Abstract
Iron deficiency is present in ∼50% of patients with heart failure (HF) and is an independent predictor of mortality. Despite growing recognition of the functional and prognostic significance of iron deficiency, randomized multicenter trials exploring the utility of oral iron supplementation in HF, a therapy that is inexpensive, readily available, and safe, have not been performed. Moreover, patient characteristics that influence responsiveness to oral iron in patients with HF have not been defined. Although results of intravenous iron repletion trials have been promising, regularly treating patients with intravenous iron products is both expensive and poses logistical challenges for outpatients. Herein we describe the rationale for the Oral Iron Repletion effects ON Oxygen UpTake in Heart Failure (IRONOUT HF) Trial. This National Institute of Health-sponsored trial will investigate oral iron polysaccharide compared to matching placebo with the primary endpoint of change in exercise capacity as measured by peak oxygen consumption at baseline and at 16 weeks
Keywords: heart failure, exercise, iron, clinical trial
Impaired exercise capacity is a cardinal manifestation of HF that is closely related to reduced quality of life and poor outcomes.1,2, 3 Therapeutic options beyond neurohormonal blockade to improve functional capacity and symptoms in HF are currently limited and must be pursued. Iron deficiency is associated with reduced functional capacity and poorer quality of life in HF. Here, a rationale for oral iron repletion in HF patients with iron deficiency is provided with an overview of the study design for the Iron Repletion effects ON Oxygen UpTake in Heart Failure (IRONOUT HF) trial, which aims to study the effect of oral iron repletion on functional capacity in HF patients with reduced ejection fraction.
Contributing factors to exercise intolerance in heart failure
In patients with HF, multiple mechanisms contribute to exercise intolerance.4–8 Abnormal central hemodynamic responses, and the degree of left ventricular systolic dysfunction (LVSD) do not adequately explain the early onset of anaerobic metabolism and impaired peak oxygen uptake (VO2) observed in HF.9–11 Prevalent anemia in HF compromises convective delivery of O2 to exercising skeletal muscle. Upon delivery of O2 to the periphery, diffusive O2 conductance and utilization is limited by impaired skeletal muscle oxidative metabolism.12 Morphologic and histochemical changes in skeletal muscle include a shift to type II fibers13 and a reduction in oxidative enzymes.13 As a result HF patients experience an early transition from oxidative to glycolytic metabolism and glycolytic end products in turn stimulate exaggerated ventilatory responses to exercise through intramuscle afferrents sensitive to products of skeletal muscle work (i.e. ergoreflex signaling).14 These findings have led to the concept of “a central role of the periphery” as a dominant mechanism to explain breathlessness and exertional intolerance in HF.9, 15 Strategies to target impaired peripheral utilization of oxygen during exercise therefore offer promise for improving functional capacity in HF.
The Central Role of Fe in O2 Delivery and Utilization
Iron plays a critical role in systemic O2 delivery and utilization (Figure 1).16–23 The contribution of iron to erythropoiesis and the role of iron deficiency in decreasing O2-carrying capacity of the blood through reduced hemoglobin are widely recognized. A less well appreciated consequence of iron deficiency is impairment in O2 storage capacity in skeletal muscle through reduced myoglobin. Additionally, iron is an obligate component of enzymes involved in cellular respiration, oxidative phosphorylation, vascular homeostasis and the citric acid cycle (Table 1).8, 9, 18, 19, 24, 25
Figure 1.
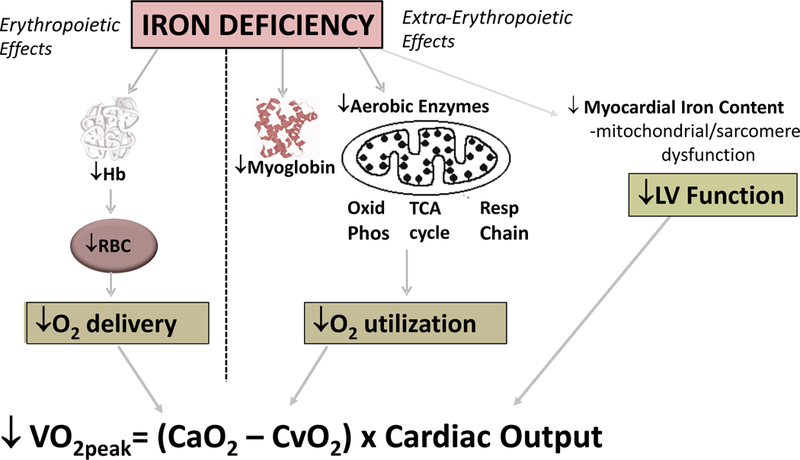
Impact of iron deficiency on components of peak VO2. Hb indicates hemoglobin; RBC red blood cell; Oxid Phos oxidative phosphorylation; TCA tricarboxylic acid; VO2peak peak oxygen uptake during exercise; CaO2 arterial content of oxygen; CvO2 mixed venous content of oxygen. Adapted from Anker et al with permission from Wiley and Sons Publishers23
Table 1:
Fe Containing Proteins that are altered in heart failure
| Name of Protein | Functional Site | Status in HF | Biological Functions |
|---|---|---|---|
| Hemoglobin | Red blood cell | ↓ | O2 transport |
| Myoglobin | Cytoplasm of muscle cells |
↓ | Facilitation of O2 transport |
| Oxidative enzymes (i.e. cytochrome oxidase) |
Mitochondrial inner membrane |
↓ | Substrate oxidation→NADH, FADH2 |
| Respiratory chain proteins | Mitochondrial inner membrane |
↓ | Electron transfer from O2→NADH, FADH2 |
| Soluble Guanylate Cyclase | Vascular smooth muscle cells, cardiomyocytes |
↓ | Nitric oxide stimulation of cGMP synthesis |
NADH indicates the reduced form of nicotinamide adenine dinucleotide; FADH2 indicates the reduced form of flavin adenine dinucleotide; cGMP indicates cyclic guanosine monophosphate. Adapted from Haas and Brownlie25 with permission of the publisher.
