Abstract
Taste or gustation is one of the 5 traditional senses including hearing, sight, touch, and smell. The sense of taste has classically been limited to the 5 basic taste qualities: sweet, salty, sour, bitter, and umami or savory. Advances from the Human Genome Project and others have allowed the identification and determination of many of the genes and molecular mechanisms involved in taste biology. The ubiquitous G protein–coupled receptors (GPCRs) make up the sweet, umami, and bitter receptors. Although less clear in humans, transient receptor potential ion channels are thought to mediate salty and sour taste; however, other targets have been identified. Furthermore, taste receptors have been located throughout the body and appear to be involved in many regulatory processes. An emerging interplay is revealed between chemical sensing in the periphery, cortical processing, performance, and physiology and likely the pathophysiology of diseases such as diabetes.
Human taste can be distilled down to the basic 5 taste qualities of sweet, sour, bitter, salty and umami or savory.1 Although the sense of taste has been viewed as a nutritional quality control mechanism, the human experience of ingesting food is the interaction of all 5 senses. The sights, sounds, and smells of food prepare the body for the next meal. Hormone levels rise, stomach rumbles, and saliva starts to flow before a bite of food is taken.2 Then, as the food is placed in the mouth, taste, temperature, and touch receptors screen for quality and intensity, stimulating the appropriate saliva in preparation for chewing, bolus formation, and swallowing, or in the case of unpalatable or toxic materials, expectorating, retching, or vomiting. Appetitive tastes, such as sweet, umami, and low levels of sodium, describe the nutrient value of the food, while bitter, sour and high sodium tastes detect the presence of toxin’s, freshness/spoilage, or high mineral content, respectively. Sandy, sharp, or painful sensations tell of the presence of potentially harmful materials that may damage the digestive system, while creamy sensations tell of consistency, physical safety, and the presence of highly desirable fats. Temperature tells if the meal is too hot, too cold, or just right. These sensations are transmitted via cranial nerves (CNs) to the central nervous system (CNS), where olfactory input and past experiences merge to give an emotional, sensory, and physiological response.3
Tongue, Papillae, and Taste Buds
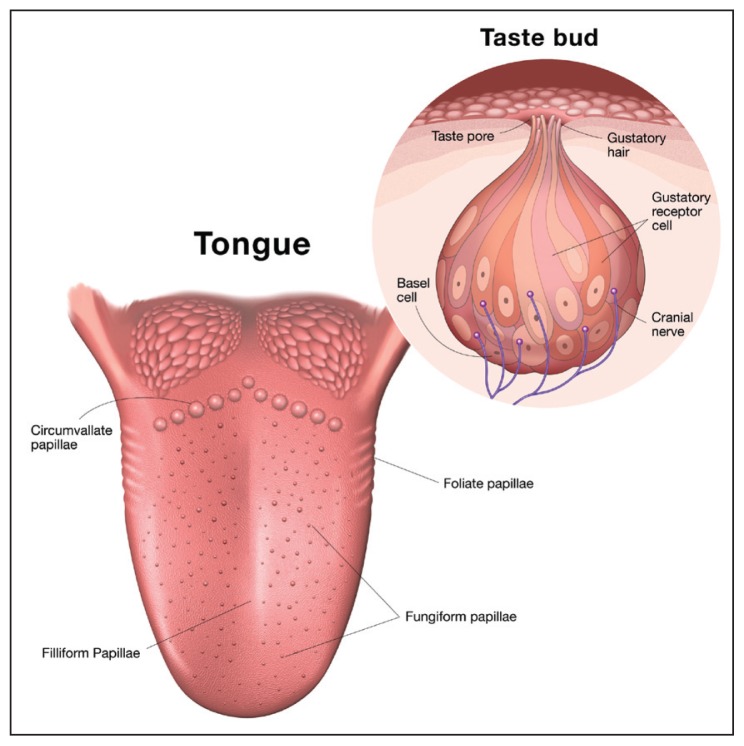
Although the human soft palate contains taste buds, the main organ of taste is classically considered the tongue and the primary structure that house the sensory endings are the papillae.4 Figure 1 shows that humans have 4 types of papillae: fungiform, foliate, and circumvallate are taste buds containing papillae, while filiform papillae transduce touch, temperature, and nociception.5,6 The fungiform papillae are mushroom-shaped structures that protrude from the surface of the tongue. Humans have on average 195 human fungiform papillae, 87% of which are located at the anterior 2 cm of the tongue.5 Foliate papillae are folds on the lateral sides of the tongue containing over 100 taste buds.5 Circumvallate papillae form an inverted V at the posterior of the tongue. These papillae are embedded into the tongue and have a moat around them. Humans have over 100 taste buds in the circumvallate papillae (Figure 1). The filiform papillae make up the bulk of the tongue papillae and contain trigeminal nerve endings that transmit information on the temperature, texture, and pain.7 Research on filiform papillae has lagged behind taste systems, but will be critical in understanding the influence of texture.
