 46 fungi were obtained from GenBank and Joint Genome Institute as part of the 1000 Fungal Genomes Project (http://1000.fungalgenomes.org) and published datasets (TABLE I). Genomes from 25 of the fungi represented all zygomycete phyla and subphyla including Mucoromycotina (12), Mortierellomycotina (2), Glomeromycota (1), Entomophthoromycotina (5), Kickellomycotina (4), and Zoopagomycotina (1). The Entomophthoromycotina fungus Pandora formica was included, but the accession is an assembled RNASeq of P. formica-infected ant and thus represents a metagenomic sample and the Zoopagomycotina fungus Piptocephalis cylindrospora was sequenced using a single-cell sequencing approach. Additional early diverging fungi included species from Chytridiomycota (6), Blastocladiomycota (2), and Cryptomycota (1). Five Ascomycota and four Basidiomycota genomes represented all major subphyla of the subkingdom Dikarya. Three outgroup species were included from the Metazoa, Choanozoa, and Ichthyosporea.
TABLE I.
List of taxa and genome data sources
| Species | GenBank accession No./JGI Web Portal/(reference) |
|---|---|
| Allomyces macrogynus ATCC 38327 v3 | ACDU00000000.1 |
| Arthrobotrys oligospora ATCC 24927 | ADOT00000000 (Yang et al. 2011) |
| Backusella circina FSU 941 | http://genome.jgi.doe.gov/Bacci1 |
| Batrachochytrium dendrobatidis JAM81 | ADAR00000000.1 |
| Basidiobolus heterosporus B8920 v1 | JNEO00000000.1 |
| Basidiobolus meristosporus CBS 931.73 | http://genome.jgi.doe.gov/Basme2finSC |
| Capsaspora owczarzaki ATCC 30864 v2 | ACFS00000000.2 (Suga et al. 2013) |
| Catenaria anguillulae PL171 | http://genome.jgi.doe.gov/Catan1 |
| Coemansia reversa NRRL 1564 | JZJC00000000 (Chang et al. 2015) |
| Conidiobolus coronatus NRRL 28638 | JXYT00000000 (Chang et al. 2015) |
| Conidiobolus thromboides FSU 785 | http://genome.jgi.doe.gov/Conth1 |
| Coprinopsis cinerea Okayama7_130 | AACS00000000.2 (Stajich et al. 2010) |
| Cryptococcus neoformans JEC21 | GCA_000149245.3 (Loftus et al. 2005) |
| Drosophila melanogaster vr6.04 | http://flybase.org (Adams et al. 2000) |
| Gonapodya prolifera JEL478 | LSZK00000000 (Chang et al. 2015) |
| Hesseltinella vesiculosa NRRL 3301 | http://genome.jgi.doe.gov/Hesve2finisherSC |
| Homoloaphlyctis polyrhiza JEL142 v1 | AFSM01000000.1 (Joneson et al. 2011) |
| Lichtheimia corymbifera FSU 9682 | CBTN000000000.1 |
| Lichtheimia hyalospora FSU 10163 | http://genome.jgi.doe.gov/Lichy1 |
| Linderina pennispora ATCC 12442 | http://genome.jgi.doe.gov/Linpe1 |
| Martensiomyces pterosporus CBS 209.56 | http://genome.jgi.doe.gov/Marpt1 |
| Monosiga brevicolis MX1 v1 | ABFJ00000000.1 (King et al. 2008) |
| Mortierella elongata AG-77 | http://genome.jgi.doe.gov/Morel2 |
| Mortierella verticillata NRRL 6337 | AEVJ00000000.1 |
| Mucor circinelloides CBS277.49 | http://genome.jgi.doe.gov/Mucci2 |
| Neurospora crassa OR74A | AABX00000000.3 (Galagan et al. 2003) |
| Orpinomyces sp. C1A | ASRE00000000.1 (Youssef et al. 2013) |
| Pandora formicae v1 | GCRV00000000.1 |
| Phycomyces blakesleeanus NRRL 1555 | http://genome.jgi.doe.gov/Phybl2 (Corrochano et al. 2016) |
| Piptocephalis cylindrospora RSA 2659 | http://genome.jgi.doe.gov/Pipcy2/Pipcy2.home.html |
| Piromyces sp. E2 | http://genome.jgi.doe.gov/PirE2_1 |
| Puccinia graminis f. sp. tritici CRL 75–36-700–3 | AAWC00000000.1 (Duplessis et al. 2011) |
| Ramicandelaber brevisporus CBS 109374 | http://genome.jgi.doe.gov/Rambr1 |
| Rhizophagus irregularis DAOM 181602 | JARB00000000.1 (Tisserant et al. 2013) |
| Rhizopus delemar RA 99–880 | AACW00000000.2 (Ma et al. 2009) |
| Rhizopus microsporus var chinensis CCTCC M201021 | CCYT00000000.1 (Wang et al. 2013) |
| Rhizopus microsporus var microsporus ATCC 52813 | http://genome.jgi.doe.gov/Rhimi1_1 |
| Rozella allomycis CSF55 | ATJD00000000.1 (James et al. 2013) |
| Saccharomyces cerevisiae S288C.vR64–2-1 | http://yeastgenome.org/(Goffeau et al. 1996) |
| Saksenaea vasiformis B4078 | JNDT00000000.1 |
| Schizosaccharomyces pombe 972h-.vASM294v2 | http://www.pombase.org/ (Wood et al. 2002) |
| Spizellomyces punctatus DAOM BR117 v1 | ACOE00000000.1 |
| Umbelopsis ramanniana NRRL 5844 | http://genome.jgi.doe.gov/Umbra1 |
| Ustilago maydis 521 v190413 | AACP00000000.2 (Kamper et al. 2006) |
| Yarrowia lipolytica CLIB122 | GCA_000002525.1 (Dujon et al. 2004) |
| Zoophthora radicans ATCC 208865 | http://genome.jgi.doe.gov/ZooradStandDraft_FD/ |
Phylogenetic analyses.
Phylogenetically informative proteins (markers) from the James et al. (2013) study of the placement of Cryptomycota and early branching fungi were used to analyze relationships. These conserved proteins were identified by comparing a pan-Eukaryotic set of species from plants, Metazoa, and Fungi. In total, 192 clusters of orthologous proteins (COPs) were aligned across the 39 eukaryotic species sampled in James et al. (2013) and built into Profile Hidden Markov Models (HMM) with TCOFFEE (Notredame et al. 2000) and HMMER3 (Eddy 2011). Each HMM was then searched against the predicted proteome from the 46 sampled species in this study with HMMSEARCH. For each marker, the highest scoring protein sequence in each species was selected by applying a significance cutoff of 1e-10 and binned to compose a file of fungal COPs for that marker. Alignments of sequences orthologous to their marker HMM were generated with HMMALIGN. The alignments were trimmed with TRIMAL (Capella-Gutiérrez et al. 2009) using the -strictplus parameter. The alignments were concatenated into a single super matrix alignment and analyzed using RAXML (Stamatakis 2006) with the ‘-f a’ fast bootstrapped tree method and 100 bootstrap replicates (FIG. 1). The PROTGAMMAAUTO option was used to determine the best model of amino acid substitution across the following models with and without empirical base frequencies: DAYHOFF, DCMUT, JTT, MTREV, WAG, RTREV, CPREV, BT, BLOSUM62, MTMAM, LG, MTART, MTZOA, PMB, HIVB, HIVW, JTTDCMUT, FLU, DUMMY, and DUMMY2. As an alternative test of the organismal tree inferred from the concatenated analysis and as a measure of potential conflict among individual sequences, a protein sequence phylogeny for each COP was inferred with RAxML using the same aforementioned parameters. The maximum likelihood tree and 100 bootstrapped trees generated by RAxML for each of the 192 individual COPs were analyzed in ASTRAL (Mirarab et al. 2014) to construct a greedy consensus tree under default settings (FIG. 2). Branch support was calculated as the percentage of bootstrap replicates that contain a particular branch. The concatenated alignment and the RAxML and ASTRAL tree files are available at TreeBASE (accession No. TB2: S18957). The individual alignments, tree files, and associated scripts are available at http://zygolife.org/home/data/.
FIG. 1.
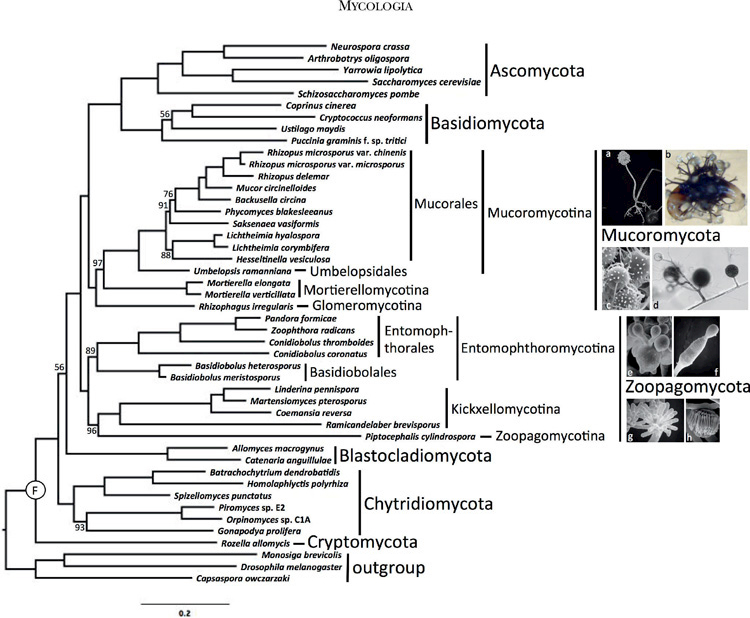
RAxML phylogenetic tree of KingdomFungi based on the concatenated alignment of 192 conserved orthologous proteins. All branches received 100%bootstrap partitions except where noted by number above or below respective branches. Example images include: a. Rhizopus sporangium(SEM). b. Phycomyces zygospore (LM). c. Mortierella chlamydospores (SEM). d. Rhizophagus spores and hyphae (LM). e. Conidiobolus secondary (replicative) conidia forming on primary conidium (SEM). f. Basidiobolus ballistosporic conidium (SEM). g. Piptocephalis merosporangia (SEM). h. Linderina merosporangium (SEM). LM: light micrograph, SEM: scanning electron micrograph.1032 MYCOLOGIA
FIG. 2.
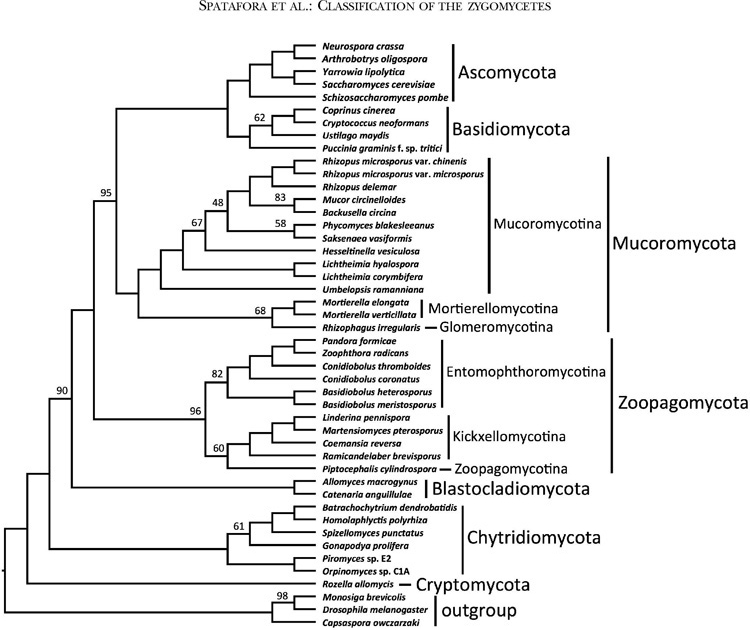
ASTRAL consensus cladogram of Kingdom Fungi based on analyses of individual bootstrap trees for each of 192 conserved orthologous proteins. All branches received 100% ASTRAL branch support except where noted by number above or below respective branches
RESULTS
The final concatenated alignment comprised 60 382 amino acid positions after trimming. Individual protein alignments ranged from 57 to 1048 positions resulting in an average alignment length of 312 positions. LG with fixed base frequencies was chosen as the best model of amino acid substitution. The inferred phylogeny from the concatenated alignment supported two clades of zygomycetes (FIG. 1). The earliest diverging lineage, which we recognize below as Zoopagomycota, comprised Entomophthoromycotina, Kickxellomycotina, and Zoopagomycotina and was recovered with 100% BP support. Despite the potential for conflict due to the mixed nature of the Pandora formica metagenomic sample and the single cell genome data from Piptocephalis, strong support was recovered for their phylum-level phylogenetic placement (FIG. 1). Entomophthoromycotina and Kickxellomycotina were supported by 89% BP and 100% BP, respectively. The clade of zygomycetes including Mucoromycotina, Mortierellomycotina, and Glomeromycota, which we recognize below as Mucoromycota, was supported by 100% BP, and it was resolved as sister to Dikarya with 100% BP. Mucoromycotina and Mortierellomycotina were both supported by 100% BP, although the latter with limited taxon sampling. The arbuscular mycorrhizal species Rhizophagus irregularis was sister to Mucoromycotina and Mortierellomycotina with 97% BP. Umbelopsis was placed outside of the core Mucorales clade with 100% BP. Internal nodes pertaining to the placement of Saksenaea and Hesseltinella within Mucorales were only moderately supported by the analyses. The phylogenetic placement of Blastocladiomycota and Chytridiomycota was not strongly supported by these analyses, and their branching order is essentially interchangeable (FIGS. 1, 2).
