Abstract
Sudden unexpected death occurs in one quarter of deaths in Rett Syndrome (RTT), a neurodevelopmental disorder caused by mutations in Methyl-CpG-binding protein 2 (MECP2). People with RTT show a variety of autonomic nervous system (ANS) abnormalities and mouse models show similar problems including QTc interval prolongation and hypothermia. To explore the role of cardiac problems in sudden death in RTT, we characterized cardiac rhythm in mice lacking Mecp2 function. Male and female mutant mice exhibited spontaneous cardiac rhythm abnormalities including bradycardic events, sinus pauses, atrioventricular block, premature ventricular contractions, non-sustained ventricular arrhythmias, and increased heart rate variability. Death was associated with spontaneous cardiac arrhythmias and complete conduction block. Atropine treatment reduced cardiac arrhythmias in mutant mice, implicating overactive parasympathetic tone. To explore the role of MeCP2 within the parasympathetic neurons, we selectively removed MeCP2 function from cholinergic neurons (MeCP2 ChAT KO), which recapitulated the cardiac rhythm abnormalities, hypothermia, and early death seen in RTT male mice. Conversely, restoring MeCP2 only in cholinergic neurons rescued these phenotypes. Thus, MeCP2 in cholinergic neurons is necessary and sufficient for autonomic cardiac control, thermoregulation, and survival, and targeting the overactive parasympathetic system may be a useful therapeutic strategy to prevent sudden unexpected death in RTT.
Introduction
RTT is an X-linked neurodevelopmental disorder that is nearly always caused by loss of function mutations in Methyl-CpG-binding protein 2 (MECP2) (1,2). The protein product, MeCP2, functions to regulate transcription both in as a repressor and activator of transcription (3). Loss of MeCP2 function leads to a complex array of neurological symptoms including developmental regression, difficulty walking, repetitive meaningless hand movements, seizures (4), breathing abnormalities (5), and a variety of autonomic problems (6) including cardiac abnormalities. Nearly 20% of individuals with RTT have a cardiac conduction defect known as QT interval prolongation, or long QT (LQT) (7–9). Furthermore, there have been case reports of severe sinus bradycardia (10), AV block (11), and possible lethal ventricular tachyarrhythmias (VT) (12). However, the aetiology of the cardiac phenotypes in RTT remains to be investigated.
About one quarter of all deaths in RTT individuals are sudden and unexpected (8). However the cause of these deaths has not been investigated, and autonomic dysfunction and cardiac arrhythmias are suspected to underlie some, if not all, of the sudden deaths (7–9,12,13). Mouse models can provide insights to a possible explanation of the sudden death. In fact, mice lacking Mecp2 function have severe autonomic dysfunction days prior to death including progressively decreasing heart rate and temperature (14). Additionally, we have previously shown that mouse models of RTT recapitulate some of cardiac phenotypes observed in RTT individuals, specifically LQT and increased susceptibility to induction of VT (9). Furthermore, the LQT and inducible VT result from loss of MeCP2 function within the nervous system (9). However, three questions remained unanswered; 1) Do RTT mice have cardiac autonomic dysfunction? 2) Do RTT mice have spontaneous arrhythmias? 3) What neuronal types are responsible for the neurogenic arrhythmias?
To address these issues, we performed ECG telemetry to analyse cardiac autonomic function in MeCP2 deficient mice and found that these mice have bradycardia, increased heart rate irregularity, and spontaneous cardiac arrhythmias. The heart rate continued to decline with increasing instances of premature ventricular contraction and AV block until complete block and asystole causing death. Treatment with pharmacological agents revealed evidence of increased parasympathetic tone and suggested overactive vagus nerve. Because of this, we hypothesized that cholinergic neurons require MeCP2 for normal cardiac autonomic function. To test this hypothesis, we have either removed or restored MeCP2 function within cholinergic neurons and found removing MeCP2 from these neurons reproduced the observed cardiac abnormalities and restoring MeCP2 within these neurons rescued the same cardiac problems. This work provides strong evidence that MeCP2 in cholinergic neurons is necessary and sufficient for cardiac autonomic control and temperature regulation that can improve survival outcome, and suggests that manipulating these systems may be a useful therapeutic approach.
Results
MeCP2 deficient mice display severe autonomic dysfunction
Previously, using a pulse oximetry device that captures heart rate over a 5 min interval from the tail vein, we found that male mice deficient in MeCP2 had decreased heart rate that showed a progressive decline in the days preceding death in male mice (14). However, the methods used did not permit detailed characterization of the heart rate variability nor allow for continuous 24-h recordings. Furthermore, although female mice lacking one copy of Mecp2 (heterozygous mutants) have decreased temperature (15), it remains unknown if they also have the same cardiac autonomic disturbances observed in male mice (14). To address these issues, we performed telemetry in both male 2-3 month old MeCP2 deficient (NULL) and 9-month-old heterozygous female mice (NULL/+). This method allows for the continuous assessment of both heart rate and temperature over a 24-h period, and comparisons of heart rate, temperature, and activity during light and dark cycles.
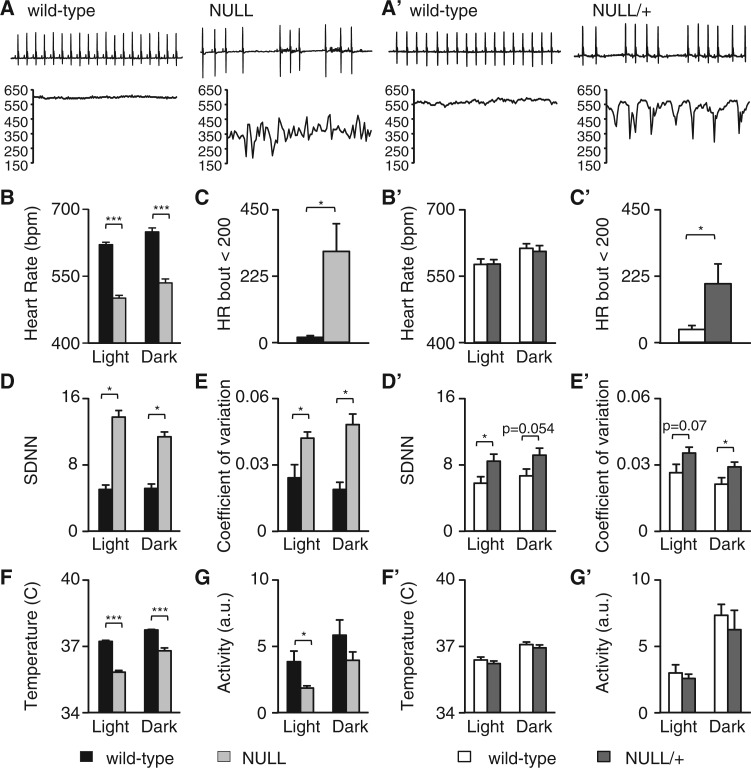
ECG recordings revealed that both male mice lacking MeCP2 (Mecp2BirdTM1.1/Y, NULL) and female heterozygous mice (Mecp2BirdTM1.1/+, NULL/+) had erratic heart rate patterns when compared to wild type littermates (Fig. 1A and A’). Inspection of very short intervals (top panels, Fig. 1A and A’) showed that both NULL and NULL/+ animals had sudden pauses in the regular rhythm of the heart rate. Analysis of longer intervals (15s, lower panel Fig. 1A and A’) showed sudden drops in the heart rate (bradycardic events) in both NULL and NULL/+ mice. Quantification of the overall heart rate during a full 24-h period revealed that male NULL mice had decreased heart rate during both the light and dark, whereas female NULL/+ mice did not show an overall change in heart rate at this level, despite having short bradycardic events (Fig. 1B and B’). We quantified these sudden bradycardic events, heart rate below 200 bpm, in both NULL and NULL/+ animals. Both male and female mice had a higher incidence of these sudden bradycardic events (Fig. 1C and C’). Additionally, both NULL and NULL/+ mice had increased heart rate variability as determined by the standard deviation of the R to R wave interval (SDNN) and coefficient of variation (Fig. 1D, 1E and 1D’, 1E’) .
