Abstract
Collet-Sicard syndrome is caused by various neoplastic and non-neoplastic lesions affecting the base of the skull with involvement of IX, X, XI and XII cranial nerves. Paraganglioma accounts for <1% of all the neoplasms in the head and neck region. They are traditionally considered as benign, slow growing, locally invasive, encapsulated and highly vascular tumors. We report a case of Collet-Sicard syndrome secondary to a large glomus jugulotympanicum in a 45-year-old woman who presented to the emergency department with complaints of recurrent episodes of a fresh bleeding from the left ear for the previous 5 days. She had pain and decreased hearing for the last 3 years and features of multiple cranial nerve palsies. A radiological diagnosis of glomus jugulotympanicum (paraganglioma) was made, which was confirmed by the biopsy tissue. At 6-month follow up, episodes of recurrent bleeding had stopped, but cranial nerve palsies persisted.
A 45-year-old female presented to the emergency department with a complaint of recurrent episodes of fresh bleeding from the left ear for the previous 5 days. There was a history of pain and hearing loss for the last 3 years for which she had consulted local practitioners with no significant benefit. She also had a history of dysphonia for the last 3 to 5 months.
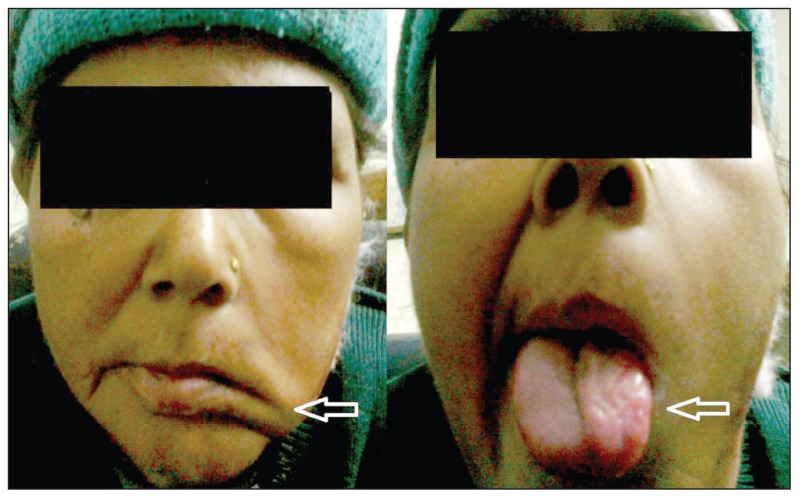
On clinical examination, the patient was alert and conscious, had mild pallor but no jaundice and her vital signs were normal. She had clinical features of left 7th and 12th cranial nerve palsy in the form of ipsilateral deviation of the angle of the mouth, an inability to blow air in the mouth and ipsilateral atrophy of tongue (Figure 1). An X-ray basal view was advised to look for a possible cause of cranial palsies which showed expansion and erosion of the jugular foramen. Based on the clinical findings of multiple cranial nerve palsies and x-ray feature, contrast enhanced CT (CECT) was advised to look for the cause of nerve palsies and ear bleeding.
Figure 1.
Clinical photograph shows ipsilateral deviation of angle of mouth (left arrow) and ipsilateral atrophy of tongue (right arrow), consistent with left 7th and 12th cranial nerve palsy.
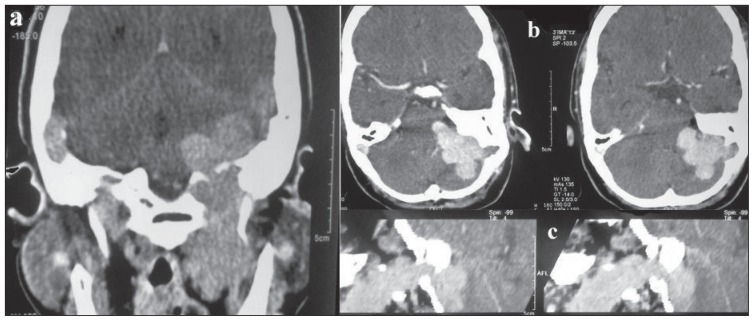
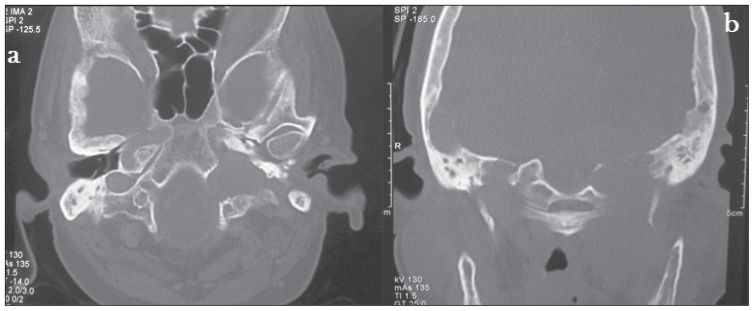
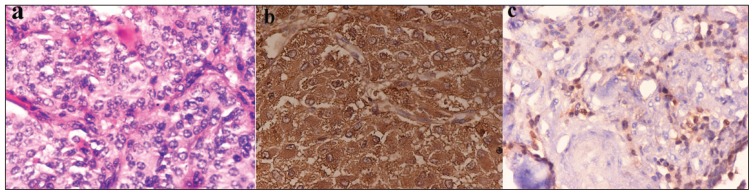
CECT neck showed a homogenous and intensely enhanced posterior skull base mass lesion centred in the left jugular foramen extending inferiorly from the infratemporal fossa to the left cerebellopontine angle superiorly leading to displacement of the left cerebellar hemisphere medially (Figure 2). A bone window CT scan image showed evidence of expansion and erosion of the jugular foramen extending up to the foramen magnum with a moth-eaten pattern of destruction of the petrous part of temporal bone with extension into and destruction of the left middle ear cavity. The occipital bone forming the skull base was also eroded (Figure 3). A radiological diagnosis of glomus jugulotympanicum (paraganglioma) was made, which was confirmed by the biopsy tissue (Figure 4).
