Abstract
BACKGROUND AND OBJECTIVES
Helicobacter pylori is a Gram negative bacteria that causes peptic ulceration and gastric adenocarcinoma. H pylori virulence factors, such as cagA and dupA, are important to study in populations as they contribute to disease risk. This study aimed to look at the distribution of the cagA and dupA genes in H pylori strains isolated from patients suffering from gastroduodenal diseases in Kurdistan region, Iraq.
DESIGN AND SETTINGS
A cross-sectional study conducted between June 2011 and January 2012. Biopsies were collected from the Endoscopy Department in Duhok and Sulaimania hospitals, Kurdistan region, northern Iraq.
PATIENTS AND METHODS
Upper gastrointestinal (GI) endoscopy examination was performed and 4 gastric biopsies (2 from the antrum and 2 from the corpus) were obtained from 204 patients. H pylori positivity was examined by CLO test; then the association between disease status and virulence factors was assessed by polymerase chain reaction.
RESULTS
154 (75%) of our samples were found to be H pylori + by CLO test. Endoscopic diagnoses for those who were positive were as follows: peptic ulcer disease (PUD) including duodenal ulcer, 45; gastric ulcer, 23; and no ulcer (NPUD), 86. The overall prevalence rates of cagA and dupA were 72.7% and 18.8%, respectively. While a significant association between cagA and PUD was observed (P ≤ .017; OR=0.4; CI=0.18–0.85), no relationship between dupA and PUD could be seen.
CONCLUSION
These data suggested that the presence of cagA may be a predictor of clinical outcome in Kurdistan region, northern Iraq.
Helicobacter pylori is a clinically important pathogen that colonises about 50% of the world’s population.1 H pylori is a Gram negative spiral-shaped organism, which prefers to live in microaerophilic conditions. It can chronically reside in the harsh environment of the stomach and causes subclinical gastritis in the majority of patients. However, infection with this bacterium may cause peptic ulcer disease (PUD). It has been found that 75% of gastric ulcers (GUs) and 90% of duodenal ulcers (DUs) are believed to be due to infection with this bacteria.1 Additionally, H pylori infection can predispose to the development of gastric cancer: adenocarcinoma and mucosa-associated lymphoid tissue lymphoma.2,3
The cytotoxin-associated gene A (cagA) is a part of the cag pathogenicity island (PAI). PAI is composed of genes that decode type IV secretion system, and this secretion system is responsible for the translocation of CagA into host cells.4 Worldwide, 60% to 80% of H pylori strains possess cagA. The presence of cagA is correlated with PUD and gastric cancer.5,6
Another H pylori virulence factor, a homologue of virB4, was identified recently.7 The initial report described a significant association between its presence and DU in various populations, hence the name DU promoting gene A (dupA).7 Subsequent reports have confirmed the association of H pylori with DU in northern India,8 and have shown a significant association with gastric adenocarcinoma in Belgium, South Africa, China, and the USA.9 The aim of this paper was to study the distribution of cagA and dupA genes in Iraqi H pylori strains and their relationship with disease status.
PATIENTS AND METHODS
Clinical samples
All patients studied were referred to the Endoscopy Department in Duhok and Sulaimania hospitals, Kurdistan region, northern Iraq. Upper gastrointestinal (GI) endoscopy examination was performed and 4 gastric biopsies (2 from the antrum and 2 from the corpus) were obtained from 204 patients. Endoscopic diagnoses were as follows: PUD, 70 and NPUD, 134.
Rapid urease test
One antral and 1 corpus biopsy were inoculated into CLO gel. The results were observed and recorded within 24 hours. A positive CLO was indicated when the color changed from yellow to pink.
DNA extraction
DNA was extracted directly from the biopsy specimens and used for polymerase chain reaction (PCR)-based H pylori typing. DNeasy tissue kits (QIAGEN, Germany) were used to extract DNA from biopsies following the manufacturer instructions.