Figure 1.
Human tongue anatomy of papillae and taste buds. Circumvallate, fungiform, and foliate papillae are the structures that house the taste buds. Taste buds are a collection of differentiated epithelial cells that respond to the 5 basic tastes and transmit that information to the CNS. Filiform papillae are trigeminal and sense touch, temperature, and pain.
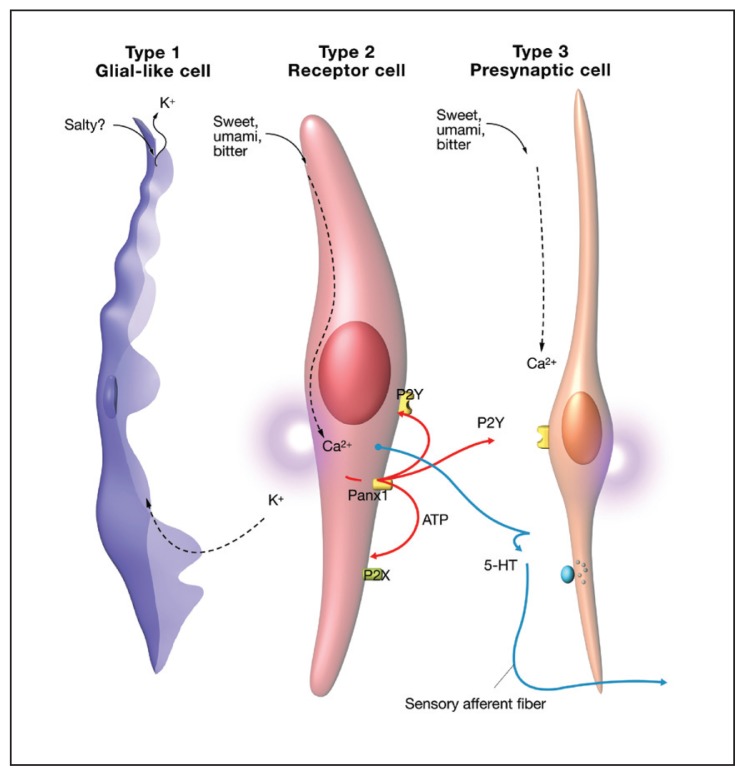
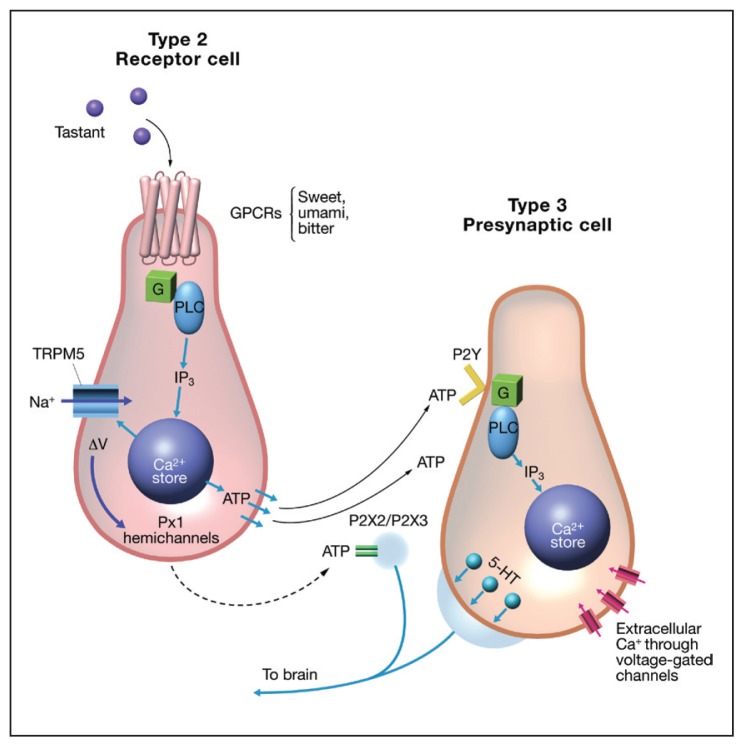
Taste buds are the primary sensory unit of the taste system and are imbedded under the keratinous layer of the papillae with a taste pore exposed to the external milieu. Figure 1 shows that taste buds are composed of 150 to 300 tightly packed cylindrical cells of epithelial origin.6 At least, 5 types of cells make up a taste bud: type 1, 2, 3 cells, basal cells, and neuronal processes (Figure 2). The various types of taste cells were originally characterized by the presence or absence of dense granules.6 Evidence now suggests that each taste modality is mutually exclusive to a subset of individual taste cells or 1 taste modality for 1 taste cell.8 For example, a type 2 sweet sensitive cell would express sweet receptors, but would not express bitter or umami receptors and vice versa. Sour is thought to be located on type 3 cells and sodium on type 1 cells.1 It has been shown that type 2 taste cells release adenosine triphosphate (ATP) in response to tastant activation.9 Figure 3 shows the current understanding of type 2 and 3 cell communication. ATP released from stimulated type 2 receptor cells activates P2Y adenosine receptors on nearby type 3 cells, releasing serotonin and stimulating afferent fibers to the CNS.10