Figure 2.
CECT neck A) Coronal image shows an intensely enhancing posterior skull base mass lesion centred in left jugular foramen extending inferiorly to infratemporal fossa. B) Axial image showing intracranial extent: Extraaxial mass lesion in left cerebellopontine angle displacing cerebellar hemisphere. C) Sagittal reformatted image shows extension of the mass lesion through jugular foramen.
Figure 3.
CT (bone window) A) Axial image shows expansion and erosion of jugular foramen extending up to foramen magnum. B) Coronal image shows erosion of left occipital condyle (base of skull) & destruction of petrous part of temporal bone.
Figure 4.
A) Shows the classical nesting pattern (Zellballen) of paraganglioma, in which nests of tumor cells are seen surrounded by thin fibrovascular stroma (Hematoxylin and eosin, 40×). B) Immunohistochemistry for chromogranin shows granular cytoplasmic positivity of Tumor cells (IHC Chromogranin, 40×). C) S-100 positivity of the surrounding sustentacular cells encompassing nests of tumour cells (IHC S100, 40×).
Based on the extent and enhancement of the lesion, recurrent ear bleeding, associated morbidity and socioeconomic status of the patient, treatment modalities were discussed including preoperative embolization followed by surgery and radiotherapy. However, radiotherapy was chosen as the treatment modality. The patient was seen at 6 months follow up. Episodes of recurrent bleeding had decreased significantly but cranial nerve palsies were still persisting.
DISCUSSION
Collet-Sicard syndrome can be caused by various neoplastic and non-neoplastic lesions at the base of the skull affecting the lower (IX, X, XI and XII) cranial nerves with signs and symptoms of dysphonia, displacement of the palate, anaesthesia of the larynx, pharynx and soft palate. Among the non-neoplastic factors, the most common are traumatic fractures at the base of the skull, inflammatory processes (osteomyelitis, Paget disease, and others ) or other alterations such as diabetes mellitus or porphyrias. However, neoplasm should always be kept in the differential diagnosis, especially in nontraumatic cases.
Glomus tumors are vascular neoplasms that arise from the paraganglia. Paraganglia are small masses of tissue composed of clusters of chief cells and are normal structures that accompany cranial nerves. The most accurate name for these tumors is therefore paragangliomas. 1 Those arising in the middle ear and mastoid are called glomus tympanicum, while those arising from jugular foramen are called as glomus jugulare. Paragangliomas account for 0.6% of all head and neck malignancies and 0.03% of all malignancies.2 Glomus jugulare originates from paraganglia located within the wall (adventitia) of the jugular bulb, Arnold nerve or Jacobson nerve. Glomus jugulotympanicum is the most common tumor of the middle ear and second only to vestibular schwannoma as the most common tumor of temporal bone.
Paragangliomas most commonly occur in the age group of 50 to 70 years with 4–6:1 female-to-male ratio. 3 The most common symptom associated with glomus jugulotympanicum is tinnitus followed by hearing loss, vertigo, hoarseness, pain and ear discharge.4,5 More than 90% of paragangliomas are non-functioning, presenting as a slowly enlarging palpable mass or pain related to local growth of the mass as was seen in our case.
Although most paragangliomas arise sporadically, approximately 10% cases are familial. The mode of inheritance in familial cases is autosomal dominant with incomplete penetrance6 and is commonly associated with MEN IIA and IIB, neurocutaneous syndromes (neurofibromatosis, tuberous sclerosis and Von Hippel-Lindau) and Carney triad. Histologically, they resemble normal paraganglia with trabecular arrangement (Zellballen) of neoplastic chief cells in a highly vascular stroma. Nuclear pleomorphism does not always predict malignancy.7 Extension to regional lymph nodes and distant metastasis are reliable criteria to diagnose malignancy.
