Abstract
1. Monitoring the response of wild mammal populations to threatening processes is fundamental to effective conservation management. This is especially true for infectious diseases, which may have dynamic and therefore unpredictable interactions with their host.
2. We investigate the long-term impact of a transmissible cancer, devil facial tumour disease (DFTD), on the endemic Tasmanian devil. We analyse trends in devil spot-light counts and density across the area impacted by the disease. We investigate the demographic parameters which might be driving these trends, and use spatial capture-recapture models to examine whether DFTD has affected home range size.
3. We found that devils have declined by an average of 77% in areas affected by DFTD, and that there is a congruent trend of ongoing small decline in spotlight counts and density estimates. Despite this, devils have persisted to date within each of nine monitoring sites. One site is showing as yet unexplained small increases in density 8–10 years after the emergence of DFTD.
4. We also found the prevalence of DFTD has not abated despite large declines in density and that diseased sites continue to be dominated by young devils. The long-term impact of the disease has been partially offset by increased fecundity in the form of precocial breeding in 1-year-old females, and more pouch young per female in diseased sites. The lower densities resulting from DFTD did not affect home range size.
5. Synthesis and applications. Transmission of devil facial tumour disease continues despite large declines in devil density over multiple generations. Plasticity in life history traits has ameliorated the impact of devil facial tumour disease, however broad-scale trends in density show ongoing decline. In light of this, devil facial tumour disease and the impact of stochastic events on the reduced densities wrought by the disease, continue to threaten devils. In the absence of methods to manage disease in wild populations, we advocate managing the low population densities resulting from disease rather than disease per se.
Keywords: devil facial tumour disease, life history traits, population trends, Sarcophilus harrisii, SCR, spatial capture–recapture, Tasmanian devil, threatened species, transmissible cancer, wildlife disease
1 |. INTRODUCTION
Questions of trend, and mechanisms for change, in animal abundance are central to ecology and conservation management (Fryxell, Sinclair, & Caughley, 2014; Krebs, 2009). There is a need to address these questions with urgency when dealing with processes that threaten the population viability of species. Infectious diseases are one such process. They can directly threaten species with extinction via demographic impacts such as increasing mortality or reducing fecundity, and they can diminish populations to the point where they become susceptible to other threats (De Castro & Bolker, 2005; McCallum, 2012). Diseases that are spread regardless of density are considered to be particularly threatening because they are transmitted even when population densities are low (De Castro & Bolker, 2005). These frequency-dependent diseases pose an even greater risk to population viability if they have high infection and mortality rates (McCallum et al., 2009).
Tasmanian devils Sarcophilus harrisii (Boitard, 1841) are marsupial carnivores endemic to the 65,000 km2 island of Tasmania. Devil facial tumour disease (DFTD) is a rare type of transmissible cancer which to-date has only been reported in devils. It presents as soft tissue masses predominantly around the head, mouth and inside the oral cavity and was first formally described from tissue samples collected in 1997 (Loh et al., 2006; Pearse & Swift, 2006). By 2005, DFTD had spread to at least 51% of the island (Hawkins et al., 2006) and within 5 years following emergence it had caused local population declines of over 80% (McCallum et al., 2007). Commensurate with declines in devil abundance in disease affected areas, the average life expectancy at birth has been reduced from 5 years to 2 (Hamede et al., 2012, 2015; Lachish, Jones, & McCallum, 2007; Pemberton, 1990). The large population declines caused by DFTD have been associated with an increase in genetic relatedness in as little as three generations (Brüniche-Olsen, Burridge, Austin, & Jones, 2013; Lachish, Miller, Storfer, Goldizen, & Jones, 2011).
Devils are considered to be under threat of extinction from DFTD based on a study showing that the prevalence of the disease depends little if at all on the density of devils (McCallum et al., 2009). This, added to the observation that the disease has been in all but rare cases fatal within 6 months of the appearance of visible symptoms (cf. Hawkins et al., 2006; Wells et al., 2017), and that it can reach 100% prevalence in devils 2 years and older (Lachish et al., 2007; McCallum et al., 2009), indicates DFTD is a frequency dependent disease with high mortality and infection rates. Indeed, early epidemiological models predicted a high chance of localized extinctions within 25–30 years of DFTD emergence (McCallum et al., 2007) and were important justification of recovery actions which included captive breeding (Lees & Andrew, 2012), work towards development of an immunotherapy (Kreiss, Brown, Tovar, Lyons, & Woods, 2015), and establishment of disease-free wild sites (Department of Primary Industries Parks Water and Environment, 2010, 2014).
The severity of predictions from these early epidemiological models may be tempered by the higher proportion of 1-year-old female devils that were later found to partake in precocial breeding as a result of reduced population densities wrought by the disease (Jones et al., 2008; Lachish, McCallum, & Jones, 2009), and the potential for a higher female sex ratio in the pouch young of diseased mothers (Lachish et al., 2009). In addition, genetic changes consistent with the evolution of resistance to the disease have been observed at three sites (Epstein et al., 2016). Potential sources of variability in the long-term impact of DFTD are not limited to life history traits or genetic responses by devils; multiple areas of research at the molecular level have shown the disease is evolving (Pearse et al., 2012; Ujvari et al., 2013, 2014). This means that the impact of the disease, via for example mortality and infection rates, may change. The appearance of a second transmissible cancer in devils that presents in a very similar way to DFTD but with a different genetic profile highlights the dynamic nature of facial disease in devils (Pye et al., 2016). Given the restricted geographic range of records for this new disease (currently less than 60 km2), this research article will focus on the impact of the first identified form of DFTD.
In light of potential changes in the interaction between DFTD and devils there is need for an assessment of the long-term impact of DFTD across the devil’s range. Here, we use two independent datasets to investigate broad-scale temporal trends in devil counts and abundance. We analyse density trends within individual monitoring sites using spatial capture-recapture (SCR) techniques (Efford, Dawson, & Borchers, 2009) and examine the demographic processes that may be driving them. We expected to see ongoing decline in response to DFTD based on a frequency dependent mode of transmission. In addition, we expected that the rate of decline of devils may have been slower than previously predicted in light of precocial breeding in 1-year-old female devils. We discuss our results in the context of recovery, ongoing decline, or persistence of devils in the wild (Caughley, 1994). More broadly, we discuss the importance of long-term monitoring in response to disease events in order to understand and visualize the results of complex interactions which determine a species’ distribution and abundance.
2 |. MATERIALS AND METHODS
2.1 |. Field methods
2.1.1 |. Distribution and year of emergence of DFTD
Cases of DFTD confirmed by histopathological examination and/or cell culture were mapped in order to visualize the latest known state-wide distribution of the disease. Tissue samples were obtained via reports from members of the public and field biologists, and were diagnosed by Mt Pleasant Laboratories, Prospect, Tasmania. The dates and locations of the nearest confirmed cases were used to infer the minimum number of years since DFTD emergence for spotlight transects. The same approach was used to calculate the minimum number of years that DFTD had been present on trap-release sites unless the disease emerged during the course of trapping surveys.
