Supplemental Digital Content is available in the text.
Keywords: adjunctive use, antipsychotic, aripiprazole, body weight, brexpiprazole, long-term treatment, major depressive disorder, schizophrenia, short-term treatment
Abstract
The aim of this analysis was to explore the effects of brexpiprazole and aripiprazole on body weight when used as monotherapy to treat schizophrenia and as adjunctive treatment to antidepressant treatment (ADT) for major depressive disorder (MDD) in short-term (4/6 weeks) and long-term (≤52 weeks) studies. Body weight data were obtained from the clinical studies of each drug (brexpiprazole and aripiprazole), in schizophrenia and adjunctive treatment of MDD. Data were pooled and analyzed to assess the mean change in body weight and to determine the incidence of a clinically relevant change in body weight from baseline (≥7% increase or decrease, at any time) in each treatment group. The overall weight profiles for brexpiprazole and aripiprazole in the short-term and long-term treatment of schizophrenia, and MDD (adjunctive to ADT), were similar. In short-term schizophrenia studies, the mean weight increase was 1.2 kg for brexpiprazole and 0.6 kg for aripiprazole. In short-term MDD studies (adjunctive to ADT), the mean weight increase was 1.5 kg for brexpiprazole and 1.6 kg for aripiprazole. In the long-term schizophrenia studies, at week 52, the mean weight increase was 2.1 kg for brexpiprazole and 3.0 kg for aripiprazole. In long-term MDD studies (adjunctive to ADT), at week 52, the mean weight increase was 3.2 kg for brexpiprazole and 4.0 kg for aripiprazole. Clinically relevant increases or decreases in body weight were also similar for brexpiprazole and aripiprazole. Overall, in the treatment of schizophrenia, and in adjunctive treatment of MDD, brexpiprazole and aripiprazole have a similar effect on body weight over the course of 1 year.
Introduction
Atypical antipsychotics are indicated for the treatment of schizophrenia as monotherapy, and some (e.g. brexpiprazole, aripiprazole, and quetiapine extended-release) are also indicated as adjunctive treatment to antidepressant treatment (ADT) for treatment of major depressive disorder (MDD).
Weight increase has been ranked among the most bothersome adverse events reported by patients with schizophrenia, by those with MDD, and by their physicians (Llorca et al., 2017). Because of its association with many of the modifiable risk factors for cardiovascular disease (e.g. obesity, diabetes, dyslipidemia) (De Hert et al., 2009), the propensity for weight increase should be considered when selecting an appropriate antipsychotic for the treatment of schizophrenia and of MDD.
The findings of a meta-analysis of randomized controlled trials showed that almost all of the antipsychotics examined resulted in an increase in body weight over time (brexpiprazole was not included) (Bak et al., 2014). Among the atypical antipsychotics, clozapine showed the greatest increase in body weight over time (4.27 kg at ≤6 weeks and 7.34 kg at >38 weeks; Bak et al., 2014). In comparison, aripiprazole showed negligible weight increase over time (0.47 kg at ≤6 weeks and 0.46 kg at >38 weeks; Bak et al., 2014). For all antipsychotics included in the meta-analysis, the proportion of patients with clinically relevant weight increase (≥7% from baseline) also increased over time (Bak et al., 2014). Among the atypical antipsychotics, another study reported that the risk of clinically relevant weight increase in patients with schizophrenia was greatest with clozapine, olanzapine, and quetiapine, and lowest with ziprasidone, amisulpride, and aripiprazole (Oh et al., 2015). In patients with MDD, a meta-analysis of short-term (4–12 weeks) randomized controlled trials evaluating antipsychotic treatment adjunctive to antidepressant medications found that clinically relevant weight increase occurred in less than 5% of patients treated with aripiprazole or quetiapine (Spielmans et al., 2013).