Figure 1.
MeCP2 deficient mice display severe autonomic dysfunction. (A) Representative 2 s ECG tracings from 10 week-old male wild-type and NULL (male Mecp2TM1.1Bird/Y) mouse. Below the tracing is a 15 s instantaneous heart rate graph showing the irregular heart rate in the NULL mouse. (A’) Representative 2 s ECG tracing of a 9 month-old female WT mouse and NULL/+ (female Mecp2TM1.1Bird/+) mouse showing the irregular heart rhythm and variable heart rate. (B and B’) The mean heart rate from telemetry recordings was binned into light and dark cycle and compared to age matched controls. (C and C’) Sudden bradycardic events defined as a heart rate below 200 bpm were quantified over a 24-h period in both male and female mice and compared to controls. (D-E and D’-E’) Heart rate irregularity was measured by the mean standard deviation of the RR interval (SDNN) and the coefficient of variation. (F-G and F’-G’) Temperature and activity levels were binned to light and dark cycles in both male and female mice and compared to controls. Data presented as mean ± SEM male n = 5–7 per genotype, female n = 8–10 per genotype *P < 0.05 **P < 0.01 ***P < 0.01.
NULL mice also showed decreased temperature during both the light and dark cycle whereas NULL/+ mice did not (Fig. 1F and F’). Finally, NULL mice had decreased activity levels during the light cycle and not the dark cycle but NULL/+ mice did not have any change in activity compared with wild-type animals (Fig. 1G and G’).
Although these results found that 9 month old female NULL/+ mice did not have decreased heart rate and temperature, we assessed whether the development of these phenotypes might be delayed in these heterozygous animals. Therefore, we performed telemetry in older female NULL/+ mice (28 months) to determine if there is an age dependent onset of these phenotypes. We found that at 28 months of age NULL/+ mice developed a decreased heart rate (Supplementary Material, Fig. S1A), and decreased temperature (Supplementary Material, Fig. S1B) compared to age matched controls. No differences in activity were observed (Supplementary Material, Fig. S1C). Thus, both male NULL and female NULL/+ mice had cardiac rhythm and temperature abnormalities, but the onset of some of these was significantly delayed in female NULL/+ animals.
Mouse Models of RTT Are Prone to Aberrant Cardiac Conduction and Ventricular Arrhythmias
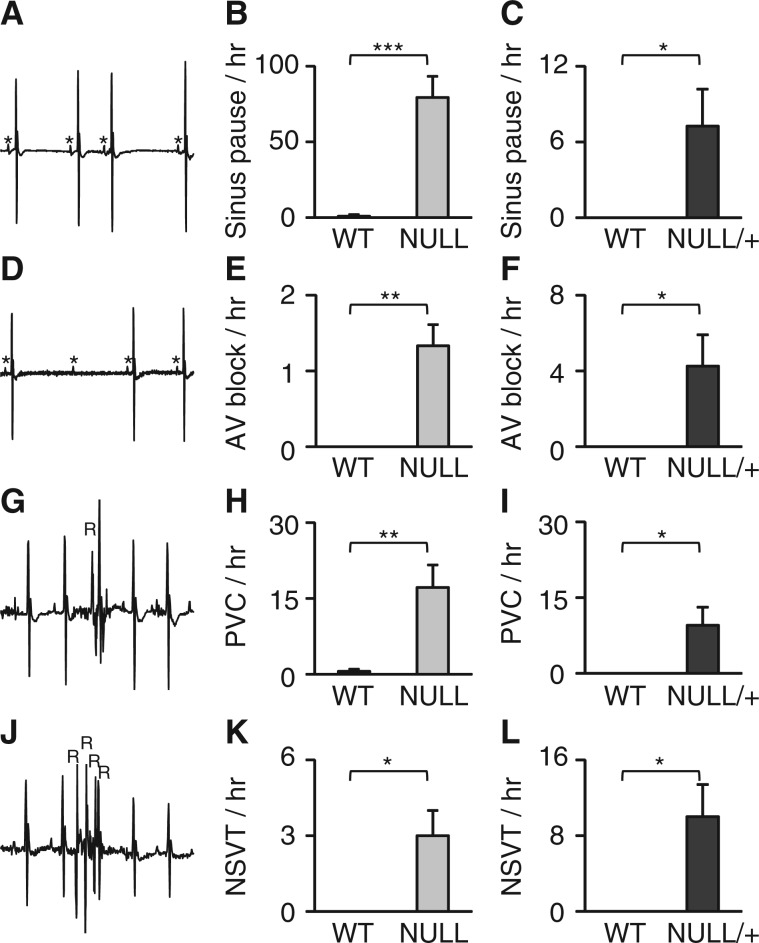
We previously found that mice lacking MeCP2 function are highly susceptible to inducible arrhythmias via programmed electrical stimulation (9), although it remains unknown if spontaneous arrhythmias in these mice. To address this, we analysed telemetry data from 2 month old male and 9 month old female MeCP2 deficient mice and their control littermates for spontaneous cardiac arrhythmias. Sinus pauses (Fig. 2A) were observed in both male NULL (Fig. 2B) and female NULL/+ (Fig. 2C) at much higher rate than control animals. Furthermore, atrioventricular block (Fig. 2D) was observed at a higher rate in male NULL (Fig. 2E) and female NULL/+ (Fig. 2F). Both male NULL and female NULL/+ were also susceptible to premature ventricular contractions (PVC, Fig. 2G–I). Finally, we observed non-sustained ventricular arrhythmias (Fig. 2G) in both genders (Fig. 2K and L). These results indicate that both male and female mouse models of RTT have a variety of spontaneous cardiac arrhythmias ranging from pauses and AV conduction block to aberrant ventricular contractions (PVC, NSVT). These findings support the idea that potentially dangerous cardiac arrhythmias may underlie sudden unexpected death in RTT.