2.1.2 |. Statewide devil counts
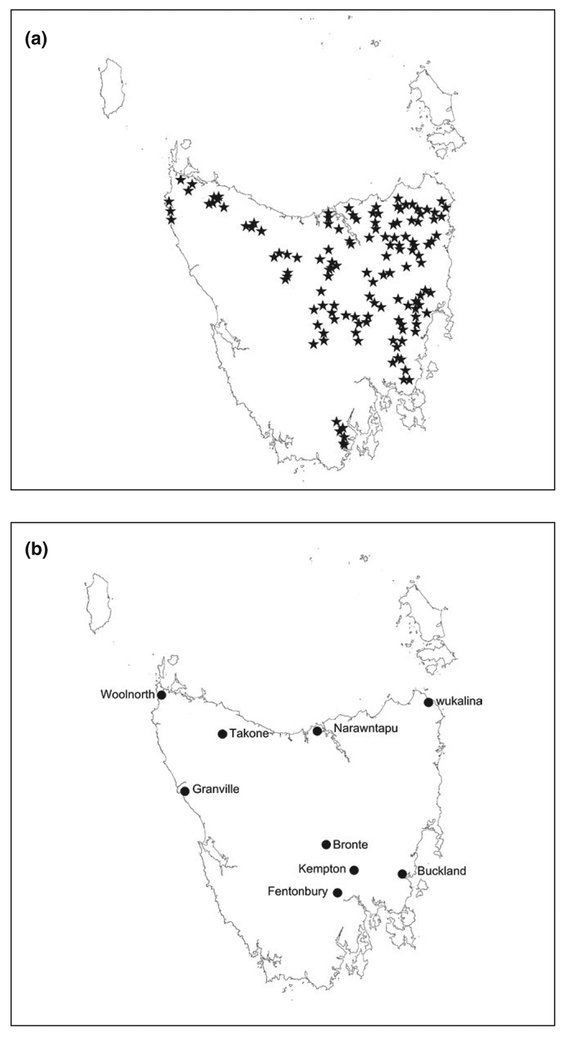
Annual spotlight surveys were conducted by the Department of Primary Industries, Parks, Water and Environment, and were established in 1975 in order to monitor browsing mammalian species subject to culling (Driessen & Hocking, 1992). Other non-domestic species, including devils, were also recorded. Spotlight survey effort was substantially increased, in conjunction with documented standardization of survey procedures, in 1985. Transects were 10 km in length on hardened and gravel roads, driven once per year between December and March, and all animals observed on both sides of the road with the aid of a hand held spotlight were recorded. Speed was held constant at 20 km/hr, and start and finish points were denoted by long-term landmarks (Driessen & Hocking, 1992). A subset consisting of 132 of these transects that have been consistently surveyed since 1985 was used to investigate broad-scale trends in devil counts (Figure 1a).
FIGURE 1.
(a) Stars mark the central point of each of 132 transects 10 km in length driven annually since 1985, and (b) Circles show the location of nine trap release sites where 40 traps were set for between 7 and 10 nights one to four times per year between 2004 and 2016
2.1.3 |. Capture–recapture
Tasmanian devils were trapped, marked, and released at nine sites across Tasmania from 2004 to 2016 (Figure 1b, see Supporting Information 1 (i). for maps of individual study sites). Up to 40 PVC pipe traps (diameter 315 mm × length 875 mm; N. Mooney and D. Ralph, unpublished) were deployed during each survey at each site over an area of c. 25 km2. Traps were baited with fresh lamb or wallaby meat and were deployed for seven to ten nights. Trap locations were selected by biologists based on the perceived likelihood of devil capture, and included crossroads, creek and road junctions, ecotones and next to latrines. Locations were limited to sites with four-wheel drive access. Repeat surveys using the same or similar individual trap locations were conducted as often as four times per year, or as infrequently as once per year, and some sites were not surveyed between 2007 and 2014 (summarized in Table 1; for full details of trap effort and area for each survey please see Supporting Information 1 part (ii)).
TABLE 1.
Summary survey effort and year of disease detection for each of nine study sites where Tasmanian devils were trapped and released. In cases where devil facial tumour disease (DFTD) was present when a site was first surveyed, minimum years since disease emergence was inferred by adopting the date of the nearest reported case of the disease
| Site | First and last year included in analysis |
Total surveys | Year disease detected |
|---|---|---|---|
| Bronte | 2004, 2016 | 13 | Present when first surveyed. Inferred 2003 |
| Buckland | 2006, 2016 | 8 | Present when first surveyed. Inferred 2005 |
| Fentonbury | 2004, 2016 | 22 | 2004 |
| Granville | 2005, 2016 | 7 | 2015 |
| Kempton | 2008, 2016 | 6 | Present when first surveyed. Inferred 2007 |
| Narawntapu | 2004, 2015 | 9 | 2011 |
| Takone | 2007, 2016 | 12 | 2009 |
| Woolnorth | 2004, 2016 | 8 | Not detected at last survey |
| wukalina | 2004, 2016 | 11 | Present when first surveyed. Inferred 1996 |
Traps were checked daily. Captured devils were individually marked with a microchip transponder (Allflex®, Palmerston North, New Zealand) which was placed under the skin on the back of the neck between shoulder blades. Weight, sex, reproductive status, and condition were recorded, and disease status and age were estimated. Scoring protocols based on a standard operating procedure were used for all assessments (Department of Primary Industries Parks Water and Environment, 2012). In brief, the reproductive status of female devils was assessed by examination of the pouch where the depth of the pouch and visual assessment of the amount of pouch oil was used to assess the stage of oestrus (Hesterman, Jones, & Schwarzenberger, 2008a). The presence of milk studs (which indicated lactation), or the size, number, and developmental stage of pouch young were recorded. There is no diagnostic test for DFTD therefore identification of the disease was limited to individuals with clear symptoms of early or late stage DFTD lesions. Where tumour samples were taken, field diagnoses of DFTD were verified by histopathological examination of tumour tissue and cell culture (Loh et al., 2006).
Age estimation was based on studies of seasonal breeding in devils (Green, 1967; Guiler, 1970; Keeley, McGreevy, & O’Brien, 2012; Pemberton, 1990), which determined the median birth date was 1st April. Tooth characteristics were used to estimate age, which was expressed as an annual age class. These characteristics included molar eruption, tooth wear, and canine over-eruption (the distance from the dentine enamel junction to the gum). This method is accurate for ageing devils until they are at least two (Lachish et al., 2007; Pemberton, 1990), and recaptures of known age devils assessed by different handlers through time have shown it to be accurate for devils 3 years and older.
Data consisting of individual measurements, trap locations and capture records for individual animals were lodged in Tasmania’s Natural Values Atlas (Natural Values Atlas version 3.4.1.3; Department of Primary Industries Parks Water and Environment, accessed 2016). Relevant data were then exported and collated to construct a capture and trap history file for each survey at each site. The capture file consisted of records for each devil capture and contained columns for survey, microchip number, night of capture, trap number, sex, age, and disease status.
2.2 |. Data analyses
2.2.1 |. Statewide devil counts and trends
Count data from spotlight transects, consisting of the number of devils sighted per 10 km route, was visualized and analysed to test for statewide decline in sightings since the first documented report of DFTD in 1996. Mean sightings per route from the 3-year period from 1993 to 1995 pre-disease were compared to the most recent 3-year survey period of 2014–2016 following the approach taken by Hawkins et al. (2006), using a one-tailed paired t test implemented in Program r (R Development Core Team, 2015). We used a one-tailed paired t test because we had a prior expectation of decline.
We estimated the trend in spotlight count by fitting generalized additive models (GAM). The models had two covariates; “year” and “minimum years since the first detection of DFTD” for each survey and transect. Transect was treated as a random factor. A quasipoisson fit was tested in order to account for the large number of zeros in the dataset. Models were fitted in r (R Development Core Team, 2015), using the mgcv package (Wood, 2011) and model selection was done based on AIC (Burnham & Anderson, 2002).