The present analysis was carried out to evaluate the effect of brexpiprazole and aripiprazole on body weight when used either as monotherapy for schizophrenia or as adjunctive treatment to ADT in the treatment of MDD. The mechanism of action for aripiprazole is qualitatively similar to that of brexpiprazole, although there are quantitative differences (relative and absolute) in the pharmacology of the two drugs. Brexpiprazole is a serotonin–dopamine activity modulator that acts as a partial agonist at serotonin 5-HT1A and dopamine D2 receptors, and as an antagonist at serotonin 5-HT2A and noradrenaline α1B/2C receptors, all with subnanomolar potency (Maeda et al., 2014). Compared with aripiprazole, brexpiprazole is more potent at 5-HT1A/2A receptors and shows lower intrinsic activity at the D2 receptor and higher affinity for the noradrenaline α1B/2C receptors (Maeda et al., 2014).
This analysis aims to characterize the body weight profile for brexpiprazole and aripiprazole, in schizophrenia and in MDD, on the basis of indirect comparisons of data from short-term and long-term clinical studies.
Materials and methods
Study design
This analysis was carried out on data obtained from previous clinical trials in patients with either schizophrenia or MDD. Overviews of the study designs and of the patient populations are presented in Supplementary Table 1 (Supplemental digital content 1, http://links.lww.com/ICP/A44) for schizophrenia and in Supplementary Table 2 (Supplemental digital content 1, http://links.lww.com/ICP/A44) for MDD. With the exception of the brexpiprazole safety study – Orion (MDD), which was ongoing at the time of this analysis – all of the studies included in the analysis have been completed and are published elsewhere. For the purpose of the analysis, the brexpiprazole data included from the Orion study are based on data used in a submission to regulatory authorities with a cutoff date of 15 May 2015.
To provide normalized data suitable for comparison, reanalysis of the aripiprazole data was required to ensure that the data analyses aligned with the brexpiprazole analyses. The aripiprazole data from published studies were reanalyzed post-hoc to ensure that the mean change in body weight was calculated from baseline to last visit (not study endpoint). The proportion of clinically relevant weight increase ‘at any time during the study’ was calculated (not just at last visit/study endpoint). The published aripiprazole long-term study in MDD presented data for 58 weeks (6 weeks of double-blind treatment followed by 52 weeks of open-label treatment; Berman et al., 2011). Therefore, the long-term data for aripiprazole in MDD were recalculated to provide 52-week data to align with the brexpiprazole definition of baseline (i.e. before the first study dose in long-term, open-label trials).
Descriptive data were used to compare brexpiprazole and aripiprazole in schizophrenia as monotherapy and in MDD as adjunctive treatment to ADT. Data from short-term and long-term studies were pooled and analyzed (pooling of long-term studies was not possible for brexpiprazole in schizophrenia and for aripiprazole in MDD because there was only one long-term study in each instance).
Assessments and statistical analyses
The descriptive analysis assessed the mean change in body weight from the study entry baseline to last visit (i.e. the last evaluable value for each patient) in the short-term studies, and from the study entry baseline to weeks 26 and 52 (observed cases) in the long-term studies.
For all studies, baseline was defined as the last evaluation of weight before the first dosing of trial medication. For the short-term studies, the last visit was defined as the visit at which the last post-baseline evaluation of weight was performed. The proportion of patients with at least 7% increase or at least 7% decrease in body weight from baseline, at any time in the study, was also calculated. Change in body weight of at least 7% is considered clinically relevant (Sachs and Guille, 1999; Spielmans et al., 2013; Bak et al., 2014) and is widely reported in antipsychotic prescribing information, as it is more indicative of clinical significance than spontaneous reports of weight increase. An increase or decrease of at least 7% from baseline was, therefore, considered to be a clinically relevant change in body weight.
The data presented are descriptive and no statistical comparison was planned or carried out.
