Abstract
Idiopathic hypoparathyroidism (IHP) is a rare endocrinopathy, characterized by the disturbances in calcium and phosphorous metabolism, owing to deficiency in parathyroid hormone, which leads to tetanic manifestations. Onset of the clinical features occurs early in life, and the severity depends on the extent of chemical imbalance. This article describes a case of 22-year-old patient undiagnosed for 12 years with this endocrinopathy (IHP). Over retained deciduous teeth, delayed eruption, impacted tooth and short roots probably resulting from untreated hypocalcemia during the developmental phase of dentition enabled us to unearth this endocrinopathy through a series of investigations. Thus the article emphasizes the importance of dental findings of this endocrinopathy.
Parathyroid hormone released from parathyroid glands plays an essential role in homeostasis of calcium and phosphate and in turn the normal mineralization of bone and teeth as well as maintenance of neuromuscular activity.1 Hyposecretion of this hormone leads to a relatively rare metabolic disorder known as hypoparathyroidism (HP), which is characterized by hypocalcemia and hyperphosphatemia.2,3 HP may occur in newborns as a result of mother’s HP status, as a result of excision of the parathyroid gland during thyroidectomy or it may develop as an isolated entity of unknown etiology, called idiopathic hypoparathyroidism (IHP).1,4 Symptoms of IHP usually appear during the first decade of life, but may become evident at any age.1 In acute form it causes hypocalcemia with consequent paresthesia, muscular spasm and seizures. Long-standing cases manifest with visual impairment from cataracts.3 Dental manifestations are enamel hypoplasia, widened pulp chambers, pulp stones, shortened roots, delayed eruption and hypodontia.5,6
To the best of our knowledge, only six studies have been published reporting the dental anomalies in IHP patients since 1966.1,3,4,7–9 This article describes the importance of analyzing the clinical presentation with dental manifestations, which led us to identify an endocrinopathy in a 22-year-old young adult, undiagnosed for about 12 years. The common dental findings of this endocrinopathy reported in the literature are summarized (Table 1).
Table 1.
Review of earlier studies with dental findings in idiopathic hypoparathyroidism.
| Study/year | Hypoplasia of teeth | Enamel defects | Unerupted/impacted teeth | Short roots |
|---|---|---|---|---|
|
| ||||
| Pisanty (1966)7 1 case | + | + | + | − |
| Riley (1969)4 52 cases | + (9%) | + (21%) | + (21%) | + (6%) |
| Frensilli et al (1971)8 3 cases | + (100%) | + (33%) | − | + (66%) |
| Myllarniemi et al (1978)9 3 cases | + | + | + | − |
| Jensen et al (1981)1 5 cases | + (20%) | + (60%) | + (40%) | + (20%) |
| Kelly et al (2009)3 1 case | + | + | + | + |
CASE
A 22-year-old male reported to the Department of Oral Medicine and Radiology, seeking advice on the absence of some teeth. The family history was unremarkable. He had discontinued his education as he could not cope with his peer group. On further inquiry the patient’s father reported the subject’s delayed milestones. His medical history revealed that he had suffered from epileptic seizures at the age of 11 years for which he was treated with anticonvulsant drugs clonazepam and carbamazepine. Seizures persisted for about 2 to 3 years despite the medication. A year previous to presentation he had been diagnosed with bilateral cataracts, which were surgically treated.