Figure 2.
Mouse models of RTT are prone to aberrant cardiac conduction and ventricular arrhythmias. (A) Representative ECG image of sinus pauses. (B and C) Sinus pauses over a 2-h period were quantified and averaged to calculate the hourly rate in both male (NULL) and female (NULL/+) mice. (D) Representative ECG image of aberrant atrioventricular block (AVB). (E and F) The mean hourly rate of AVB was quantified in both male and female (NULL/+) mice. (G) An example of an isolated pre mature contraction (PVC). (H-I) Quantification of PVC’s per hour in male (NULL) and female (NULL/+) mice. (J) ECG trace of an example of non-sustained ventricular tachycardia. (K-L) The mean hourly rate of NSVT in male (NULL) and female (NULL/+) mice. Data presented as mean ± SEM. male n = 5–7 per genotype, female n = 7 per genotype *P < 0.05 **P < 0.01 ***P < 0.01.
Sudden Cardiac Death Observed in a Mouse Model of RTT
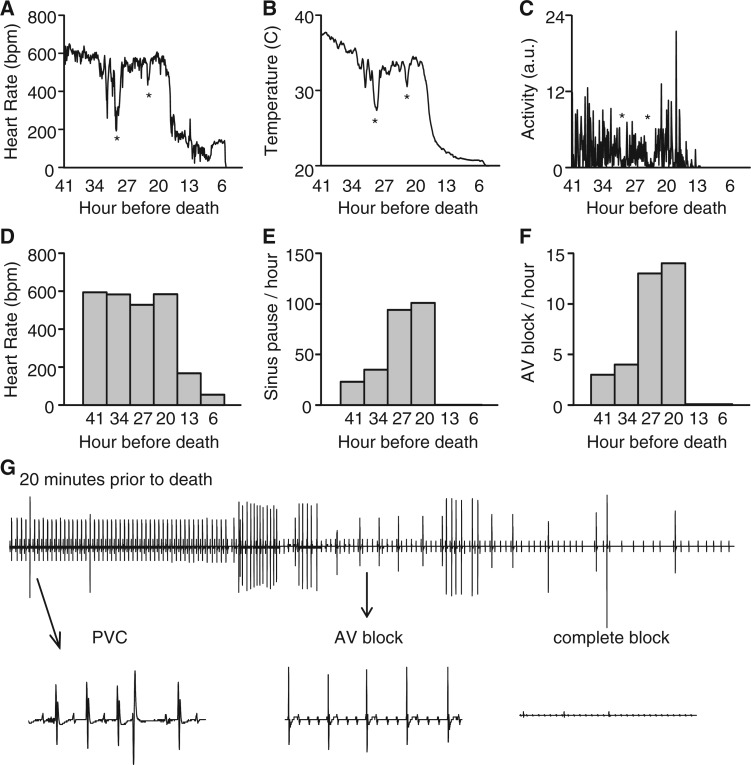
The identification of spontaneous cardiac arrhythmias in the mouse models of RTT raised the question whether at least some of the deaths observed in these mice are due to severe cardiac arrhythmias. During the collection of telemetry data on male mice containing a Mecp2 allele with a stop-flox (Mecp2TM2Bird) that eliminates nearly all MeCP2 protein expression (non-rescue) (16) and results in a mouse model that is essentially indistinguishable from NULL mice, we were able to capture sudden deaths in 2 of 7 mice (Fig. 3). In these two mutant mice, we observed abnormal fluctuations in heart rate and temperature that coincided with a sudden decrease in activity (indicated by * in Fig. 3A–C, plotted as 5 min bins for a representative animal). These mice have previously been reported to have seizure-like events that may cause these events. Interestingly, the heart rate and temperature recovered after the first two sudden drops, but at the third event the heart rate and temperature remained low and continued to decline, despite normal or even increased activity (Fig. 3C). When the data were viewed in 7 h bins, heart rate declined dramatically to severe bradycardia in the last 13 h before death (Fig. 3D). Interestingly, we observed a marked increase in sinus pauses and AV block events up until 13 h before death, but once the heart rate dropped to the bradycardic level these arrhythmias ceased (Fig. 3E and F). However, 20 min prior to death there was an onset of PVC’s followed by AV block (Fig. 3G). This representative mouse developed AVB for 18 min before complete block, asystole, and death (Fig. 3G). Thus, death in this mouse model of RTT can be caused by the observed spontaneous cardiac arrhythmias.
Figure 3.
Sudden cardiac death observed in a mouse model of RTT. Telemetry recordings from male mice containing a Mecp2 allele with a stop-flox (non-rescue, Mecp2TM2Bird/Y), an allele that removes detectable MeCP2 expression in the absence of Cre, revealed sudden abnormal cardiac activity (A), associated with thermoregulation abnormalities (B) and hypoactivity (C) signified in all plots by *. (D) Heart rate decreased with time approaching death. (E and F) Incidence of sinus pauses and AV block increased with time approaching death until the final 1 h before death. (G) 2-min representative ECG from a non-rescue mouse 20 min prior death showed PVC’s and AVB leading to complete heart block.