Animal studies have suggested that during iron repletion, improvements in hemoglobin levels and peak VO2 evolve in parallel, whereas enhancements in endurance track with the increase in aerobic enzyme activity.18, 19, 26 Studies in animals and humans without HF have demonstrated that iron deficiency anemia reduces indices of work capacity (i.e. peak VO2) by 10–50%.19, 25, 27 The correction of iron deficiency in both anemic and non-anemic patients without HF improves symptoms, quality of life, and exercise performance.19
Iron homeostasis in heart failure
There are multiple factors that predispose patients with HF to iron deficiency (Figure 2). Reduced nutrient intake is common in HF which may result in failure to meet the recommended 8–18mg of daily oral intake of elemental iron.28 Impairment in iron homeostasis in HF is partially attributable to pro-inflammatory processes.21 Hepcidin, a hepatically-derived peptide that is increased by pro-inflammatory cytokines, blocks intestinal absorption of iron and impairs iron delivery by diverting iron into the reticuloendothelial system. Gut edema may also impair the absorption of oral iron and contribute to iron deficiency in HF. Finally, frequent use of anticoagulants and anti-platelet agents predisposes HF patients to blood loss and associated depletion in iron stores.
Figure 2.
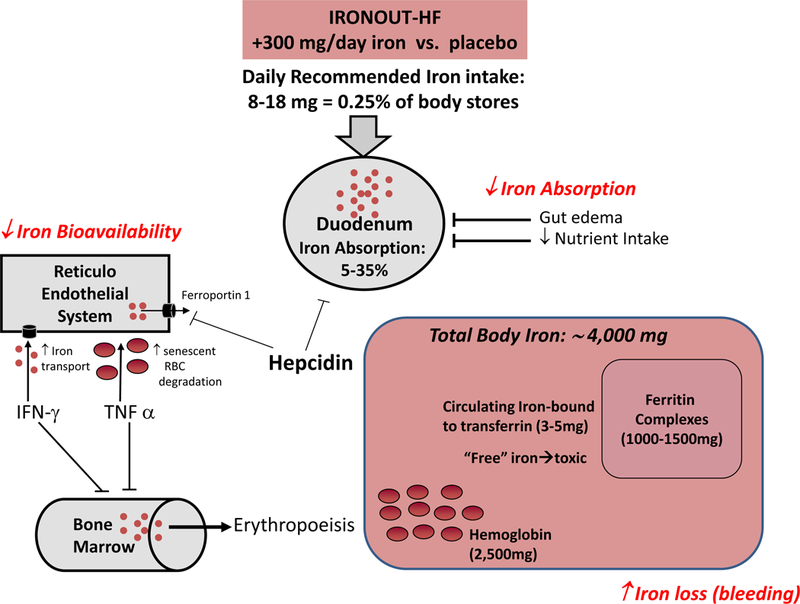
Iron homeostasis in heart failure. RBC indicates red blood cell; IFN-γ interferon gamma; TNFα tumor necrosis factor alpha.
Definition, Prevalence, and Significance of Iron Deficiency in HF
Historically, assessment of iron levels in HF patients has been performed as part of the evaluation of anemia, which is often multifactorial in HF.29 However, recent studies have focused on the importance of iron deficiency independent from anemia in HF pathophysiology.5–7
Definition:
The gold standard for measurement of iron stores is a bone marrow biopsy. However, due to the invasiveness of this procedure, blood biomarkers, including ferritin and transferrin saturation, can be used instead to reflect iron bioavailability in patients.9 A ferritin value <30 ng/mL has historically been used as a cutoff for defining iron deficiency. However, the inflammatory processes in HF and their tendency to increase serum ferritin concentration cause iron-deficiency in HF patients to be widely under recognized using this definition.29,30 Therefore, for patients with HF, iron deficiency has been defined as either ferritin less than 100 ng/mL, indicating a deficiency in iron stores, or ferritin between 100–300 ng/mL with transferrin saturation less than 20%, suggesting a disruption in iron delivery.10–13
Circulating levels of hepcidin and soluble transferrin receptor levels complement measurements of ferritin, iron and total iron binding capacity (TIBC). Hepcidin inhibits iron absorption and bioavailability and is downregulated in primary iron deficiency, whereas soluble transferrin receptor levels are upregulated and released into the bloodstream by cells avid for iron during states of iron deficiency. Jankowska et al. recently reported that hepcidin levels <14.5 ng/ml coupled with sTfR >1.6mg/L are indicators of iron deficiency that independently predict poor prognosis in HF.31
Prevalence:
Approximately 70% of patients with HF with reduced ejection fraction (HFrEF) have reduced bone marrow iron stores29 and 37–67% meet criteria for have iron deficiency14,15,32 Although circulating ferritin levels can increase as an acute phase reactant, and thereby potentially mask iron deficiency in patients admitted with acute HF, a recent study found that 72% of hospitalized patients met diagnostic criteria for iron deficiency.33 Notably, 25–42% of HF patients with iron deficiency are not anemic,32 which highlights the importance of considering iron deficiency even when hemoglobin levels are normal.
Functional and Prognostic Significance:
Iron deficiency reduces indices of functional and endurance capacity in patients with HF which likely reflects its broad impact on oxygen transport and cellular oxidative capacity.12,16–18 HF patients with iron deficiency had a worse health-related quality of life (as assessed by Minnesota Living with Heart Failure questionnaire scores) than those without iron deficiency.34 In addition, iron deficiency was associated with poorer outcomes in patients with systolic HF, independent of other well-established prognostic factors, including anemia, New York Heart Association (NYHA) class, LVEF, and NT-proBNP levels.15,18,19
Iron deficiency has also been associated with ultrastructural changes in cardiomyocytes including mitochondrial swelling and abnormal sarcomere structure.21 HF patients have lower myocardial iron content and transferrin receptor concentrations than controls,35 and severe iron deficiency leads to LV systolic dysfunction.36, 37 In a recent study of patients with non-ischemic cardiomyopathy, reduced myocardial iron content (as defined by MRI-based T2 star values in excess of 25) was associated with increased LVEDV, decreased LVEF, and poorer outcomes.38 Based on the multiple deleterious effects of iron deficiency on cardiac and peripheral performance, iron deficiency represents an attractive target to improve functional capacity in HF patients.