The ASTRAL analyses provided an additional assessment of organismal phylogeny and identified nodes that may be affected by ancient incomplete lineage sorting (FIG. 2). Despite low bootstrap values, the node placing Blastocladiomycota as sister group to the nonflagellated fungi was supported by 90% ASTRAL branch support (ABS). The clades defined below as Zoopagomycota and Mucoromycota were supported by 96% and 100% ABS, respectively, and the monophyly of Mucoromycota plus Dikarya was supported by 95% ABS. Within Zoopagomycota, lower levels of ABS characterized the placement of Piptocephalis (60%) and the branch defining Entomophthoromycotina (82%). Within Mucoromycotina, low levels of ABS characterized the placement of Rhizophagus (68%) and Hesseltinella and Saksenea within Mucorales.
TAXONOMY
Our classification follows the principles promoted in Hibbett et al.’s (2007) phylogenetic classification of Kingdom Fungi. All taxa are either demonstrated or presumed to be monophyletic and are autotypified by validly published genera. The name Zygomycota Moreau is rejected as a name for either clade of zygomycetes. Its taxonomic and nomenclatural use is in reference to the zygote, i.e. zygospore, formed through gametangial conjugation in the sexual reproductive phase. The zygospore, however, is not a synapomorphy for either clade of zygomycete fungi; rather it is a sympleisiomorphic trait inherited from the common ancestor of Zoopagomycota, Mucoromycota, and Dikarya (FIG. 1). As such, these findings support the discontinued use of Zygomycota to avoid confusion and misrepresentation of a more recent common ancestor between Zoopagomycota and Mucoromycota as opposed to Mucoromycota with Dikarya. Descriptions of new taxa follow phylogenetic nomenclature (Cantino 2010) and define the least inclusive monophyletic lineage as illustrated in a reference phylogenetic tree (FIG. 1). The classification presented here is restricted to fungi historically classified as zygomycetes, except where they have been demonstrated not to be members of Kingdom Fungi (e.g. the traditional ‘trichomycete’ orders Eccrinales and Amoebidiales; Benny and O’Donnell 2000, Cafaro 2005). Unnecessary intercalary taxa are avoided, and the classification does not treat taxa below the level of order. The proposed classification includes two phyla, six subphyla, four classes, and 16 orders (TABLE II).
TABLE II.
Phylogenetic classification of zygomycete fungi
| Mucoromycota Doweld (2001) |
| Glomeromycotina (C. Walker & A. Schüßler) Spatafora & Stajich, subphylum and stat. nov. |
| Glomeromycetes Caval.-Sm. (1998) |
| Archaeosporales C. Walker & A. Schüßler (2001) |
| Diversisporales C. Walker & A. Schüßler (2004) |
| Glomerales J. B. Morton & Benny (1990) |
| Paraglomerales C. Walker & A. Schüßler (2001) |
| Mortierellomycotina Kerst. Hoffm., K. Voigt & P.M. Kirk (2011) |
| Mortierellales Caval.-Sm. (1998) |
| Mucoromycotina Benny (2007) |
| Endogonales Moreau ex R.K. Benj. (1979) |
| Mucorales Fr. (1832) |
| Umbelopsidales Spatafora & Stajich, ord. nov. |
| Zoopagomycota Gryganskyi, M.E. Smith, Stajich & Spatafora, phylum nov. |
| Entomophthoromycotina Humber (2007) |
| Basidiobolomycetes Doweld (2001) |
| Basidiobolales Jacz. & P.A. Jacz. (1931) |
| Entomophoromycetes Humber (2012) |
| Entomophthorales G. Winter (1880) |
| Neozygitomycetes Humber (2012) |
| Neozygitales Humber (2012) |
| Kickxellomycotina Benny (2007) |
| Asellariales Manier ex Manier & Lichtw. (1978) |
| Dimargaritales R.K. Benj. (1979) |
| Harpellales Lichtw. & Manier (1978) |
| Kickxellales Kreisel ex R.K. Benj. (1979) |
| Zoopagomycotina Benny (2007) |
| Zoopagales Bessey ex R.K. Benj. (1979) |
Phylum: Mucoromycota Doweld, Prosyllabus Tracheophytorum, Tentamen systematis plantarum vascularium (Tracheophyta): LXXVII. 2001, emend. Spatafora & Stajich.
Synonym: Zygomycota F. Moreau, Encyclopédie Mycologique 23:2035. 1954 (pro parte).
Type: Mucor P. Micheli ex L. (1753).
Emendation:
Phylum Mucoromycota is emended here to apply to all descendants of the node defined in the reference phylogeny (FIG. 1) as the terminal Mucoromycota clade. It is the least inclusive clade containing Mucoromycotina, Mortierellomycotina, and Glomeromycotina. Characters associated with sexual reproductive states, where known, include zygospore production by gametangial conjugation. Asexual reproductive states can involve chlamydospores and spores produced in sporangia and sporangioles.
Commentary.
The name Mucoromycota Doweld (2001) formally specifies the group referred to as zygomycetes I in the INTRODUCTION. It is preferred to Glomeromycota C. Walker & A. Schüßler (2001) because it is more representative of the taxa that comprise the phylum. Mucoromycota shares a most recent common ancestor with Dikarya and it is characterized by plant-associated nutritional modes (e.g. plant symbionts, decomposers of plant debris, plant pathogens etc.) and only rare or derived ecological interactions with animals (e.g. primarily associated with opportunistic infections). Zygospores tend to be globose, smooth or ornamented, and produced on opposed or apposed suspensor cells with or without appendages. Asexual reproduction typically involves the production of sporangiospores in sporangia or sporangioles, or chlamydospores. Hyphae tend to be large diameter and coenocytic with the exception of the delimitation of reproductive structures by adventitious septa.
Subphylum: Glomeromycotina (C. Walker & A. Schüßler) Spatafora & Stajich, subphylum and stat. nov.
MycoBank MB816301
Replaced name: Glomeromycota C. Walker & A. Schüßler, in Schüßler et al., Mycol. Res. 105:1416. 2001.
Type: Glomus Tul. & C. Tul. 1845.
Description: Subphylum Glomeromycotina is erected here for the least inclusive clade containing Archaeosporales, Diversisporales, Glomerales, and Paraglomerales (Redecker & Schüßler 2014). Sexual reproduction is unknown and asexual reproduction is by specialized spores that resemble azygospores or chlamydospores.
Class: Glomeromycetes Caval.-Sm., Biol. Rev. 73:246. 1998. (as “Glomomycetes”).
Orders: Archaeosporales C. Walker & A. Schüßler, in Schüßler et al., Mycol. Res. 105:1418. 2001; Diversisporales C. Walker & A. Schüßler, Mycol. Res. 108:981. 2004; Glomerales J.B. Morton & Benny, Mycotaxon 37:473. 1990. (as “Glomales”); Paraglomerales C. Walker & A. Schüßler, in Schüßler et al., Mycol. Res. 105:1418. 2001.
Commentary.
Glomeromycotina includes all fungi that form arbuscular mycorrhizae and Geosiphon,a symbiont of cyanobacteria in the genus Nostoc. Sexual reproduction is unknown but supported by genome evidence (Ropars et al. 2016). Asexually formed chlamydospore-like spores are borne terminally, laterally, or intercalary on specialized hyphae. Most species produce spores directly in soil or roots, but several species in different lineages make macroscopic sporocarps (Gerdemann and Trappe 1974). Arbuscules, the site of bidirectional nutrient transfer in arbuscular mycorrhizae, are modified, highly branched haustorium-like cells that are produced in cortical plant root cells. Some taxa also produce darkly staining, intercellular, and intracellular vesicles. Species of Glomeromycotina produce coenocytic hyphae that can harbor bacterial endosymbionts (Bianciotto et al. 2003, Torres-Cortés and Ghignone 2015). These fungi were previously treated as a family within Endogonales (Glomeraceae, Gerdemann and Trappe 1974), an order within the class Zygomycetes (Glomales, Morton and Benny 1990) and as a phylum more closely related to Dikarya (Glomeromycota, Schüßler et al. 2001). Its membership in Mucoromycota is supported by genome-scale phylogenetic analyses (FIG. 1) and by gene content analyses (Tisserant et al. 2013).
Subphylum: Mortierellomycotina Kerst. Hoffm., K. Voigt & P.M. Kirk, in Hoffmann, Voigt & Kirk, Mycotaxon 115:360. 2011.
Order: Mortierellales Caval.-Sm., Biol. Rev. 73: 246. 1998.
Commentary.
Mortierellomycotina reproduce asexually by sporangia that either lack or have a highly reduced columella. Mortierella was historically classified within Mucorales, but molecular phylogenetic (Hoffmann et al. 2011) and phylogenomic analyses (Tisserant et al. 2013) rejected this hypothesis. Rather, Mortierella is best treated in its own subphylum related to Mucoromycotina and Glomeromycotina (Hoffmann et al. 2011). Molecular phylogenetic analyses reveal considerable diversity within Mortierellomycotina (Wagner et al. 2013) and environmental sampling supports a diversity of taxa associated with soils, rhizosphere, and plant roots (Summerbell 2005, Nagy et al. 2011, Shakya et al. 2013). Mortierella species are known as prolific producers of fatty acids, especially arachidonic acid (Higashiyama et al. 2002) and they frequently harbor bacterial endosymbionts (Sato et al. 2010). Most species of Mortierellomycotina only form microscopic colonies, but at least two species in the genus Modicella make multicellular sporocarps (Smith et al. 2013).
Subphylum: Mucoromycotina Benny, in Hibbett et al., Mycol. Res. 111:517. 2007.
Orders: Endogonales Moreau ex R.K. Benj., in Kendrick, ed., Whole Fungus 2:599. 1979. Emend. Morton & Benny, Mycotaxon 37:473. 1990; Mucorales Fr., Syst. Mycol. 3:296. 1832; Umbelopsidales Spatafora, Stajich & Bonito, ord. nov.
Commentary.