Figure 2.
Taste bud cell types. Type 1 taste bud cells are glial-like and are thought to tranduce salty taste. Type 2 taste bud cells contain the GPCR receptors and are thought to mediate sweet, umami and bitter tastes. Type 3 presynaptic cells are through to transduce sour taste and mediate communication from the type 2 cells via P2Y adenosine receptors. The type 3 cell then signals to the afferent neurons via release of serotonin to neurons.
Figure 3.
Communication between type 2 and type 3 taste receptor cells. Type 2 cells contain the GPCRs for sweet, bitter, and umami. Activation by ligands stimulates a G protein cascade, releasing intracellular stores of calcium that causes release of ATP. ATP then binds to P2Y receptors on the type 3 presynaptic cells, resulting in serotonin release to stimulate afferent neurons.
Although sensory processing at the level of the taste bud is complex, the transfer of information to the CNS seems to be via a labeled line.11 Three CNs innervate the tongue: the chorda tympani (CN-VII), glossopharyngeal (CN-IX), and trigeminal (CN-V). The chorda tympani innervate the anterior fungiform papillae of the tongue. The glossopharyngeal innervates the circumvallate and foliate papillae of the posterior portion of the tongue. The trigeminal nerve receives information from the filiform papillae and from various nerve endings throughout the oral cavity. Taste information is projected to the insula of the gustatory cortex, where a gustotopic map has been created.12 Each individual taste has a “hot spot” in the insular that responds to a particular taste.