Genotyping of H pylori
Thermal cycling for amplifying cagA was 95°C for 30 seconds, 50°C for 1 minute, and 72°C for 2 minutes, for a total of 35 cycles. PCR amplification of cagA used previously described primers cag2 (5′-GGAACCCTAGTCGGTAATG-3′) and cag4 (5′-ATCTTTGAGCTTGTCTATCG-3′) to amplify about 500-base pair (bp) product from the middle of cagA (PAI).10 The dupA was amplified by using standard protocols with the following block cycler conditions: 35 cycles, each consisting of denaturation at 95C for 30 seconds, annealing at 50°C for 1 minute, and elongation at 72°C for 2 minutes. Two primers were used: DupAF113 (5′-GACGATTGAGCGATGGGAATAT-3′) and DupAR1083(5′-CTGAGAAGCCTTATTATCTT GTTGG-3′).11 Amplification of these genes started with an initial denaturation at 95°C for 60 seconds and a final elongation step of 5 minutes at 72°C. Reactions were performed in 25 μL volume containing 1 μL of H pylori genomic DNA, 1 μL of primer, 0.5 μL of Taq DNA polymerase, 0.5 μL of dNTP, and 2.5 10× PCR buffer. Then 5 μL of the PCR products were electrophoresed in 1.5% agarose gels for 40 minutes at 80 V in 1× TAE buffer. All gels were stained with ethidium bromide (1 mg/L) and photographed under UV light. A 100-bp DNA ladder (Gibco, Paisley, UK) was used as a size marker (M) in all gels. For cagA, and dupA, 8823 and J99 strains were used as a positive control, respectively.
The study protocol was approved by the Ethics and Research Committees of the individual hospitals, and all patients were given informed consent to the study.
Data analysis
The statistical analysis of data was performed by using chi-square test with significance set at a P value of <.05 using Minitab 15 software (Minitab Inc, Pennsylvania, USA).
RESULTS
The mean age (standard deviation) of our patients was 36 (17) years. A total of 154 (75%) of our samples were found to be H pylori+ by CLO test. Endoscopic diagnoses for those who were positive were as follows: PUD including DU, 45; GU, 23; and no ulcer (NPUD), 86. No cancer was observed.
The cagA gene was observed in 112 (72.7%) isolates (Table 1, Figure 1). Among our samples, 56/68 (82%) peptic ulcer patients carried cagA+ strains, significantly more than the 56/86 (65%) non-ulcer patients (P ≤ ..017; OR=0.4; CI=0.18–0.85). Considering duodenal and GU separately in this population, 21 (91%) patients with GU had cagA+ strains, compared with 56 (65%) with no ulcer (P<.014; OR=0.18; CI=0.03–0.8). A total of 35 (78%) Iraqi DU patients had cagA+ strains (P=not significant [ns] compared with no ulcer).
Table 1.
cagA status among H pylori strains from unselected Iraqi patients with dyspepsia. A significant association was found between gastric ulcer and cagA.
| GU | DU | PUD (DU and GU) | NPUD | |
|---|---|---|---|---|
|
| ||||
| cagA+ (n) | 21 | 35 | 56 | 56 |
| cagA− (n) | 2 | 10 | 12 | 30 |
| Total | 23 | 45 | 68 | 86 |
GU: Gastric ulcer, DU: duodenal ulcer, PUD: peptic ulcer disease, NPUD: no peptic ulcer disease.
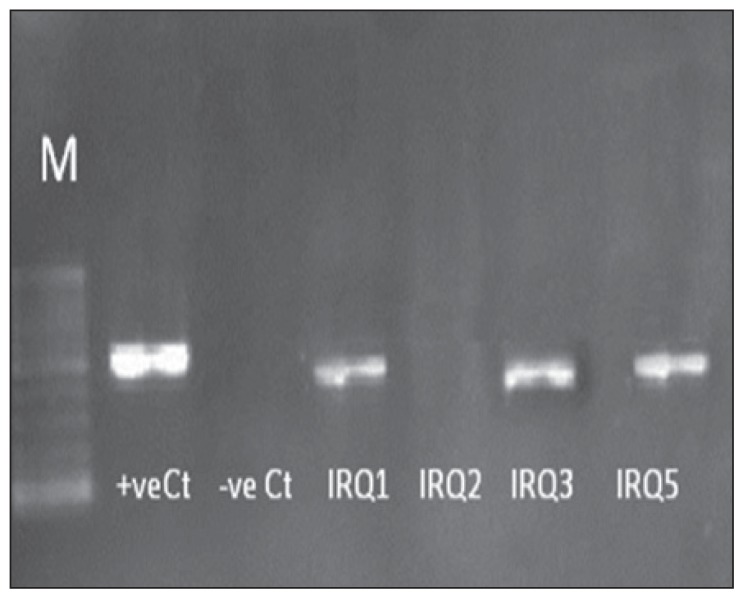
Figure 1.