Mucoromycotina has the largest number of described species of Mucoromycota and includes the well-known model species Mucor mucedo and Phycomyces blakesleeanus. It also includes industrially important species of Rhizopus and other genera. Where known, sexual reproduction within Mucoromycotina is by prototypical zygospore formation and asexual reproduction typically involves the copious production of sporangia and/or sporangioles. Species are frequently isolated from soil, dung, plant debris, and sugar-rich plant parts (e.g. fruits). As such, fungi in the Mucoromycotina represent the majority of zygomycetous fungi in pure culture. Endogonales includes both ectomycorrhizal and saprobic species (Bidartondo et al. 2011). Sexual reproduction involves the production of zygospores by apposed gametangia within a simple, often sequestrate or enclosed sporocarp that may be hypogeous, embedded in heavily decayed wood, or produced among foliage of mosses or liverworts. Recent studies suggest that ectomycorrhizae have probably evolved twice within Endogonales (Tedersoo and Smith 2013). Endogonales represents an independent origin of mycorrhizae relative to the arbuscular mycorrhizae of Glomeromycotina and ectomycorrhizae of Dikarya (Bidartondo et al. 2011, Tedersoo and Smith 2013, Dickie et al. 2015) and like many of Mucoromycota, they harbor endohyphal bacteria (Desiro et al. 2014).
Order: Umbelopsidales Spatafora, Stajich & Bonito, ord. nov.
MycoBank MB816302
Type: Umbelopsis Amos & H.L. Barnett (1966)
Description: Umbelopsidales is erected here to apply to all descendants of the node defined in the reference phylogeny (FIG. 1) as the terminal Umbelopsidales clade. It is the least inclusive clade containing the genus Umbelopsis. Asexual reproduction is by sporangia and chlamydospores. Sporangiophores may be branched in a cymose or verticillate fashion. Sporangia are typically pigmented red or ochre, multi-or single-spored and with or without conspicuous columella. Sporangiospores are globose, ellipsoidal, or polyhedral and pigmented like sporangia. Chlamydospores are filled with oil globules and often abundant in culture. Sexual reproduction is unknown.
Commentary:
Species in the Umbelopsidales were previously classified in Mucorales (e.g. U. isabellina) or Mortierellales (e.g. Micromucor [5Umbelopsis] ramanniana). Phylogenetic analyses of genome-scale data resolve this as a distant sister group to Mucorales, consistent with ordinal status. Like Mortierellales, species of Umbelopsidales are frequently isolated from rhizosphere soils, with increasing evidence that these fungi occur as root endophytes (Hoff et al. 2004, Terhonen et al. 2014).
Phylum: Zoopagomycota Gryganskyi, M.E. Smith, Spatafora & Stajich, phylum nov.
MycoBank MB816300
Synonym: Zygomycota F. Moreau, Encyclopédie Mycologique 23:2035. 1954 (pro parte).
Type: Zoopage Drechsler (1935).
Description: Phylum Zoopagomycota is erected here to apply to all descendants of the node defined in the reference phylogeny (FIG. 1) as the terminal Zoopagomycota clade. It is the least inclusive clade containing Entomophthoromycotina, Kickellomycotina, and Zoopagomycotina. Sexual reproduction, where known, involves the production of zygospores by gametangial conjugation. Morphologies associated with asexual reproductive states include sporangia, merosporangia, conidia, and chlamydospores.
Commentary.
Zoopagomycota represents the earliest diverging clade of zygomycetous fungi and formally applies to the group referred to as zygomycetes II in the INTRODUCTION. It comprises three subphyla in which associations with animals (e.g. pathogens, commensals, mutualists) form a common ecological theme, although species from several lineages are mycoparasites (e.g. Syncephalis, Piptocephalis, and Dimargaritales). Because of its broader and more inclusive meaning, the name Zoopagomycota (Gr.: zoo = animal, pago = frozen, ice or unite) is preferred to other possible names for the clade including Trichomycota R.T. Moore (1994), Basidiobolomycota Doweld (2001), Entomophthoromycota Humber (2012), and Harpellomycota Doweld (2013). All of these alternative names were originally proposed to refer to a particular clade within Zoopagomycota; therefore, use of these alternative names would probably cause confusion. Although some of the fungi in Zoopagomycota can be maintained in axenic culture, most species are more difficult to maintain in pure culture than species of Mucoromycota. Accordingly, species of Zoopagomycota are most frequently observed growing in association with a host organism. Haustoria are produced by some of the animal pathogens and mycoparasites. Zoopagomycota hyphae may be compartmentalized by septa that may be complete or uniperforate; in the latter, bifurcate septa contain electron opaque lenticular plugs. Zygospore formation typically involves modified hyphal tips, thallus cells, or hyphal bodies (yeast-like cells) that function as gametangia.
Subphylum: Entomophthoromycotina Humber, in Hibbett et al. Mycol. Res. 111:517. 2007.
Synonym: Entomophthoromycota Humber, Mycotaxon 120:481. 2012.
Classes: Basidiobolomycetes Doweld, Prosyllabus Tracheophytorum, Tentamen systematis plantarum vascularium (Tracheophyta): LXXVII. 2001; Entomophthoromycetes Humber, Mycotaxon 120:486. 2012; Neozygitomycetes Humber, Mycotaxon 120:485. 2012. Orders: Basidiobolales Jacz. & P.A. Jacz., Opredelitel’ Gribov, (edn 3) I Ficomiţeti (Leningrad):8. 1931; Entomophthorales G. Winter, Rabenh. Krypt.-Fl. 1:74. 1880; Neozygitales Humber, Mycotaxon 120:486. 2012.
Commentary.
Entomophthoromycotina includes three classes and three orders of saprobic and insect pathogenic fungi. The thallus may consist of coenocytic or septate hyphae, which may fragment to form hyphal bodies, or it may comprise only hyphal bodies. Asexual reproduction is by conidiogenesis from branched or unbranched conidiophores; primary conidia are forcibly discharged and secondary conidia are either forcibly or passively released. Sexual reproduction involves the formation of either zygospores by gametangial copulation, involving hyphal compartments or hyphal bodies (Humber 2012).
Subphylum: Kickxellomycotina Benny, in Hibbett et al. Mycol. Res. 111:518. 2007.
Synonym: Trichomycota R.T. Moore, Identification and Characterization of Pest Organisms:250. 1994 (pro parte).
Orders: Asellariales Manier ex Manier & Lichtw., Mycotaxon 7:442. 1978; Dimargaritales R.K. Benj., in Kendrick (ed.), Whole Fungus 2:607. 1979; Harpellales Lichtw. & Manier, Mycotaxon 7:441. 1978; Kickxellales Kreisel ex R.K. Benj., in Kendrick (ed.), Whole Fungus 2:610. 1979; R.K. Benj., in Kendrick, ed., Whole Fungus 2:607. 1979.
Commentary.
Mycelium is regularly divided into compartments by bifurcate septa that often have lenticular occlusions. Sexual reproduction involves the formation of variously shaped zygospores by gametangial conjugation of relatively undifferentiated sexual hyphal compartments (Lichtwardt 1986). Sporophores may be produced from septate, simple, or branched somatic hyphae. Asexual reproduction involves the production of uni- or multispored merosporangia arising from a specialized vesicle (i.e. sporocladium), sporiferous branchlets, or an undifferentiated sporophore apex. Species may be saprobes, mycoparasites, and symbionts of insects; the latter includes Harpellales that are typically found within the hindguts of aquatic life history stages.
Subphylum: Zoopagomycotina Benny, in Hibbett et al. Mycol. Res. 111:518. 2007.
Order: Zoopagales Bessey ex R.K. Benj., in Kendrick, ed., Whole Fungus 2:590. 1979.
Commentary.
Zoopagomycotina include mycoparasites and predators or parasites of small invertebrates and amoebae. The hyphal diameter is characteristically narrow in thalli that are branched or unbranched; sometimes specialized haustoria are produced in association with hosts. Only a handful of species have been successfully maintained in axenic culture. Sexual reproduction, where known, is by gametangial conjugation, forming globose zygospores on apposed differentiated or undifferentiated suspensor cells (Dreschler 1935). Asexual reproduction is by arthrospores, chlamydospores, conidia, or multispored merosporangia that may be simple or branched.
DISCUSSION
Overview of Kingdom Fungi.
In the concatenated RAxML analyses, we resolve and recognize seven clades that we classify as phyla of Kingdom Fungi (FIG. 1), with zoosporic fungi comprising the three earliest diverging lineages. Cryptomycota, represented by the genus Rozella, is the earliest diverging lineage of Fungi followed by Chytridiomycota and Blastocladiomycota. The branching order of the latter two taxa is weakly supported and both have been resolved as sharing a most recent common ancestor (MRCA) with the nonflagellated fungi of Zoopagomycota, Mucoromycota, and Dikarya (James et al. 2006, Chang et al. 2015). Within Chytridiomycota we recognize three classes, including Chytridiomycetes Caval.-Sm. (1998), Monoblepharidomycetes J.H. Schaffner (1909), and Neocallimastigomycetes M.J. Powell (2007). The remaining phyla of Fungi include the nonflagellated phyla Zoopagomycota, Mucoromycota, Basidiomycota, and Ascomycota. Because to the absence of genomic data, we could not assess the validity of the newly erected phylum Entorrhizomycota (Bauer et al 2015).
The 192 protein clusters incorporated into these analyses are encoded by single to low-copy genes that are conserved throughout eukaryotes (James et al. 2013). As such, these genes tend to be ubiquitously distributed in Fungi and arguably less susceptible to errors associated with orthology assignment. The interpretation of bootstrap support for branches in genome-scale phylogenies is still poorly understood given that some genes within a genome may have different evolutionary histories (e.g. Salichos et al. 2014). We attempted to alleviate this problem through the use of conservative orthologs, but we cannot currently discount issues associated with ancient lineage sorting events, whole genome duplications, and inadvertent biases associated with taxon sampling (e.g. unsampled taxa, extinction events, etc.). In an attempt to characterize the effect of ancient lineage sorting events, ASTRAL analyses were performed on the bootstrap trees derived from the RAxML analyses of each protein sequence alignment. The placement of Blastocladiomycota as sister group to the nonflagellated lineages of Kingdom Fungi was supported by 56% BP and 90% ABS values, suggesting that the node is not characterized by high levels of ancient incomplete lineage sorting but low levels of phylogenetic signal present in the current dataset; a finding consistent with the results of Chang et al. (2015). The effect of adding taxa to fill the gaps among unsampled lineages is more difficult to predict, but it is reasonable to assume that it might increase support for long, relatively isolated branches, such as Blastocladiomycota (Wiens and Morrill 2011). At this time we consider the placement of Blastocladiomycota unresolved.
Paraphyly of zygomycetes and support for major clades.
Both the concatenated RAxML (FIG. 1) and the ASTRAL (FIG. 2) analyses reject zygomycete monophyly and resolve two clades, Zoopagomycota and Mucoromycota, which form a paraphyletic grade from which Dikarya are derived. Although this finding is consistent with rDNA analyses (White et al. 2006) and multigene phylogenies (James et al. 2006, Chang et al. 2015), it provides greater clarity on clade membership and relationship to other major clades of Kingdom Fungi. By not resurrecting the abandoned name Zygomycota Moreau, we propose names for each of the two monophyletic phyla and we expand the use of autotypification based on validly published genera as espoused by Hibbett et al. (2007). Because the International Code for algae, fungi, and plants (McNeill et al. 2012) does not require adherence to the principle of priority above the rank of family, we have selected names that communicate taxa or traits that are characteristic of the majority of species contained within the two phyla. In addition, the names Zoopagomycota and Mucoromycota avoid taxonomic confusion stemming from previous use of other names that are linked to alternative evolutionary hypotheses. For example, Glomeromycota has been used over the last 15 y to refer to the monophyletic group of arbuscular mycorrhizal fungi (Schüßler et al. 2001); the use of this name for a wider group of fungi would likely be problematic and confusing. Finally, we recognize the minimum number of phylum-level clades necessary to name monophyletic clades of zygomycetes to produce a classification system that is easier to teach and reduces the use of redundant taxa.