2.2.2 |. Density estimates
We used SCR models (Borchers & Efford, 2008) to estimate the density of devils through time and across sites. Surveys were 7–10 nights in duration therefore sites were considered to be closed to births, deaths, immigration and emigration for the duration of each survey. SCR models utilize the spatial information of the captures of an individual to determine two parameters: the probability of capture at the centre of the home range (g0), and the spatial scale over which capture probability declines with increasing distance from home range centre which can be translated into a 95% home range radius (σ; Borchers & Efford, 2008; Efford et al., 2009). This means that density estimates can be compared directly between sites or surveys, and that changes in trap layout or study animal space use can be incorporated into analyses. In addition, survey and individual level covariates can be modelled within a maximum likelihood framework (Borchers & Efford, 2008; Efford et al., 2009).
For our analyses we combined the data from all sites and years into a single model. Our model set consisted of parameters that we hypothesized a priori to potentially affect g0 and σ. These hypotheses were based on knowledge of the biology of devils and other carnivores and included that g0 and σ could vary by site, season or individual covariates of disease status, sex, or age. Given the small count of individuals within any one age class that results from surveys in long-term diseased sites, age was expressed as one of two levels: up to 2 years of age, or 3 years and over. This grouping corresponds with the change in age class structure that is typically wrought by DFTD, where devils 3 years and older are rare (Lachish et al., 2007). We analysed the models in a maximum likelihood framework, using the secr package version 3.0.1 (Efford, 2015) in r (R Development Core Team, 2015). We used a conditional likelihood model with a half-normal detection function and model selection was based on Akaike information criterion adjusted for finite sample sizes (AICc; Burnham & Anderson, 2002). We used a buffer of 10 km, which was estimated by multiplying the largest estimate of σ by three, around the trap area (Efford, 2015). Oceanic areas associated with coastal sites were excluded from the area over which density was estimated by applying a habitat mask (Efford, 2017).
We used a different modelling approach to test if σ was density dependent. To do this we excluded individual covariates and fitted models by maximizing the full likelihood. This allowed us to include density as a parameter and therefore test for density dependent σ.
The broad-scale trend in density across the disease zone was estimated by fitting a GAM to pooled density estimates across all sites. The model had “minimum years since the first detection of DFTD” as a covariate. Site was treated as a random factor. Models were fitted in r (R Development Core Team, 2015), using the mgcv package (Wood, 2011) and model selection was done based on AIC.
2.2.3 |. Demographic responses to DFTD and density
We used generalized linear mixed models to investigate how density, age structure (up to 2 years of age, or 3 years and over), disease prevalence (the proportion of all devils, and the proportion of 1-year-old devils, in any one survey showing symptoms consisted with DFTD), sex ratio, and fecundity changed with increasing time after DFTD emergence. Fecundity was investigated using two approaches; the percentage of females breeding, and the number of pouch young per breeding female with increasing time after the first occurrence of DFTD. Given earlier reports of an increase in precocial breeding in 1-year-old devils (Jones et al., 2008; Lachish et al., 2009), we explored three aspects of the percentage of females breeding and these were the number of females breeding as a proportion all females in a survey regardless of age, the proportion of 1-year-old females that bred, and the proportion of all breeding females that were 1-year-old. Demographic parameters were estimated for each survey at each site. We used a logistic model for all variables except for the number of pouch young per breeding female where we used a Poisson model (Bolker et al., 2009). We included study site as a random effect in order to account for any site based differences for all models and modelled “minimum years after DFTD” as a linear fixed effect. To account for possible drastic changes once DFTD was present in a population, we evaluated models that included an additional binary factor indicating whether DFTD was present during a survey or not. We also tested for seasonal variation, using a fixed effect with four levels. All models were implemented in R (R Development Core Team, 2015) using the lme4 package (Bates, Maechler, Bolker, & Walker, 2015) and model selection was done based on AIC.
We used two sets of data for each question excluding prevalence and fecundity: one that included all site surveys, and another that was limited to site surveys three or more years after the first confirmed case of DFTD at diseased sites. Demographic responses to DFTD in the first 3 years post-disease have been well documented for a small number of sites (Hamede et al., 2012; Lachish et al., 2007; McCallum et al., 2009), however longer-term responses have not. We were able to separate the marked changes that are known to occur in the short term from the potentially more subtle longer-term changes, using these two datasets. We limited modelling prevalence as a dependent variable to site surveys after disease had been recorded based on the same two datasets. The proportions of all females breeding, 1-year-old’s breeding, and all breeding females that were 1 year-old, and the number of pouch young per breeding female with increasing time after the first occurrence of DFTD were modelled as a dependent variable on two reduced datasets. One dataset consisted of surveys between autumn and spring when pouch young and/or early stage lactation is occurring and before weaning commences, and the other was based on surveys conducted in late autumn to winter when pouch young can be counted. For age and disease prevalence we adjusted percentages based on the results from the SCR model to account for differences in g0 and σ of different groups.
3 |. RESULTS
3.1 |. Distribution of DFTD and statewide devil counts
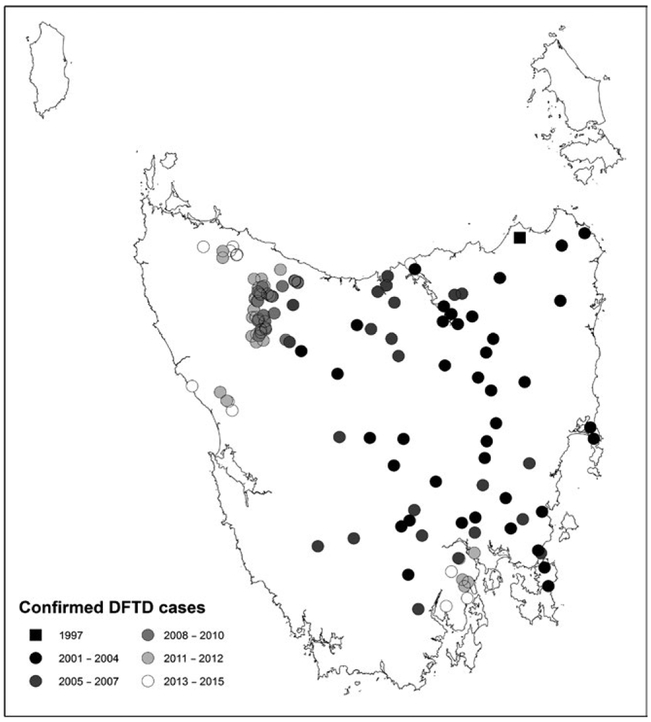
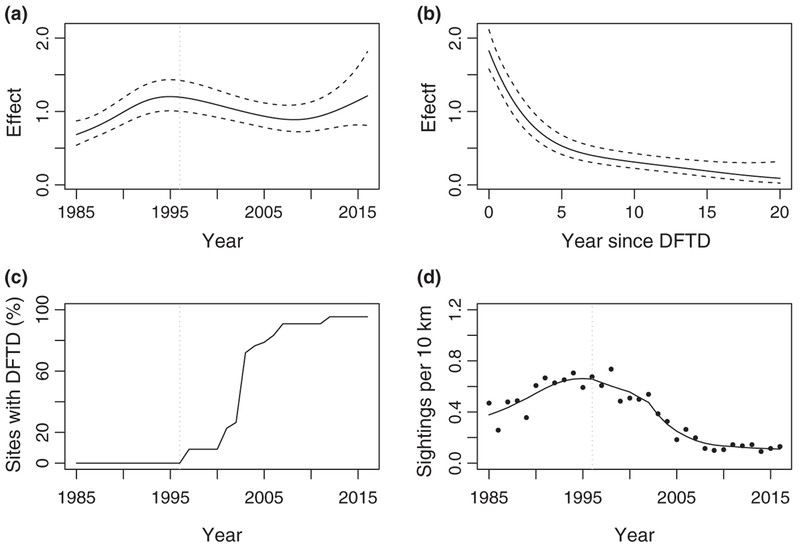
As of September 2017, a minimum convex polygon around confirmed cases indicated DFTD covered over 80% percent of the state of Tasmania, excluding offshore islands (Figure 2). Average devil sightings per spotlighting route increased until the mid 1990s, followed by a significant decline of 83% from counts conducted immediately prior to the first report of DFTD between 1993 and 1995, compared to average post-disease counts from 2014 to 2016 (paired t test t = 12.18, df = 131, p = <.0001). The GAM allowed us to separate temporal trends from the effect of DFTD. When the effect of DFTD was excluded, counts increased until 1995 and then stabilized (Figure 3a). The impact of DFTD on the count of devils in diseased areas (Figure 3b) in combination with the spread of the disease (Figure 3c) showed that counts declined rapidly within 5 years of DFTD emergence (Figure 3d).