Taste Mechanisms
Sweet
Sweet taste is one of the most hedonically pleasurable senses. The goal of sweet taste is to detect highly calorific saccharides for ingestion. Sweet taste is hardwired into our genes known as tas1R2 and tas1R3.13 Even newborn children will show positive stereotypical behavior when exposed to sugar solutions.14
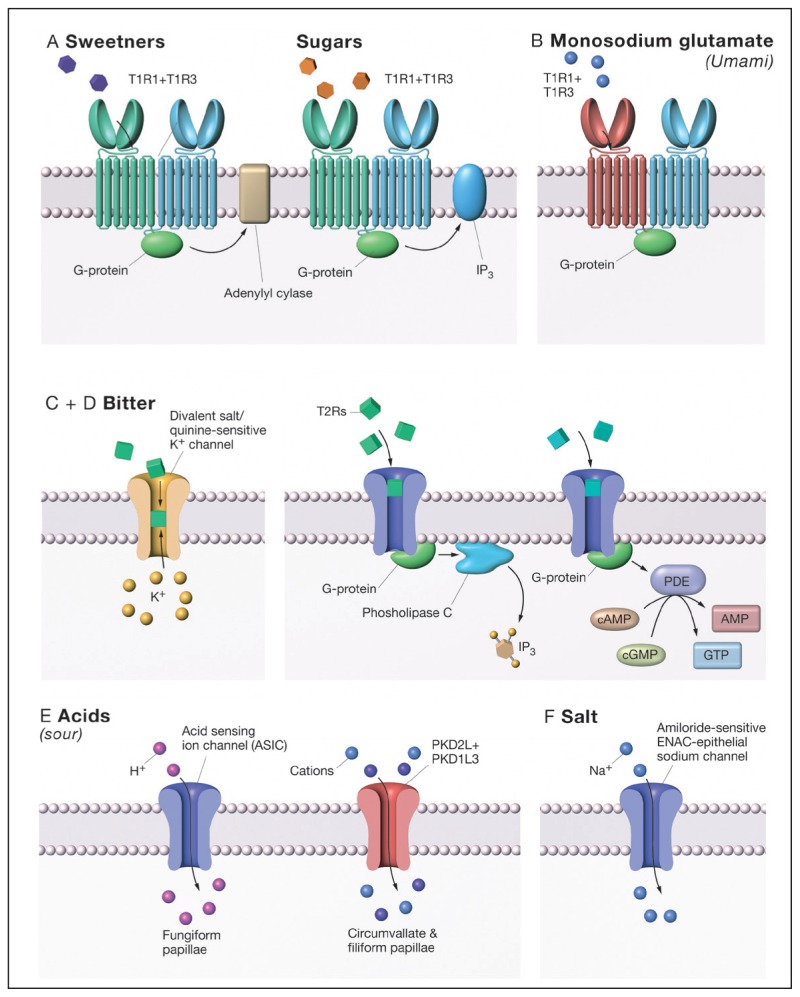
Figure 4A shows that sweet responsive type 2 taste cells express the C class receptors, G protein–coupled receptors (GPCRs) T1R2 + T1R3.15 See Urwyler, 2011, for an excellent review of C Class GPCRs.16 GPCRs are a ubiquitous class of proteins that function to detect extracellular signals and transmit that information to the cell.17 It is estimated that GPCRs make up 1% of the human genome and that 50% of drugs target these proteins.17 C class GPCRs are heterodimers with a large extracellular domain, a cysteine rich hinge, region and 7 transmembrane domain (7-TMD). The 7-TMD is composed of 7 α-helices per subunit, which thread through the cell membrane. When a molecule binds to a GPCR, a conformational change occurs in the protein, resulting in the activation of an intracellular heterotrimeric G protein composed of alpha, beta, and gamma and subunits can stimulate multiple downstream pathways. Well-characterized pathways exist via mobilization of calcium stories from the endoplasmic reticulum by activation of phospholipase beta 3, modulation of phosphodiesterase or adenylyl cyclase (AC)/guanylyl cyclase.18,19 These pathways modulate the second messengers inositol triphosphate and cAMP, cyclic guanosine monophosphate (cGMP) resulting in depolarization of the taste cell and release of neurotransmitter.
Figure 4.
Taste receptor mechanisms for the 5 basic tastes. A) Sweeteners and sugars stimulate the sweet taste receptor, T1R2/3, and activate downstream pathways. It has been suggested that sugars activate a phospholipase C–dependent pathway, while artificial sweeteners activate an adenylyl cyclase (AC) pathway. B) Monosodium glutamate activates the umami receptor, T1R1/3, and subsequently a phospholipase C pathway. C) Bitter signal transduction is mediated by 25 T2R receptors. Like the sweet receptors, bitter receptors are thought to activate both phospholipase C and AC pathways. D) Evidence suggests that a potassium channel maybe responsible for mediating or enhancing bitter tastes. E) Sour taste is thought to be mediated by PKD and ASIC channels, which are distributed on different populations of papillae. F) Salt taste is mediated by an amiloride-sensitive ENAC channel in rodents, but humans appeared to downregulated ENAC and have a less sensitive amiloride-insensitive system that has yet to be identified.