Atropine Improves Heart Rate and Sinus Pauses in Null Mice
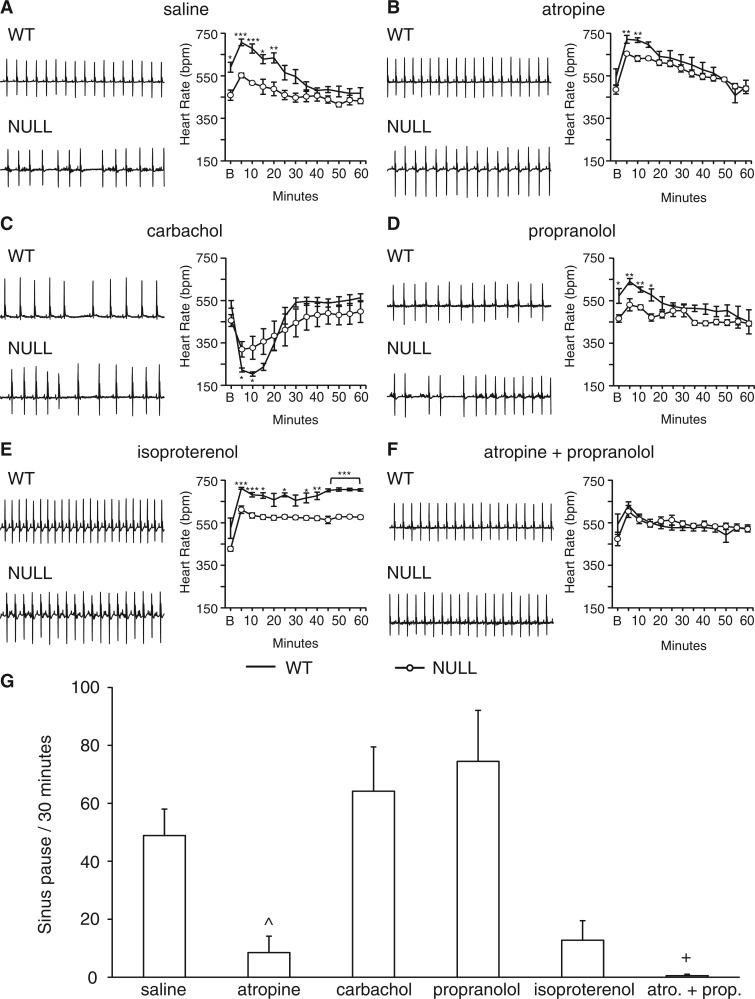
To further explore the origin of cardiac rhythm abnormalities in mice lacking MeCP2 function, we treated animals with pharmacological agents that activate or inhibit the two components of the autonomic nervous system. We probed the role of the sympathetic nervous system by treating either with an agonist (isoproterenol) and an antagonist (propranolol), and the parasympathetic nervous system with either an agonist (carbachol) or an antagonist (atropine). Additionally, we treated mice with both the sympathetic antagonist (propranolol) and the parasympathetic antagonist (atropine) to induce a "chemical denervation" that reveals the intrinsic heart rate. For all treatments we monitored heart rate response and quantified sinus pauses after drug. We restricted our treatment to male NULL mice as they show a robust cardiac phenotype at an earlier age compared to the heterozygous female mice.
Despite having been subjected to repeated injections to habituate to manipulation, saline injection into wild-type mice caused an initial heart rate increase that was blunted in NULL animals (Fig. 4A). Parasympathetic blockade with atropine nearly normalized the initial heart rate increase in NULL animals (Fig. 4B), but had little effect on wild-type animals. In contrast, parasympathetic activation with carbachol caused a dramatic decline in heart rate in wild-type animals but a less robust response in NULL animals (Fig. 4C). These results indicate that NULL mice are sensitive to parasympathetic blockade but have a blunted response to parasympathetic activation.
Figure 4.
Atropine improves heart rate and sinus pauses in NULL mice. Mice were treated acutely with pharmacological agents that activate or inhibit the sympathetic or parasympathetic nervous system. The average heart rate response was binned in 5 min intervals and recorded over a 1-h period. Representative 2 s ECG signals and heart rate response plotted over a 1-h period in both male NULL and wild-type mice after injected with saline (A), 1 mg/kg atropine (B), 0.5 mg/kg carbachol (C), 4mg/kg propranolol (D), 3.3 mg/kg isoproterenol (E), 4 mg/kg propranolol + 1 mg/kg atropine (F). The effect on sinus pauses was quantified over a 30-min period post injection to characterize the change in number of sinus pauses after injection with these drugs or saline (G). Data presented as mean ± SEM. WT mice n = 5 per treatment and NULL mice 4–6 per treatment. *P < 0.05, **P < 0.01, ***P < 0.01; NULL saline vs. NULL atropine ^ < 0.05; NULL saline vs. NULL atropine and propranolol + <0.01.
Acute sympathetic blockade with propranolol blunted the heart rate increase in wild-type animals but had little effect on the already blunted response of NULL animals (Fig. 4D). Similarly, sympathetic activation with isoproterenol resulted in a greater persistent increase in heart rate in wild-type mice but NULL mice did not achieve the same level of persistent heart rate increase (Fig. 4E). Thus, NULL mice have a blunted response to either sympathetic blockade or activation. In combination with the parasympathetic manipulation results, it appears that NULL mice have an excessive parasympathetic tone that blunts the responsiveness to pharmacological manipulations aside from the direct parasympathetic blockade.
To determine the intrinsic heart rate without sympathetic or parasympathetic stimulation, we performed a "chemical denervation" by treating animals simultaneously with atropine and propranolol (Fig. 4F). The heart rate response to chemical denervation revealed no differences in basal heart rate post injection (Fig. 3F), reinforcing the idea that abnormal heart rate in NULL animals is due to abnormal neuronal input to the heart and not a function of the heart itself.
Sinus pauses, previously observed to occur spontaneously (Fig. 2A), were present at high frequency in NULL saline injected animals, however, treatment with atropine caused a dramatic drop in the frequency of these arrhythmias (Fig. 4G). Similarly, increasing the heart rate with isoproterenol also decreased the rate of sinus pauses. In contrast, treatment of NULL animals with either propranolol or carbachol did not reduce the rate of sinus pauses and in fact numerically (but not significantly) increased the frequency of sinus pauses compared to saline treatment. These results may suggest that sinus pauses are merely a reflection of heart rate because any treatment expected to increase heart rate (atropine or isoproterenol) causes decreased sinus pauses and any treatment expected to decrease heart rate (propranolol or carbachol) causes increased sinus pauses. However, careful inspection reveals that treatment with propranolol does not cause a marked decline of heart rate in NULL animals (Fig. 4D) but does numerically increase sinus pauses (Fig. 4G). Furthermore, simultaneous treatment of NULL animals with atropine and propranolol dramatically decreases the rate of sinus pauses (Fig. 4G) but has little effect on the overall heart rate (Fig. 4F), which is near identical to wild-type heart rate. The results obtained here from pharmacological manipulation of NULL animals strongly points towards excessive vagal nerve activity as a key underlying cause of decreased heart rate and cardiac arrhythmia in animals lacking MeCP2 function.