FIGURE 2.
Cases of devil facial tumour diseased confirmed via histopathological examination to May 2017. The black square represents the first confirmed case at Waterhouse. Each circle represents the first confirmed record from an area, with later subsequent records from the same area not shown
FIGURE 3.
Results form a generalized additive model for state-wide spotlight sightings of devils along 132 transects 10 km in length surveyed annually from 1985 to 2016. (a and b) The estimated smoothing curves with 95% confidence intervals of year and years since devil facial tumour disease (DFTD) respectively (y-axis values were back transformed). (c) The percentage of transects where DFTD was present and (d) the observed (dots) and estimated (line) mean sightings for all transects over time. The dashed vertical line represents the first documented report of DFTD
3.2 |. SCR models
The full capture-recapture dataset included nine sites, 96 surveys across all sites, 34,853 trap nights, 3,814 individual devils, and 7,288 captures (see Supporting Information 1 part (ii) for details of trap effort and animal captures from each survey at each site). The highest-ranking SCR model included site, age, and disease covariates for g0 (the probability of being captured at the home range centre). The highest-ranking model also included site and age covariates for σ (spatial scale over which capture probability declines with increasing distance from home range centre which can be translated into a 95% home range radius; see Supporting Information 1 part (iii) for model rankings). Healthy devils, and devils 3 years and older, were more likely to be caught at the centre of their home range compared to devils showing signs of DFTD and devils under the age of three (average g0 across all sites healthy devils under three = 0.0257 ± SE0.0036, healthy devils 3 years and over = 0.0357 ± 0.0050, diseased devils under three = 0.0206 ± 0.0029, diseased devils 3 years and over = 0.0287 ± 0.0040). Granville Harbour had the highest, and Woolnorth the lowest values of g0 (see Supporting Information 1 part (iv) for model outputs for each site and covariate for g0). Devils under the age of three had a larger estimated home range (average σ across all sites devils under three = 1,666 ± 153 m, 3 years and older = 1,377 ± 127), with devils at Narawntapu and Woolnorth having the highest values and devils at Fentonbury and Granville the lowest (see Supporting Information 1 part (v) for model outputs for each site and covariate for σ). Full likelihood models provided no support for density dependence in σ.
3.3 |. Density trends
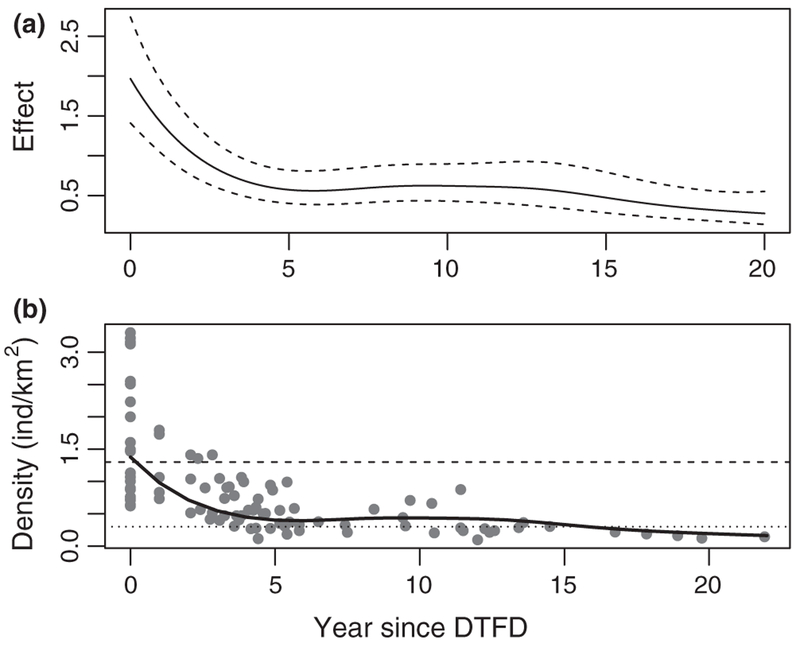
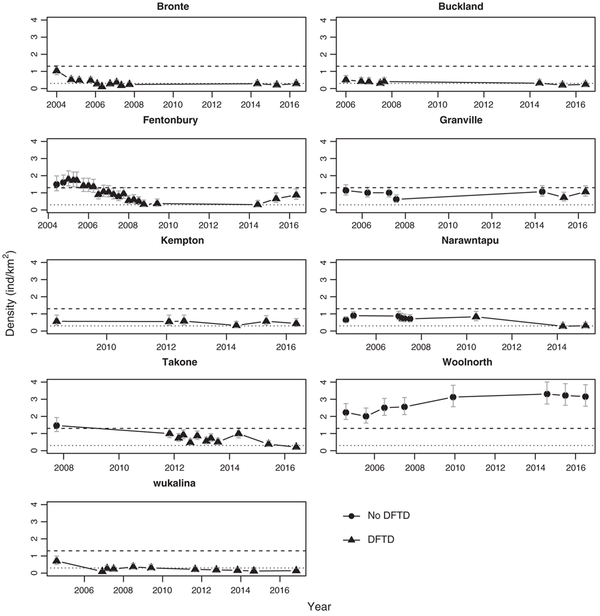
Pooled density estimates across all sites showed that there was ongoing small decline in devil densities associated with years since DFTD emergence (Figure 4). Despite this broad-scale trend, devils persisted within individual monitoring sites (Figure 5; see Supporting Information 1 part (vi) for density estimates for each site and survey). Median density in healthy populations was 1.3 ind. per km2 (range 0.62–3.30 ind. per km2) and declined by 77% to a median density of 0.3 ind. per km2 5 years after DFTD emergence. There was a consistent decline in response to DFTD emergence within all sites. Following this it was not clear whether density stabilized or there was ongoing small decline. One site, Fentonbury, showed an atypical response of small ongoing increases in density 8–10 years after DFTD emergence. Woolnorth was the only study site where the disease had not been recorded, and density showed an increase over the first 5 years after which it stabilized at a level markedly higher than the pre-disease levels of other sites.
FIGURE 4.