The sweet receptor functions as a dimer with an active site described as a venus fly trap module (VFTM).20 VFTM has been shown to close upon ligand binding and activate their respective G proteins. Small sugars, such as glucose, are thought to bind into the VFTM of the sweet taste receptor. T1R2 and T1R3 each have a VFTM and are thought to bind to different sweeteners. 21 This may be the basis for synergies and allosteric modulation of sweet taste. Furthermore, the large extracellular domain, the cysteine-rich linking region and the 7-TMD collectively allow for the binding of a rich array of modulators, agonists, and antagonist.16
Evidence has shown that T1R2/3 is expressed more widely than just the taste buds. Sweet taste receptors have been shown to be expressed in the K and L cells of the intestine, the beta-cells of the pancreas, bladder and hippocampus of the brain.22–25 The functional significance of the sweet taste receptor expression is hypothesized to be the requirement for sugar sensing in these tissues and the implication for a wider role in regulating metabolism for T1R2/3. Initial evidence came from studies with the immortalized enteroendocrine cell line NCI-H716.26 These cells express T1R2/3 and the machinery to release glucagon-like peptide-1 (GLP-1) in response to sweet stimuli. GLP-1, peptide tyrosine tyrosine (PYY), and gastrointestinal inhibitory peptide are key hormones mediating the incretin effect, or the gut stimulation of insulin release from beta-cells of the pancreas.27,28 T1R2/3 regulation of incretin secretion was examined in rats and humans. It was shown that enteroendocrine L-cells co-expressed T1R2/3, GLP-1 and PYY.29 Furthermore, data show that glucose-stimulated GLP-1 and PYY secretion was blocked by the T1R2/3 blocker lactisole.29 In additional studies, the incretin effect was isolated by intragastric or intraduodenal perfusion of glucose or mixed meal (17% protein, 30% fat, and 53% carbohydrate), again with or without lactisole.30 Intragastric infusion of glucose showed a significant stimulatory effect on GLP-1 and PYY secretions in vivo and this effect was blocked by lactisole, suggesting a role for T1R2/3. However, no effect was observed for liquid-mixed meal perfusion, suggesting the sweet taste receptor alone was not responsible for hormone release. Additional work is needed to determine if cephalic phase signals primes incretin effects.30
A major question arising from this research is as follows: Do artificial sweeteners alter the incretin effect and if so what are the physiological consequences? Past studies looking at the effect of artificial sweeteners on a variety of hormone levels in the blood have shown little effect from artificial sweeteners in vivo.31 However, pronounced effects have been reported in vitro.31 Studies suggest that local activation of sweet taste receptor from L-cells are thought to cause paracrine release of GLP-1 resulting in translocation of glucose transporters of the brush border in preparation for sugar absorption and distribution. Further evidence for the role of T1R2/3 in metabolism comes from the research showing that the loss of the incretin effect is a specific and early marker for type 2 diabetes.32 Moreover, studies with T1R2 or T1R3 knockout mice showed similar phenotypes to gastric bypass mice.33 Since gastric bypass surgery has the potential to reverse type 2 diabetes, the inference is that aberrant T1R2/3 function is a major factor in the progress on type 2 diabetes and that T1R2/3 is a potential drug target.34 In fact, the Gymnema sylvestre plant from India is a potent sweet taste blocker and has several thousand years of use as a homeopathic treatment for diabetes.35 The potential role for the sweet taste receptor in obesity and diabetes is provocative and will require much study, but the benefits of preventing or curing these costly diseases is clearly justified.
Umami
Umami is the Japanese word for the savory taste of amino acids, such as monosodium glutamate (MSG). Umami taste was first described in Japan by Kikunae Ikeda in 1908. Controversy surrounded the idea of umami as a primary taste until tas1R1 + tas1R3 was shown to code for the umami receptor.36 Figure 4B illustrates that the umami taste receptor is also a C class GPCR and contains a common subunit T1R3 with the sweet taste receptor and a unique subunit T1R1. Umami receptors show classical allosteric modulation of MSG response by GMP and IMP.37,38 The affinity of MSG can be lowered by an order of magnitude by the addition nucleotides, and this fact has been used in the food industries for over a hundred years to enhance the flavor of savory meals.39 Although the human umami receptor is very promiscuous, responding to all 20 ammo acids, it has the highest affinity for glutamate.40
Bitter
Currently 25 T2R bitter receptors have been identified in humans and belong to the A class GPCRs family. Figure 4C illustrates the A class receptors function as monomers and have small extracellular domains.8, 41 It is amazing to note that ~25 bitter receptors can identify a seemingly endless array of compounds.42 Many pharmacologically active compounds are bitter, which can be a major hindrance to oral compliance.