Characterization of Iraqi strains for cagA gene. Two per cent gel electrophoresis of H pylori genotypes showing polymerase chain reaction results of cagA gene. Lane M is a 100-bp ladder. IRQ1, IRQ3, and IRQ4 typed as cagA+, while IRQ3 typed as cagA−. Negative Ct is negative control and positive Ct is positive control.
The overall prevalence of the dupA+ genotype was 18.8% (29/154 isolates) (Figure 2). The difference in the prevalence of dupA positivity was not significant between patients with NUD (16%) and PUD (28%) (P=ns) (Table 2).
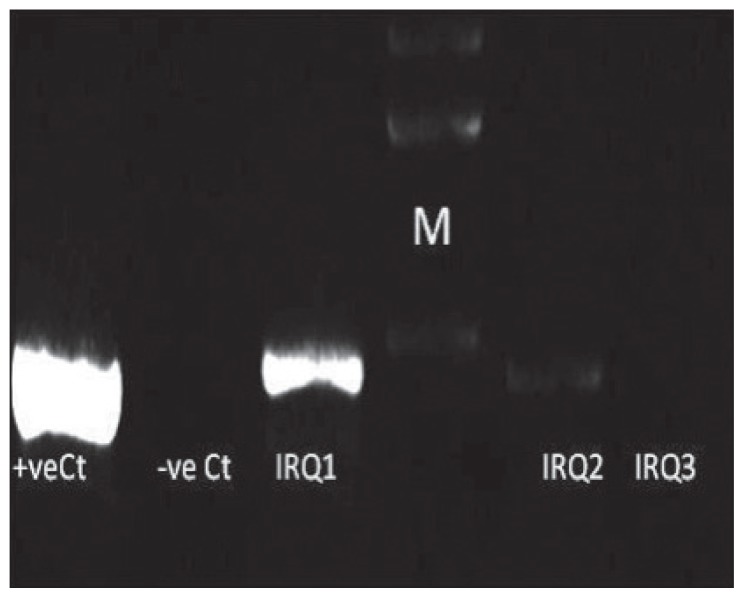
Figure 2.
Characterisation of Iraqi strains for dupA gene. Two per cent gel electrophoresis of H pylori genotypes showing polymerase chain reaction results of dupA gene. Lane M is a 100-bp ladder. IRQ1 and IRQ2 typed as dupA+, while IRQ3 typed as dupA−. Negative Ct is negative control and positive Ct is positive control.
Table 2.
dupA status among H pylori strains from unselected Iraqi patients with dyspepsia. No association was found between clinical outcome and dupA, cagA, cytotoxin-associated A gene.
| GU | DU | PUD (DU and GU) | NPUD | |
|---|---|---|---|---|
|
| ||||
| dupA+ (n) | 4 | 11 | 15 | 14 |
| dupA− (n) | 19 | 34 | 53 | 72 |
| Total | 23 | 45 | 68 | 86 |
GU: Gastric ulcer, DU: duodenal ulcer, PUD: peptic ulcer disease, NPUD: no peptic ulcer disease, dupA: duodenal ulcer promoting A gene.
DISCUSSION
The prevalence of H pylori infection ranged from more than 70% in developing countries such as Bangladesh, India, and Mexico to around 20% in developed countries such as Netherland and Australia.12 It was previously found that inadequate sanitation practices, low social class, and crowded or high-density living conditions seem to be related to a higher prevalence of H pylori infection.13 Hence, this high prevalence of H pylori infection in our population may be due to poor hygiene and crowded conditions.