Loss of MECP2 Function in Cholinergic Neurons Leads to Cardiac Abnormalities and Death
The evidence of increased parasympathetic tone and excessive vagal nerve activity led us to hypothesize that the cardiac abnormalities observed in RTT are caused by the loss of MeCP2 function within cholinergic neurons. To test this hypothesis, we generated conditional knock-out animals (17) in which MeCP2 function has been specifically removed from cholinergic neurons targeted by a transgenic animal containing Cre recombinase under the control of choline acetyl transferase (ChAT) promoter sequences (18). This ChAT-Cre line has previously been used to target cholinergic neurons in the dorsal medial hypothalamus known to modulate temperature (19) and dorsal nucleus of the vagus nerve known to modulate vagal nerve activity (20).
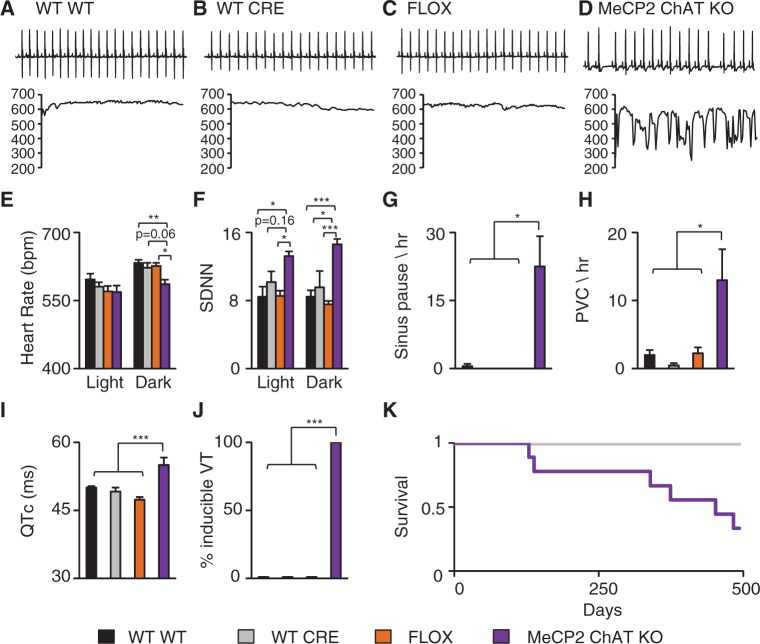
MeCP2 cholinergic neuron knock-out (MeCP2 ChAT KO) mice had an irregular heart rhythm and sudden drops in heart rate when compared to control mice (Fig. 5A–D), very similar to the abnormalities observed in complete NULL animals (Fig. 1). Quantification of the average heart rate during the light and dark cycles revealed that the heart rate during the dark cycle in MeCP2 ChAT KO was decreased compared with control animals (Fig. 5E) but was not different during the light cycle. Additionally, we observed an increase in heart rate variability during both the light and dark cycles in MeCP2 ChAT KO when compared to the control animals (Fig. 5F). The MeCP2 ChAT KO mice also displayed decreased temperature during the dark cycle but not the light cycle (Supplementary Material, Fig. S2A). Finally, there was no significant difference in activity during the light or dark cycle (Supplementary Material, Fig. S2B). Thus, MeCP2 function is necessary in cholinergic neurons for normal autonomic function, as manifested by decrease heart rate and temperature, but the cholinergic MeCP2 function is not critical for normal activity.
Figure 5.
Loss of MeCP2 function in cholinergic neurons leads to cardiac abnormalities. Telemetry recordings were collected from WT (Mecp2+/Y; No Cre), CRE (Mecp2+/Y; ChAT-Cre), FLOX (Mecp2TM1Jae/Y; No Cre), and MeCP2 cholinergic neuron specific knock-out (MeCP2 ChAT KO, Mecp2TM1Jae/Y; ChAT-Cre) mice. Data from 24-h recording were binned into light and dark cycles and compared. (A-C) Representative 2s ECG trace from WT, CRE, and FLOX mice and corresponding 15s instantaneous heart rate showing a normal heart rate and stable pattern. (D) Representative 2s ECG trace from a MeCP2 ChAT KO mouse and corresponding 15s instantaneous heart rate showing an irregular heart rate. (E) MeCP2 ChAT KO mice showed decreased heart rate during the dark cycle. (F) MeCP2 ChAT KO mice had increased heart rate variability during the light and dark cycle. (G and H) MeCP2 ChAT KO mice display a high incidence of sinus pauses and PVCs per hour. MeCP2 ChAT KO had prolonged corrected QT interval (QTc) (I) and a high incidence of inducible arrhythmias (J). MeCP2 ChAT KO mice had reduced lifespan with a median survival of 14 months (K). No significant differences in activity were found. Data presented as mean ± SEM. *P < 0.05 **P < 0.01, ***P < 0.001. Telemetry: WT n = 4, CRE n = 3, FLOX n = 4, MeCP2 ChAT KO n = 4; ECG and PES: WT n = 5, CRE n = 4, FLOX n = 5, MeCP2 ChAT KO n = 6; Survival: WT n = 4, CRE n = 3, FLOX n = 9, MeCP2 ChAT KO n = 9.
Because loss of MeCP2 function from cholinergic neurons caused the decreased heart rate and increased variability observed in complete NULL animals, we assessed whether this cell-type specific removal of MeCP2 would also lead to spontaneous arrhythmias. MeCP2 ChAT KO had a high incidence of sinus pauses (Fig. 5G) and a non-significant but numerically increased incidence of AV block (Supplementary Material, Fig. S2C). Additionally, MeCP2 ChAT KO mice showed an increased rate of spontaneous ventricular arrhythmias including PVC’s and NSVT (Fig. 5H and Supplementary Material, Fig. S2D). Interestingly, removal of MeCP2 function specifically from cardiomyocytes using an αMHC promoter driven Cre (MeCP2 MCH KO) (21) did not cause increased sinus pauses, PVC, AV block, or NSVT (Supplementary Material, Fig. S3A–D).
Previously, we demonstrated that both individuals with RTT and mouse models of RTT are susceptible to having LQT (3). Furthermore, LQT and increased susceptibility towards induced ventricular arrhythmias were found to be neuronal mediated (9). However, the neuronal type responsible for the phenotype has not been determined. Because MeCP2 ChAT KO mice have spontaneous arrhythmias, we hypothesized that cholinergic neurons play a role in the development of the neuronal mediated LQT and susceptibility to induced arrhythmias. Surface ECG intervals were quantified to determine if there were any abnormalities in cardiac conduction (Supplementary Material, Table S1). MeCP2 ChAT KO animals had increased QTc duration compared to controls (Fig. 5I) and were highly susceptible to inducible arrhythmias (Fig. 5J). In contrast, MeCP2 MHC KO did not show QTc prolongation (Supplementary Material, Fig. S3E) or increased susceptibility to induced arrhythmias (Supplementary Material, Fig. S3F). MeCP2 MHC KO animal also did not show any changes in temperature or activity in either the light or dark phases (Supplementary Material, Fig. S3G and H).