The change in density of devils with years since devil facial tumour disease (DFTD) emergence across nine sites. Density was estimated for a total of 96 surveys using spatial capture-recapture analysis, and the trend in density estimates with years since DFTD was modelled with a generalized additive model. (a) Estimated smoothing curve of years since DFTD with 95% confidence intervals (y-axis values were back transformed), and (b) the observed densities (dots) and the estimated mean (line) as a function of years since the emergence of DFTD. The dotted dashed line represents the median post disease density (5 and more years after first case) across the nine monitoring sites, and the dashed line the median pre-diseased density across sites
FIGURE 5.
Trends in the estimated density of devils through time at nine sites. Densities were estimated with a spatial capture-recapture model. Light dashed lines represent the average post disease density (5 and more years after first case) across the nine monitoring sites, and heavier dashed line the average pre-diseased density across sites. Error bars represent 95% confidence intervals
3.4 |. Demographic responses to DFTD and density
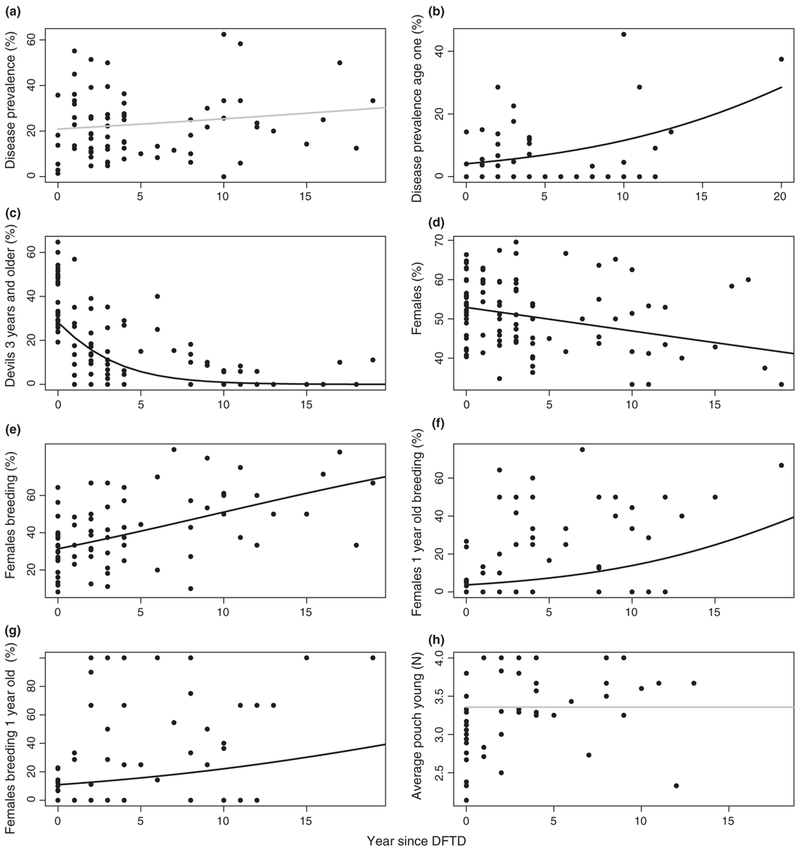
We found a weak increase in disease prevalence irrespective of devil age class that was not significant with increasing time after the first case of DFTD (βYearDFTD = 0.025 ± SE0.019, p = .187) and lower prevalence in the autumn and winter (βAutumn = −0.352 ± 0.170, p = .039; βWinter = −0.311 ± 0.164, p = .020; Figure 6a). On the contrary, there was a significant increase in the prevalence of DFTD in 1-year-old devils with years since DFTD (βYearDFTD = 0.112 ± 0.040, p = .005) and prevalence was higher in spring and summer (βSpring = 0.745 ± 0.415, p = .073; βSummer = 1.619 ± 0.377, p = <.005). The proportion of devils 3 years and older was lower when the disease was present and drastically declined over time (βDFTD = −0.372 ± 0.144, p = .010; βYearDFTD = −0.248 ± 0.036, p < .0001). It was also lower in the winter (βWinter = −0.273 ± 0.125, p = .029). When evaluating data from 3 years and more the linear trend disappeared indicating a stable age structure with mainly young devils at long-term disease sites, but there was a seasonal pattern with a slightly higher proportion of older devils during the summer (βAutumn = −1.330 ± 0.621, p = .034; βWinter = −0.918 ± 0.635, P = .149; βSpring = −1.416 ± 0.651, p = .030; Figure 6b).
FIGURE 6.
Trends in demographic parameters calculated for individual surveys at each of nine sites as a function of number of years since the emergence of devil facial tumour disease (DFTD) estimated by generalized linear mixed models. Graphs represent (a) disease prevalence expressed as a percentage and based on the proportion of all devils showing symptoms consistent with DFTD, (b) disease prevalence in 1-year-old devils, (c) the percentage of devils 3 years and older, (d) the percentage of female devils, (e) the percentage of females breeding across all age classes, (f) the percentage of 1-year-old females that bred (as a proportion of all 1-year-old females), (g) the percentage of all breeding females that were 1-year-old, and (h) the average number of pouch young per breeding female. Data were combined from nine study sites and site was included in the models as a random effect. Black lines indicate significant trends, grey lines non-significant trends
The proportion of females decreased over the years after DFTD (βYearDFTD = −0.024 ± 0.013, p = .055), but that relationship was weak and there was a lot of variation across surveys. There was no significant relationship when only looking at data from 3 years and more (Figure 6c). There was a strong positive relationship between the proportion of female devils breeding and the number of years after DFTD (βYearDFTD = 0.083 ± 0.021, p < .0001; Figure 6d). The percentage of 1-year-old females that were breeding as a proportion of all 1-year-old females significantly increased with years after DFTD (βYearDFTD = 0.131 ± 0.035, p = .0002; Figure 6e) and so did the percentage of 1-year-old females as a proportion of breeding females across all ages (βYearDFTD = 0.084 ± 0.039, p = .030; Figure 6f). There were significantly more pouch young per female in surveys where DFTD was present but there was no linear trend over time (βDFTD = 0.137 ± 0.061, p = .003; Figure 6g).
4 |. DISCUSSION
Devil facial tumour disease has spread. As of September 2017 the disease covered over 80% percent of the state of Tasmania, which is a 30% increase on that reported in Hawkins et al. (2006). Our expectation, based on the frequency dependent mode of transmission of DFTD, was that devil densities would continue to decline in areas affected by the disease. Spotlight counts across the disease zone, and pooled density estimates from nine trap-release sites confirmed this by showing a congruent trend of ongoing decline through time following the emergence of DFTD. There was also a similar magnitude of decline of 83% and 77% between average pre- and post-disease spotlight and density estimates respectively. Despite this overall trend, devils persisted at low density within individual trap-release monitoring sites. One site even showed small increases in density over the 2-year period between 2014 and 2016.
Devils may have persisted within sites despite indications of ongoing small decline at a broader scale because the trend beyond 12 years of DFTD emergence is limited to one site, wukalina. We note that our estimation of years since DFTD emergence represents the minimum years that the disease may have been present in an area because it may not have been detected when it first emerged. Despite this, there is clearly potential for site-based variability in the impact of DFTD as illustrated by the small and sustained increase in densities observed at Fentonbury between 2014 and 2016. In comparison, there have been no indications of an increase in density at wukalina across any of the years that the site has been monitored. The clear trend of ongoing small decline in devil counts across spotlight transects with years since DFTD lends weight to the concept that at least some monitoring sites may experience ongoing small decline in devil densities as the longer-term impact of the disease unfolds. Ecological theory, and observations of decline and extinction in other species, suggests the cumulative impact of stochastic events on reduced densities can result in inexorable decline (Belovsky, Mellison, Larson, & Van Zandt, 1999).
