Abstract
BACKGROUND AND OBJECTIVES
Mucolipidosis II (MLII) is characterized by severe global developmental delay, coarse facial features, skeletal deformities, and other systemic involvement. It is caused by a deficiency in N-acetylglucosamine-1 phosphotransferase.
DESIGN AND SETTINGS
This is a case series study conducted at King Abdulaziz Medical City in Riyadh, Saudi Arabia, between 2008–2012.
PATIENTS AND METHODS
We described three unrelated Saudi children who presented with neonatal hyperparathyroidism, microcephaly, craniosynostosis, coarse facial features, cardiac involvement, and skeletal deformities.
RESULTS
The MLII diagnosis was confirmed by assaying enzyme activities in fibroblasts, which showed a severe reduction in hydrolyzed substrates compared to controls, and by identifying a pathogenic homozygous GNPTAB gene mutation. One of the children died at 2 months of age due to severe pulmonary hypertension, and the other two children were still alive at 12 months and 18 months of age, respectively. Both surviving children had severe global developmental delay at 2 months of age.
CONCLUSION
Clinicians should investigate any child presenting with neonatal hyperparathyroidism, craniosynostosis, skeletal deformities, and coarse facial features for MLII.
Mucolipidosis II (MLII), also known as I-cell disease (MIM#252500), is an autosomal recessive inborn error of metabolism that begins at birth. It has a grave prognosis in early childhood. 1–3 Clinically, it is characterized by severe global developmental delay, marked postnatal growth delay and arrest, coarse facial features, skeletal deformities, and other systemic involvement.1–5 Biochemically, it is caused by a deficiency in the lysosomal enzyme N-acetylglucosamine-1 phosphotransferase (GlcNAc-phosphotransferase), which transfers phosphate to function as a marker for the uptake and transport of lysosomal enzymes. This is an essential step in hydrolases trafficking to lysosomes. Without this marker, the hydrolases are instead secreted outside the cells, and their activities are strikingly increased in the serum and decreased in cultured fibroblasts.2,6 Furthermore, there is the excessive secretion of oligosaccharides, the presence of several inclusion bodies in the cytoplasm of fibroblasts, and the absence of mucopolysacchariduria.1,2 The diagnosis is confirmed by DNA molecular testing of the GNPTAB gene. GNPTAB gene defects can also cause mucolipidosis III (MIM #252600), which is a multisystem disorder that can be differentiated from MLII by its later onset, milder symptoms, and slower progressive course.2,3,7–9 MLII is a panethnic disease. Natural history and radiological and molecular findings have been reported in different ethnic groups, including Indian, Portuguese, Japanese, Dutch, and Chinese patients.2,5,7,9–13 Although, it is not uncommon to see MLII patients in Saudi Arabia, there are no previous studies regarding the natural history and radiological and molecular findings in the Saudi population. In this report, we describe the natural history and clinical, radiological, and molecular findings in 3 unrelated Saudi children with MLII. To the best of our knowledge, this is the first report on MLII in Saudi patients.
PATIENTS AND METHODS
Patient 1
This girl was born by cesarian section (C/S) at 40 weeks of gestation to a healthy G1, 21-year-old mother with an uneventful antenatal history. Her birth weight was 1880 gm (<5th percentile), and the Apgar scores were 6, 7, and 10 at 1, 5, and 10 minutes, respectively. Directly after birth, it was noticed that the newborn was in respiratory distress. The baby was intubated, ventilated, and admitted to the neonatal intensive care unit. She continued to be ventilator-dependent for 2 months and failed extubation 3 times. She lost weight, and the postnatal growth subsequently stopped. On examination at 2 months of age, her weight was 1.5 kg (<5th percentile), and her length was 44 cm (<5th percentile). She appeared dysmorphic, with coarse facial features, including a flat face, depressed nasal bridge, micrognathia, fair hair, and gum hypertrophy. She was microcephalic, and her head circumference was 39.5 cm (<5th percentile); neurologically, she had central hypotonia. Additionally, she had a small chest, hepatomegaly, and elbow and knee joint contractures bilaterally. The ophthalmological exam and hearing tests were normal. The subsequent investigations showed the following complete blood count profile: thrombocytopenia, parathyroid hormone (PTH) elevated to 140.1 pmol/L (1.60–7.20 pmol/L), alkaline phosphatase (ALP) increased to 1071 U/L (95–368 U/L), and serum calcium and phosphorus were within normal ranges. The echocardiogram showed patent ductus arteriosus (PDA) and severe pulmonary hypertension. The skeletal survey showed a severe periosteal reaction, osteopenia, and bowing of the long bones. The skull x-ray showed craniosynostosis, and the abdominal ultrasound showed hepatomegaly and cholesterol polyps in the gall bladder. At 2.5 months of age, the baby continued to be intubated and ventilated, with reduced motor activity and respiratory depression despite full support. Subsequently, a decision was made to withdraw support, and the baby died a few minutes after extubation.