Results
Patients
Schizophrenia
The baseline demographics and clinical characteristics were comparable between the brexpiprazole and aripiprazole studies (short-term and long-term) (Supplementary Table 3, Supplemental digital content 1, http://links.lww.com/ICP/A44). Patient disposition data for brexpiprazole and aripiprazole in the schizophrenia studies are presented in Supplementary Table 4 (Supplemental digital content 1, http://links.lww.com/ICP/A44).
Major depressive disorder (adjunctive use with antidepressants)
The baseline demographics and clinical characteristics were comparable between the brexpiprazole and aripiprazole studies (short-term and long-term); patients were moderately depressed at study entry (Supplementary Table 5, Supplemental digital content 1, http://links.lww.com/ICP/A44). Patient disposition data for the MDD studies are presented in Supplementary Table 6 (Supplemental digital content 1, http://links.lww.com/ICP/A44).
Change in body weight
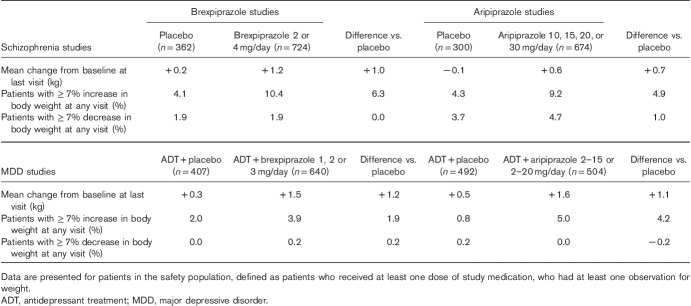
Table 1 shows the mean change in body weight from baseline to last visit, and the proportion of patients with at least 7% increase or decrease in body weight, at any study visit, for brexpiprazole and aripiprazole, in the short-term studies.
Table 1.
Short-term mean and clinically relevant (≥7%) changes in body weight with brexpiprazole and aripiprazole from schizophrenia and major depressive disorder studies
Figure 1 shows the mean change in body weight, and the proportion of patients with at least 7% increase or decrease in body weight, at any study visit, for brexpiprazole and aripiprazole, in the long-term studies.
Fig. 1.
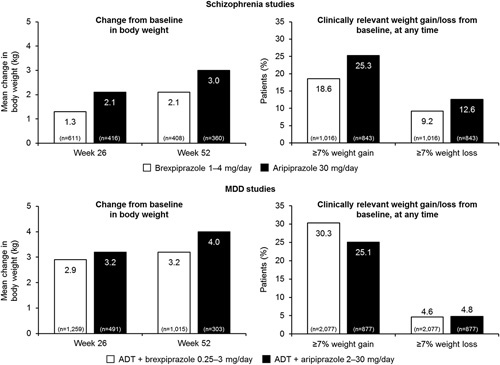
Long-term mean and clinically relevant (≥7%) changes in body weight with brexpiprazole and aripiprazole from schizophrenia and MDD studies. Data are presented for patients in the safety population, defined as patients who received at least one dose of study medication, who had at least one observation for weight. ADT, antidepressant treatment; MDD, major depressive disorder.
Schizophrenia
The analysis of the short-term schizophrenia studies showed that the mean weight increase from baseline to last visit for brexpiprazole 2 or 4 mg was 1.2 kg (placebo: 0.2 kg). The mean weight increase for aripiprazole was 0.6 kg (placebo: −0.1 kg) (Table 1). An increase in body weight of at least 7%, at any time, was observed in 10.4% of patients treated with brexpiprazole; the corresponding value for aripiprazole was 9.2% (Table 1). The proportion of patients experiencing at least 7% decrease in body weight, at any time, was 1.9% for brexpiprazole and 4.7% for aripiprazole (Table 1).