The mechanism for the small ongoing increase in the density of devils at Fentonbury 8–10 years after DFTD emerged at the site is not known at this stage. DFTD is still present on the site. If resistance to the disease was evolving (Epstein et al., 2016) then we would expect to see restoration of devils 3 years and older. Older devils are still rare at Fentonbury however it may take 2–3 years for this demographic signal to change as the 1-year-old devils that typically constitute the bulk of the devils at long-term diseased sites mature into 3-year olds. One site, called West Pencil Pine, which is not included in this analysis, experienced a temporary reduction in the impact of DFTD attributed to a tetraploid strain of the disease which had lower infection rate (Hamede et al., 2015). There is ongoing analysis of tumour samples collected from all monitoring sites (L. Murchison, pers. comm.) and those collected from Fentonbury have not indicated that a less infectious form of the disease has emerged in recent years. Immigration is a potential mechanism for the increase, however, it seems unlikely given devil densities are low in surrounding areas. Ongoing monitoring at the site will aid interpretation of the density trend, and whether it is caused by a change in the disease, such as a less virulent form, a change in devils, such as evolution of resistance, or it is a cumulation of fortuitous stochastic events.
Measures of demographic variables were pooled across all sites and surveys and used to assess the mechanisms driving past, and signal future change or stability in density trends. Estimated variables were limited to data that was collected within each trapping session, because the recapture rate between yearly trapping sessions following the emergence of DFTD was too low to effectively model rates such as survival and recruitment. We found there was no reduction in the prevalence of DFTD or restoration of older age classes. The female bias in pouch young of diseased mothers observed by Lachish et al. (2009) did not result in a change towards a female bias in sex ratio with years since DFTD across the sites that we monitored. The impact of the disease was at least partially offset by precocial breeding in 1-year-old females, and more pouch young per female in diseased sites, however these offsets were concomitant with an increase in disease prevalence in 1-year-old devils. Taken together, these results indicate that plasticity in devil life history traits, that are triggered by reduced densities, have allowed them to persist with the disease to date.
The prevalence of DFTD across the eight diseased sites that we monitored did not abate with declining densities. This observation affirms the conclusion that DFTD is a frequency-dependent disease (McCallum et al., 2009). Our results gave no indication of a density threshold for the disease (McCallum, 2012), however estimates of disease prevalence were variable. This variability is not surprising as chance events could have a large impact on the small sample sizes we were dealing with in long-term disease sites. Previous research has found the prevalence of DFTD to be lower in 1- to 2-year-old devils in the first 5 years following disease emergence (Lachish et al., 2009; McCallum et al., 2009), however our results show that prevalence increased in 1-year-old devils with years since DFTD. Our results support the idea that the frequency of encounter in young devils increases as they breed younger (McCallum et al., 2009) and therefore increases their chances of contracting DFTD. In addition, there may be less social inhibitions on interactions in long-term diseased sites with fewer, potentially socially dominant, older devils around (Hamede, McCallum, & Jones, 2013; Wells et al., 2017).
We observed a new form of population compensation to DFTD with females in diseased sites having on average more pouch young, and we confirmed a higher rate of precocial breeding in 1-year-old devils with years since emergence of the disease. The higher proportion of females breeding was caused by an increase in the proportion of 1-year-old females breeding as the years since DFTD unfolded and devil density decreased. This precocial breeding in response to DFTD is believed to result from a reduction in competition for food (Jones et al., 2008; Lachish et al., 2009), which results in young female devils reaching sexual maturity earlier (Hesterman, Jones, & Schwarzenberger, 2008b). It may also result from less inhibitions to sexual maturity and mating because older devils are rare if not absent from diseased sites (Lachish et al., 2007). The mechanisms for precocial breeding might also explain the greater number of pouch young observed in females at diseased sites.
The degree to which population compensation can reduce the long-term impact of DFTD is unclear, but population growth rate is known to increase exponentially in mammals as the age of first reproduction decreases (Hone, Duncan, & Forsyth, 2010). This means that precocial breeding may be a powerful offset to DFTD. We suspect that the rate of precocial breeding and the number of pouch young per female may be near maximum capacity in response to the reduced densities caused by DFTD (Lachish et al., 2009), with some variability in compensation caused by stochastic events. In light of this we are unlikely to see any ongoing increases in density as a result of these forms of population compensation in the future.
Spatial capture-recapture modelling facilitated our analysis of a large dataset collected over many years. It did this by accommodating small changes in trap layout, allowing for potential changes in space use between sites and surveys which may have affected the trappability of devils, having the capacity to include covariates within a model selection framework (Muneza et al., 2016), and excluding oceanic areas from the area over which density was estimated in coastal sites. This meant that estimates of density were directly comparable between sites.
Spatial capture-recapture modelling indicated that trap-deduced home range size was site-specific, and that it did not change in response to the reduced densities caused by DFTD. One of the determinants of home range size is food availability (Gittleman & Harvey, 1982). Lower densities resulting from DFTD are likely to have resulted in more abundant food resources over smaller areas, therefore it is surprising but encouraging home range size did not decrease. The maintenance of home range sizes indicates that although reduced densities have reduced nightly opportunities for social interaction between devils and other species, the interactions that remain are still occurring over the same spatial scale. This result, however, is limited to trap-deduced home range sizes outside of the breeding season. We are unable to comment on the impact of DFTD on the dispersal distances or the size of mating forays which have the potential to affect genetic diversity (Brüniche-Olsen et al., 2013; Lachish et al., 2011).
There were significant but small differences in g0 (the probability of being captured at the home range centre), and σ (spatial scale over which capture probability declines with increasing distance from home range centre which can be translated into a 95% home range radius) between different sites, and devils of different age and disease status. Devils 3 years and older, and healthy devils, had higher g0 values. Younger devils had larger σ values. We suspect that older devils were less cautious in entering traps because they may have had more opportunities to experience them as younger devils during previous surveys. Healthy devils may have had better olfactory senses compared to diseased devils with late stage lesions, therefore they were able to locate traps more efficiently. Devils under the age of three may have tended towards larger home range sizes because this group included devils that were recently weaned and were yet to establish a home range.
5 |. CONCLUSIONS
The mode of transmission of DFTD remains frequency-dependent, with no sign of a density threshold. Devil densities have declined on average by 77% in areas affected by disease, and broad scale trends in spotlight counts and density indicate ongoing small decline. Despite this, devils have persisted in individual monitoring sites, at least partly because populations have compensated for low densities by increasing fecundity. There are early signs of variability in the long-term impact of DFTD at monitoring sites which cannot be attributed to disease virulence, but the exact mechanism for this variability is not currently known.
5.1 |. Management implications
The interaction between devils and DFTD highlights how important it is to monitor the realization of the complex and potentially unpredictable interplay between diseases and their hosts. DFTD and the impact of stochastic events on the reduced densities wrought by the disease continue to threaten devils. These impacts are likely to be compounded across larger areas of the devil’s range as the demographic and genetic consequences of low densities affects their metapopulation dynamics. There are currently no effective known methods for managing DFTD in wild populations. Research into the development of an immunotherapy is ongoing (Kreiss et al., 2015) and removal of known infected individuals from a semi-isolated site was trialled and was ineffective (Beeton & McCallum, 2011; Lachish, McCallum, Mann, Pukk, & Jones, 2010). This means that recovery effort in wild populations should focus on the low densities resulting from DFTD, rather than the disease per se. Such recovery efforts should include reducing the impact of other threats such as road mortality, and maintenance of genetic diversity. Research that investigates methods to increase population viability in diseased areas is a priority. Ultimately management actions should aim to maximize the ability of devils to fulfil their ecological function.