Zoopagomycota is resolved as the earliest diverging lineage of zygomycetes. Although genomic sampling included representatives from all three subphyla, a further increase in taxon sampling will undoubtedly reveal additional phylogenetic diversity. Kickxellomycotina is represented by four taxa that are all from Kickxellales. Entomophthoromycotina is represented by five taxa, three from Entomophthorales (Conidiobolus spp., Pandora formicae, Zoophthora radicans) and two from Basidiobolales (B. heterosporus and B. meristosporus). Branch support (BP 5 89, ABS 5 82) for Entomophthoromycotina is the lowest of the subphyla, which is in part a result of the topological instability of Basidiobolus. This finding is similar to observations in previous multigene studies (Gryganskyi et al. 2012) and suggests that more robust support for the placement of Basidiobolus will not be achieved by the addition of sequence data alone but will instead require additional taxon sampling, consideration of episodic events associated with rare genomic changes, and possibly the use of models of evolution that are not strictly bifurcating (Than et al. 2008). The sole representative of Zoopagomycotina is Piptocephalis cylindrospora, for which the sequence data were generated based on single-cell genomics methods (Rinke et al. 2013). Its membership in Zoopagomycota is strongly supported by these analyses, but its placement within the phylum is less well supported (MLBS 5 96, ABS 5 60). This is possibly a consequence of the nature of the data from single-cell sequencing and sparse taxon sampling for the subphylum. As most species of Zoopagomycotina are obligate symbionts, additional sampling will require the use of advanced equencing and computational techniques, use of dual-organism cultures and novel approaches to establish axenic cultures.
Mucoromycota is resolved as the clade of zygomycetes that diverged most recently from a shared ancestor with Dikarya. The most significant change from previous molecular-based classifications of zygomycetes (Schüßler et al. 2001) is the inclusion of Glomeromycotina in Mucoromycota. Although Glomeromycotina (=Glomeromycota) was previously resolved as more closely related to Dikarya than Mucoromycotina and Mortierellomycotina using nuclear SSU rDNA and multigene sequence data (Schüßler et al. 2001, James et al. 2006), this was not supported by the present analyses. Rather, the topology presented here is consistent with recent mitochondrial phylogenies (Nadimi et al. 2012, Pelin et al. 2012), genome-scale phylogenies, and gene content analyses (Tisserant et al. 2013, Chang et al. 2015), as well as with traditional morphology-based classifications (Gerdemann and Trappe 1974, Morton and Benny 1990). As in previous studies (Chang et al. 2015), the position of Glomeromycotina is equivocal and it appears alternatively as the earliest diverging lineage of the Mucoromycota (FIG. 1, MLBS =97) or as a sister group to Mortierellomycotina (FIG. 2, ABS = 68). Mortierellomycotina is represented by the genomes of two species of Mortierella; their placement is consistent with being phylogenetically distinct from Mucoromycotina. Because of the ease of their maintenance in axenic culture, the strongly supported Mucoromycotina is sampled more and is represented by 11 taxa, two orders, and eight families. Although represented only by a single taxon, Umbelopsidales is supported as the sister clade to Mucorales, a finding consistent with multigene phylogenetic analyses (Sekimoto et al. 2011, Hoffmann et al. 2013). Suggestive of phylogenetic conflict among protein-sequence trees within the Mucorales, several nodes within the order are resolved differently between the RAxML and ASTRAL analyses. Expanding the sampling density throughout the Mucoromycota is needed to better understand processes underlying molecular evolution (e.g. possible genome duplications) around these potentially problematic nodes.
Evolution of host association and nutritional modes.
Our phylogenomic analysis shows a striking contrast between the host associations and trophic modes of Zoopagomycota and Mucoromycota (TABLE III). Most species of Zoopagomycota are pathogens, parasites, or commensals of animals and other fungi, whereas a few species are considered to be more generalized saprobes (Benny et al. 2014). Associations with living plants are rare for the phylum. In contrast, Mucoromycota includes multiple mycorrhizal lineages (Glomeromycotina, Endogonales; Bidartondo et al. 2011, Redecker and Schüßler 2014), root endophytes (Mortierellomycotina, Umbelopsidales; Hoff et al. 2004, Summerbell 2005, Terhonen et al. 2014) and decomposers of plant-based carbon sources (Mucorales; Benny et al. 2014). Members of both Mucoromycotina and Glomeromycotina can also form mycorrhiza-like relationships with nonvascular plants (Field et al. 2015a). All species of Mucoromycotina known as mycoparasites (e.g. Spinellus fusiger, Syzygites megalocarpa) or putative parasites of arthropods (e.g. Sporodiniella umbellata) are evolutionarily derived and closely related to saprobes (Hoffman et al. 2013). In rare cases when species in Mucoromycota infect humans or other animals, they are interpreted as opportunistic pathogens, typically of immunocompromised individuals.
TABLE III.
Taxonomic distribution of selected morphological and ecological characters of zygomycete fungi
| Zoopagomycotina | Kickxellomycotina | Entomophthoromycotina | Mucoromycotina | Mortierellomycotina | Glomeromycotina | |
|---|---|---|---|---|---|---|
| Sexual Reproduction | Zygospore | Zygospore | Zygospore | Zygospore | Zygospore | Unknown |
| Asexual Reproduction | Sporangia, conidia | Trichospores, sporangia, merosporangia |
Conidia | Sporangia, sporangioles |
Sporangia | Chlamydospore-like |
| Hyphae | Coenocytic | Bifurcate septa w/ lenticular plug |
Complete septa, bifurcate septa, or coenocytic; hyphal bodies |
Coenocytic | Coenocytic | Coenocytic |
| MTOCa | —b | Centriole-like | Centriole-like | Spindle pole body | — | — |
| Hyphal tip structure | — | AVCc | Spitzenkörper | AVC | AVC | AVC |
| Fruiting body production | Absent | Absent | Absent | Present (rare) | Present (rare) | Present (rare) |
| Major host/substrate | Amoeba, animal, fungi |
Animal, fungi | Animal | Plant | Plant | Plant |
Microtubular Organizing Center.
Unsampled.
Apical Vesicle Crescent.
The phylogenetic distribution of these nutritional associations illuminates two elements of fungal evolution that shape the development of evolutionary hypotheses of early diverging fungi. First, deep divergences among Zoopagomycota point to an early origin for animal-and fungus-associated nutritional relationships. Ancient associations with animals, other fungi, and non-plant organisms are poorly documented in the known fossil record (Taylor et al. 2014) and our results predict hidden fungal associations yet to be detected through analysis of animal fossils. The second major point of emphasis from these analyses is the sister-group relationship of Mucoromycota and Dikarya and the diversification of fungi in association with land plants. Dikarya clearly diversified with land plants in terrestrial ecosystems (Selosse and Le Tacon 1998, Berbee 2001). It is now reasonable to consider that nutrition from land plants had a deeper origin in fungal evolutionary history, extending back to the common ancestor of Mucoromycota and Dikarya. This is consistent with studies that considered ancient fungal relationships with algae and the land plant lineage (Chang et al. 2015, Field et al. 2015a). Furthermore, it is consistent with the record of fossil fungi from some of the earliest 407 million year old land plants. Such fossils include arbuscules characteristic of the Glomeromycotina (Glomites rhyniensis; Taylor et al. 1995), swellings and hyphae reminiscent of Mucoromycotina (Strullu-Derrien et al. 2014) and sporocarps suggestive of Dikarya (Paleopyrenomycites devonicus; Taylor et al. 2005). It has been hypothesized that symbioses with heterotrophic fungi played a role the evolution of land plants (Bidartondo et al. 2011, Field et al. 2015b). Our results specify the plant-associated, terrestrial MRCA of Mucoromycota plus Dikarya as the species that gave rise to independent and parallel origins of important plant-fungal symbioses from endophytes to mycorrhizae.
Evolution of morphology.
Interpretation of morphology in the context of this genome-scale phylogeny highlights the importance of Zoopagomycota, Mucoromycota, and their MRCA in understanding the evolution of fungal traits associated with the flagellum, hyphae, reproduction, and multicellularity. We provide a brief summary of these traits with an emphasis on development and refinement of evolutionary hypotheses, but direct readers to more comprehensive treatments for detailed discussions (Humber 2012, Benny et al. 2014, Redecker and Schüßler 2014, McLaughlinet al. 2015).
Although these analyses resolve a single loss of the flagellum in the MRCA of Zoopagomycota, Mucoromycota, and Dikarya, it should be noted that numerous lineages were not sampled here and their inclusion would indicate additional losses of the flagellum among early diverging fungi. Microsporidia are sister group to Cryptomycota and represent the loss of the flagellum in the earliest diverging lineage of Fungi (James et al. 2013). Similarly, Hyaloraphidium is a nonflagellated member of Chytridiomycota and represents a loss of the flagellum among the core clade of zoosporic fungi (James et al. 2006). Relevant to the zygomycete fungi is Olpidium, a genus of zoosporic fungi that has been hypothesized to be closely related to Zoopagomycota based on multigene phylogenies (Sekimoto et al. 2001, James et al. 2006). Analysis of genomic data for this genus is crucial to more accurately estimate the number of losses of flagellum, their placement on the fungal tree of life, and to test alternative hypotheses of a single loss of the flagellum (Liu et al. 2006). Furthermore, the placement of Zoopagomycota as the earliest diverging lineage of nonflagellated fungi is intriguing because some of its species have retained what may be relicts of a flagellum in the form of cylindrical, centriole-like organelles. Centriole-like organelles are associated with the nuclei of Basidiobolus of Entomophthoromycotina (McKerracher and Heath 1985, Roberson et al. 2011) and Coemansia of Kickxellomycotina (McLaughlin et al. 2015). In contrast to these centriole-like organelles, Mucoromycotina and Dikarya share discoidal to hemispherical spindle pole bodies. Although spindle pole bodies function as microtubule organizing centers, as do centrioles, they lack any obvious remnant of the centrioles’ characteristic 9+2 microtubule arrangement (reviewed in McLaughlin et al. 2015). Broader analyses are needed, but the distribution of putative relict centrioles is consistent with flagellum loss occurring shortly before or during the diversification of Zoopagomycota.
Hyphae vary among species and clades in Mucoromycota and Zoopagomycota. Species of Zoopagomycotina typically produce small diameter coenocytic hyphae and haustoria in association with parasitism of hosts. Species of Kickxellomycotina produce hyphae that are regularly compartmentalized by uniperforate, bifurcate septa occluded by lenticular plugs (Jeffries and Young 1979, Saikawa 1989). Species of Entomophthoromycotina produce either coenocytic hyphae, hyphae with complete septa that may disarticulate into one or two-celled hyphal bodies (reviewed in Humber 2012), or with septa similar to those of Kickxellomycotina (Saikawa 1989). Species of Mucoromycotina and Mortierellomycotina produce large diameter, coenocytic hyphae characteristic of textbook zygomycetes, as do Glomeromycotina, which in addition make highly branched, narrow hyphal arbuscules in host cells. Where septations do occur in Mucoromycota they tend to be adventitious and formed at the base of reproductive structures.
The Spitzenkörper is associated with hyphal growth in Dikarya but has been elucidated for only a few species of zygomycetes. Roberson et al. (2011) documented an apical spherical organization of microvesicles in Basidiobolus (Zoopagomycota) consistent with a Spitzenkörper. In contrast, hyphae of Coemansia (Zoopagomycota) and Gilbertella, Mortierella, and Mucor (Mucoromycota) (Fisher and Roberson 2016) and the germ tubes of Gigaspora (Mucoromycota) (Bentivenga et al. 2013) lack a classical Spitzenkörper, but instead possess a hemispherical organization of vesicles, the apical vesicle crescent, which in some taxa has been demonstrated to be mandatory for hyphal growth (Fisher and Roberson 2016).
Asexual reproduction by sporangia is present in all subphyla of Zoopagomycota and Mucoromycota with three notable exceptions (Benny et al. 2014). Entomophthoromycotina is characterized by the production of conidia with the formation of forcibly discharged primary conidia that may undergo germination to form passively dispersed secondary conidia (Humber 2012). Conidia are also described for species in Zoopagomycotina that are pathogenic to amoebae and nematodes (Dreschler 1935, 1936), but mycoparasitic lineages produce reduced sporangia, sporangioles, and merosporangia (Benny et al. 2014). Presumably, conidiogenesis in Zoopagomycota and Dikarya arose independently, but closer analysis may yet reveal homologies at the level of molecular development. Glomeromycotina are known to reproduce only asexually via unique spores that resemble chlamydospores or azygospores.