Patient 2
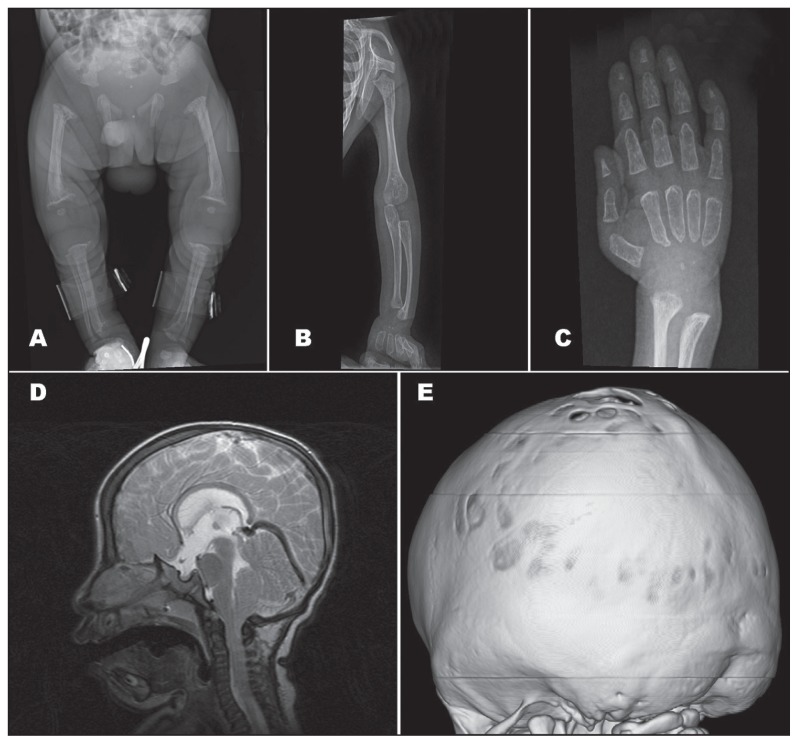
This 18-month-old girl was born by C/S at 40 weeks of gestation to a G2P1, 26-year-old mother. The pregnancy had been complicated by gestational diabetes and polyhydramnios. At birth, the baby was noted to be small for gestational age, with a birth weight of 2 kg (<10th percentile), birth length of approximately 45 cm (<10th percentile), and a head circumference of 30 cm (<10th percentile). The Apgar scores were 9 and 9 at 1 and 5 minutes, respectively. She had moderate respiratory distress requiring supplemental oxygen but was not intubated. She was admitted to the special care nursery because of being small for gestational age to rule out sepsis. During her 5-day stay in the special care nursery, she developed jaundice, which responded to a single phototherapy session. After sepsis was ruled out and the jaundice was resolved, she was discharged home in good condition. She was readmitted at 4 months of age due to pneumonia and found to have coarse facial features, poor growth, and global developmental delay; therefore, she was referred to the biochemical genetics clinic for further investigations. At the age of 14 months, her weight was 6.1 kg (<5th percentile), and her length was 65 cm (<5th percentile). She appeared dysmorphic with coarse facial features, including the following: flat face, depressed nasal bridge, micrognathia, and gum hypertrophy (Figure 1). She was microcephalic, with a head circumference of 40 cm (<5th percentile), and had craniosynostosis; neurologically, she had central hypotonia, diminished power, and increased reflexes +3. Her postnatal growth ceased, and her motor development was severely delayed. She could not fix or follow or sit or roll over, and she lay in the bed with a weak, hoarse cry. Additionally, she had a small chest, hepatomegaly, and brachydactyly of the hands and feet. The ophthalmological exam and hearing tests were normal. The subsequent investigations showed a normal echocardiogram. The skeletal survey showed decreased bone density in the lower limbs, with a thin periosteal reaction of both femora (Figure 2). The brain CT scan showed Chiari I malformation with generalized craniosynostosis. The brain MRI confirmed Chiari I malformation associated with brain herniation, ventriculomegaly, and delayed myelination (Figure 2). The abdominal ultrasound showed hepatomegaly.