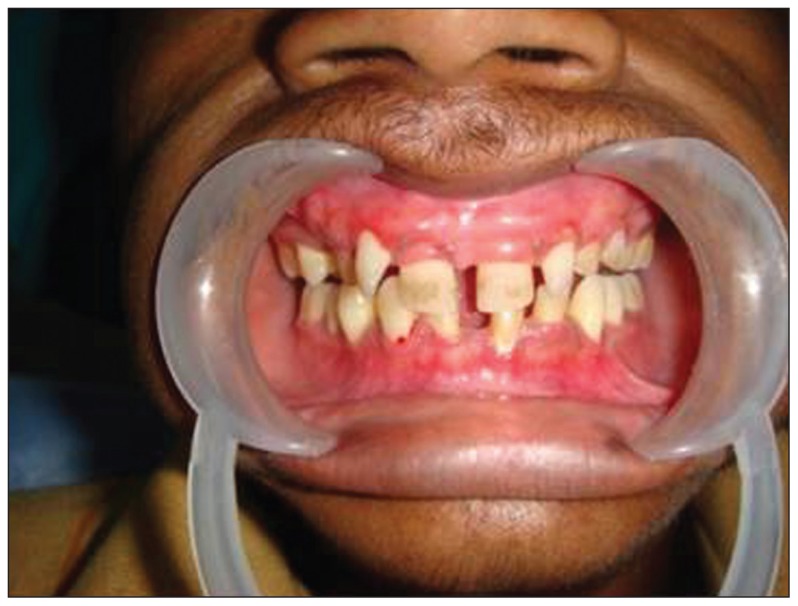
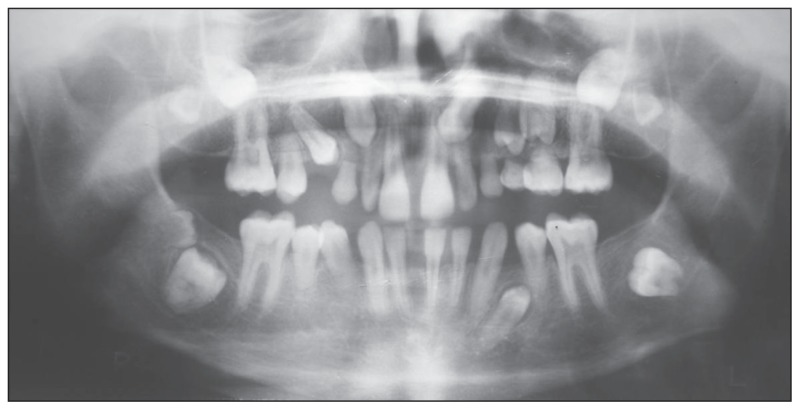
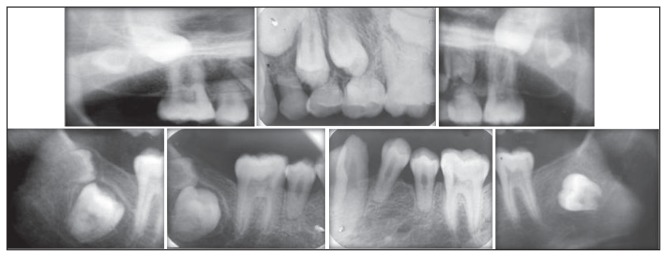
The patient was of short stature, and was subnormal in his alertness and response to questions. An intraoral examination revealed normal soft tissues. However, the dentition revealed diastema in the upper and lower arch, multiple retained deciduous teeth (#53, #63, #64, and #65) and missing permanent teeth (#13, #14, #17, #18, #23, #24, #25, #27, #28, #34, #37, #38, #42, #47, and #48) (Figure 1). Panoramic and periapical radiographs showed multiple impacted teeth, shortened roots in the premolars, retarded root development in the second molars and microdontia associated with enamel hypoplasia in the impacted third molars (Figures 2,3). There were no gross changes in the bone trabecular pattern. Based on the history, and clinical and radiological findings, the possibility of endocrine disturbance pertaining to thyroid, pituitary, parathyroid and vitamin D deficiency state was considered in the differential diagnosis.
Figure 1.
Clinical photographs reveling diastema in upper and lower arch; retained deciduous teeth (#53, #63, #64, and #65), missing permanent teeth (#13, #14, #17, #18, #23, #24, #25, #27, #28) and multiple missing permanent teeth (#34, #37, #38, #42, #47, #48).
Figure 2.
Orthopantomograph revealing multiple impacted teeth, short premolars roots, retarded root development of second molars, microdontia of third molars. Normal bone architecture is also noted.