Supplementary Material
ACKNOWLEDGEMENTS
Trapping and handling of devils was conducted under permits issued by the Department of Primary Industries, Parks, Water, and Environment for animal ethics, scientific research and standard operating procedures. Volunteers and landowners generously provided assistance with surveys and access to land. The authors also acknowledge Nick Mooney who instigated collection of public records for disease spread and provided significant input into the design of the monitoring program, Jane McGee for management of public reports, Save the Devil Program members, and University of Tasmania students past and present for collection of data. Ron Swaisgood was instrumental in facilitating the collaboration between the Save the Devil Program and San Diego Zoo Global, without which the analyses presented would not have been possible. This work was funded by the Australian Commonwealth Government, Tasmanian State Government, and Toledo Zoo.
Funding information
Toledo Zoo and Aquarium; Australian Government; Tasmanian State Government
Footnotes
Handling Editor: Margaret Stanley
AUTHORS’ CONTRIBUTION
B.L., M.T., C.H., G.H., M.J., H.W., S.F. and D.P. conceived the ideas and designed the experiment; B.L., W.B., C.H., C.L., G.H., F.H., S.H., P.I., S.T., P.W. and S.F. collected the data; M.T. and B.L. analysed and interpreted the data; B.L., M.T. and D.P. drafted the article. All authors contributed critically to the drafts and gave final approval for publication.
DATA ACCESSIBILITY
Capture histories and trap locations for SCR analysis, and devil spotlight counts: Dryad Digital Repository https://doi.org/10.5061/dryad.j1sb5 (Lazenby et al., 2018).
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- Bates D, Maechler M, Bolker B, & Walker S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67, 1–48. [Google Scholar]
- Beeton N, & McCallum H (2011). Models predict that culling is not a feasible strategy to prevent extinction of Tasmanian devils from facial tumour disease. Journal of Applied Ecology, 48, 1315–1323. 10.1111/j.1365-2664.2011.02060.x [DOI] [Google Scholar]
- Belovsky GE, Mellison C, Larson C, & Van Zandt PA (1999). Experimental studies of extinction dynamics. Science, 286, 1175–1177. 10.1126/science.286.5442.1175 [DOI] [PubMed] [Google Scholar]
- Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, & White JSS (2009). Generalized linear mixed models: A practical guide for ecology and evolution. Trends in Ecology & Evolution, 24, 127–135. 10.1016/j.tree.2008.10.008 [DOI] [PubMed] [Google Scholar]
- Borchers DL, & Efford MG (2008). Spatially explicit maximum likelihood methods for capture–recapture studies. Biometrics, 64, 377–385. 10.1111/j.1541-0420.2007.00927.x [DOI] [PubMed] [Google Scholar]
- Brüniche-Olsen A, Burridge CP, Austin JJ, & Jones ME (2013). Disease induced changes in gene flow patterns among Tasmanian devil populations. Biological Conservation, 165, 69–78. 10.1016/j.biocon.2013.05.014 [DOI] [Google Scholar]
- Burnham KP, & Anderson DR (2002). Model selection and multimodel inference: A practical information-theoretic approach. Berlin, Germany: Springer Science & Business Media. [Google Scholar]
- Caughley G (1994). Directions in conservation biology. Journal of Animal Ecology, 63, 215–244. 10.2307/5542 [DOI] [Google Scholar]
- De Castro F, & Bolker B (2005). Mechanisms of disease induced extinction. Ecology Letters, 8, 117–126. [Google Scholar]
- Department of Primary Industries Parks Water and Environment. (2010). Draft recovery plan for the Tasmanian devil (Sarcophilus harrisii). Australia: Tasmanian Government. [Google Scholar]
- Department of Primary Industries Parks Water and Environment. (2012). Standard operating procedure: Trapping and handling wild Tasmanian devils v 1.1. Australia: Tasmanian Government. [Google Scholar]
- Department of Primary Industries Parks Water and Environment. (2014). Save the Tasmanian Devil Program business plan 2014–2019. Australia: Tasmanian Government. [Google Scholar]
- Driessen MM, & Hocking G (1992). Review and analysis of spotlight surveys in Tasmania: 1975–1990. Australia: Tasmanian Government. [Google Scholar]
- Efford MG (2015). Package ‘secr’. Retrieved from https://cran.r-project.org/web/packages/secr/secr.pdf
- Efford M (2017). Habitat masks in the package secr. Retrieved from http://www.otago.ac.nz/density/pdfs/secr-habitatmasks.pdf
- Efford MG, Dawson DK, & Borchers DL (2009). Population density estimated from locations of individuals on a passive detector array. Ecology, 90, 2676–2682. 10.1890/08-1735.1 [DOI] [PubMed] [Google Scholar]
- Epstein B, Jones M, Hamede R, Hendricks S, McCallum H, Murchison EP, … Storfer A (2016). Rapid evolutionary response to a transmissible cancer in Tasmanian devils. Nature Communications, 7, 12684 10.1038/ncomms12684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryxell JM, Sinclair AR, & Caughley G (2014). Wildlife ecology, conservation, and management. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- Gittleman JL, & Harvey PH (1982). Carnivore home-range size, metabolic needs and ecology. Behavioral Ecology and Sociobiology, 10, 57–63. 10.1007/BF00296396 [DOI] [Google Scholar]
- Green RH (1967). Notes on the Devil (Sarcophilus harrisii) and the Quoll (Dasyurus viverrinus) in north-eastern Tasmania. Records of the Queen Victoria Museum, 27, 1–12. [Google Scholar]
- Guiler ER (1970). Observations on the Tasmanian devil Sarcophilus harrisii, Marsupialia, Dasyuridae. Part 2 Reproduction, breeding, and growth of pouch young. Australian Journal of Zoology, 18, 63–70. 10.1071/Z09700063 [DOI] [Google Scholar]
- Hamede R, Lachish S, Belov K, Woods G, Kreiss A, Pearse A-M., … McCallum H (2012). Reduced effect of Tasmanian devil facial tumor disease at the disease front. Conservation Biology, 26, 124–134. 10.1111/j.1523-1739.2011.01747.x [DOI] [PubMed] [Google Scholar]
- Hamede RK, McCallum H, & Jones M(2013). Biting injuries and transmission of Tasmanian devil facial tumour disease. Journal of Animal Ecology, 82, 182–190. 10.1111/j.1365-2656.2012.02025.x [DOI] [PubMed] [Google Scholar]
- Hamede RK, Pearse AM, Swift K, Barmuta LA, Murchison EP, & Jones ME (2015). Transmissible cancer in Tasmanian devils: Localized lineage replacement and host population response. Proceedings of the Royal Society of London Series B, Biological Sciences, 282, 20151468–20151468. 10.1098/rspb.2015.1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins CE, Baars C, Hesterman H, Hocking GJ, Jones ME, Lazenby B, … Wiersma J (2006). Emerging disease and population decline of an island endemic, the Tasmanian devil Sarcophilus harrisii. Biological Conservation, 131, 307–324. 10.1016/j.biocon.2006.04.010 [DOI] [Google Scholar]