The analysis of the long-term schizophrenia studies showed that the mean increases in body weight at weeks 26 and 52 were 1.3 and 2.1 kg for adjunctive brexpiprazole, respectively, and 2.1 and 3.0 kg for adjunctive aripiprazole, respectively (Fig. 1). The difference between the mean increase in body weight at weeks 26 and 52 was ~0.8 kg for brexpiprazole and 0.9 kg for aripiprazole (Fig. 1). Therefore, most of the weight increase occurred over the first 26 weeks. The proportion of patients experiencing at least 7% increase in body weight, at any time, was 18.6% for brexpiprazole and 25.3% for aripiprazole (Fig. 1). The proportion of patients experiencing at least 7% decrease in body weight, at any time, was 9.2% for brexpiprazole and 12.6% for aripiprazole (Fig. 1).
Major depressive disorder (adjunctive use with antidepressants)
The analysis of the short-term MDD studies showed that the mean weight increase from baseline to last visit was 1.5 kg for adjunctive treatment with brexpiprazole 1, 2, or 3 mg (placebo: 0.3 kg), and 1.6 kg with aripiprazole (placebo: 0.5 kg) (Table 1). The proportion of patients with at least 7% increase in body weight, at any study visit, was 3.9% for adjunctive brexpiprazole and 5.0% for adjunctive aripiprazole (Table 1). A decrease in body weight of at least 7%, at any time, was observed in 0.2% of patients treated with adjunctive brexpiprazole, and not in any patients receiving adjunctive aripiprazole (Table 1).
The analysis of the long-term MDD studies showed that the mean increases in body weight at weeks 26 and 52 were 2.9 and 3.2 kg for adjunctive brexpiprazole, respectively, and 3.2 and 4.0 kg for adjunctive aripiprazole, respectively (Fig. 1). The proportion of patients with at least 7% increase in body weight, at any time, was 30.3% for brexpiprazole and 25.1% for aripiprazole (Fig. 1). The proportion of patients with at least 7% decrease in body weight, at any time, was 4.6% for adjunctive brexpiprazole and 4.8% for adjunctive aripiprazole (Fig. 1).
Discussion
This present analysis aimed to explore the effects of brexpiprazole and aripiprazole on body weight in patients receiving either of these compounds as treatments for schizophrenia or as adjunctive treatment to ADT in MDD. Weight changes with aripiprazole have been reported previously (Kane et al., 2002; Kasper et al., 2003; Potkin et al., 2003; McEvoy et al., 2007). The aripiprazole data were recalculated to facilitate comparison with brexpiprazole.
Overall, changes in weight for brexpiprazole and aripiprazole in short-term studies were similar. In the short-term (4/6 weeks) schizophrenia studies, the placebo-corrected difference in weight increase (0.3 kg) between the two treatments is small, yet it may be important to note that the brexpiprazole and aripiprazole studies were carried out at different times. When the aripiprazole studies were carried out (1997–2002), antipsychotics that caused weight increase were more commonly used and, therefore, the aripiprazole studies potentially enrolled patients who had previously been receiving antipsychotics that caused weight increase. However, it is not possible to determine if the discontinuation of previous, potentially weight-inducing, antipsychotics when switching to aripiprazole influenced the results of the short-term (or even the long-term) studies.
Short-term adjunctive treatment with brexpiprazole and aripiprazole in MDD was associated with a weight increase of 1.2 and 1.1 kg, respectively, over the increase observed for antidepressant monotherapy (ADT+placebo). The increases observed with adjunctive brexpiprazole are similar to the increase in body weight of 1 kg reported for adjunctive treatment with aripiprazole (6 weeks), and with quetiapine (6–8 weeks), in a meta-analysis of adjunctive atypical antipsychotic medications (Spielmans et al., 2013).
In the long-term studies, most of the weight increase observed with brexpiprazole and aripiprazole occurred during the earlier period (first 6 months) of treatment, leveling off at 1 year; similar observations have been seen in some long-term studies of other atypical antipsychotics (Peuskens et al., 2007; Durgam et al., 2016). In the long-term MDD studies, the proportion of patients who experienced a clinically relevant increase in body weight (≥7% from baseline), at any time, may be considered to be within the range of variability expected for patients followed over the long term (Michelson et al., 1999; Sussman et al., 2001; Andersen et al., 2005), with the weight gain potentially reflecting recovery (Michelson et al., 1999). The relative contributions to weight gain of adjunctive treatment to ADT, ADT alone, or improvement from the depressive episode are difficult to determine.