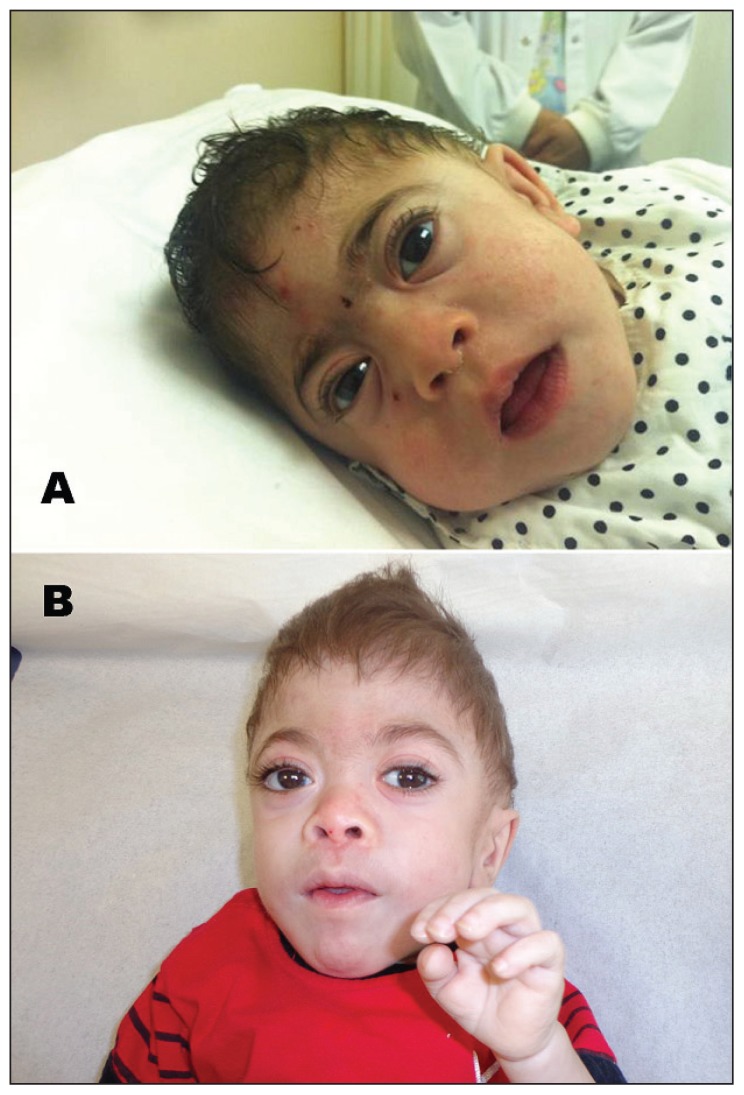
Figure 1.
Coarse facial features. A) Patient 2: microcephaly, craniocynostosis, flat face, round eyebrow, proptosis, depressed nasal bridge, and micrognathia. B) Patient 3: same as patient 2 but have fair hair.
Figure 2.