Because LQT and cardiac arrhythmias can predispose to sudden death, we assessed the survival of MeCP2 ChAT KO mice and found that 6 of 9 MeCP2 ChAT KO mice (67%) die early and the median life span of these animals is 16 months of age (Fig. 5K), with three mice alive past 500 days. This demonstrates that the MeCP2 function in cholinergic neurons is necessary for a normal heart rhythm and loss of MeCP2 in cholinergic neurons leads to neuronal mediated LQT, arrhythmias, and early death in a subset of animals.
Restoration of MeCP2 in Cholinergic Neurons Ameliorates Cardiac Dysfunction and Improves Survival
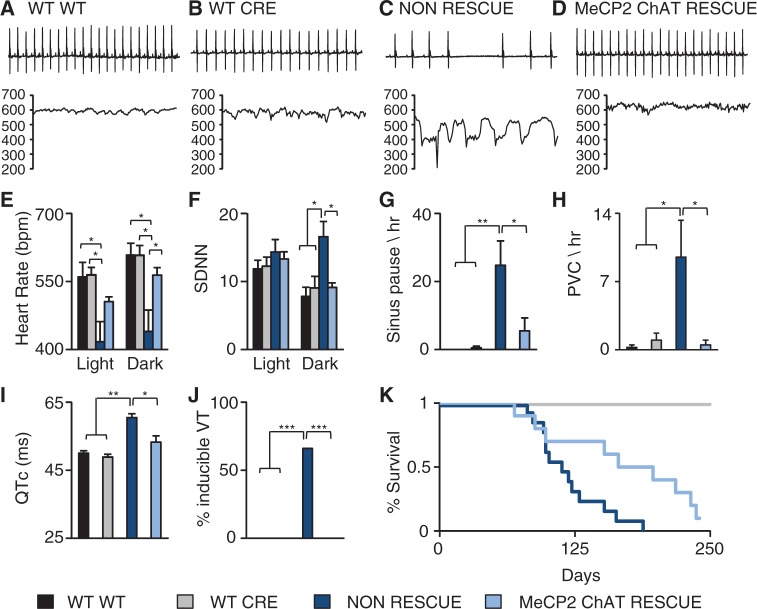
Removing MeCP2 function solely from cholinergic neurons revealed that the MeCP2 function in cholinergic neurons in necessary for normal cardiac function and survival, as well as temperature regulation. To determine whether a MeCP2 function within cholinergic neurons is sufficient for normal cardiac function, temperature regulation, and survival, we restored MeCP2 only within cholinergic neurons using a stop-flox or conditional rescue allele of Mecp2 (16) and crossed to the same ChAT Cre. Mice lacking Cre and containing the stop-flox allele are phenotypically similar to NULL mice.
Representative ECGs and instantaneous heart rate tracings revealed that although the WT and CRE mice had a normal heart rate and a stable rhythm (Fig. 6A and B), the non-rescue mice displayed an irregular rhythm with sudden bradycardic events (Fig. 6C) similar to that seen in complete NULL animals (Fig. 1). In contrast, the MeCP2 ChAT rescue mice showed a stable, regular heart rate comparable to WT animals (Fig. 6D). During the dark cycle, non-rescue animals showed decreased heart rate in both the light and dark cycle, which was restored to normal levels during the dark cycle in MeCP2 ChAT rescue mice (Fig. 6E). Non-rescue animals also had increased heart rate variability during the dark cycle compared to WT, CRE (Fig. 6F), which was also rescued in MeCP2 ChAT rescue mice (Fig. 6F). Non-rescue mice displayed decreased temperature during both the light and dark cycle (Supplementary Material, Fig. S4A). Restoring MeCP2 function in cholinergic neurons normalized the temperature during the light and dark cycle (Supplementary Material, Fig. S4A). Finally, we did not observe any significant differences in activity between any of the genotypes (Supplementary Material, Fig. S4B). These data clearly indicate that MeCP2 function within cholinergic neurons is sufficient for normal cardiac rhythm and thermoregulation.
Figure 6.
Restoration of MeCP2 in cholinergic neurons rescues cardiac dysfunction. Telemetry recordings were collected from WT (Mecp2+/Y: No-Cre), CRE (Mecp2+/Y: ChAT-Cre), non-rescue (Mecp2TM2Bird/Y: No-Cre) and MeCP2 cholinergic neuron specific rescue (MeCP2 ChAT rescue, Mecp2TM2Bird/Y: ChAT-Cre) mice. Data from 24-h recording were binned into light and dark cycles and compared to controls. (A-B) Representative 2s ECG trace from a WT and CRE mice and its corresponding 15s instantaneous heart rate showed a normal heart rate. (C) Representative 2s ECG trace from a non-rescue mouse and its corresponding 15s instantaneous heart rate showed an irregular heart rate. (D) Representative 2s ECG trace from a MeCP2 ChAT rescue mouse and its corresponding 15s instantaneous heart rate showed a more regular heart rate. (E) Non-rescue mice had decreased heart rate during the light and dark cycle and the decreased heart rate was rescued in MeCP2 ChAT rescue mice during the dark cycle. (F) Restoring MeCP2 in cholinergic neurons normalized the heart rate variability. Restoring MeCP2 in cholinergic neurons was sufficient to rescue the incidence of sinus pauses (G) and PVCs (H). (I) Non-rescue mice had a prolonged QTc which is rescued in the MeCP2 ChAT rescue. (J) Non-rescue mice had a high incidence of inducible arrhythmias that is rescued in MeCP2 ChAT rescue. (K) Restoring MeCP2 in cholinergic neurons improved survival outcome. Data presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001; Telemetry: WT n = 4, CRE n = 4, non-rescue n = 5, MeCP2 ChAT rescue n = 5; ECG and PES: WT n = 3, CRE n = 4, non-rescue n = 6, MeCP2 ChAT rescue n = 6; Survival: WT n = 10, CRE n = 13, non-rescue n = 13, MeCP2 ChAT rescue n = 10.
Because autonomic dysregulation was rescued by restoring MeCP2 function solely within cholinergic neurons, we performed a detailed analysis of arrhythmias in these mice. As in complete NULL animals, non-rescue mice had a higher incidence of sinus pauses and PVC’s (Fig. 6G and H). The spontaneous arrhythmias were rescued by restoring MeCP2 function in the cholinergic neurons (Fig. 6G and H). Furthermore, non-rescue mice had longer QTc than controls (Fig. 6I) and showed increased susceptibility to induction of arrhythmias (Fig. 6J), both of which were rescued in MeCP2 ChAT rescue mice (Fig. 6I and J). As we have previously shown (14), non-rescue animals die early (Fig. 6K), with a median survival of 16 weeks. Restoring the MeCP2 function in cholinergic neurons improved survival to a median lifespan of 26 weeks (Fig. 6K), and 40% of the MeCP2 ChAT rescue mice were able to live past 30 weeks of age. MeCP2 function in cholinergic neurons is sufficient to rescue spontaneous and induced cardiac arrhythmias, LQT, and significantly impacts survival.