Efficacy and Safety of Treatment of Iron Deficiency in Heart Failure
Clinical studies of intravenous iron repletion in HF22, 39–43 are summarized in Table 2. Initial single-center trials focused on patients with HFrEF and iron deficiency anemia, and consistently observed improvements in peak VO2, patient global assessment (PGA) scores, and NT-BNP levels.19,20 Correction of iron deficiency has also been shown to have direct effects on myocardial contractility.7 Two meta-analyses44, 45 reported significant improvement in echocardiography-derived LVEF with iron therapy (mean improvement +5%). In another single-center study,46 an improvement in LV strain rate was reported after 3 months of IV iron therapy in 40 patients with iron deficiency and HFrEF.47, 48 These findings reinforce the hypothesis that iron repletion may directly improve cardiac function in addition to promoting improved peripheral oxygen delivery and utilization.
Table 2.
Trials evaluating intravenous iron supplementation for treatment of heart failure.
| Drug | Authors/ Journal |
N | Subjects Studied |
Iron Deficiency Definition |
Time | Primary Endpoint |
Findings |
|---|---|---|---|---|---|---|---|
| IV Iron Sucrose |
Bolger39 JACC 2006 |
16 | NYHA 2–3 LVEF <0.35 |
Ferritin<400 mg/ml | 12 wks |
∆ 6MWT | ↑ 6MWD, ↓NYHA ↓ MLHF Scores |
| IV Iron Sucrose |
Tobilli22 JACC 2007 |
40 | NYHA 3–4 LVEF<0.35 |
Ferritin<100ng/ml and/or Tsat<20% |
5 wks |
∆ Global Assessment score |
↑ PGAS, ↑ 6MWD ↓ NT-BNP, ↑ LVEF |
| IV iron Sucrose |
Usmanov40 J Nephrol 2008 |
32 | NYHA 3–4 LVEF<0.35 |
Hb < 11g/dl Iron indices not specified |
26 wks |
∆ NYHA | ↓ NYHA, ↑ LVEF |
| IV Iron Sucrose |
Okonko41 JACC 2008 |
35 | NYHA 2–3 LVEF<0.35 |
Ferritin<100 ng/ml or 100–300 with Tsat<20% |
16 wks |
Δ peak VO2 | ↑ PGAS, ↓ NYHA ↑ Peak VO2 α Δ Tsat |
| IV Iron Carboxy maltose |
Anker42 NEJM 2009 |
45 | NYHA 2–3 LVEF<0.4 Hb 9.5–13.5 |
Ferritin<100 ng/ml or 100–300 with Tsat <20% |
24 wks |
∆ Global Assessment Score |
↑ PGAS, ↓NYHA ↑ 6MWD Similar benefit Hb<>12 |
| IV Iron Carboxy maltose |
Ponikowski43 EHJ 2014 |
30 | NYHA 2–3 LVEF<0.45 Hb <15 |
Ferritin<100 ng/ml or 100–300 with Tsat<20% |
52 wk |
∆ 6MWD | ↑ 6MWD ↑ PGAS, ↓NYHA Similar benefit Hb<>12 ↓ HF hospitalization |
Tsat, transferrin saturation; 6MWD, 6-minute walk distance; PGAS, patient global assessment score; NT-BNP, N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association functional class; VO2, oxygen consumption; Hb, hemoglobin; HF, heart failure.
The landmark randomized trial of iron repletion in HFrEF patients with iron deficiency, FAIR-HF, demonstrated significant improvements in PGA scores and decreases in NYHA class with the administration of 200 mg IV ferric carboxymaltose weekly until repletion.42 Improvements in PGA score and 6MW distance were similar in patients with and without anemia. The CONFIRM-HF Trial, which assessed long-term therapy with IV ferric carboxymaltose in HFrEF, extended findings from FAIR-HF by demonstrating improvement in 6MW distance and quality of life in patients with hemoglobin values up to 15 g/dl while also demonstrating reduction in HF hospitalizations.43 The fact that iron repletion demonstrates similar efficacy in anemic and non-anemic patients clearly distinguishes this strategy from that of treatment with anemia alone with erythropoiesis-stimulating agents, which has been shown to not improve HF outcomes.49
ORAL IRON
With the exception of one truncated study that included 7 individuals randomized to oral iron,50 trials exploring the utility of oral iron supplementation in HF patients with iron deficiency have not been performed. A recent retrospective observational study of 105 HFrEF patients with iron deficiency showed that oral iron supplementation improved ferritin (median 39 to 75ng/ml), Tsat (10 to 21%), iron (34 to 69µg/dL), and hemoglobin (10.4 to 11.6 g/dL) values (all p<0.0001) during a median follow up period of 164 days. In non-HF populations, IV and oral Fe repletion have resulted in similar sustained increases in circulating ferritin levels, transferrin saturations, and hemoglobin concentrations.51 A study in iron-deficient patients with congenital heart disease demonstrated functional improvements with oral iron supplementation after 3 months of iron fumarate (600 mg total daily dose).52 However, further investigation is clearly needed to determine the utility of oral therapies in iron deficient HF populations.
Rationale for IRONOUT
Despite growing recognition of the functional and prognostic significance of iron deficiency, current HF guidelines in the United States do not specify when, or whether, to assess for and treat iron deficiency. European guidelines have recently recommended evaluation of iron studies without specifically addressing whether to implement treatment strategies.