Radiological findings. A) X-ray of lower limbs showed shortening of the limbs, decreased bone density, and bowing of the legs. B) Bowing of ulna and radius bones. C) Brachydactyly of the hands with periosteal reaction and tapering of distal phalanges. D) Sagittal T2 brain MRI showed chiari I malformation with small posterior fossa. E) 3D volume rendering of brain CT scan showed diffuse craniocynostosis.
Patient 3
This 1-year-old boy was born normally at 37 weeks of gestation to a G1, healthy 24-year-old mother with no significant antenatal history. At birth, the baby was noted to be small for gestational age, with a birth weight of 1.8 kg (<10th percentile), birth length of 43 cm (<10th percentile), and head circumference of 30 cm (<10th percentile). The Apgar scores were 9 and 9 at 1 and 5 minutes, respectively. He had moderate respiratory distress requiring supplemental oxygen but was not intubated. He was admitted to the special care nursery because of being small for gestational age to rule out sepsis. During his 1-week stay in the special care nursery, he was found to have jaundice, which was easily corrected by a single phototherapy session. Additionally, he had thrombocytopenia, skeletal dysplasia with osteopenia, hypospadias, and bilateral hydrocele; therefore, he was referred to a biochemical genetics clinic for further investigations. On examination, he appeared microcephalic and dysmorphic with coarse facial features, including the following: flat face, depressed nasal bridge, micrognathia and gum hypertrophy (Figure 1). Neurologically, he had central hypotonia, diminished power, and increased reflexes +3. After discharge, he continued to be followed up in the biochemical genetics clinics. At 16 months of age, his postnatal growth ceased. His length was 61 cm (<5th percentile), weight was 4.6 kg (<5th percentile), and head circumference was 41 cm (<5th percentile). The ophthalmological exam and hearing tests were normal. His PTH was elevated to 84.27 pmol/L (1.60–7.20 pmol/L), his ALP was increased to 1126 U/L (95-368 U/L), and his serum calcium and phosphorus were within normal ranges. The echocardiogram revealed left ventricular hypertrophy with moderate depressed function. The skeletal survey showed a periosteal reaction with shortening of the limbs, decreased bone density, and bowing of the legs (Figure 2). Abdominal ultrasound showed renal nephrocalcinosis with no organomegaly.
All 3 children had an unremarkable acylcarnitine profile, organic acids in the urine, mucopolysaccharides in the urine, and chromosomal analysis. Oligosaccharides in the urine were excessive in the second patient but normal in the others. After getting informed consent, a skin biopsy was performed in all 3 children, and the enzyme activities in fibroblasts showed a drastic reduction in hydrolyzed substrates compared to controls. In Patient 2, an assay of enzymes in the serum showed very high activities of all lysosomal enzymes.
After obtaining informed consent from the parents, the GNPTAP gene was analyzed by polymerase chain reaction and sequencing of both the DNA strand of the entire coding region and the highly conserved exon-intron splice junctions. The diagnosis of MLII was confirmed in all 3 children after a homozygous nonsense mutation was identified in exon 02 of the GNPTAB gene, c.136C>T (p.R46X).3 The parents were tested and found to be carriers for this mutation.
DISCUSSION
This is the first report from Saudi Arabia about mucolipidosis II. The probands were clinically comparable to other patients described in the published reports. All were small for gestational age, had severe global developmental delay, had postnatal growth arrest, were microcephalic, and had striking coarse facial features, including a flat face, full round cheeks, round eyebrows, slight proptosis of the eyes, depressed nasal bridge, micrognathia, fair hair, and gum hypertrophy (Figure 1). All had characteristic skeletal deformities with a characteristic periosteal reaction, also called periosteal cloaking, which was reminiscent of rickets, osteopenia, bowing of the long bones, and joint contractures. Interestingly but not surprisingly, two of the presented children had increased serum PTH and ALP activities but normal serum calcium concentrations. The presentation of MLII with neonatal hyperparathyroidism has been reported previously.9,14 The mechanism is unknown, but it has been hypothesized that it is due to a targeting defect in MLII that would result in the impairment of transplacental calcium transport to the fetus, which would lead to the stimulation of the parathyroid response to maintain normal concentrations of extracellular calcium.14 Two of the 3 children had cardiac involvement, including PDA and left ventricular hypertrophy with moderately depressed function. Cardiac manifestations reported in the published reports include cardiomyopathy, right and generalized ventricular hypertrophy, valvular thickening, and regurgitation, with the mitral valve, and, less commonly, the aortic valve being the most frequent findings.2–4,12 Two out of the 3 patients had craniosynostosis. The association of craniosynostosis and MLII has been well described in the published reports.15–17 It was thought to be a sign of a primary skeletal disorder rather than due to microcephaly.17 Other systemic involvements included hepatomegaly, rarely splenomegaly, inguinal and umbilical hernia, corneal haziness, recurrent otitis media, and pulmonary hypertension,1–3 which was one of the characteristics observed in one of the presented patients.