Where sexual reproduction is known in species of both Zoopagomycota and Mucoromycota, it is by the formation of zygospores via gametangial conjugation (Drechsler 1935, Lichtwardt 1986, Humber 2012). In Mucoromycota, sexual reproduction is under the control of mating type genes, sexP and sexM, which regulate the production of pheromones required for the maturation of hyphae into gametangia (Idnurm et al. 2008) and confer + and – mating-type identity, respectively (reviewed in Lee et al. 2010). Recent genomic studies have revealed numerous mating genes in the genomes of Glomeromycotina (Riley et al. 2013) and a Dikarya-like mating processes in R. irregularis (Ropars et al. 2016), suggesting that they may have a cryptic sexual cycle. In Zoopagomycota, the genetic basis and physiological control of mating has not been characterized. From commonalities across fungal phyla (Cassleton 2008), we assume that genetic systems in Zoopagomycota and Mucoromycota might be similar, but detailed studies are needed.
Multicellular sporocarps are not produced by Zoopagomycota and though rare, they are present within Mucoromycota through independent origins in Endogone (Mucoromycotina; Bidartondo et al. 2011), Modicella (Mortierellomycotina; Smith et al. 2013) and as aggregations of spore-producing hyphae and spores in species of Glomeromycotina (Gerdemann and Trappe 1974, Redecker and Schüßler 2014). Along with the multicellular sporocarps in Agaricomycotina (Basidiomycota) and Pezizomycotina (Ascomycota), multicellular sporocarps within Mucoromycota have been derived independently, suggesting that while the genetic and metabolic potential for complex thallus diversity did not arise until the MRCA of Mucoromycota and Dikarya, it then resulted in multiple independent origins of complex spore-producing structures involving hyphal differentiation (Stajich et al. 2009).
ACKNOWLEDGMENTS
This paper is dedicated to our colleague and coauthor Thomas N. Taylor who passed away during the final preparation of this manuscript. The authors thank the following persons for access to unpublished genomes: Santiago Torres Martínez for Mucor circinelloides, Teresa Pawlowska and Stephen Mondo for Rhizopus microsporus var. microsporus, Vincent Bruno for Basidiobolus heterosporus and Saksenaea vasiformis, and Francis Martin for Mortierella elongata. This material is based upon work supported by the National Science Foundation (DEB-1441604 to JWS, DEB-1441715 to JES, DEB-1441677 to TYJ, DEB-1441728 to RWR), the French National Research Agency through the Laboratory of Excellence ARBRE (grant No. ANR–11–LBX–0002–01 to JWS) and the Canadian National Science and Engineering Research Council grants (412318–11 and 138427–11 to MLB). Any opinions, findings and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. USDA is an equal opportunity provider and employer.
Contributor Information
Joseph W. Spatafora, Department of Botany and Plant Pathology, Oregon State University, Corvallis, Oregon 97331.
Ying Chang, Department of Botany and Plant Pathology, Oregon State University, Corvallis, Oregon 97331.
Gerald L. Benny, Department of Plant Pathology, University of Florida, Gainesville, Florida 32611
Katy Lazarus, Department of Plant Pathology, University of Florida, Gainesville, Florida 32611.
Matthew E. Smith, Department of Plant Pathology, University of Florida, Gainesville, Florida 32611
Mary L. Berbee, Department of Botany, University of British Columbia, Vancouver, British Columbia, V6T 1Z4 Canada
Gregory Bonito, Department of Plant, Soil, and Microbial Sciences, Michigan State University, East Lansing, Michigan 48824.
Nicolas Corradi, Department of Biology, University of Ottawa, Ottawa, Ontario, K1N 6N5 Canada.
Igor Grigoriev, US Department of Energy (DOE) Joint Genome Institute, 2800 Mitchell Drive, Walnut Creek, California 94598.
Andrii Gryganskyi, L.F. Lambert Spawn Co., Coatesville, Pennsylvania 19320.
Timothy Y. James, Department of Ecology and Evolutionary Biology, University of Michigan, Ann Arbor, Michigan 48103
Kerry O’Donnell, Mycotoxin Prevention and Applied Microbiology Research Unit, NCAUR-ARS-USDA, 1815 N. University Street, Peoria, Illinois 61604.
Robert W. Roberson, School of Life Sciences, Arizona State University, Tempe, Arizona 85287
Thomas N. Taylor, Department of Ecology and Evolutionary Biology, and Natural History Museum and Biodiversity Research Center, University of Kansas, Lawrence, Kansas 66045.
Jessie Uehling, Biology Department, Box 90338, Duke University, Durham, North Carolina 27708.
Rytas Vilgalys, Biology Department, Box 90338, Duke University, Durham, North Carolina 27708.
Merlin M. White, Department of Biological Sciences, Boise State University, Boise, Idaho 83725
Jason E. Stajich, Department of Plant Pathology & Microbiology and Institute for Integrative Genome Biology, University of California–Riverside, Riverside, California 92521
LITERATURE CITED
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, George RA, Lewis SE, Richards S, Ashburner M, Henderson SN, Sutton GG, Wortman JR, Yandell MD, Zhang Q, Chen LX, Brandon RC, Rogers YH, Blazej RG, Champe M, Pfeiffer BD, Wan KH, Doyle C, Baxter EG, Helt G, Nelson CR, Gabor GL, Abril JF, Agbayani A, An HJ, Andrews-Pfannkoch C, Baldwin D, Ballew RM, Basu A, Baxendale J, Bayraktaroglu L, Beasley EM, Beeson KY, Benos PV, Berman BP, Bhandari D, Bolshakov S, Borkova D, Botchan MR, Bouck J, Brokstein P, Brottier P, Burtis KC, Busam DA, Butler H, Cadieu E, Center A, Chandra I, Cherry JM, Cawley S, Dahlke C, Davenport LB, Davies P, de Pablos B, Delcher A, Deng Z, Mays AD, Dew I, Dietz SM, Dodson K, Doup LE, Downes M, Dugan-Rocha S, Dunkov BC, Dunn P, Durbin KJ, Evangelista CC, Ferraz C, Ferriera S, Fleischmann W, Fosler C, Gabrielian AE, Garg NS, Gelbart WM, Glasser K, Glodek A, Gong F, Gorrell JH, Gu Z, Guan P, Harris M, Harris NL, Harvey D, Heiman TJ, Hernandez JR, Houck J, Hostin D, Houston KA, Howland TJ, Wei MH, Ibegwam C, Jalali M, Kalush F, Karpen GH, Ke Z, Kennison JA, Ketchum KA, Kimmel BE, Kodira CD, Kraft C, Kravitz S, Kulp D, Lai Z, Lasko P, Lei Y, Levitsky AA, Li J, Li Z, Liang Y, Lin X, Liu X, Mattei B, McIntosh TC, McLeod MP, McPherson D, Merkulov G, Milshina NV, Mobarry C, Morris J, Moshrefi A, Mount SM, Moy M, Murphy B, Murphy L, Muzny DM, Nelson DL, Nelson DR, Nelson KA, Nixon K, Nusskern DR, Pacleb JM, Palazzolo M, Pittman GS, Pan S, Pollard J, Puri V, Reese MG, Reinert K, Remington K, Saunders RD, Scheeler F, Shen H, Shue BC, Sidén-Kiamos I, Simpson M, Skupski MP, Smith T, Spier E, Spradling AC, Stapleton M, Strong R, Sun E, Svirskas R, Tector C, Turner R, Venter E, Wang AH, Wang X, Wang ZY, Wassarman DA, Weinstock GM, Weissenbach J, Williams SM, Woodage T, Worley KC, Wu D, Yang S, Yao QA, Ye J, Yeh RF, Zaveri JS, Zhan M, Zhang G, Zhao Q, Zheng L, Zheng XH, Zhong FN, Zhong W, Zhou X, Zhu S, Zhu X, Smith HO, Gibbs RA, Myers EW, Rubin GM, Venter JC. The genome sequence of Drosophila melanogaster. Science 287:2185–2195, doi: 10.1126/science.287.5461.2185 [DOI] [PubMed] [Google Scholar]
- Bauer R, Garnica S, Oberwinkler F, Reiss K, Weiß M, Begerow D. 2015. Entorrhizomycota: a new fungal phylum reveals new perspectives on the evolution of fungi. PLoS One 10:e0128183, doi: 10.1371/journal.pone.0128183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer R, Humber RA, Voigt K. 2014. Zygomycetous fungi: phylum Entomophthoromycota and subphyla Kickxellomycotina, Mortierellomycotina, Mucoromycotina, and Zoopagomycotina. In: McLaughlin DJ, Spatafora JW, eds. Systematics and evolution. Part A New York: Springer-Verlag. The Mycota; VII:209–250. [Google Scholar]
- Benny GL, Humber RA, Voigt K. 2014. Zygomycetous fungi: phylum Entomophthoromycota and subphyla Kickxellomycotina, Mortierellomycotina, Mucoromycotina, and Zoopagomycotina. In: McLaughlin DJ, Spatafora JW, eds. Mycota VII, part A. Systematics and evolution New York: Springer-Verlag; p 209–250. [Google Scholar]
- Benny GL, O’Donnell K 2000. Amoebidium parasiticum is a protozoan, not a Trichomycete. Mycologia 92:1133–1137, doi: 10.2307/3761480 [DOI] [Google Scholar]
- Bentivenga SP, Kumar TKA, Kumar L, Roberson RW, McLaughlin DJ. 2013. Cellular organization in germ tube tips of Gigaspora and its phylogenetic implications. Mycologia 105:1087–1099, doi: 10.3852/12-291 [DOI] [PubMed] [Google Scholar]
- Berbee ML. 2001. The phylogeny of plant and animal pathogens in the Ascomycota. Physiol Mol Plant Pathol 59:165–187, doi: 10.1006/pmpp.2001.0355 [DOI] [Google Scholar]
- Berbee ML, Taylor JW. 2010. Dating the molecular clock in fungi—how close are we? Fungal Biol Rev 24:1–16, doi: 10.1016/j.fbr.2010.03.001 [DOI] [Google Scholar]
- Bianciotto V, Lumini E, Bonfante P, Vandamme P. 2003. “Candidatus Glomeribacter gigasporarum” gen. nov., sp. nov., an endosymbiont of arbuscular mycorrhizal fungi. Int J Syst Evol Micrbiol 53:121–124, doi: 10.1099/ijs.0.02382-0 [DOI] [PubMed] [Google Scholar]
- Bidartondo MI, Read DJ, Trappe JM, Merckx V, Ligrone R, Duckett JG. 2011. The dawn of symbiosis between plants and fungi. Biol Lett 7:574–577, doi: 10.1098/rsbl.2010.1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafaro MJ. 2005. Eccrinales (Trichomycetes) are not fungi, but a clade of protists at the early divergence of animals and fungi. Mol Phylogenet Evol 35:21–34, doi: 10.1016/j.ympev.2004.12.019 [DOI] [PubMed] [Google Scholar]
- Cantino P 2010. International Code of phylogenetic nomenclature 102 p. [Google Scholar]
- Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T. 2009. TrimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25: 1972–1973, doi: 10.1093/bioinformatics/btp348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casselton LA. 2008. Fungal sex genes—searching for the ancestors. Bioessays 30:711–714, doi: 10.1002/bies.20782 [DOI] [PubMed] [Google Scholar]