Tumor spread within the mastoid bone follows the path of least resistance via air cells, vascular channels, eustachian tube and skull base neural foramina.8 Tumor spread leads to a moth-eaten pattern of destruction in the region of jugular fossa and postero-inferior petrous bone followed by the mastoid and adjacent occipital bone. Tumor extension into the jugular foramen leads to motor paralysis of the IX, X, XI cranial nerves resulting in jugular foramen (Vernet) syndrome. Vernet syndrome with additional involvement of the XII cranial nerve leads to Collet-Sicard syndrome.9,10
Thin section CT shows the extent of bone destruction with an enhancing mass seen on postcontrast scans. MRI is better than CT for evaluation of extent and location of these tumors. They have a classical ‘salt and pepper’ appearance on MR. The “salt” is the enhancing tumor stroma (on T1W images after contrast material enhancement), and the “pepper” is the signal voids of the tumor vessels on T2W. Carotid arteriography shows an intense tumor blush with enlarged feeding arteries. It has diagnostic as well as therapeutic benefit, as it guides the preoperative embolization. Surgery is the method of choice with complete resection if possible, having excellent prognosis.11 In patients with unresectable tumors, residual tumors, those who refuse surgery or those who are not surgical candidates, radiotherapy has been used with limited success.12,13 In our patient also radiotherapy was used because of large size of the tumor, associated morbidity of the surgery and poor socioeconomic status.
REFERENCES
- 1.Weissman JL, Hirsch BE. Beyond the promontory: the multifocal origin of glomus tympanicum tumors. AJNR Am J Neuroradiol. 1998;19:119–122. [PMC free article] [PubMed] [Google Scholar]
- 2.Borba LA, Al-Mefty O. Intravagal paragangliomas: report of four cases. Neurosurgery. 1996 Mar;38(3):569–75. doi: 10.1097/00006123-199603000-00030. [DOI] [PubMed] [Google Scholar]
- 3.Alford BR, Guildford FOR. A comprehensive study of tumors of the glomus jugulare. Laryngoscope. 1962;72:765–787. doi: 10.1097/00005537-199609000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Weissman JL, Hirsch BE. Imaging of tinnitus – a review. Radiology. 2000 Aug;216:342–349. doi: 10.1148/radiology.216.2.r00au45342. [DOI] [PubMed] [Google Scholar]
- 5.Remley KB, Coit WE, Harnsberger HR, Smoker WORK, Jacobs JM, McIff EB. Pulsatile tinnitus and the vascular tympanic membrane: CT, MR, and angiographic findings. Radiology. 1990;174:383–389. doi: 10.1148/radiology.174.2.2296650. [DOI] [PubMed] [Google Scholar]
- 6.Young AL, Baysal BE, Deb A, Young WF., Jr Familial malignant catecholamine-secreting paraganglioma with prolonged survival associated with mutation in the succinate dehydrogenase B gene. J Clin Endocrinol Metab. 2002;87:4101–4105. doi: 10.1210/jc.2002-020312. [DOI] [PubMed] [Google Scholar]
- 7.Lack EE, Cubilla AL, Woodruff JM. Paragangliomas of the head and neck region: a pathologic study of tumors from 71 patients. Hum Pathol. 1979;10:191–218. doi: 10.1016/s0046-8177(79)80008-8. [DOI] [PubMed] [Google Scholar]
- 8.Gulya AJ. The glomus tumor and its biology. Laryngoscope. 1993;103:7–1. doi: 10.1002/lary.1993.103.s60.7. [DOI] [PubMed] [Google Scholar]
- 9.Spector GJ, Druck NS, Gado M. Neurologic manifestations of glomus tumors in the head and neck. Arch Neurol. 1976;33:270–274. doi: 10.1001/archneur.1976.00500040054008. [DOI] [PubMed] [Google Scholar]
- 10.Makek M, Franklin DJ, Zhao JC, Fisch U. Neural infiltration of glomus temporale tumors. Am J Otol. 1990 Jan;11(1):1–5. [PubMed] [Google Scholar]
- 11.Dickinson PH, Griffin SM, Guy AJ, McNeil IF. Carotid body tumor: 30 years experience. Br J Surg. 1986;73:14–16. doi: 10.1002/bjs.1800730107. [DOI] [PubMed] [Google Scholar]
- 12.Anand VK, Leonetti JP, al-Mefty O. Neurovascular considerations in surgery of glomus tumors with intracranial extensions. Laryngoscope. 1993;103:722–728. doi: 10.1288/00005537-199307000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Gardner G, Cocke EW, Jr, Robertson JH, Palmer RE, III, Bellott AL, Jr, Hamm CW. Skull base surgery for glomus jugulare tumors. Am J Otol. 1985;(Suppl):126–134. [PubMed] [Google Scholar]