All 3 children carried the same pathogenic missense mutation in the GNPTAB gene, c.136C>T (p.R46X). This mutation appears to have a poor outcome, as one of the probands died at 2 months of age, and the other two had severe global developmental delay at 2 months of age. Cathey et al (2010) reported 51 different GNPTAB pathogenic mutations in the largest cohort published so far describing clinical and molecular findings in 61 mucolipidosis cases.3 The most common mutation was the frameshift mutation c.3503delTC. The mutation c.136C>T (p.R46X) was reported in 1 patient in a heterozygous state,3 but in our cohort, it was found in the homozygous state.
In conclusion, our report highlights the natural history, clinical findings and molecular mutations in 3 unrelated Saudi children with MLII. Clinicians should investigate any child presenting with neonatal hyperparathyroidism, craniosynostosis, skeletal deformities, and coarse facial features for MLII.
Acknowledgments
We are grateful to the families and patients reported in this article for their genuine support. Also, we would like to thank King Abdullah International Medical Research Center for their unlimited support.
REFERENCES
- 1.Paik KH, Song SM, Ki CS, Yu HW, Kim JS, Min KH, Chang SH, Yoo EJ, Lee IJ, Kwan EK, Han SJ, Jin DK. Identification of mutations in the GNPTA (MGC4170) gene coding for GlcNAc-phosphotransferase alpha/beta subunits in Korean patients with mucolipidosis type II or type IIIA. Human mutation. 2005 Oct;26(4):308–314. doi: 10.1002/humu.20205. [DOI] [PubMed] [Google Scholar]
- 2.Leroy JG, Cathey S, Friez MJ. Mucolipidosis II. In: Pagon RA, Bird TD, Dolan CR, Stephens K, Adam MP, editors. GeneReviews. Seattle (WA): 1993. [Google Scholar]
- 3.Cathey SS, Leroy JG, Wood T, Eaves K, Simensen RJ, Kudo M, Stevenson RE, Friez MJ. Phenotype and genotype in mucolipidoses II and III alpha/beta: a study of 61 probands. Journal of medical genetics. 2010 Jan;47(1):38–48. doi: 10.1136/jmg.2009.067736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leroy JG, Martin JJ. Mucolipidosis II (I-cell disease): present status of knowledge. Birth defects original article series. 1975;11(6):283–293. [PubMed] [Google Scholar]
- 5.Kovacevic A, Schranz D, Meissner T, Pillekamp F, Schmidt KG. Mucolipidosis II complicated by severe pulmonary hypertension. Molecular genetics and metabolism. 2011 Sep-Oct;104(1–2):192–193. doi: 10.1016/j.ymgme.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 6.Kudo M, Brem MS, Canfield WM. Mucolipidosis II (I-cell disease) and mucolipidosis IIIA (classical pseudo-hurler polydystrophy) are caused by mutations in the GlcNAc-phosphotransferase alpha/beta -subunits precursor gene. American journal of human genetics. 2006 Mar;78(3):451–463. doi: 10.1086/500849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma GC, Ke YY, Chang SP, Lee DJ, Chen M. A compound heterozygous GNPTAB mutation causes mucolipidosis II with marked hair color change in a Han Chinese baby. American journal of medical genetics. Part A. 2011 Apr;155A(4):931–934. doi: 10.1002/ajmg.a.33834. [DOI] [PubMed] [Google Scholar]