Potassium bitter taste may not be mediated by a T2R-dependent mechanism, but by a potassium-sensitive ion channel on type 2 cells (Figure 4D).43 The identity of this channel is currently under investigation.
Sour
Previously it was show that the polycystic kidney disease (PKD) channel PKD1L2 + PKD3L1 mediates sour taste (Figure 4F).44 These receptors have been found on type III taste cells and appear to function by allowing protons to traverse the membrane. The depolarization of the taste cell then stimulates the release of neurotransmitter. Cholecystokinin and neuropeptide Y are candidate neurotransmitters for the sour signal transduction. A study has suggested that PKD channels, mediates sour taste from circumvallate and foliate papillae.45 In a study of PKD knockout mice, sour taste response was inhibited only 25% to 45%, suggesting a secondary mechanism. Acid sensing ion channel (ASIC) were proposed as the mechanism for the fungiform papillae of the tongue.45,46 The researchers found that ACIS were present on taste cells and were sour active.
Salt
Salt mechanism has been quite controversial over the last decade. Epithelial sodium channel (ENaC) was an ideal candidate for the salt taste channel, and in rodents ENaC plays a central role in sodium taste; however, in humans ENaC seems to play very little role (Figure 4E).47 The sodium channel blocker, amiloride, effectively blocks sodium-dependent evoke potentials from rodents and blocks sodium preference, but in humans, amiloride has little to no effect in humans. Some have shown evidence for a second salt detection system.48,49 This alternative salt detection system mediates sodium and potassium tastes and is thought to be the ASIC and is much less sensitive to salt concentration; however, today no definitive evidence has identified the human protein responsible for salt taste.
In conclusion, an understanding of human taste biology has exploded over the last decade, but many questions still remain. It is remarkable that food quality and intensity can be coded by just 5 basic tastes; however, when combined with retronasal input from the approximately 300 human olfactory receptors, we perceive a large variety of flavors. The rich flavors of our human diet are the sum of taste, olfaction, and trigeminal input, but the synthesis of perception is the sum of peripheral input modulated by emotion, physiological and metabolic state, and learning. It is now becoming clear that taste receptors have a deeper role then just quality control. They also play an important role in maintaining nutrient homeostasis. How these sensory modalities interplay to regulate weight and satiety will be the next challenge in human nutrition.
Acknowledgments
We would like to thank George Retseck for the illustrations.
REFERENCES
- 1.Lindermann B. Taste Reception. Physiol Rev. 1996:718–766. doi: 10.1152/physrev.1996.76.3.719. [DOI] [PubMed] [Google Scholar]
- 2.Sarles H, et al. Cephalic phase of pancreatic secretion in man. Gut. 1968;9(2):214–21. doi: 10.1136/gut.9.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaudhari N, Roper SD. The cell biology of taste. The Journal of Cell Biology. 2010;190(3):285–296. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azzali G, et al. Vallate, foliate and fungiform human papillae gustatory cells. An immunocytochemical and ultrastructural study. Minerva Stomatol. 1996;45(9):363–79. [PubMed] [Google Scholar]
- 5.Miller IJ, Jr, Preslar AJ. Spatial distribution of rat fungiform papillae. Anat Rec. 1975;181(3):679–84. doi: 10.1002/ar.1091810309. [DOI] [PubMed] [Google Scholar]
- 6.Brouwer JN, Wiersma A. Location of taste buds in intact taste papillae by a selective staining method. Histochemistry. 1978;58(3):145–51. doi: 10.1007/BF00495713. [DOI] [PubMed] [Google Scholar]