- Chang Y, Wang S, Sekimoto S, Aerts AL, Choi C, Clum A, LaButti KM, Lindquist EA, Yee Ngan C, Ohm RA, Salamov AA, Grigoriev IV, Spatafora JW, Berbee ML. 2015. Phylogenomic analyses indicate that early fungi evolved digesting cell walls of algal ancestors of land plants. Genome Biol Evol 7:1590–1601, doi: 10.1093/gbe/evv090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrochano LM, Kuo A, Marcet-Houben M, Polaino S, Salamov A, Villalobos-Escobedo JM, Grimwood J, Álvarez MI, Avalos J, Bauer D, Benito EP, Benoit I, Burger G, Camino LP, Cánovas D, Cerdá-Olmedo E, Cheng JF, Domínguez A, Eliáš M, Eslava AP, Glaser F, Gutiérrez G, Heitman J, Henrissat B, Iturriaga EA, Lang BF, Lavín JL, Lee SC, Li W, Lindquist E, López-García S, Luque EM, Marcos AT, Martin J, McCluskey K, Medina HR, Miralles-Durán A, Miyazaki A, Muñoz-Torres E, Oguiza JA, Ohm RA, Olmedo M, Orejas M, Ortiz-Castellanos L, Pisabarro AG, Rodríguez-Romero J, Ruiz-Herrera J, Ruiz-Vázquez R, Sanz C, Schackwitz W, Shahriari M, Shelest E, Silva-Franco F, Soanes D, Syed K, Tagua VG, Talbot NJ, Thon MR, Tice H, de Vries RP, Wiebenga A, Yadav JS, Braun EL, Baker SE, Garre V, Schmutz J, Horwitz BA, Torres-Martínez S, Idnurm A, HerreraEstrella A, Gabaldón T, Grigoriev IV. Expansion of signal transduction pathways in fungi by extensive genome duplication. Curr Biol 26:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desiro A, Faccio A, Kaech A, Bidartondo MI, Bonfante P. 2014. Endogone, one of the oldest plant-associated fungi, host unique Mollicutes-related endobacteria. New Phytol 205:1464–1472, doi: 10.1111/nph.13136 [DOI] [PubMed] [Google Scholar]
- Dickie IA, Alexander I, Lennon S, Öpik M, Selosse MA, van der Heijden MGA, Martin FM. 2015. Evolving insights to understanding mycorrhizas. New Phytol 205:1369– 1374, doi: 10.1111/nph.13290 [DOI] [PubMed] [Google Scholar]
- Doggett JS, Wong B. 2014. Mucormycosis. In: Loriaux L, ed. Endocrine emergencies New York: Humana Press; p 57–63. [Google Scholar]
- Dreschler C 1935. Some conidial phycomycetes destructive to terricolous amoebae. Mycologia 27:6–40, doi: 10.2307/3754021 [DOI] [Google Scholar]
- Doggett JS, Wong B. 1936. New conidial phycomycetes destructive to terricolous amoebae. Mycologia 28:363–389, doi: 10.2307/3754001 [DOI] [Google Scholar]
- Dujon B, Shermann D, Fischer G, Durrens P, et al. 2004. Genome evolution in yeasts. Nature 430:35–44, doi: 10.1038/nature02579 [DOI] [PubMed] [Google Scholar]
- Duplessis S, Cuoma CA, Lin YC, Aerts A, et al. 2011. Obligate biotrophy features unraveled by the genomic analysis of rust fungi. Proc Natl Acad Sci USA 108:9166–9171, doi: 10.1073/pnas.1019315108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersberger I, de Matos Simoes R, Kupczok A, Gube M, Kothe E, Voigt K, Haeseler von A. 2012. A consistent phylogenetic backbone for the fungi. Mol Biol Evol 29:1319–1334, doi: 10.1093/molbev/msr285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy SR. 2011. Accelerated profile HMM searches. PLoS Comput Biol 7:e1002195, doi: 10.1371/journal.pcbi.1002195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field KJ, Pressel S, Duckett JG, Rimington WR, Bidartondo MI. 2015b. Symbiotic options for the conquest of land. Trends Ecol Evol 30:477–486, doi: 10.1016/j.tree.2015.05.007 [DOI] [PubMed] [Google Scholar]
- Field KJ, Rimington WR, Bidartondo MI, Allinson KE, Beerling DJ, Cameron DD, Duckett JG, Leake JR, Pressel S. 2015a. First evidence of mutualism between ancient plant lineages (Haplomitriopsida liverworts) and Mucoromycotina fungi and its response to simulated Palaeozoic changes in atmospheric CO2. New Phytol 205:743–756, doi: 10.1111/nph.13024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher KE, Roberson RW. 2016. Hyphal tip cytoplasmic organization in four zygomycetous fungi. Mycologia 108: 533–542, doi: 10.3852/15-226 [DOI] [PubMed] [Google Scholar]
- Floudas D, Binder M, Riley R, Barry K, Blanchette RA, Henrissat B, Martínez AT, Otillar R, Spatafora JW, Yadav JS, Aerts A, Benoit I, Boyd A, Carlson A, Copeland A, Cou-tinho PM, de Vries RP, Ferreira P, Findley K, Foster B, Gaskell J, Glotzer D, Górecki P, Heitman J, Hesse C, Hori C, Igarashi K, Jurgens JA, Kallen N, Kersten P, Kohler A, Kües U, Kumar TK, Kuo A, LaButti K, Larrondo LF, Lindquist E, Ling A, Lombard V, Lucas S, Lundell T, Martin R, McLaughlin DJ, Morgenstern I, Morin E, Murat C, Nagy LG, Nolan M, Ohm RA, Patyshakuliyeva A, Rokas A, Ruiz-Dueñas FJ, Sabat G, Salamov A, Samejima M, Schmutz J, Slot JC, St John F, Stenlid J, Sun H, Sun S, Syed K, Tsang A, Wiebenga A, Young D, Pisabarro A, Eastwood DC, Martin F, Cullen D, Grigoriev IV, Hibbett DS. 2012. The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 336:1715–1719, doi: 10.1126/science.1221748 [DOI] [PubMed] [Google Scholar]
- Galagan JE, Calvo SE, Borkovich KA, Selker EU, Read ND, Jaffe D, FitzHugh W, Ma LJ, Smirnov S, Purcell S, Rehman B, Elkins T, Engels R, Wang S, Nielsen CB, Butler J, Endrizzi M, Qui D, Ianakiev P, Bell-Pedersen D, Nelson MA, Werner-Washburne M, Selitrennikoff CP, Kinsey JA, Braun EL, Zelter A, Schulte U, Kothe GO, Jedd G, Mewes W, Staben C, Marcotte E, Greenberg D, Roy A, Foley K, Naylor J, Stange-Thomann N, Barrett R, Gnerre S, Kamal M, Kamvysselis M, Mauceli E, Bielke C, Rudd S, Frishman D, Krystofova S, Rasmussen C, Metzenberg RL, Perkins DD, Kroken S, Cogoni C, Macino G, Catcheside D, Li W, Pratt RJ, Osmani SA, DeSouza CP, Glass L, Orbach MJ, Berglund JA, Voelker R, Yarden O, Plamann M, Seiler S, Dunlap J, Radford A, Aramayo R, Natvig DO, Alex LA, Mannhaupt G, Ebbole DJ, Freitag M, Paulsen I, Sachs MS, Lander ES, Nusbaum C, Birren B. 2003. The genome sequence of the filamentous fungus Neurospora crassa. Nature 422:859–868, doi: 10.1038/nature01554 [DOI] [PubMed] [Google Scholar]
- Gerdemann J, Trappe JM. 1974. The Endogonaceae in the Pacific Northwest. Mycol Mem 5:1–76. [Google Scholar]
- Goffeau A, Barrell BG, Bussey H, Davis RW, Dujon B, Feldmann H, Galibert F, Hoheisel JD, Jacq C, Johnston M, Louis EJ, Mewes HW, Murakami Y, Philippsen P, Tettelin H, Oliver SG. Life with 6000 genes. Science 274:546–567, doi: 10.1126/science.274.5287.546 [DOI] [PubMed] [Google Scholar]
- Gryganskyi AP, Humber RA, Smith ME, Miadlikowska J, Miadlikovska J, Wu S, Voigt K, Walther G, Anishchenko IM, Vilgalys R. 2012. Molecular phylogeny of the Entomophthoromycota. Mol Phylogenet Evol 65:682–694, doi: 10.1016/j.ympev.2012.07.026 [DOI] [PubMed] [Google Scholar]
- Gryganskyi AP, Muszewska A 2014. Whole genome sequencing and the Zygomycota. Fungal Genom Biol 4:e116,doi: 10.4172/2165-8056.1000e116 [DOI] [Google Scholar]
- Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, Huhndorf S, James T, Kirk PM, Lücking R, Thorsten Lumbsch H, Lutzoni F, Matheny PB, McLaughlin DJ, Powell MJ, Redhead S, Schoch CL, Spatafora JW, Stalpers JA, Vilgalys R, Aime MC, Aptroot A, Bauer R, Begerow D, Benny GL, Castlebury LA, Crous PW, Dai YC, Gams W, Geiser DM, Griffith GW, Gueidan C, Hawksworth DL, Hestmark G, Hosaka K, Humber RA, Hyde KD, Ironside JE, Kõljalg U, Kurtzman CP, Larsson KH, Lichtwardt R, Longcore J, Miadlikowska J, Miller A, Moncalvo JM, Mozley-Standridge S, Oberwinkler F, Parmasto E, Reeb V, Rogers JD, Roux C, Ryvarden L, Sampaio JP, Schüssler A, Sugiyama J, Thorn RG, Tibell L, Untereiner WA, Walker C, Wang Z, Weir A, Weiss M, White MM, Winka K, Yao YJ, Zhang N. 2007. A higher-level phylogenetic classification of the Fungi. Mycol Res 111:509–547, doi: 10.1016/j.mycres.2007.03.004 [DOI] [PubMed] [Google Scholar]
- Higashiyama K, Fujikawa S, Park EY. 2002. Production of arachidonic acid by Mortierella fungi. Biotechnol Bioprocess Eng 7:252–262, doi: 10.1007/BF02932833 [DOI] [Google Scholar]
- Hoff JA, Klopfenstein NB, McDonald GI. 2004. Fungal endophytes in woody roots of Douglas‐fir (Pseudotsuga menziesii) and ponderosa pine (Pinus ponderosa). For Pathol 34:255–271, doi: 10.1111/j.1439-0329.2004.00367.x [DOI] [Google Scholar]
- Hoffmann K, Voigt K, Kirk PM. 2011. Mortierellomycotina subphyl. nov., based on multi-gene genealogies. Mycotaxon 115:353–363, doi: 10.5248/115.353 [DOI] [Google Scholar]
- Hoffmann K, Pawłowska J, Walther G. 2013. The family structure of the Mucorales: a synoptic revision based on comprehensive multigene-genealogies. Persoonia 30:57–76, doi: 10.3767/003158513X666259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humber RA. 2012. Entomophthoromycota: a new phylum and reclassification for entomophthoroid fungi. Mycotaxon 120:477–492, doi: 10.5248/120.477 [DOI] [Google Scholar]
- Idnurm A, Walton FJ, Floyd A, Heitman J. 2008. Identification of the sex genes in an early diverged fungus. Nature 451:193–196, doi: 10.1038/nature06453 [DOI] [PubMed] [Google Scholar]
- James TY, Kauff F, Schoch CL, Matheny PB, Hofstetter V, Cox CJ, Celio G, Gueidan C, Fraker E, Miadlikowska J, Lumbsch HT, Rauhut A, Reeb V, Arnold AE, Amtoft A, Stajich JE, Hosaka K, Sung GH, Johnson D, O’Rourke B, Crockett M, Binder M, Curtis JM, Slot JC, Wang Z, Wilson AW, Schüssler A, Longcore JE, O’Donnell K, Mozley-Standridge S, Porter D, Letcher PM, Powell MJ, Taylor JW, White MM, Griffith GW, Davies DR, Humber RA, Morton JB, Sugiyama J, Rossman AY, Rogers JD, Pfister DH, Hewitt D, Hansen K, Hambleton S, Shoemaker RA, Kohlmeyer J, Volkmann-Kohlmeyer B, Spotts RA, Serdani M, Crous PW, Hughes KW, Matsuura K, Langer E, Langer G, Untereiner WA, Lücking R, Büdel B, Geiser DM, Aptroot A, Diederich P, Schmitt I, Schultz M, Yahr R, Hibbett DS, Lutzoni F, McLaughlin DJ, Spatafora JW, Vilgalys R. 2006. Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature 443:818–822, doi: 10.1038/nature05110 [DOI] [PubMed] [Google Scholar]