CagA protein, which is encoded by cagA gene with in the cag PAI, is produced by the vast majority of H pylori strains. The cagA has been found to associate significantly with an increased risk for the development of atrophic gastritis, PUD, and gastric cancer.5,6 We looked within our population for associations between virulence factors and PUD. Among our strains, we observed an association between cagA+ status and PUD. Reports from neighboring countries, Turkey and Saudi, have shown similar results to ours.14–16 However, conflicting results have been reported in Iran, probably due to the difference between cagA prevalence among Iranian regions.14,17–19 Additionally, we showed that cagA is not associated with DU, which may be a type II error due to the sample size. More research is needed to investigate the role of H pylori virulence factors and their role in upper GI diseases.
In a study conducted using samples from South Korea, Japan, and Colombia, it was shown that the presence of dupA was significantly associated with DU and negatively associated with gastric cancer. In the same study, dupA appeared to increase interleukin-8 secretion from gastric mucosa.7 Additionally, this correlation between dupA and DU was shown in a population from northern India.8 However, no significant association between dupA prevalence and ulceration or cancer was found in populations from Brazil.20 Alternatively, it was found that dupA was significantly associated with gastric cancer development in populations from Belgium, South Africa, China, and the USA, but was not significantly associated with DU.9 Previously, it was also shown that the presence of dupA was associated with more intense antral neutrophil infiltration in populations from East Asia and South America7 but not from Iraq.21 In this project, no association was found between dupA and PUD. Again, this may be due to the sample size or due to the presence of polymorphisms in the dupA, such as the one described by Hussein et al.11 These differences may be similar to the differences found in vacA and cagA phosphorylation motifs.
In conclusion, while a significant association between cagA and PUD was observed, no relationship between dupA and PUD was seen. Studies from different parts of Iraq including different ethnic groups are needed to reach solid conclusions about the relationships between H pylori virulence factors and clinical outcomes.
REFERENCES
- 1.Atherton J. The Pathogenesis of H pylori–Induced Gastro-Duodenal Diseases. Annual Review of: Mechanisms of Disease. 2006;1:63–96. doi: 10.1146/annurev.pathol.1.110304.100125. [DOI] [PubMed] [Google Scholar]
- 2.Bandipalliam P. MALT lymphomas--a closer look in the genomics era. South Med J. 2006;99(12):1322–4. doi: 10.1097/01.smj.0000224741.24827.b1. [DOI] [PubMed] [Google Scholar]
- 3.Forman D, Newell DG, Fullerton F, Yarnell JW, Stacey AR, Wald N, Sitas F. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. Bmj. 1991;302(6788):1302–5. doi: 10.1136/bmj.302.6788.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akopyants NS, Clifton SW, Kersulyte D, Crabtree JE, Youree BE, Reece CA, Bukanov NO, Drazek ES, Roe BA, Berg DE. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol. 1998;28(1):37–53. doi: 10.1046/j.1365-2958.1998.00770.x. [DOI] [PubMed] [Google Scholar]
- 5.Atherton JC. H pylori virulence factors. Br Med Bull. 1998;54(1):105–120. doi: 10.1093/oxfordjournals.bmb.a011662. [DOI] [PubMed] [Google Scholar]
- 6.Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, Stemmermann GN, Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55(10):2111–5. [PubMed] [Google Scholar]