Figure 3.
Intra-oral periapical [IOPA] radiographs revealing multiple impacted teeth, short premolars roots, retarded root development of second molars, enamel hypoplasia and microdontia of third molars. Pulp stones in the maxillary molars are also noted.
The patient underwent hematological and serological analysis. Hematological studies showed normal readings. Thyroid panel by chemiluminescence disclosed a T3 of 155.6ng/dl (normal, 60–180ng/dl), T4 of 8.4 μg/dl (normal, 4.5–12.60 μg/dl), TSH of 1.52 μIU/ml (normal, 0.35–5.5 μIU/mL) and growth hormone of 1.1 ng/mL (normal, 0.01–1 ng/mL). However, chemical analyses revealed a serum calcium of 5.1 mg/dL (normal, 9–11 mg/dL) and serum phosphate of 6.3 mg/dL (normal, 3–5 mg/dL) with alkaline phosphatase in the normal range (118 U/L; normal range-30–120 U/L).
A history of tetanic spasms (possibly misdiagnosed earlier as seizures), cataracts, radiographic details and the picture of hypocalcemia and hyperphosphatemia with normal thyroid and growth hormone assays and normal bone morphology prompted us to consider the possibility of parathyroid gland disturbance.
An endocrinologist opinion was obtained to rule out hypoparathyroidism or pseudohypoparathyroidism as the possible cause for the patient’s systemic and dental manifestations. Examination of the patient’s hands and limbs ruled out pseudohypoparathyroidism, which revealed short metacarpals and metatarsals.1 Hand wrist radiographs revealed normal findings. A further serum parathyroid hormone assay was carried out with a reported value of <2.50 pg/mL (normal, 14–72 pg/mL). Finally, based on chemical analysis and radiographic features, a diagnosis of hypoparathyroidism was made. The patient was advised to take calcium gluconate (24 g/day) and calcitrol (1 μg/day). After one month, calcium levels were slightly increased along with decreased levels of phosphate.
DISCUSSION
The clinical presentation of hypocalcemia and hyperphosphatemia along with dental changes is the sequelae of parathyroid hormone deficiency (hypoparathyroidism) or inability of the target organs (kidneys and bones) to respond to this hormone leading to pseudohypoparathyroidism (PTH).1 PTH is a rare metabolic disorder with clinical findings as similar as IHP. Distinction is made by the serum parathyroid hormone levels and by testing renal resistance to exogenous hormone (Ellsworth-Howard test).1
The most common presenting symptom of IHP is tetany, which occurs as a result of a low serum level of calcium, which if left untreated causes increased neuromuscular activity.1 This could be the possible cause in the present case, resulting in tetany mistaken and treated as epilepsy for about 12 years. Hence, even with the treatment of antiepileptic drugs, the patient suffered with tetanic spasms. Ectodermal disorders are most common in the patients with IHP. These disorders include scaling of the skin, deformities of the nails, opacities of the cornea and lens, keratoconjunctivitis and dental abnormalities.3 Dental findings in the previous six studies were enamel hypoplasia as the most frequent finding, and short rounded roots, hypodontia, lack or delayed tooth eruption; partial anodontia and microdontia were also noted.5,6,10 Most of these findings were observed in the present case.
Evidence from the previous studies emphasized that dental abnormality in IHP was the result of a calciotraumatic response, which coincides with the age of onset and reflects periods of hypocalcemia during tooth development.11,12 In the present case, the chemical imbalance which might have occurred at around the age of 10–11 years presented with enamel hypoplasia and microdontia with respect to the third molars (mean age of calcification for third molars), and impairment of root development of the premolars, the second molars and the third molars. These manifestations have been attributed to a disturbance in mineralization, alterations in the formation of the Hertwig epithelial root sheath coupled with other ectodermal disorders, lack of differentiation of odontoblasts, or are due to resorptive processes.1
As patients with this endocrinopathy go to the dental clinic with a complaint of discolored (hypoplastic) teeth or delayed eruption of teeth, treatment and the prognosis will remain uncertain unless the root cause is diagnosed. Thus, a thorough evaluation of the dental and clinical findings aided with relevant investigations can help in the early detection of this endocrine-deficiency syndrome. Hence prompt intervention during the early stages of the disease can not only minimize the dental anomalies, but also improve the overall health of the patient.