- 7.Triantafyllou A, Coulter P. Structural organization of subgemmal neurogenous plaques in foliate papillae of tongue. Hum Pathol. 2004;35(8):991–9. doi: 10.1016/j.humpath.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, et al. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112(3):293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 9.Finger TE, et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310(5753):1495–9. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 10.Kataoka S, et al. A2BR adenosine receptor modulates sweet taste in circumvallate taste buds. PLoS One. 2012;7(1):10. doi: 10.1371/journal.pone.0030032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellekant G, Ninomiya Y, Danilova V. Taste in chimpanzees. III: Labeled-line coding in sweet taste. Physiol Behav. 1998;65(2):191–200. doi: 10.1016/s0031-9384(97)00532-5. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, et al. A gustotopic map of taste qualities in the mammalian brain. Science. 2011;333(6047):1262–6. doi: 10.1126/science.1204076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson G, et al. Mammalian sweet taste receptors. Cell. 2001;106(3):381–90. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 14.Rosenstein D, Oster H. Differential facial responses to four basic tastes in newborns. Child Dev. 1988;59(6):1555–68. [PubMed] [Google Scholar]
- 15.Zhao GQ, et al. The receptors for mammalian sweet and umami taste. Cell. 2003;115(3):255–66. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- 16.Urwyler S. Allosteric modulation of family C G-protein-coupled receptors: from molecular insights to therapeutic perspectives. Pharmacol Rev. 2011;63(1):59–126. doi: 10.1124/pr.109.002501. [DOI] [PubMed] [Google Scholar]
- 17.Lundstrom K. The future of G protein-coupled receptors as targets in drug discovery. IDrugs. 2005;8(11):909–13. [PubMed] [Google Scholar]
- 18.Gilbertson TA, Damak S, Margolskee RF. The molecular physiology of taste transduction. Curr Opin Neurobiol. 2000;10(4):519–27. doi: 10.1016/s0959-4388(00)00118-5. [DOI] [PubMed] [Google Scholar]
- 19.Clapp TR, et al. Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction. BMC Neurosci. 2001;2(6):23. doi: 10.1186/1471-2202-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bessis AS, et al. Closure of the Venus flytrap module of mGlu8 receptor and the activation process: Insights from mutations converting antagonists into agonists. Proc Natl Acad Sci U S A. 2002;99(17):11097–102. doi: 10.1073/pnas.162138699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masuda K, et al. Characterization of the modes of binding between human sweet taste receptor and low-molecular-weight sweet compounds. PLoS One. 2012;7(4):20. doi: 10.1371/journal.pone.0035380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haid D, Widmayer P, Breer H. Nutrient sensing receptors in gastric endocrine cells. J Mol Histol. 2011;42(4):355–64. doi: 10.1007/s10735-011-9339-1. [DOI] [PubMed] [Google Scholar]
- 23.Kyriazis GA, Soundarapandian MM, Tyrberg B. Sweet taste receptor signaling in beta cells mediates fructose-induced potentiation of glucose-stimulated insulin secretion. Proc Natl Acad Sci U S A. 2012;109(8):6. doi: 10.1073/pnas.1115183109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elliott RA, Kapoor S, Tincello DG. Expression and distribution of the sweet taste receptor isoforms T1R2 and T1R3 in human and rat bladders. J Urol. 2011;186(6):2455–62. doi: 10.1016/j.juro.2011.07.083. [DOI] [PubMed] [Google Scholar]
- 25.Ren X, et al. Sweet taste signaling functions as a hypothalamic glucose sensor. Front Integr Neurosci. 2009;3(12):19. doi: 10.3389/neuro.07.012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kokrashvili Z, Mosinger B, Margolskee RF. Taste signaling elements expressed in gut enteroendocrine cells regulate nutrient-responsive secretion of gut hormones. Am J Clin Nutr. 2009;90(3):1. doi: 10.3945/ajcn.2009.27462T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev. 2008;60(4):470–512. doi: 10.1124/pr.108.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young RL. Sensing via intestinal sweet taste pathways. Front Neurosci. 2011;5(23):00023. doi: 10.3389/fnins.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinert RE, et al. The functional involvement of gut-expressed sweet taste receptors in glucose-stimulated secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) Clin Nutr. 2011;30(4):524–32. doi: 10.1016/j.clnu.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Gerspach AC, et al. The role of the gut sweet taste receptor in regulating GLP-1, PYY, and CCK release in humans. Am J Physiol Endocrinol Metab. 2011;301(2):3. doi: 10.1152/ajpendo.00077.2011. [DOI] [PubMed] [Google Scholar]