Although results of IV iron repletion trials have been promising, regularly treating patients with IV iron products is both expensive and poses logistical challenges for outpatients. In FAIR-HF, 8–12 weekly injections of IV iron (200mg) were required to achieve iron repletion, followed by monthly injections during the maintenance phase. Intravenous iron infusions are expensive (~$4,000/injection for the least expensive preparation, iron sucrose 200mg) and pose logistical challenges for outpatients based on the frequency of required visits and need for the infrastructure within HF clinics to administer infusions. While a higher-dose IV iron preparation is now available (Ferumoxytol [Feraheme], 510 mg elemental iron), this preparation has been associated with hypersensitivity reactions in 3.7% of patients, including anaphylaxis, cardiac arrest, and hypotension.53 Randomized multicenter trials exploring the utility of oral iron supplementation in HF, a therapy that is inexpensive, readily available, and safe, have not been performed. There is also a need to understand patient characteristics that influence responsiveness to oral iron. A recent retrospective study showed lack of relationship between dose of elemental iron and change in iron stores, suggesting host factors play an important role.54 There may be subsets of HFrEF patients (i.e. lack of right heart failure and intestinal edema, low hepcidin levels) that benefit from oral iron supplementation even if the strategy is not effective in all.
STUDY DESIGN
Oral Iron Repletion effects ON Oxygen UpTake in Heart Failure (IRONOUT HF) is an NIH-sponsored multi-center, randomized, double-blinded, placebo-controlled trial of oral iron polysaccharide compared to matching placebo with the primary endpoint of change in peak VO2 measured by cardiopulmonary exercise testing (CPET) at baseline and at 16 weeks (clinicaltrials.gov, NCT02188784, Figure 3). A total of 220 patients with HFrEF meeting the eligibility criteria (Table 3) are being enrolled and randomized in the study. Screening is being conducted in outpatients with chronic symptomatic HFrEF. Willing participants who are found to have iron deficiency and meet other entry criteria are being consented for the trial.
Figure 3.
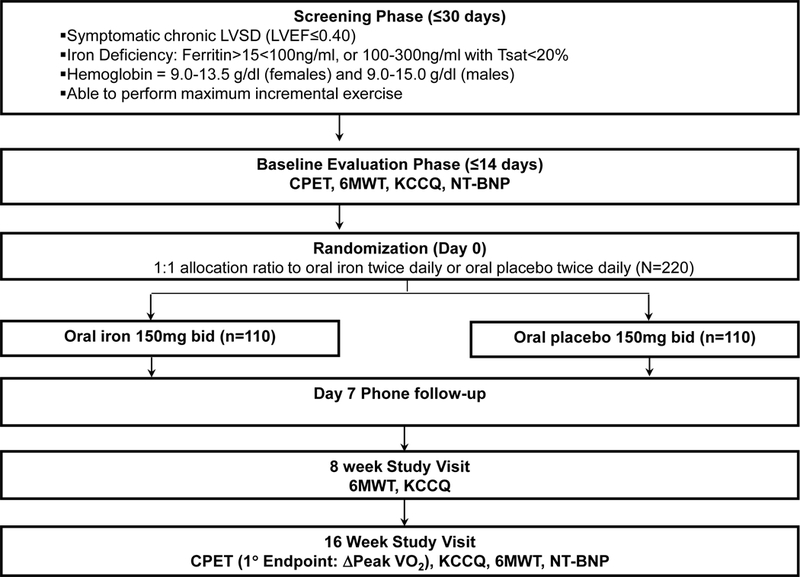
Flow diagram of IRONOUT-HF Trial. NT-BNP, N-terminal pro B-type natriuretic peptide; CPET, cardiopulmonary exercise test; 6MWT, six minute walk test; KCCQ, Kansas City Cardiomypoathy Questionnaire.
Table 3.
IRONOUT HF Inclusion and Exclusion Criteria
|
Inclusion Criteria 1. Age ≥18 years 2. Previous clinical diagnosis of heart failure with current NYHA Class II-IV symptoms, LVEF≤0.40 within 2 years prior to consent, and ≥3 months after a major change in cardiac status (i.e. CABG or CRT). 3. Serum ferritin between 15–100 ng/ml or serum ferritin between 100–299 ng/ml with transferrin saturation <20% 4. Hemoglobin 9.0–15.0 g/dL (males), 9.0–13.5 (females) at time of enrollment 5. Evidence-based medical therapy for HF (including beta-blocker and ACE-inhibitor/ARB unless previously deemed intolerant and diuretics as necessary) with ≤100% change in dose for 30 days prior to randomization. Changes in diuretic dose guided by a patient-directed flexible dosing program are considered stable medical therapy 6. Willingness to provide informed consent Exclusion Criteria 1. Presence of a neuromuscular, orthopedic or other non-cardiac condition that prevents the patient from exercise testing on a cycle/treadmill ergometer and/or inability to achieve an RER ≥ 1.0 on screening/baseline CPET 2. Severe renal dysfunction (eGFR< 20 ml/min/1.73m2) 3. Severe liver disease (ALT or AST > 3x normal, alkaline phosphatase or bilirubin >2x normal) 4. Gastrointestinal conditions known to impair Fe absorption (i.e. inflammatory bowel disease) 5. Known active infection as defined by current use of oral or intravenous antimicrobial agents 6. Documented active gastrointestinal bleeding 7. Active malignancy other than non-melanoma skin cancers 8. Anemia with known cause other than Iron deficiency or chronic disease 9. Fe overload disorders (i.e. hemochromatosis or hemosiderosis) 10. History of erythropoietin, IV or oral Fe therapy, or blood transfusion in previous 3 months. 11. Current ventricular assist device 12. Anticipated cardiac transplantation within the next 4 months 13. Primary hypertrophic cardiomyopathy, infiltrative cardiomyopathy, acute myocarditis, constrictive pericarditis or tamponade 14. Previous adverse reaction to study drug or other oral Fe preparation 15. Known or anticipated pregnancy in the next 4 months |
Randomization and stratification
After providing written informed consent, research participants complete all baseline procedures, including clinical evaluation, blood samples, and CPET. Eligible participants are randomized with a 1:1 allocation ratio to either oral iron polysaccharide 150 mg twice daily or matching placebo. Randomization is stratified by anemia status (anemia is defined as hemoglobin <12g/dL). A permuted block randomization method stratified by site is used to ensure relatively equal distribution of subjects to each arm within each clinical site.