Acknowledgments
I owe a great deal of respect and gratitude to Dr. Amit Rastogi, Assistant Professor, M.D (Med), D.M (Endocrinology), Department of Endocrinology, Subharti Medical College and Hospital, for his suggestions and help during the investigative trials and helping in diagnosis.
REFERENCES
- 1.Jensen SB, Illum F, Dupont E. Nature and frequency of dental changes in idiopathic hypoparathyroidism and pseudohypoparathyroidism. Scand J Dent Res. 1981;89:26–37. doi: 10.1111/j.1600-0722.1981.tb01274.x. [DOI] [PubMed] [Google Scholar]
- 2.Neville WB, Damm DD, Allen CM, Bouquot EJ. Text book of Oral and Maxillofacial pathology. 2nd ed. Philadelphia, Pennsylvania: WB Saunders Company, Elsevier Ind Pvt. Ltd., India; 2004. pp. 722–723. [Google Scholar]
- 3.Kelly A, Pomarico L, de Souza R. Cessation of dental development in a child with idiopathic hypoparathyroidism: a 5-year follow up. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:673–677. doi: 10.1016/j.tripleo.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 4.Riley DJ. Dental changes in patients with Idiopathic hypoparathyroidism: report of two cases. J Oral Surg. 1969;27:44–47. [PubMed] [Google Scholar]
- 5.Greenberg MS, Brightman VJ, Lynch MA, Ship II. Idiopathic hypoparathyroidism, chronic candidiasis, and dental hypoplasia. Oral Surg Oral Med Oral Pathol. 1969;28(1):42–53. doi: 10.1016/0030-4220(69)90192-3. [DOI] [PubMed] [Google Scholar]
- 6.Pindborg JJ. Pathology of the dental hard tissues. Copenhagen: Munksgaurd; 1970. pp. 182–184. [Google Scholar]
- 7.Pisanty S. Primary idiopathic juvenile hypoparathyroidism. Oral Surg Oral Med Oral Pathol. 1966;21:294–298. doi: 10.1016/0030-4220(66)90060-0. [DOI] [PubMed] [Google Scholar]
- 8.Frensilli JA, Stoner RE, Hinrichs EH. Dental changes of idiopathic hypoparathyroidism: report of three cases. J Oral Surg. 1971;29:727–731. [PubMed] [Google Scholar]
- 9.Myllarniemi S, Perheentupa J. Oral findings in the autoimmune polyendocrinopathy-candidosis syndrome (APECS) and other forms of hypoparathyroidism. Oral Surg Oral Med Oral Pathol. 1978;45:721–729. doi: 10.1016/0030-4220(78)90147-0. [DOI] [PubMed] [Google Scholar]
- 10.Nally FF. Idiopathic juvenile hypoparathyroidism with superficial moniliasis. Report of a case. Oral Surg Oral Med Oral Pathol. 1970;30(3):356–365. doi: 10.1016/0030-4220(70)90313-0. [DOI] [PubMed] [Google Scholar]
- 11.Sjoberg KH. Moniliasis – An Internal Disease? Three cases of Idiopathic hypoparathyroidism with moniliasis, steatorrhea, primary amenorrhea and pernicious anemia. Acta Med Scand. 1966;179:159. [PubMed] [Google Scholar]
- 12.Bronsky D, Kushner DS, Dubin A, Snapper I. Idiopathic hypoparathyroidism and pseudohypoparathyroidism: Case reports and review of literature. Medicine. 1958;37:317–352. doi: 10.1097/00005792-195812000-00003. [DOI] [PubMed] [Google Scholar]