The results of the present post-hoc analysis suggest that the weight increases observed in patients with schizophrenia and in those with MDD, in short-term and in long-term studies, were similar following treatment with either brexpiprazole or aripiprazole. Antagonism of the histamine H1 receptor has been associated with a greater risk of weight increase induced by antipsychotic treatment, mediated by activation of hypothalamic adenosine monophosphate-activated protein (AMP)-kinase (Kim et al., 2007; He et al., 2013). Hypothalamic AMP-kinase stimulation has been shown to parallel the appetite-stimulating actions of antipsychotic drugs, with clozapine and olanzapine producing the most marked effects; the appetite-stimulating potencies of the drugs paralleled their H1 receptor affinities (Kim et al., 2007). During a 1-week exposure, olanzapine induced weight gain, and brain imaging data showed changes indicative of enhanced anticipatory desire for food, enhanced reward experience of consuming the anticipated food, and a compromised satiety-related mechanism, providing plausible mechanisms for the orexigenic effects of olanzapine (Mathews et al., 2012). In contrast to olanzapine, brexpiprazole and aripiprazole show lower D2 : H1 receptor occupancy ratios (olanzapine=1.57; brexpiprazole=0.016; aripiprazole=0.0056), which may be indicative of a low propensity to induce weight increase (European Medicines Agency EMA, 2004; Eli Lilly and Company, 2017; Otsuka Pharmaceutical Co. Ltd., 2017a, 2017b). Roles for serotonin, dopamine, and noradrenaline in obesity have also been proposed, but the evidence for the association with H1 receptor antagonism appears to be more compelling (Kim et al., 2007). Indeed, by itself, H1 receptor affinity correctly classified 15 of 17 typical and atypical antipsychotics as to their potential to induce weight gain (Kroeze et al., 2003). However, the mechanisms of antipsychotic-induced weight gain appear to be complex – antipsychotics that have a more selective receptor profile (e.g. amisulpride) can still cause weight gain (Manu et al., 2015). Likewise, synergistic effects of D2 receptor antagonism and 5-HT2A or 5-HT2C receptors might play a key role in triggering the cascade of events that lead to increased food consumption and weight gain (Correll et al., 2011; Manu et al., 2015). Taken together, the available preclinical and human data indicate that no single one neurotransmitter system is responsible for antipsychotic-related weight gain (Correll et al., 2011).
In the context of previously conducted meta-analyses (Spielmans et al., 2013; Oh et al., 2015), the weight changes described in this present analysis position brexpiprazole among the group of atypical antipsychotics that have the lowest propensity to induce weight increase in schizophrenia and in MDD. The benefits of brexpiprazole with regard to weight increase are supported by a favorable metabolic profile, in terms of blood glucose and lipid levels (Kane et al., 2016; Nelson et al., 2016).
A limitation of the present analysis is that the data have not been obtained from a head-to-head comparative study. The long-term aripiprazole studies in schizophrenia and in MDD, were double-blind; the long-term brexpiprazole studies in schizophrenia and in MDD were open-label. However, given the objective nature of weight change, an open-label study design is considered unlikely to have affected the results. The analysis included a large number of patients, with over 1000 individuals allocated to each drug, in each indication, treated for up to 52 weeks. Analysis of such a large dataset over a long period of time supports the robustness of the data reported.
Conclusion
This comparison using descriptive data has shown that brexpiprazole and aripiprazole have a similar propensity to influence body weight over the course of 1 year, in patients with schizophrenia and in those with MDD (adjunctive use).
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.intclinpsychopharm.com.