Polysaccharide Iron Complex is being studied as the oral iron preparation of choice for this study because it is relatively nontoxic, thus permitting a higher therapeutic dosage (150 – 300 mg elemental iron daily) than other iron preparations.55 Polysaccharide Iron Complex capsules are a highly water soluble complex of iron and a low molecular weight polysaccharide. Iron polysaccharide is considered to be a dietary supplement, and although it has a human over-the-counter drug label, it is not an FDA approved drug. From previous studies using inclusion criteria based on the same iron study results, we estimated an average iron deficit of ~1g using the Ganzoni Formula.56 Assuming 5% absorption of iron polysaccharide, a period of 10 weeks of 300 mg daily dosing is required to completely make up this deficit.
Participants are started on Polysaccharide Iron Complex 150 mg or placebo administered twice daily in a double-blinded fashion. Instructions are provided to take pills separately from meals and to avoid taking antacids, dairy products, tea, or coffee within 2 hours before or after this medication because they will decrease effectiveness. Drug administration with orange juice or other products rich in Vitamin C may enhance absorption and therefore is encouraged.
Primary endpoint
Impaired exercise capacity is a cardinal manifestation of HF and it can be objectively and reproducibly measured by quantifying peak oxygen uptake (peak VO2) through cardiopulmonary exercise testing (CPET). The multiple mechanisms by which iron repletion is expected to improve systemic O2 delivery and utilization (Figure 1) are captured with assessment of peak VO2, making this measure ideally suited to be the primary endpoint. Unlike changes in alternative trial end points, such as circulating biomarkers or echocardiographic parameters, there is significant intrinsic value to patients associated with improving exercise capacity.
For assessment of changes in exercise capacity in response to iron repletion, there are several advantages of CPET in comparison to 6 minute walk tests used in previous multicenter trials of intravenous iron repletion.42, 43 First, CPET permits precise assessment of volitional effort based on whether the respiratory exchange ratio (VCO2/VO2) exceeds 1.0 during exercise; second, CPET provides insights into the organ system limiting gas exchange; third, CPET-derived peak VO2 has been shown to outperform 6MW distance in predicting HF outcomes57; and finally, CPET permits measurement of an array of variables that reflect metabolic responses to low-level, intermediate, and maximum exercise and thereby permits comprehensive assessment of the impact of a therapy that is expected to impact oxygen utilization via multiple mechanisms.
Secondary Endpoints and Exploratory Studies
Secondary endpoints of IRONOUT-HF include assessments of the impact of oral Fe repletion on (1) submaximal exercise capacity, as measured by O2 uptake kinetics upon initiation of exercise;58 (2) ventilatory efficiency, as measured by minute ventilation relative to CO2 production throughout exercise; (3) 6 minute walk distance; (4) plasma NT-pro BNP levels; and (5) Health Status: Kansas City Cardiomyopathy Questionnaire (KCCQ). The following exploratory objectives, for which the trial is not primarily powered, seek to determine if specific subgroups of patients derive differential benefit from oral Fe polysaccharide: (1) patients with or without anemia; (2) patients with or without venous congestion, based on JVP>10cm or lower extremity edema; and (3) patients with and without an RER>1.1 during maximum incremental exercise. Other exploratory objectives include whether iron repletion influences clinical outcomes: time to death and HF hospitalization, or renal function (creatinine, cystatin C). Exploratortrial is powered to detect differences in the primary Iron studies (iron, TIBC, ferritin) will be measured at baseline and after 16 weeks of study medication to determine the extent to which oral iron leads to iron repletion in HF patients.
Statistical Considerations
All primary analyses are based on the intention-to-treat principle. A general linear model with the change in peak VO2 measured at 16 weeks as the response variable and predictor variables including a treatment indicator and the baseline measure of peak VO2 are used in the primary analysis. Using a minimally important difference for peak VO2 of 1.0 ml/kg/min, and an estimate of 2.0 ml/kg/min for the standard deviation for peak VO2 a sample size of 172 subjects (86 per group) provides 90% power to detect the minimally important difference with a two-sided type I error of 0.05. Allowing for 20% missing data results in a sample size of 108 per group, or a total sample size of approximately 220 subjects.
A secondary analysis of the peak VO2 outcome uses a repeated measures analysis. For this analysis all measurement of peak VO2 (including baseline) are treated as response variables. The following covariates are included in the regression model: treatment group, time period, treatment group * time period interaction, baseline VO2 and baseline Fe level. An unstructured correlation matrix is assumed for the repeated measures within subjects.
General linear models and nonparametric approaches are used to analyze the continuous outcomes. For binary outcomes, Chi-square tests and Fisher’s exact test are used for unadjusted comparisons. For adjusted comparisons, logistic regression analysis is used to compare oral iron vs. placebo with the estimated odds ratio and associated 95% confidence interval.
Conclusions
Iron deficiency is present in ~50% of patients with HFrEF and predicts poor prognosis independently of anemia.32 Despite these recent observations and trials suggesting benefits from IV iron repletion, current HF guidelines do not specify when or if to assess for and treat iron deficiency. The IRONOUT-HF trial design has several strengths, including entry criteria that permit assessment of effects of oral iron repletion on patients with and without anemia, objective measurements of submaximum and maximum exercise capacity, novel secondary endpoints (i.e. assessment of oxygen uptake kinetics) that directly reflect mechanisms of action of iron repletion, and careful patient phenotyping to aid in identification of subgroups that may benefit most from oral iron repletion.
While the sample size and short duration of this trial precludes definitive assessment of patient outcomes, the results of IRONOUT-HF could lead to guideline recommendations for use of oral iron to improve functional capacity in HF. Based on the low cost and widespread availability of this therapy, the trial is anticipated to impact the clinical practice of administering oral iron to patients with HF whether the results are positive or not. If HFrEF patients derive no benefit from oral iron repletion it would mitigate polypharmacy that is nearly ubiquitous in HF patients. However, if iron repletion is shown to improve exercise capacity beyond its effects on erythropoiesis, it will profoundly impact the treatment of HF.