- Hesterman H, Jones SM, & Schwarzenberger F (2008a). Pouch appearance is a reliable indicator of the reproductive status in the Tasmanian devil and the spotted-tailed quoll. Journal of Zoology, 275, 130–138. 10.1111/j.1469-7998.2008.00419.x [DOI] [Google Scholar]
- Hesterman H, Jones SM, & Schwarzenberger F (2008b). Reproductive endocrinology of the largest dasyurids: Characterization of ovarian cycles by plasma and fecal steroid monitoring. Part I. The Tasmanian devil (Sarcophilus harrisii). General and Comparative Endocrinology, 155, 234–244. 10.1016/j.ygcen.2007.05.013 [DOI] [PubMed] [Google Scholar]
- Hone J, Duncan RP, & Forsyth DM (2010). Estimates of maximum annual population growth rates (rm) of mammals and their application in wildlife management. Journal of Applied Ecology, 47, 507–514. 10.1111/j.1365-2664.2010.01812.x [DOI] [Google Scholar]
- Jones ME, Cockburn A, Hamede R, Hawkins C, Hesterman H, Lachish S, … Pemberton D (2008). Life-history change in disease-ravaged Tasmanian devil populations. Proceedings of the National Academy of Sciences of the United States of America, 105, 10023–10027. 10.1073/pnas.0711236105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley T, McGreevy PD, & O’Brien JK (2012). The effects of season and devil facial tumour disease on the reproductive physiology of the male Tasmanian devil (Sarcophilus harrisii). Reproduction Fertility and Development, 24, 999–1007. 10.1071/RD11134 [DOI] [PubMed] [Google Scholar]
- Krebs CJ (2009). Ecology: The experimental analysis of distribution and abundance (6th ed.). San Francisco, CA: Benjamin Cummings. [Google Scholar]
- Kreiss A, Brown GK, Tovar C, Lyons AB, & Woods GM (2015). Evidence for induction of humoral and cytotoxic immune responses against devil facial tumor disease cells in Tasmanian devils (Sarcophilus harrisii) immunized with killed cell preparations. Vaccine, 33, 3016–3025. 10.1016/j.vaccine.2015.01.039 [DOI] [PubMed] [Google Scholar]
- Lachish S, Jones M, & McCallum H (2007). The impact of disease on the survival and population growth rate of the Tasmanian devil. Journal of Animal Ecology, 76, 926–936. 10.1111/j.1365-2656.2007.01272.x [DOI] [PubMed] [Google Scholar]
- Lachish S, McCallum H, & Jones M (2009). Demography, disease and the devil: Life history changes in a disease-affected population of Tasmanian devils (Sarcophilus harrisii). Journal of Animal Ecology, 78, 427–436. 10.1111/j.1365-2656.2008.01494.x [DOI] [PubMed] [Google Scholar]
- Lachish S, McCallum H, Mann D, Pukk CE, & Jones ME (2010). Evaluation of selective culling of infected individuals to control Tasmanian devil facial tumor disease. Conservation Biology, 24, 841–851. 10.1111/j.1523-1739.2009.01429.x [DOI] [PubMed] [Google Scholar]
- Lachish S, Miller KJ, Storfer A, Goldizen AW, & Jones ME (2011). Evidence that disease-induced population decline changes genetic structure and alters dispersal patterns in the Tasmanian devil. Heredity, 106, 172–182. 10.1038/hdy.2010.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazenby BL, Tobler MW, Brown WE, Hawkins CE, Hocking GJ, Fume E, … Pemberton D (2018). Data from: Density trends and demographic signals uncover the long-term impact of a transmissible cancer in Tasmanian Devils. Dryad Digital Repository, 10.5061/dryad.j1sb5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees C, & Andrew P (2012). The Tasmanian devil insurance meta-population: 2012 evaluation and review. Apple Valley, MN: IUCN/SSC Conservation Breeding Specialist Group. [Google Scholar]
- Loh R, Bergfeld J, Hayes D, O’Hara A, Pyecroft S, Raidal S, & Sharpe R (2006). The pathology of devil facial tumor disease (DFTD) in Tasmanian devils (Sarcophilus harrisii). Veterinary Pathology, 43, 890–895. 10.1354/vp.43-6-890 [DOI] [PubMed] [Google Scholar]
- McCallum H (2012). Disease and the dynamics of extinction. Philosophical Transactions of the Royal Society B-Biological Sciences, 367, 2828–2839. 10.1098/rstb.2012.0224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum H, Jones M, Hawkins C, Hamede R, Lachish S, Sinn DL, … Lazenby B (2009). Transmission dynamics of Tasmanian devil facial tumor disease may lead to disease-induced extinction. Ecology, 90, 3379–3392. 10.1890/08-1763.1 [DOI] [PubMed] [Google Scholar]
- McCallum H, Tompkins DM, Jones M, Lachish S, Marvanek S, Lazenby B, … Hawkins CE (2007). Distribution and impacts of Tasmanian devil facial tumor disease. EcoHealth, 4, 318–325. 10.1007/s10393-007-0118-0 [DOI] [Google Scholar]
- Muneza AB, Linden DW, Montgomery RA, Dickman AJ, Roloff GJ, Macdonald DW, & Fennessy JT (2016). Examining disease prevalence for species of conservation concern using non-invasive spatial capture–recapture techniques. Journal of Applied Ecology, 54, 709–717. [Google Scholar]
- Pearse AM, & Swift K (2006). Allograft theory: Transmission of devil facial-tumour disease. Nature, 439, 549 10.1038/439549a [DOI] [PubMed] [Google Scholar]
- Pearse A-M, Swift K, Hodson P, Hua B, McCallum H, Pyecroft S, … Belov K (2012). Evolution in a transmissible cancer: A study of the chromosomal changes in devil facial tumor (DFT) as it spreads through the wild Tasmanian devil population. Cancer Genetics, 205, 101–112. 10.1016/j.cancergen.2011.12.001 [DOI] [PubMed] [Google Scholar]
- Pemberton D (1990). Social organisation and behaviour of the Tasmanian devil Sarcophilus harrisii (PhD thesis). University of Tasmania. [Google Scholar]
- Pye RJ, Pemberton D, Tovar C, Tubio JMC, Dun KA, Fox S, … Woods GM (2016). A second transmissible cancer in Tasmanian devils. Proceedings of the National Academy of Sciences of the United States of America, 113, 374–379. 10.1073/pnas.1519691113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. (2015). R: A language and environment for statistical computing. Retrieved from https://www.r-project.org/
- Ujvari B, Pearse A-M, Peck S, Harmsen C, Taylor R, Pyecroft S, … Belov K (2013). Evolution of a contagious cancer: Epigenetic variation in Devil Facial Tumour Disease. Proceedings of the Royal Society Biological Sciences Series B, 280, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujvari B, Pearse A-M, Swift K, Hodson P, Hua B, Pyecroft S, … Madsen T (2014). Anthropogenic selection enhances cancer evolution in Tasmanian devil tumours. Evolutionary Applications, 7, 260–265. 10.1111/eva.12117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells K, Hamede RK, Kerlin DH, Storfer A, Hohenlohe PA, Jones ME, & McCallum H (2017). Infection of the fittest: Devil facial tumour disease has greatest effect on individuals with highest reproductive output. Ecology Letters, 20, 770–778. 10.1111/ele.12776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SN (2011). Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society: Series B (Statistical Methodology), 73, 3–36. 10.1111/j.1467-9868.2010.00749.x [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.