Acknowledgements
Writing support was provided by Emma Court, PhD, assisted by her colleagues at Cambridge Medical Communication Ltd (Cambridge, UK), and funded by Otsuka Pharmaceutical Development and Commercialization Inc. (Princeton, New Jersey, USA) and H. Lundbeck A/S (Valby, Denmark).
Conflicts of interest
C.W., R.A.B., R.A.D., P.Z., and R.D.M. are full-time employees of Otsuka Pharmaceutical Development and Commercialization Inc. At the time that this work was undertaken, E.W. was a full-time employee of H. Lundbeck A/S. K.K.G. is a full-time employee of Lundbeck LLC.
References
- Andersen SW, Clemow DB, Corya SA. (2005). Long-term weight gain in patients treated with open-label olanzapine in combination with fluoxetine for major depressive disorder. J Clin Psychiatry 66:1468–1476. [DOI] [PubMed] [Google Scholar]
- Bak M, Fransen A, Janssen J, van Os J, Drukker M. (2014). Almost all antipsychotics result in weight gain: a meta-analysis. PLoS One 9:e94112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Thase ME, Trivedi MH, Hazel JA, Marler SV, McQuade RD, et al. (2011). Long-term safety and tolerability of open-label aripiprazole augmentation of antidepressant therapy in major depressive disorder. Neuropsychiatr Dis Treat 7:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU, Lencz T, Malhotra AK. (2011). Antipsychotic drugs and obesity. Trends Mol Med 17:97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hert M, Dekker JM, Wood D, Kahl KG, Holt RIG, Möller HJ. (2009). Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC). Eur Psychiatry 24:412–424. [DOI] [PubMed] [Google Scholar]
- Durgam S, Earley W, Li R, Li D, Lu K, Laszlovszky I, et al. (2016). Long-term cariprazine treatment for the prevention of relapse in patients with schizophrenia: A randomized, double-blind, placebo-controlled trial. Schizophr Res 176:264–271. [DOI] [PubMed] [Google Scholar]
- Eli Lilly and Company (2017). ZYPREXA (olanzapine) US prescribing information. Eli Lilly and Company. Available at: http://pi.lilly.com/us/zyprexa-pi.pdf. [Accessed 16 November 2017].
- European Medicines Agency (EMA) (2004). Scientific discussion. Zyprexa. CPMP/0646/96. European Medicines Agency. Available at:http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000115/WC500055204.pdf. [Accessed 16 November 2017].
- He M, Deng C, Huang XF. (2013). The role of hypothalamic H1 receptor antagonism in antipsychotic-induced weight gain. CNS Drugs 27:423–434. [DOI] [PubMed] [Google Scholar]
- Kane JM, Carson WH, Saha AR, McQuade RD, Ingenito GG, Zimbroff DL, et al. (2002). Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry 63:763–771. [DOI] [PubMed] [Google Scholar]
- Kane JM, Skuban A, Hobart M, Ouyang J, Weiller E, Weiss C, Correll C. (2016). Overview of short- and long-term tolerability and safety of brexpiprazole in patients with schizophrenia. Schizophr Res 174:93–98. [DOI] [PubMed] [Google Scholar]
- Kasper S, Lerman MN, McQuade RD, Saha A, Carson WH, Ali M, et al. (2003). Efficacy and safety of aripiprazole vs. haloperidol for long-term maintenance treatment following acute relapse of schizophrenia. Int J Neuropsychopharmacol 6:325–337. [DOI] [PubMed] [Google Scholar]
- Kim SF, Huang AS, Snowman AM, Teuscher C, Snyder SH. (2007). Antipsychotic drug-induced weight gain mediated by histamine H1 receptor-linked activation of hypothalamic AMP-kinase. Proc Natl Acad Sci USA 104:3456–3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeze WK, Hufeisen SJ, Popadak BA, Renock SM, Steinberg S, Ernsberger P, et al. (2003). H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology 28:519–526. [DOI] [PubMed] [Google Scholar]