Supplementary Material
Acknowledgments
Sources of Funding: This work was supported by grants from the National Heart, Lung, and Blood Institute (NHLBI) (coordinating center: U10 HL084904; regional clinical centers: U10 HL110312, U10 HL110337, U10 HL110342, U10 HL110262, U10 HL110297, U10 HL110302, U10 HL110309, U10 HL110336, U10 HL110338).
Disclosures: All authors acknowledge grant support from NHLBI during the conduct of this study. Dr. Hernandez reports research funding from Amgen, AstraZeneca; Merck, Novartis and honorarium from Amgen; AstraZeneca; Luitpold; Merck, and Novartis. Dr. Braunwald reports grant support from Duke University during the conduct of this study.
REFERENCES
- 1.O’Neill JO, Young JB, Pothier CE, Lauer MS. Peak oxygen consumption as a predictor of death in patients with heart failure receiving beta-blockers. Circulation 2005;111:2313–2318 [DOI] [PubMed] [Google Scholar]
- 2.Francis DP, Shamim W, Davies LC, Piepoli MF, Ponikowski P, Anker SD, Coats AJ. Cardiopulmonary exercise testing for prognosis in chronic heart failure: Continuous and independent prognostic value from ve/vco(2)slope and peak vo(2). European heart journal 2000;21:154–161 [DOI] [PubMed] [Google Scholar]
- 3.Szlachcic J, Massie BM, Kramer BL, Topic N, Tubau J. Correlates and prognostic implication of exercise capacity in chronic congestive heart failure. Am J Cardiol 1985;55:1037–1042 [DOI] [PubMed] [Google Scholar]
- 4.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, Keteyian SJ, Lavie CJ, Macko R, Mancini D, Milani RV. Clinician’s guide to cardiopulmonary exercise testing in adults: A scientific statement from the american heart association. Circulation 2010;122:191–225 [DOI] [PubMed] [Google Scholar]
- 5.Arena R, Myers J, Williams MA, Gulati M, Kligfield P, Balady GJ, Collins E, Fletcher G. Assessment of functional capacity in clinical and research settings: A scientific statement from the american heart association committee on exercise, rehabilitation, and prevention of the council on clinical cardiology and the council on cardiovascular nursing. Circulation 2007;116:329–343 [DOI] [PubMed] [Google Scholar]
- 6.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA : the journal of the American Medical Association 2002;288:2144–2150 [DOI] [PubMed] [Google Scholar]
- 7.Corra U, Piepoli MF, Adamopoulos S, Agostoni P, Coats AJ, Conraads V, Lambrinou E, Pieske B, Piotrowicz E, Schmid JP, Seferovic PM, Anker SD, Filippatos G, Ponikowski PP. Cardiopulmonary exercise testing in systolic heart failure in 2014: The evolving prognostic role: A position paper from the committee on exercise physiology and training of the heart failure association of the esc. Eur J Heart Fail 2014;16:929–941 [DOI] [PubMed] [Google Scholar]
- 8.Guazzi M, Adams V, Conraads V, Halle M, Mezzani A, Vanhees L, Arena R, Fletcher GF, Forman DE, Kitzman DW, Lavie CJ, Myers J. Eacpr/aha scientific statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2012;126:2261–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark AL, Poole-Wilson PA, Coats AJ. Exercise limitation in chronic heart failure: Central role of the periphery. Journal of the American College of Cardiology 1996;28:1092–1102 [DOI] [PubMed] [Google Scholar]
- 10.Pina IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, Fletcher BJ, Fleg JL, Myers JN, Sullivan MJ. Exercise and heart failure: A statement from the american heart association committee on exercise, rehabilitation, and prevention. Circulation 2003;107:1210–1225 [DOI] [PubMed] [Google Scholar]
- 11.Massie BM, Conway M, Rajagopalan B, Yonge R, Frostick S, Ledingham J, Sleight P, Radda G. Skeletal muscle metabolism during exercise under ischemic conditions in congestive heart failure. Evidence for abnormalities unrelated to blood flow. Circulation 1988;78:320–326 [DOI] [PubMed] [Google Scholar]
- 12.Drexler H, Faude F, Hoing S, Just H. Blood flow distribution within skeletal muscle during exercise in the presence of chronic heart failure: Effect of milrinone. Circulation. 1987;76:1344–1352 [DOI] [PubMed] [Google Scholar]
- 13.Sullivan MJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation 1990;81:518–527 [DOI] [PubMed] [Google Scholar]
- 14.Bennett FM. A role for neural pathways in exercise hyperpnea. J Appl Physiol 1984;56:1559–1564 [DOI] [PubMed] [Google Scholar]
- 15.Harrington D, Anker SD, Chua TP, Webb-Peploe KM, Ponikowski PP, Poole-Wilson PA, Coats AJ. Skeletal muscle function and its relation to exercise tolerance in chronic heart failure. Journal of the American College of Cardiology 1997;30:1758–1764 [DOI] [PubMed] [Google Scholar]
- 16.Dallman PR. Iron deficiency: Does it matter? J Intern Med 1989;226:367–372 [DOI] [PubMed] [Google Scholar]
- 17.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med 2005;352:1011–1023 [DOI] [PubMed] [Google Scholar]
- 18.Dunn LL, Rahmanto YS, Richardson DR. Iron uptake and metabolism in the new millennium. Trends Cell Biol 2007;17:93–100 [DOI] [PubMed] [Google Scholar]
- 19.Davies KJ, Maguire JJ, Brooks GA, Dallman PR, Packer L. Muscle mitochondrial bioenergetics, oxygen supply, and work capacity during dietary iron deficiency and repletion. Am J Physiol 1982;242:E418–427 [DOI] [PubMed] [Google Scholar]
- 20.Petering DH, Stemmer KL, Lyman S, Krezoski S, Petering HG. Iron deficiency in growing male rats: A cause of development of cardiomyopathy. Annals of nutrition & metabolism 1990;34:232–243 [DOI] [PubMed] [Google Scholar]