- 31.Renwick AG, Molinary SV. Sweet-taste receptors, low-energy sweeteners, glucose absorption and insulin release. Br J Nutr. 2010;104(10):1415–20. doi: 10.1017/S0007114510002540. [DOI] [PubMed] [Google Scholar]
- 32.Holst JJ, et al. Loss of incretin effect is a specific, important, and early characteristic of type 2 diabetes. Diabetes Care. 2011;34(2) doi: 10.2337/dc11-s227. p. dc11-s227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geraedts MC, et al. Transformation of postingestive glucose responses after deletion of sweet taste receptor subunits or gastric bypass surgery. Am J Physiol Endocrinol Metab. 2012;303(4):5. doi: 10.1152/ajpendo.00163.2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Dixon JB, et al. Gastric bypass in Type 2 diabetes with BMI < 30: weight and weight loss have a major influence on outcomes. Diabet Med. 2012;28(10):12107. doi: 10.1111/dme.12107. [DOI] [PubMed] [Google Scholar]
- 35.Liu B, et al. Characterisation of the insulino-tropic activity of an aqueous extract of Gymnema sylvestre in mouse beta-cells and human islets of Langerhans. Cell Physiol Biochem. 2009;23(1–3):125–32. doi: 10.1159/000204101. [DOI] [PubMed] [Google Scholar]
- 36.Nelson G, et al. An amino-acid taste receptor. Nature. 2002;416(6877):199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 37.Mouritsen OG, Khandelia H. Molecular mechanism of the allosteric enhancement of the umami taste sensation. Febs J. 2012;279(17):3112–20. doi: 10.1111/j.1742-4658.2012.08690.x. [DOI] [PubMed] [Google Scholar]
- 38.Zhang F, et al. Molecular mechanism for the umami taste synergism. Proc Natl Acad Sci U S A. 2008;105(52):20930–4. doi: 10.1073/pnas.0810174106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mallick HN. Understanding safety of glutamate in food and brain. Indian J Physiol Pharmacol. 2007;51(3):216–34. [PubMed] [Google Scholar]
- 40.Li X, et al. Human receptors for sweet and umami taste. Proc Natl Acad Sci U S A. 2002;99(7):4692–6. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mueller KL, et al. The receptors and coding logic for bitter taste. Nature. 2005;434(7030):225–9. doi: 10.1038/nature03352. [DOI] [PubMed] [Google Scholar]
- 42.Behrens M, Meyerhof W. Bitter taste receptor research comes of age: From characterization to modulation of TAS2Rs. Semin Cell Dev Biol. 2012;27(12):00147–4. doi: 10.1016/j.semcdb.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Liu L, et al. Expression and characterization of delayed rectifying K+ channels in anterior rat taste buds. Am J Physiol Cell Physiol. 2005;289(4):1. doi: 10.1152/ajpcell.00115.2005. [DOI] [PubMed] [Google Scholar]
- 44.Huang AL, et al. The cells and logic for mammalian sour taste detection. Nature. 2006;442(7105):934–8. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamoto K, et al. Genetic tracing of the gustatory neural pathway originating from Pkd1l3-expressing type III taste cells in circumvallate and foliate papillae. J Neurochem. 2011;119(3):497–506. doi: 10.1111/j.1471-4159.2011.07443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimada S, et al. Acid-sensing ion channels in taste buds. Arch Histol Cytol. 2006;69(4):227–31. doi: 10.1679/aohc.69.227. [DOI] [PubMed] [Google Scholar]
- 47.Chandrashekar J, et al. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464(7286):297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lyall V, et al. The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. J Physiol. 2004;558(Pt 1):147–59. doi: 10.1113/jphysiol.2004.065656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lyall V, et al. A novel vanilloid receptor-1 (VR-1) variant mammalian salt taste receptor. Chem Senses. 2005;30(1):i42–3. doi: 10.1093/chemse/bjh104. [DOI] [PubMed] [Google Scholar]