Restoring MeCP2 in Cholinergic Neurons Does Not Improve Abnormal Breathing Patterns
Although restoring MeCP2 function within cholinergic neurons completely rescued cardiac function, it only partially rescued the early lethality observed in non-rescue animals (Fig. 6K). Another phenotype observed in both people with RTT and mouse models of RTT is abnormal breathing patterns, which could contribute to mortality. We found that neither apneas (Supplementary Material, Fig. S5A) nor abnormal hypoxic breathing response (Supplementary Material, Fig. S5B) was rescued in MeCP2 ChAT rescue mice. Therefore, we can conclude that MeCP2 function within the cholinergic neurons is not sufficient to rescue breathing and that this breathing dysfunction may contribute to the incomplete rescue of survival observed in MeCP2 ChAT rescue mice. Meanwhile, MeCP2 ChAT KO mice do not have the increased basal apneas (Supplementary Material, Fig. S6A) or the abnormal hypoxic response (Supplementary Material, Fig. S6B), which may explain why we observe an extended or delayed death in these mice.
Discussion
Sudden unexpected death causes approximately 25% of all deaths in RTT and a number of potential proximate causes of sudden death exist in RTT, including seizures, cardiac rhythm abnormalities, and breathing problems. Previously, we have shown that mouse models of RTT have LQT and are susceptible to induced VT (9,22). Here we extended the analysis and determined that both male and female RTT mice have a number of cardiac rhythm abnormalities. Notably, these animals developed spontaneous cardiac rhythm abnormalities that become progressively worse prior to progression to death, which in some animals result from complete cardiac conduction block. Importantly, the type of spontaneous arrhythmias we observed in these mouse models (sinus bradycardia, AVB, PVCs, and NSVT) have previously been described in patients with RTT (10–12), supporting the validity of these models in understanding pathophysiology in RTT. Pharmacological manipulation of the autonomic nervous system revealed increased parasympathetic tone and an overactive vagus nerve. Using genetic manipulation methods, we demonstrated that the cardiac rhythm abnormalities observed were the result of loss of MeCP2 function within cholinergic neurons, and these deficits, including early lethality, could be rescued by restoring MeCP2 function within cholinergic neurons. Additionally, we found that the decreased temperature observed in NULL mice is due to MeCP2 function within the cholinergic system. These data suggest that the inhibition of cholinergic activity may be a potential approach for therapeutic intervention and preventative measure for sudden death in individuals with RTT.
The discovery that low heart rate and sinus pauses appear to be driven by an excess of parasympathetic activity was somewhat unexpected, as in many situations loss of MeCP2 function appears to lead to decreased neuronal activity rather than increased. However, recent work has indicated that in some neurons loss of MeCP2 is associated with an overall increase in neuronal activity including in regions of the brainstem important for cardiorespiratory function (23). A growing body of evidence suggests that excessive parasympathetic activity underlies cardiac arrhythmias and sudden death in mouse models of epilepsy (24–27). For example, loss of function of SCN1A in the brain, but not in the heart, can lead to seizures that cause an excess of parasympathetic flow leading to severe bradycardia and death (25). Notably, anticholinergic therapy was sufficient to reduce the incidence of sudden death and aberrant conduction in these SCN1A knockout mice (21). Recent work also has shown that loss of sentrin/SUMO-specific protease 2 (SENP2) caused seizures that led to bradycardia, AVB and asystole (26). This cardiac conduction block can be prevented with atropine but not propranolol (26) similar to our finding that propranolol is not effective in a mouse model of RTT to correct the observed LQT (9,22). Testing whether long term treatment of mice lacking MeCP2 function with anticholinergic agents such as atropine or scopolamine is beneficial to survival and cardiac function is an important next step, however the marked constipation (28) and incidence of urinary retention (J. Neul, personal observation) in people with RTT raise concern that the chronic use of anticholinergic agents in RTT may worsen these symptoms.
Previously, we have shown that sodium channel blockers can prevent cardiac abnormalities by blocking the persistent sodium current (9,22). However, we do not know the exact cause of the sodium channel abnormalities in cardiomyoctes. We have previously shown that cardiomyocytes isolated from Mecp2 neuronal KO mice also present with the sodium channel abnormalities so we can speculate that cardiac ion channel remodelling might be due to increased aberrant parasympathetic tone. Alternatively, it is possible that these sodium channel abnormalities are also found in neurons, explaining the efficacy of treatment with sodium channel blockers. Future experiments should focus on what changes are occurring in cardiomyocytes due to the increased vagal activity and how MeCP2 may alter sodium channel activity in both neurons and cardiomyocytes.
The activity data obtained from telemetry show that the Mecp2 mutant mice do not have decreased activity as previously reported from open field activity assays. This may be in part due to the small sample size and heterogeneity of onset of behavioural phenotypes in the Mecp2 mutant mice. However, there is a possibility that environmental features such as the novel environment provided by the open field assay might affect performance. Meanwhile, the mouse activity will not be affected by being in a less threatening environment provided by the home-cage containing familiar scents, food, and water. As an example, Fyffe etal. have previously shown that mice that do not express Mecp2 in Sim1 neurons manifested aggression when stressed by an unfamiliar mouse (29). However, this aggression was not detected in the home cage (29). Therefore, it is possible that activity may also be dependent on the environmental setting and stressors associated with novel environments that will need to be addressed with future experiments.
An important aspect of the cardiac rhythm abnormalities found in these mouse models of epilepsy are that seizures appear to be the driving force behind the sudden increase in parasympathetic tone. Although seizures are common in people with RTT (4) and mice lacking MeCP2 function have seizures (14,30,31), in this work we did not perform simultaneous EEG and ECG that would have allowed us to determine if a similar mechanism underlies the cardiac rhythm abnormalities found in these RTT mouse models. We observed that RTT mouse models have sudden brief episodes of severe bradycardia that could represent short seizure activity; furthermore, in the animals in which telemetry happened to capture death we observed that the sudden bouts of bradycardia and temperature declines coincided with sudden drops in activity that could represent seizure activity. This cycling of bradycardia and temperature decline is reminiscent of the phenomena of spreading depolarization (SD) within the brainstem that causes autonomic disruption and was recently linked to sudden unexplained death in epilepsy (24). Future work using simultaneous EEG/ECG to conclusively determine whether the seizure activity is driving cardiac rhythm problems in RTT is needed. Interestingly, treating SENP2 knock out animals with retigabine, an antiepileptic drug that acts to increase the potassium channel opening, stopped the seizures and thus prevented development of cardiac rhythm abnormalities (23). This may be an additional therapeutic avenue to explore in RTT.