- 21.Dong F, Zhang X, Culver B, Chew HG Jr., Kelley RO, Ren J. Dietary iron deficiency induces ventricular dilation, mitochondrial ultrastructural aberrations and cytochrome c release: Involvement of nitric oxide synthase and protein tyrosine nitration. Clin Sci (Lond) 2005;109:277–286 [DOI] [PubMed] [Google Scholar]
- 22.Toblli JE, Lombrana A, Duarte P, Di Gennaro F. Intravenous iron reduces nt-pro-brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. Journal of the American College of Cardiology 2007;50:1657–1665 [DOI] [PubMed] [Google Scholar]
- 23.Anker SD, Colet JC, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Luscher TF, Mori C, von Eisenhart Rothe B, Pocock S, Poole-Wilson PA, Ponikowski P, committees F-H, investigators. Rationale and design of ferinject assessment in patients with iron deficiency and chronic heart failure (fair-hf) study: A randomized, placebo-controlled study of intravenous iron supplementation in patients with and without anaemia. European journal of heart failure 2009;11:1084–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maguire JJ, Davies KJ, Dallman PR, Packer L. Effects of dietary iron deficiency of iron-sulfur proteins and bioenergetic functions of skeletal muscle mitochondria. Biochim Biophys Acta 1982;679:210–220 [DOI] [PubMed] [Google Scholar]
- 25.Haas JD, Brownlie Tt. Iron deficiency and reduced work capacity: A critical review of the research to determine a causal relationship. The Journal of nutrition 2001;131:676S–688S; discussion 688S-690S [DOI] [PubMed] [Google Scholar]
- 26.Davies KJ, Donovan CM, Refino CJ, Brooks GA, Packer L, Dallman PR. Distinguishing effects of anemia and muscle iron deficiency on exercise bioenergetics in the rat. Am J Physiol 1984;246:E535–543 [DOI] [PubMed] [Google Scholar]
- 27.Ohira Y, Edgerton VR, Gardner GW, Senewiratne B, Barnard RJ, Simpson DR. Work capacity, heart rate and blood lactate responses to iron treatment. Br J Haematol 1979;41:365–372 [DOI] [PubMed] [Google Scholar]
- 28.Herbert V Recommended dietary intakes (rdi) of iron in humans. The American journal of clinical nutrition 1987;45:679–686 [DOI] [PubMed] [Google Scholar]
- 29.Nanas JN, Matsouka C, Karageorgopoulos D, Leonti A, Tsolakis E, Drakos SG, Tsagalou EP, Maroulidis GD, Alexopoulos GP, Kanakakis JE, Anastasiou-Nana MI. Etiology of anemia in patients with advanced heart failure. Journal of the American College of Cardiology 2006;48:2485–2489 [DOI] [PubMed] [Google Scholar]
- 30.Weiss G Iron metabolism in the anemia of chronic disease. Biochim Biophys Acta 2009;1790:682–693 [DOI] [PubMed] [Google Scholar]
- 31.Jankowska EA, Kasztura M, Sokolski M, Bronisz M, Nawrocka S, Oleskowska-Florek W, Zymlinski R, Biegus J, Siwolowski P, Banasiak W, Anker SD, Filippatos G, Cleland JG, Ponikowski P. Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. European heart journal 2014;35:2468–2476 [DOI] [PubMed] [Google Scholar]
- 32.Klip IT, Comin-Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, Lok DJ, Rosentryt P, Torrens A, Polonski L, van Veldhuisen DJ, van der Meer P, Jankowska EA. Iron deficiency in chronic heart failure: An international pooled analysis. American heart journal 2013;165:575–582 e573 [DOI] [PubMed] [Google Scholar]
- 33.Cohen-Solal A, Damy T, Terbah M, Kerebel S, Baguet JP, Hanon O, Zannad F, Laperche T, Leclercq C, Concas V, Duvillie L, Darne B, Anker S, Mebazaa A. High prevalence of iron deficiency in patients with acute decompensated heart failure. European journal of heart failure 2014;16:984–991 [DOI] [PubMed] [Google Scholar]
- 34.Enjuanes C, Klip IT, Bruguera J, Cladellas M, Ponikowski P, Banasiak W, van Veldhuisen DJ, van der Meer P, Jankowska EA, Comin-Colet J. Iron deficiency and health-related quality of life in chronic heart failure: Results from a multicenter european study. International journal of cardiology 2014;174:268–275 [DOI] [PubMed] [Google Scholar]
- 35.Maeder MT, Khammy O, dos Remedios C, Kaye DM. Myocardial and systemic iron depletion in heart failure implications for anemia accompanying heart failure. Journal of the American College of Cardiology 2011;58:474–480 [DOI] [PubMed] [Google Scholar]
- 36.Georgieva Z, Georgieva M. Compensatory and adaptive changes in microcirculation and left ventricular function of patients with chronic iron-deficiency anaemia. Clinical hemorheology and microcirculation 1997;17:21–30 [PubMed] [Google Scholar]
- 37.Jankowska EA, Ponikowski P. Molecular changes in myocardium in the course of anemia or iron deficiency. Heart failure clinics 2010;6:295–304 [DOI] [PubMed] [Google Scholar]
- 38.Nagao M, Matsuo Y, Kamitani T, Yonezawa M, Yamasaki Y, Kawanami S, Abe K, Mukai Y, Higo T, Yabuuchi H, Takemura A, Yoshiura T, Sunagawa K, Honda H. Quantification of myocardial iron deficiency in nonischemic heart failure by cardiac t2* magnetic resonance imaging. The American journal of cardiology 2014;113:1024–1030 [DOI] [PubMed] [Google Scholar]
- 39.Bolger AP, Bartlett FR, Penston HS, O’Leary J, Pollock N, Kaprielian R, Chapman CM. Intravenous iron alone for the treatment of anemia in patients with chronic heart failure. Journal of the American College of Cardiology 2006;48:1225–1227 [DOI] [PubMed] [Google Scholar]