- 8.Cathey SS, Kudo M, Tiede S, Raas-Rothschild A, Braulke T, Beck M, Taylor HA, Canfield WM, Leroy JG, Neufeld EF, McKusick VA. Greenwood Genetic Center, Greenwood, South Carolina 29646, USA. scathey@ggc.orgMolecular order in mucolipidosis II and III nomenclature. American journal of medical genetics. Part A. 2008 Feb 15;146A(4):512–513. doi: 10.1002/ajmg.a.32193. [DOI] [PubMed] [Google Scholar]
- 9.David-Vizcarra G, Briody J, Ault J, Fietz M, Fletcher J, Savarirayan R, Wilson M, McGill J, Edwards M, Munns C, Alcausin M, Cathey S, Sillence D. The natural history and osteodystrophy of mucolipidosis types II and III. Journal of paediatrics and child health. 2010 Jun;46(6):316–322. doi: 10.1111/j.1440-1754.2010.01715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coutinho MF, da Santos LS, Girisha KM, Satyamoorthy K, Lacerda L, Prata MJ, Alves S. Mucolipidosis type II alpha/beta with a homozygous missense mutation in the GNPTAB gene. American journal of medical genetics. Part A. 2012 May;158A(5):1225–1228. doi: 10.1002/ajmg.a.35295. [DOI] [PubMed] [Google Scholar]
- 11.Pinto R, Caseiro C, Lemos M, Lopes L, Fontes A, Ribeiro H, Pinto E, Silva E, Rocha S, Marcão A, Ribeiro I, Lacerda L, Ribeiro G, Amaral O, Sá Miranda MC. Prevalence of lysosomal storage diseases in Portugal. European journal of human genetics : EJHG. 2004 Feb;12(2):87–92. doi: 10.1038/sj.ejhg.5201044. [DOI] [PubMed] [Google Scholar]
- 12.Okada S, Owada M, Sakiyama T, Yutaka T, Ogawa M. I-cell disease: clinical studies of 21 Japanese cases. Clinical genetics. 1985 Sep;28(3):207–215. doi: 10.1111/j.1399-0004.1985.tb00388.x. [DOI] [PubMed] [Google Scholar]
- 13.Poorthuis BJ, Wevers RA, Kleijer WJ, Groener JE, de Jong JG, van Weely S, Niezen-Koning KE, van Diggelen OP. The frequency of lysosomal storage diseases in The Netherlands. Human genetics. 1999 Jul-Aug;105(1–2):151–156. doi: 10.1007/s004399900075. [DOI] [PubMed] [Google Scholar]
- 14.Unger S, Paul DA, Nino MC, McKay CP, Miller S, Sochett E, Braverman N, Clarke JT, Cole DE, Superti-Furga A. Mucolipidosis II presenting as severe neonatal hyperparathyroidism. European journal of pediatrics. 2005 Apr;164(4):236–243. doi: 10.1007/s00431-004-1591-x. [DOI] [PubMed] [Google Scholar]
- 15.Pazzaglia UE, Beluffi G, Castello A, Coci A, Zatti G. Bone changes of mucolipidosis II at different ages. Postmortem study of three cases. Clinical orthopaedics and related research. 1992 Mar;(276):283–290. [PubMed] [Google Scholar]
- 16.Yamada H, Ohya M, Higeta T, Kinoshita S. Craniosynostosis and hydrocephalus in I-cell disease (mucolipidosis II) Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1987;3(1):55–57. doi: 10.1007/BF00707197. [DOI] [PubMed] [Google Scholar]
- 17.Aynaci FM, Cakir E, Aynaci O. A case of I-cell disease (mucolipidosis II) presenting with craniosynostosis. Child’s nervous system: ChNS : official journal of the International Society. doi: 10.1007/s00381-002-0627-7. [DOI] [PubMed] [Google Scholar]