- James TY, Pelin A, Bonen L, Ahrendt S, Sain D, Corradi N, Stajich JE. 2013. Shared signatures of parasitism and phylogenomics unite Cryptomycota and microsporidia. Curr Biol 23:1548–1553, doi: 10.1016/j.cub.2013.06.057 [DOI] [PubMed] [Google Scholar]
- Jeffries P, Young T. 1979. Ultrastructure of septa in Dimargaris cristalligena RK Benjamin. J Gen Microbiol 111: 303–311, doi: 10.1099/00221287-111-2-303 [DOI] [Google Scholar]
- Jennessen J, Schnürer J, Olsson J, Samson RA, Dijksterhuis J. 2008. Morphological characteristics of sporangiospores of the tempe fungus Rhizopus oligosporus differentiate it from other taxa of the R. microsporus group. Mycol Res 112:547–563, doi: 10.1016/j.mycres.2007.11.006 [DOI] [PubMed] [Google Scholar]
- Joneson S, Stajich JE, Shiu SH, Rosenblum EB. 2011. Genomic transition to pathogenicity in chytrid fungi. PLoS Pathog 7:e1002338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamper J, Kahmann R, Bolker M, Ma LJ, Brefort T, Saville BJ, Banuett F, Kronstad JW, Gold SE, Müller O, Perlin MH, Wösten HA, de Vries R, Ruiz-Herrera J, Reynaga-Peña CG, Snetselaar K, McCann M, Pérez-Martín J, Feldbrügge M, Basse CW, Steinberg G, Ibeas JI, Holloman W, Guzman P, Farman M, Stajich JE, Sentandreu R, González-Prieto JM, Kennell JC, Molina L, Schirawski J, Mendoza-Mendoza A, Greilinger D, Münch K, Rössel N, Scherer M, Vranes M, Ladendorf O, Vincon V, Fuchs U, Sandrock B, Meng S, Ho EC, Cahill MJ, Boyce KJ, Klose J, Klosterman SJ, Deelstra HJ, Ortiz-Castellanos L, Li W, Sanchez-Alonso P, Schreier PH, Häuser-Hahn I, Vaupel M, Koopmann E, Friedrich G, Voss H, Schlüter T, Margolis J, Platt D, Swimmer C, Gnirke A, Chen F, Vysotskaia V, Mannhaupt G, Güldener U, Münsterkötter M, Haase D, Oesterheld M, Mewes HW, Mauceli EW, DeCaprio D, Wade CM, Butler J, Young S, Jaffe DB, Calvo S, Nusbaum C, Galagan J, Birren BW. 2006. Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature 444:97–101, doi: 10.1038/nature05248 [DOI] [PubMed] [Google Scholar]
- King N, Westbrook MJ, Young SL, Kuo A, Abedin M, Chapman J, Fairclough S, Hellsten U, Isogai Y, Letunic I, Marr M, Pincus D, Putnam N, Rokas A, Wright KJ, Zuzow R, Dirks W, Good M, Goodstein D, Lemons D, Li W, Lyons JB, Morris A, Nichols S, Richter DJ, Salamov A, Sequencing JG, Bork P, Lim WA, Manning G, Miller WT, McGinnis W, Shapiro H, Tjian R, Grigoriev IV, Rokhsar D. The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature 451: 783–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler A, Kuo A, Nagy LG, Morin E, Barry KW, Buscot F, Canbäck B, Choi C, Cichocki N, Clum A, Colpaert J, Copeland A, Costa MD, Doré J, Floudas D, Gay G, Girlanda M, Henrissat B, Herrmann S, Hess J, Högberg N, Johansson T, Khouja HR, LaButti K, Lahrmann U, Levasseur A, Lindquist EA, Lipzen A, Marmeisse R, Martino E, Murat C, Ngan CY, Nehls U, Plett JM, Pringle A, Ohm RA, Perotto S, Peter M, Riley R, Rineau F, Ruytinx J, Salamov A, Shah F, Sun H, Tarkka M, Tritt A, Veneault-Fourrey C, Zuccaro A; Mycorrhizal Genomics Initiative Consortium, Tunlid A, Grigoriev IV, Hibbett DS, Martin F. 2015. Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nat Genet 47:410–415, doi: 10.1038/ng.3223 [DOI] [PubMed] [Google Scholar]
- Lee SC, Ni M, Li W, Shertz C, Heitman J. 2010. The evolution of sex: a perspective from the fungal kingdom. Microbiol Mol Biol Rev 74:298–340, doi: 10.1128/MMBR.00005-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtwardt RW. 1986. The Trichomycetes: fungal associates of arthropods New York: Springer-Verlag; 343 p. [Google Scholar]
- Liu Y, Hodson MC, Hall BD. 2006. Loss of flagellum happened only once in the fungal lineage: phylogenetic structure of Kingdom Fungi inferred from RNA polymerase II subunit genes. BMC Evol Biol 6:74, doi: 10.1186/1471-2148-6-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Steenkamp ET, Brinkmann H, Forget L, Philippe H, Lang BF. 2009. Phylogenomic analyses predict sistergroup relationship of nucleariids and fungi and paraphyly of zygomycetes with significant support. BMC Evol Biol 9:272, doi: 10.1186/1471-2148-9-272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus BJ, Fung E, Roncaglia P, Rowley D, et al. 2005. The genome of the basidiomycetous yeast and human pathogen Cryptococcus neoformans. Science 307:1321–1324, doi: 10.1126/science.1103773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma LJ, Ibrahim AS, Skory C, Grabherr MG, Burger G, Butler M, Elias M, Idnurm A, Lang BF, Sone T, Abe A, Calvo SE, Corrochano LM, Engels R, Fu J, Hansberg W, Kim JM, Kodira CD, Koehrsen MJ, Liu B, Miranda-Saavedra D, O’Leary S, Ortiz-Castellanos L, Poulter R, RodriguezRomero J, Ruiz-Herrera J, Shen YQ, Zeng Q, Galagan J, Birren BW, Cuomo CA, Wickes BL. 2009. Genomic analysis of the basal lineage fungus Rhizopus oryzae reveals a whole-genome duplication. PLoS Genet 5:e1000549, doi: 10.1371/journal.pgen.1000549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerracher LJ, Heath IB, 1985. The structure and cycle of thenucleus-associated organelle in two species of Basidiobolus. Mycologia 77:412–417, doi: 10.2307/3793197 [DOI] [Google Scholar]
- McLaughlin DJ, Healy RA, Celio GJ, Roberson RW, Kumar TKA. 2015. Evolution of zygomycetous spindle pole bodies: evidence from Coemansia reversa mitosis. Am J Bot 102:707–717, doi: 10.3732/ajb.1400477 [DOI] [PubMed] [Google Scholar]
- McNeill J, Barrie FF, Buck WR, Demoulin V, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Marhold K, Prado J, Prud’homme van Reine WF, Smith GF, Wier-sema JH, Turland N, eds. 2012. International code of nomenclature for algae, fungi, and plants (Melbourne code). [Regnum Vegetabile no. 154.] Königstein: Koeltz Scientific Books; 208 p. [Google Scholar]
- Mirarab S, Reaz R, Bayzid MS, Zimmermann T. 2014. ASTRAL: Genome-scale coalescent-based species tree estimation. Bioinformatics 30:i541–i548, doi: 10.1093/bioinformatics/btu462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau F 1954. [‟1953”] Les Champignons. Physiologie, morphologie, développment et systématique Vol. 2 Paris: Lechevalier; 1179 p. [Google Scholar]
- Morton JB, Benny GL. 1990. Revised classification of arbuscular mycorrhizal fungi (Zygomycetes): a new order, Glomales, two new suborders, Glomineae and Gigasporineae, and two new families, Acaulosporaceae and Gigasporaceae, with an emendation of Glomacae. Mycotaxon 37:471–491. [Google Scholar]
- Nadimi M, Beaudet D, Forget L, Hijri M, Lang BF. 2012. Group I intron-mediated trans-splicing in mitochondria of Gigaspora rosea and a robust phylogenetic affiliation of arbuscular mycorrhizal fungi in Mortierellales. Mol Biol Evol 29:2199–2012, doi: 10.1093/molbev/mss088 [DOI] [PubMed] [Google Scholar]
- Nagy LG, Ohm RA, Kovács GM, Floudas D, Riley R, Gácser A, Sipiczki M, Davis JM, Doty SL, de Hoog GS, Lang BF, Spatafora JW, Martin FM, Grigoriev IV, Hibbett DS. 2014. Latent homology and convergent regulatory evolution underlies the repeated emergence of yeasts. Nat Commun 5:4471, doi: 10.1038/ncomms5471 [DOI] [PubMed] [Google Scholar]
- Nagy LG, Petkovits T, Kovács GM, Voigt K, Vágvölgyi C, Papp T. 2011. Where is the unseen fungal diversity hidden? A study of Mortierella reveals a large contribution of reference collections to the identification of fungal environmental sequences. New Phytol 191:789–794, doi: 10.1111/j.1469-8137.2011.03707.x [DOI] [PubMed] [Google Scholar]
- Notredame C, Higgins DG, Heringa J. 2000. T-Coffee: a novel method for fast and accurate multiple sequence alignment. J Molec Biol 302:205–217, doi: 10.1006/jmbi.2000.4042 [DOI] [PubMed] [Google Scholar]
- Papanikolaou S, Panayotou MG. 2007. Lipid production by oleaginous Mucorales cultivated on renewable carbon sources. Eur J Lipid Sci Tech 109:1060–1070, doi: 10.1002/ejlt.200700169 [DOI] [Google Scholar]
- Pelin A, Pombert JF, Salvioli A, Bonen L, Bonfante P, Corradi N. 2012. The mitochondrial genome of the arbuscular mycorrhizal fungus Gigaspora margarita reveals two unsuspected trans-splicing events of group I introns. New Phytol 194:836–845, doi: 10.1111/j.1469-8137.2012.04072.x [DOI] [PubMed] [Google Scholar]
- Redecker D, Schüßler A. 2014. Glomeromycota. In: McLaughlin DJ, Spatafora JW, eds. Mycota VII. Part A. Systematics and Evolution New York: Springer-Verlag; p 251–269. [Google Scholar]
- Riley R, Charron P, Idnurm A, Farinelli L, Dalpé Y, Martin F, Corradi N. 2013. Extreme diversification of the mating type-high-mobility group (MATA-HMG) gene family in a plant-associated arbuscular mycorrhizal fungus. New Phytol 201:254–268, doi: 10.1111/nph.12462 [DOI] [PubMed] [Google Scholar]
- Rinke C, Schwientek P, Sczyrba A, Ivanova NN, Anderson IJ, Cheng JF, Darling A, Malfatti S, Swan BK, Gies EA, Dodsworth JA, Hedlund BP, Tsiamis G, Sievert SM, Liu WT, Eisen JA, Hallam SJ, Kyrpides NC, Stepanauskas R, Rubin EM, Hugenholtz P, Woyke T. 2013. Insights into the phylogeny and coding potential of microbial dark matter. Nature 499:431–437, doi: 10.1038/nature12352 [DOI] [PubMed] [Google Scholar]
- Roberson RW, Saucedo E, Maclean D, Propster J, Unger B, Oneil TA, Parvanehgohar K, Cavanaugh C, Lowry D. 2011. The hyphal tip structure of Basidiobolus sp.: a zygomycete fungus of uncertain phylogeny. Fungal Biol 115:485–492, doi: 10.1016/j.funbio.2011.02.012 [DOI] [PubMed] [Google Scholar]
- Ropars J, Toro KS, Noel J, Pelin A, Charron P, Farinelli L, Marton T, Krüger, Fuchs J, Brachmann A, Corradi N. 2016. Evidence for the sexual origin of heterokaryosis in arbuscular mycorrhizal fungi. Nat Microbiol 1: art. no.16033. [DOI] [PubMed] [Google Scholar]
- Saikawa M 1989. Ultrastructure of the septum in Ballocephala verrucospora (Entomophthorales, Zygomycetes). Can J Bot 67:2484–2488, doi: 10.1139/b89-318 [DOI] [Google Scholar]
- Salichos L, Stamatakis A, Rokas A. 2014. Novel information theory-based measures for quantifying incongruence among phylogenetic trees. Mol Biol Evol 31:1261–1271, doi: 10.1093/molbev/msu061 [DOI] [PubMed] [Google Scholar]