- 40.Usmanov RI, Zueva EB, Silverberg DS, Shaked M. Intravenous iron without erythropoietin for the treatment of iron deficiency anemia in patients with moderate to severe congestive heart failure and chronic kidney insufficiency. Journal of nephrology 2008;21:236–242 [PubMed] [Google Scholar]
- 41.Okonko DO, Grzeslo A, Witkowski T, Mandal AK, Slater RM, Roughton M, Foldes G, Thum T, Majda J, Banasiak W, Missouris CG, Poole-Wilson PA, Anker SD, Ponikowski P. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency ferric-hf: A randomized, controlled, observer-blinded trial. Journal of the American College of Cardiology 2008;51:103–112 [DOI] [PubMed] [Google Scholar]
- 42.Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Luscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart Rothe B, Pocock SJ, Poole-Wilson PA, Ponikowski P. Ferric carboxymaltose in patients with heart failure and iron deficiency. The New England journal of medicine 2009;361:2436–2448 [DOI] [PubMed] [Google Scholar]
- 43.Ponikowski P, van Veldhuisen DJ, Comin-Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD, Investigators C-H. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiencydagger. European heart journal 2015;36:657–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Avni T, Leibovici L, Gafter-Gvili A. Iron supplementation for the treatment of chronic heart failure and iron deficiency: Systematic review and meta-analysis. European journal of heart failure 2012;14:423–429 [DOI] [PubMed] [Google Scholar]
- 45.Kapoor M, Schleinitz MD, Gemignani A, Wu WC. Outcomes of patients with chronic heart failure and iron deficiency treated with intravenous iron: A meta-analysis. Cardiovascular & hematological disorders drug targets 2013;13:35–44 [DOI] [PubMed] [Google Scholar]
- 46.Gaber R, Kotb NA, Ghazy M, Nagy HM, Salama M, Elhendy A. Tissue doppler and strain rate imaging detect improvement of myocardial function in iron deficient patients with congestive heart failure after iron replacement therapy. Echocardiography (Mount Kisco, N.Y 2012;29:13–18 [DOI] [PubMed] [Google Scholar]
- 47.Motoki H, Borowski AG, Shrestha K, Troughton RW, Tang WH, Thomas JD, Klein AL. Incremental prognostic value of assessing left ventricular myocardial mechanics in patients with chronic systolic heart failure. Journal of the American College of Cardiology 2012;60:2074–2081 [DOI] [PubMed] [Google Scholar]
- 48.Bertini M, Ng AC, Antoni ML, Nucifora G, Ewe SH, Auger D, Marsan NA, Schalij MJ, Bax JJ, Delgado V. Global longitudinal strain predicts long-term survival in patients with chronic ischemic cardiomyopathy. Circulation. Cardiovascular imaging. 2012;5:383–391 [DOI] [PubMed] [Google Scholar]
- 49.Swedberg K, Young JB, Anand IS, Cheng S, Desai AS, Diaz R, Maggioni AP, McMurray JJ, O’Connor C, Pfeffer MA, Solomon SD, Sun Y, Tendera M, van Veldhuisen DJ, Committees R-H, Investigators R-H. Treatment of anemia with darbepoetin alfa in systolic heart failure. The New England journal of medicine 2013;368:1210–1219 [DOI] [PubMed] [Google Scholar]
- 50.Beck-da-Silva L, Piardi D, Soder S, Rohde LE, Pereira-Barretto AC, de Albuquerque D, Bocchi E, Vilas-Boas F, Moura LZ, Montera MW, Rassi S, Clausell N. Iron-hf study: A randomized trial to assess the effects of iron in heart failure patients with anemia. International journal of cardiology 2013;168:3439–3442 [DOI] [PubMed] [Google Scholar]
- 51.Van Wyck DB, Martens MG, Seid MH, Baker JB, Mangione A. Intravenous ferric carboxymaltose compared with oral iron in the treatment of postpartum anemia: A randomized controlled trial. Obstetrics and gynecology 2007;110:267–278 [DOI] [PubMed] [Google Scholar]
- 52.Tay EL, Peset A, Papaphylactou M, Inuzuka R, Alonso-Gonzalez R, Giannakoulas G, Tzifa A, Goletto S, Broberg C, Dimopoulos K, Gatzoulis MA. Replacement therapy for iron deficiency improves exercise capacity and quality of life in patients with cyanotic congenital heart disease and/or the eisenmenger syndrome. International journal of cardiology 2011;151:307–312 [DOI] [PubMed] [Google Scholar]
- 53.Bailie GR. Comparison of rates of reported adverse events associated with i.V. Iron products in the united states. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists 2012;69:310–320 [DOI] [PubMed] [Google Scholar]
- 54.Niehaus ED, Malhotra R, Cocca-Spofford D, Semigran M, Lewis GD. Repletion of iron stores with the use of oral iron supplementation in patients with systolic heart failure. Journal of cardiac failure 2015;21:694–697 [DOI] [PubMed] [Google Scholar]
- 55.Coe EM, Bowen LH, Speer JA, Bereman RD. Comparison of polysaccharide iron complexes used as iron supplements. Journal of inorganic biochemistry 1995;57:287–292 [DOI] [PubMed] [Google Scholar]
- 56.Ganzoni AM. [intravenous iron-dextran: Therapeutic and experimental possibilities]. Schweizerische medizinische Wochenschrift 1970;100:301–303 [PubMed] [Google Scholar]
- 57.Guazzi M, Dickstein K, Vicenzi M, Arena R. Six-minute walk test and cardiopulmonary exercise testing in patients with chronic heart failure: A comparative analysis on clinical and prognostic insights. Circulation. Heart failure 2009;2:549–555 [DOI] [PubMed] [Google Scholar]
- 58.Chatterjee NA, Murphy RM, Malhotra R, Dhakal BP, Baggish AL, Pappagianopoulos PP, Hough SS, Semigran MJ, Lewis GD. Prolonged mean vo2 response time in systolic heart failure: An indicator of impaired right ventricular-pulmonary vascular function. Circulation. Heart failure 2013;6:499–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