- 7.Lu H, Hsu PI, Graham DY, Yamaoka Y. Duodenal ulcer promoting gene of Helicobacter pylori. Gastroenterology. 2005;128(4):833–848. doi: 10.1053/j.gastro.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arachchi HS, Kalra V, Lal B, Bhatia V, Baba CS, Chakravarthy S, Rohatgi S, Sarma PM, Mishra V, Das B, Ahuja V. Prevalence of duodenal ulcerpromoting gene (dupA) of Helicobacter pylori in patients with duodenal ulcer in North Indian population. Helicobacter. 2007;12(6):591–7. doi: 10.1111/j.1523-5378.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- 9.Argent RH, Burette A, Miendje Deyi VY, Atherton JC. The presence of dupA in Helicobacter pylori is not significantly associated with duodenal ulceration in Belgium, South Africa, China, or North America. Clin Infect Dis. 2007;45(9):1204–6. doi: 10.1086/522177. [DOI] [PubMed] [Google Scholar]
- 10.Rudi J, Kolb C, Maiwald M, Kuck D, Sieg A, Galle PR, Stremmel W. Diversity of Helicobacter pylori vacA and cagA genes and relationship to VacA and CagA protein expression, cytotoxin production, and associated diseases. J Clin Microbiol. 1998;36(4):944–948. doi: 10.1128/jcm.36.4.944-948.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussein NR, Argent RH, Marx CK, Patel SR, Robinson K, Atherton JC. Helicobacter pylori dupA Is Polymorphic, and Its Active Form Induces Proinflammatory Cytokine Secretion by Mononuclear Cells. Journal of Infectious Diseases. 2010;202(2):261–269. doi: 10.1086/653587. [DOI] [PubMed] [Google Scholar]
- 12.Khalifa M, Sharaf R, Aziz R. Helicobacter pylori: a poor man’s gut pathogen? Gut Pathogens. 2010;2(1):2. doi: 10.1186/1757-4749-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown LM. Helicobacter Pylori: Epidemiology and Routes of Transmission. Epidemiologic Reviews. 2000;22(2):283–297. doi: 10.1093/oxfordjournals.epirev.a018040. [DOI] [PubMed] [Google Scholar]
- 14.Hussein NR, Mohammadi M, Talebkhan Y, Doraghi M, Letley DP, Muhammad MK, Argent RH, Atherton JC. Differences in virulence markers between Helicobacter pylori strains from Iraq and those from Iran: potential importance of regional differences in H pylori-associated disease. J Clin Microbiol. 2008;46(5):1774–9. doi: 10.1128/JCM.01737-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Momenah AM, Tayeb MT. Helicobacter pylori cagA and iceA genotypes status and risk of peptic ulcer in Saudi patients. Saudi Med J. 2007;28(3):382–385. [PubMed] [Google Scholar]
- 16.Saribasak HSB, Yamaoka Y, Sander E. Analysis of Helicobacter pylori genotypes and correlation with clinical outcome in Turkey. J Clin Microbiol. 2004;42:1648–1651. doi: 10.1128/JCM.42.4.1648-1651.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dabiri H, Maleknejad P, Yamaoka Y, Feizabadi MM, Jafari F, Rezadehbashi M, Nakhjavani FA, Mirsalehian A, Zali MR. Distribution of Helicobacter pylori cagA, cagE, oipA and vacA in different major ethnic groups in Tehran, Iran. Journal of Gastroenterology and Hepatology. 2009;24(8):1380–1386. doi: 10.1111/j.1440-1746.2009.05876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salehi Z, Jelodar M, Rassa M, Ahaki M, Mollasalehi H, Mashayekhi F. Helicobacter pylori cagA Status and Peptic Ulcer Disease in Iran. Digestive Diseases and Sciences. 2009;54(3):608–613. doi: 10.1007/s10620-008-0378-8. [DOI] [PubMed] [Google Scholar]
- 19.Talebkhan Y, Mohammadi M, Mohagheghi M, Vaziri H, Eshagh Hosseini M, Mohajerani N, Oghalaei A, Esmaeili M, Zamaninia L. cagA Gene and Protein Status Among Iranian Helicobacter pylori Strains. Digestive Diseases and Sciences. 2008;53(4):925–932. doi: 10.1007/s10620-007-9978-y. [DOI] [PubMed] [Google Scholar]
- 20.Gomes LI, Rocha GA, Rocha AM, Soares TF, Oliveira CA, Bittencourt PF, Queiroz DM. Lack of association between Helicobacter pylori infection with dupA-positive strains and gastroduodenal diseases in Brazilian patients. Int J Med Microbiol. 2007;298:223–230. doi: 10.1016/j.ijmm.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Hussein NR, Napaki S, Atherton J. A study of Helicobacter pylori -associated gastritis patterns in Iraq and their association with strain virulence. Saudi J Gastroenterol. 2009;15(2):125–127. doi: 10.4103/1319-3767.48971. [DOI] [PMC free article] [PubMed] [Google Scholar]