- Sato Y, Narisawa K, Tsuruta K, Umezu M. 2010. Detection of Betaproteobacteria inside the mycelium of the fungus Mortierella elongata. Microbes Environ 25:321–324, doi: 10.1264/jsme2.ME10134 [DOI] [PubMed] [Google Scholar]
- Schüßler A, Schwarzott D, Walker C. 2001. A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycol Res 105:1413–1421, doi: 10.1017/S0953756201005196 [DOI] [Google Scholar]
- Sekimoto S, Rochon D, Long JE, Dee JM, Berbee ML. 2011. A multigene phylogeny of Olpidium and its implications for early fungal evolution. BMC Evol Biol 11:331, doi: 10.1186/1471-2148-11-331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selosse MA, Le Tacon F. 1998. The land flora: a phototroph-fungus partnership? Trends Ecol Evol 13:15–20, doi: 10.1016/S0169-5347(97)01230-5 [DOI] [PubMed] [Google Scholar]
- Shakya M, Gottel N, Castro H, Yang ZK, Gunter L, Labbà J, Muchero W, Bonito G, Vilgalys R, Tuskan G, Podar M, Schadt CW. 2013. A multifactor analysis of fungal and bacterial community structure in the root microbiome of mature Populus deltoides trees. PLoS One 8:e76382, doi: 10.1371/journal.pone.0076382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME, Gryganskyi A, Bonito G, Nouhra E. 2013. Phylogenetic analysis of the genus Modicella reveals an independent evolutionary origin of sporocarp-forming fungi in the Mortierellales. Fungal Genet Biol 61:61–68, doi: 10.1016/j.fgb.2013.10.001 [DOI] [PubMed] [Google Scholar]
- Stajich JE, Berbee ML, Blackwell M, Hibbett DS, James TY, Spatafora JW, Taylor JW. 2009. The fungi. Curr Biol 19:R840–R845, doi: 10.1016/j.cub.2009.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stajich JE, Wilke SK, Ahrén D, Au CH, Birren BW, Borodovsky M, Burns C, Canbäck B, Casselton LA, Cheng CK, Deng J, Dietrich FS, Fargo DC, Farman ML, Gathman AC, Goldberg J, Guigó R, Hoegger PJ, Hooker JB, Hug-gins A, James TY, Kamada T, Kilaru S, Kodira C, Kües U, Kupfer D, Kwan HS, Lomsadze A, Li W, Lilly WW, Ma LJ, Mackey AJ, Manning G, Martin F, Muraguchi H, Natvig DO, Palmerini H, Ramesh MA, Rehmeyer CJ, Roe BA, Shenoy N, Stanke M, Ter-Hovhannisyan V, Tunlid A, Velagapudi R, Vision TJ, Zeng Q, Zolan ME, Pukkila PJ. 2010. Insights into evolution of multicellular fun‐ gi from the assembled chromosomes of the mush‐ room Coprinopsis cinerea (Coprinus cinereus). Proc Natl Acad Sci USA 107:11889–11894, doi: 10.1073/pnas.1003391107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690, doi: 10.1093/bioinformatics/btl446 [DOI] [PubMed] [Google Scholar]
- Strullu-Derrien C, Kenrick P, Pressel S, Duckett JG, Rioult J-P, Strullu D-G. 2014. Fungal associations in Horneophy-ton ligneri from the Rhynie Chert (c. 407 million year old) closely resemble those in extant lower land plants: novel insights into ancestral plant-fungus symbioses. New Phytol 203:964–979, doi: 10.1111/nph.12805 [DOI] [PubMed] [Google Scholar]
- Suga H, Chen Z, de Mendoza A, Sebé-Pedrós A, Brown MW, Kramer E, Carr M, Kerner P, Vervoort M, Sánchez-Pons N, Torruella G, Derelle R, Manning G, Lang BF, Russ C, Haas BJ, Roger AJ, Nusbaum C, Ruiz-Trillo I. 2013. The Capsaspora genome reveals a complex unicellular prehis-tory of animals. Nat Comm 4:2325, doi: 10.1038/ncomms3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerbell RC. 2005. Root endophyte and mycorrhizo-sphere fungi of black spruce. Stud Mycol 53:121–145, doi: 10.3114/sim.53.1.121 [DOI] [Google Scholar]
- Taylor TN, Hass H, Kerp H, Krings M, Hanlin RT. 2005. Peri-thecial ascomycetes from the 400 million year old Rhy-nie chert: an example of ancestral polymorphism. Mycologia 97:269–285, doi: 10.3852/mycologia.97.1.269 [DOI] [PubMed] [Google Scholar]
- Taylor TN, Krings M, Taylor E. 2014. Fossil Fungi Amsterdam: Academic Press, Elsevier; 382 p. [Google Scholar]
- Taylor TN, Remy W, Hass H, Kerp H. 1995. Fossil arbuscular mycorrhizae from the Early Devonian. Mycologia 87:560–573, doi: 10.2307/3760776 [DOI] [Google Scholar]
- Tedersoo L, Smith ME. 2013. Lineages of ectomycorrhizal fungi revisited: foraging strategies and novel lineages revealed by sequences from belowground. Fungal Biol Rev 27:83–99, doi: 10.1016/j.fbr.2013.09.001 [DOI] [Google Scholar]
- Terhonen E, Keriö S, Sun H, Asiegbu FO. 2014. Endophytic fungi of Norway spruce roots in boreal pristine mire, drained peatland and mineral soil and their inhibitory effect on Heterobasidion parviporum in vitro. Fungal Ecol 9:17–26, doi: 10.1016/j.funeco.2014.01.003 [DOI] [Google Scholar]
- Than C, Ruths D, Nakhleh L. 2008. PhyloNet: a software package for analyzing and reconstructing reticulate evolutionary relationships. BMC Bioinformatics 9:322, doi: 10.1186/1471-2105-9-322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisserant E, Malbreil M, Kuo A, Kohler A, Symeonidi A, Balestrini R, Charron P, Duensing N, Frei dit Frey N, Gianinazzi-Pearson V, Gilbert LB, Handa Y, Herr JR, Hijri M, Koul R, Kawaguchi M, Krajinski F, Lammers PJ, Masclaux FG, Murat C, Morin E, Ndikumana S, Pagni M, Petitpierre D, Requena N, Rosikiewicz P, Riley R, Saito K, San Clemente H, Shapiro H, van Tuinen D, Bécard G, Bonfante P, Paszkowski U, Shachar-Hill YY, Tuskan GA, Young JP, Sanders IR, Henrissat B, Rensing SA, Grigoriev IV, Corradi N, Roux C, Martin F. 2013. Genome of an arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. Proc Natl Acad Sci USA 110:20117–20122, doi: 10.1073/pnas.1313452110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Cortés G, Ghignone S. 2015. Mosaic genome of endo-bacteria in arbuscular mycorrhizal fungi: transkingdom gene transfer in an ancient mycoplasma-fungus association. Proc Natl Acad Sci USA 112:7785–7790, doi: 10.1073/pnas.1501540112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter ED, Johnson EM, Benny GL, Lichtwardt RW, Wang Y, Kandel P, Novak SJ, Smith JF, White MM. 2014. An eight-gene molecular phylogeny of the Kickxellomyco-tina, including the first phylogenetic placement of Asel-lariales. Mycologia 106:912–935, doi: 10.3852/13-253 [DOI] [PubMed] [Google Scholar]
- Wagner L, Stielow B, Hoffmann K. 2013. A comprehensive molecular phylogeny of the Mortierellales (Mortierello-mycotina) based on nuclear ribosomal DNA. Persoonia 30:77–93, doi: 10.3767/003158513X666268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Wu R, Xu Y, Li M. 2013. Draft genome sequence of Rhizopus chinensis CCTCCM201021, used for brewing traditional Chinese alcoholic beverages. Genome Announc 1:e0019512, doi: 10.1128/genomeA.00195-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Chen W, Feng Y, Ren Y, Gu Z, Chen H, Wang H, Thomas MJ, Zhang B, Berquin IM, Li Y, Wu J, Zhang H, Song Y, Liu X, Norris JS, Wang S, Du P, Shen J, Wang N, Yang Y, Wang W, Feng L, Ratledge C, Zhang H, Chen YQ. 2011. Genome characterization of the ole-aginous fungus Mortierella alpina. PLoS One 6:e28319, doi: 10.1371/journal.pone.0028319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MM, James TY, O’Donnell K, Cafaro MJ, Tanabe Y, Sugiyama J. 2006. Phylogeny of the Zygomycota based on nuclear ribosomal sequence data. Mycologia 98: 872–884, doi: 10.3852/mycologia.98.6.872 [DOI] [PubMed] [Google Scholar]
- Wiens JJ, Morrill MC. 2011. Missing data in phylogenetic analysis: reconciling results from simulations and empirical data. Syst Biol 60:719–731, doi: 10.1093/sysbio/syr025 [DOI] [PubMed] [Google Scholar]
- Wood V, Gwilliam R, Rajandream MA, Lyne M, Lyne R, Stew-art A, Sgouros J, Peat N, Hayles J, Baker S, Basham D, Bowman S, Brooks K, Brown D, Brown S, Chillingworth T, Churcher C, Collins M, Connor R, Cronin A, Davis P, Feltwell T, Fraser A, Gentles S, Goble A, Hamlin N, Harris D, Hidalgo J, Hodgson G, Holroyd S, Hornsby T, Howarth S, Huckle EJ, Hunt S, Jagels K, James K, Jones L, Jones M, Leather S, McDonald S, McLean J, Mooney P, Moule S, Mungall K, Murphy L, Niblett D, Odell C, Oliver K, O’Neil S, Pearson D, Quail MA, Rabbinowitsch E, Rutherford K, Rutter S, Saunders D, Seeger K, Sharp S, Skelton J, Simmonds M, Squares R, Squares S, Stevens K, Taylor K, Taylor RG, Tivey A, Walsh S, Warren T, Whitehead S, Woodward J, Volckaert G, Aert R, Robben J, Grymonprez B, Weltjens I, Vanstreels E, Rieger M, Schäfer M, Müller-Auer S, Gabel C, Fuchs M, Düsterhöft A, Fritzc C, Holzer E, Moestl D, Hilbert H, Borzym K, Langer I, Beck A, Lehrach H, Reinhardt R, Pohl TM, Eger P, Zimmermann W, Wedler H, Wambutt R, Purnelle B, Goffeau A, Cadieu E, Dréano S, Gloux S, Lelaure V, Mottier S, Galibert F, Aves SJ, Xiang Z, Hunt C, Moore K, Hurst SM, Lucas M, Rochet M, Gaillardin C, Tallada VA, Garzon A, Thode G, Daga RR, Cruzado L, Jimenez J, Sánchez M, del Rey F, Benito J, Domínguez A, Revuelta JL, Moreno S, Armstrong J, Forsburg SL, Cerutti L, Lowe T, McCombie WR, Paulsen I, Potashkin J, Shpakovski GV, Ussery D, Barrell BG, Nurse P. 2002. The genome sequence of Schizosaccharomyces pombe. Nature 415:871–880, doi: 10.1038/nature724 [DOI] [PubMed] [Google Scholar]
- Yang J, Wang L, Ji X, Feng Y, Li X, Zou C, Xu J, Ren Y, Mi Q, Wu J, Liu S, Liu Y, Huang X, Wang H, Niu X, Li J, Liang L, Luo Y, Ji K, Zhou W, Yu Z, Li G, Liu Y, Li L, Qiao M, Feng L, Zhang KQ. 2011. Genomic and proteomic analyses of the fungus Arthrobotrys oligospora provide insights into nematode-trap formation. PLoS Pathog 7: e1002179, doi: 10.1371/journal.ppat.1002179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef NH, Couger MB, Struchtemeyer CG, Liggenstoffer AS, Prade RA, Najar FZ, Atiyeh HK, Wilkins MR, Elshahed MS. 2013. The genome of the anaerobic fungus Orpinomyces sp. strain C1A reveals the unique evolutionary history of a remarkable plant biomass degrader. Appl Environ Microbiol 79:4620–4634, doi: 10.1128/AEM.00821-13 [DOI] [PMC free article] [PubMed] [Google Scholar]