- Llorca PM, Lançon C, Hartry A, Brown TM, DiBenedetti DB, Kamat SA, François C. (2017). Assessing the burden of treatment-emergent adverse events associated with atypical antipsychotic medications. BMC Psychiatry 17:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Sugino H, Akazawa H, Amada N, Shimada J, Futamura T, et al. (2014). Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin–dopamine activity modulator. J Pharmacol Exp Ther 350:589–604. [DOI] [PubMed] [Google Scholar]
- Manu P, Dima L, Shulman M, Vancampfort D, De Hert M, Correll CU. (2015). Weight gain and obesity in schizophrenia: epidemiology, pathobiology, and management. Acta Psychiatr Scand 132:97–108. [DOI] [PubMed] [Google Scholar]
- Mathews J, Newcomer JW, Mathews JR, Fales CL, Pierce KJ, Akers BK, et al. (2012). Neural correlates of weight gain with olanzapine. Arch Gen Psychiatry 69:1226–1237. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Daniel DG, Carson WH, Jr, McQuade RD, Marcus RN. (2007). A randomized, double-blind, placebo-controlled, study of the efficacy and safety of aripiprazole 10, 15 or 20 mg/day for the treatment of patients with acute exacerbations of schizophrenia. J Psychiatr Res 41:895–905. [DOI] [PubMed] [Google Scholar]
- Michelson D, Amsterdam JD, Quitkin FM, Reimherr FW, Rosenbaum JF, Zajecka J, et al. (1999). Changes in weight during a 1-year trial of fluoxetine. Am J Psychiatry 156:1170–1176. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Zhang P, Skuban A, Hobart M, Weiss C, Weiller E, Thase ME. (2016). Overview of short-term and long-term safety of brexpiprazole in patients with major depressive disorder and inadequate response to antidepressant treatment. Curr Psychiatry Rev 12:278–290. [Google Scholar]
- Oh GH, Yu JC, Choi KS, Joo EJ, Jeong SH. (2015). Simultaneous comparison of efficacy and tolerability of second-generation antipsychotics in schizophrenia: mixed-treatment comparison analysis based on head-to-head trial data. Psychiatry Investig 12:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka Pharmaceutical Co. Ltd. (2017a). ABILIFY (aripiprazole) US prescribing information. Otsuka America Pharmaceutical Inc. Available at: https://www.otsuka-us.com/media/static/Abilify-PI.pdf. [Accessed 16 November 2017].
- Otsuka Pharmaceutical Co. Ltd. (2017b). REXULTI (brexpiprazole) US prescribing information. Otsuka America Pharmaceutical Inc. Available at: https://www.otsuka-us.com/media/static/Rexulti-PI.pdf. [Accessed 16 November 2017].
- Peuskens J, Trivedi J, Malyarov S, Brecher M, Svensson O, Miller F, et al. (2007). Prevention of schizophrenia relapse with extended release quetiapine fumarate dosed once daily: a randomized, placebo-controlled trial in clinically stable patients. Psychiatry (Edgmont) 4:34–50. [PMC free article] [PubMed] [Google Scholar]
- Potkin SG, Saha AR, Kujawa MJ, Carson WH, Ali M, Stock E, et al. (2003). Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry 60:681–690. [DOI] [PubMed] [Google Scholar]
- Sachs GS, Guille C. (1999). Weight gain associated with use of psychotropic medications. J Clin Psychiatry 60:16–19. [PubMed] [Google Scholar]
- Spielmans GI, Berman MI, Linardatos E, Rosenlicht NZ, Perry A, Tsai AC. (2013). Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes. PLoS Med 10:e1001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman N, Ginsberg DL, Bikoff J. (2001). Effects of nefazodone on body weight: a pooled analysis of selective serotonin reuptake inhibitor- and imipramine-controlled trials. J Clin Psychiatry 62:256–260. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.intclinpsychopharm.com.