In this study, we linked both the cardiac rhythm abnormalities and the decreased body temperature to MeCP2 function within the cholinergic neurons, and there are some mechanisms by which this may occur. The cholinergic system is a key component of all autonomic function and the Cre line we utilized targets the dorsal motor nucleus of the vagus (DMV), which gives rise to preganglionic parasympathetic fibres (20). The observed bradycardia may be caused by the hyperexcitability of DMV neurons when they lack MeCP2 function. Similarly, the observed hypothermia may be a result of increased activity within the cholinergic neurons in the dorsal medial hypothalamus (DMH) (19). Recent work has identified that these cholinergic neurons project onto serotonergic sympathetic premotor neurons in the raphe pallidus that modulate brown adipose tissue (BAT) thermogenesis (19), and elevated activity of cholinergic neurons in the DMH causes a reduction of serotoninergic activity which in turn decreases BAT temperature (19). An alternative explanation for the bradycardic and hypothermic bouts is intermittent seizure activity, as outlined above. If this is indeed the mechanism, the question is how alterations of MeCP2 function within the cholinergic neurons lead to changes in seizure frequency. Although a general emphasis in seizure genesis is placed on the excitatory glutamatergic neurons and the inhibitor GABAergic neurons, it is known that alterations in the cholinergic system can also modulate seizure frequency. For example, a commonly used model of temporal lobe epilepsy is induced by injecting the cholinergic agonist pilocarpine (32), which causes an excitatory/inhibitory imbalance leading to status epilepticus (32). Similarly, chemical warfare agents such as Sarin and organophosphate pesticides act to increase cholinergic neurotransmission by inhibiting cholinesterases (33) and rapidly induce seizures. Finally, gain-of-function mutations in nicotinic acetylcholine receptors are associated with distinct forms of epilepsy (34). Thus, modulation of MeCP2 function within the cholinergic system may lead to changes in seizure frequency, and subsequent acute changes in heart rate and temperature. A shortcoming of the current work is the lack of combined EEG/ECG analysis, which will be the focus of future work.
Although removal of MeCP2 function within cholinergic neurons faithfully reproduced the cardiac rhythm abnormalities observed in complete null animals and caused early death, the survival of the cholinergic knock out animals was much longer than that observed for complete NULL animals (14). Comparing the MeCP2 ChAT KO to other previously reported conditional KO mice, we observe that the MeCP2 ChAT KO mice have a prolonged median lifespan as well. For example, Viaat MeCP2 KO mice have a median lifespan of 26 weeks (35) while the MeCP2 Vglut2 KO mice were recently reported to have a median lifespan of 10 weeks (36). Both of these mice were on a 129S6SvEVTac/FVB mixed background and using the Mecp2tm1.1Bird allele, while the MeCP2 ChAT KO mice were on a C57BL6 background and used the Mecp2TM1Jae allele. Previously, it was reported that some phenotypes such as weight (37) and lipid metabolism (38) can depend on the genetic background strain and allele which presents as potential caveat when comparing survival across studies. It is important to note that our cardiac data from NULL data are from a mixed B6129S6F1, while the conditional KO and rescue were on a pure C57BL/6, indicating that the cardiac phenotypes are observed across strains and alleles.
Similarly, restoring MeCP2 function in cholinergic neurons completely rescued the cardiac rhythm abnormalities, however, only a partial rescue of early lethality was observed. Thus, there must be other factors that contribute to early lethality in animals lacking MeCP2. We found that restoring MeCP2 function within cholinergic neurons did not rescue breathing abnormalities, and this may contribute to some of the observed lethality.
In conclusion, this study demonstrates that mice lacking MeCP2 function reproduce many of the cardiac rhythm abnormalities observed in people with RTT and provide insight into the pathogenic mechanisms underlying the observed sudden unexpected death in RTT. Abnormal function in the cholinergic nervous system underlies these cardiac abnormalities and blocking the hyperexcitability of this system may offer a meaningful therapeutic approach. Alternatively, the recognition that lethality is likely the result of severe bradycardia and cardiac conduction block suggests that implantable cardiac pacemakers may be a suitable therapy. Important questions remain. First, what is the relationship between seizures and cardiac rhythm abnormalities? Second, what molecular and cellular changes occur in cholinergic neurons lacking MeCP2 function? Third, what secondary ion channel remodelling occurs in the heart when MeCP2 is absent from neurons? The robust validity of the mouse models of RTT provides a useful substrate and an opportunity to test new therapeutic approaches to treat cardiac arrhythmias in RTT using anticholinergic drugs, sodium channel blockers, or potassium channel openers, and to additionally address these important pathophysiological questions.
Material and Methods
Experimental animals
All animals were housed at Baylor College of Medicine’s AAALAC approved facilities. Mecp2 genomic knockout mice were generated by mating female Mecp2TM1.1Bird (JAX #003890) obtained from Jackson Laboratory to C57Bl/6J (JAX #000664) males. Male (NULL) and female (NULL/+) mutant and wild type isogenic B6129S6F1 mice were used for the following experiments. FLOX animals, Mecp2TM1Jae (011918-UCD), were acquired from the University of California-Davis Mouse Mutant Regional Resource Center and maintained on a C57BL/6J. Mecp2TM2Bird (JAX #006849) (non-rescue) were obtained from the Jackson Laboratory and maintained in a C57BL/6J. Female Mecp2TM1Jae and Mecp2TM2Bird were crossed to ChAT Cre mice on a 129S6/SvEvTac background obtained from Jackson Laboratory that was backcrossed 4 generations to C57BL/6J. The αMHC cre obtained from M. D. Schneider and maintained on C57BL/6J (21).
Telemetry
Mice were implanted with a DSI ETA F-10 telemeter (Data Sciences International, St. Paul, MN) and allowed a week to recover from the surgery, as previously reported (39). ECG output was recorded by using a receiver matrix coupled to data acquisition software provided by DSI (Data Sciences International, St. Paul, MN). This allowed heart rate recordings in freely moving mice. Mice were allowed to acclimate to single housing for 24 h and the following 24 h were used for detailed heart rate analysis. NULL male mice were tested at 2–3 months of age while NULL/+ female mice were tested at 9 months and 28 months. MeCP2 ChAT KO, MeCP2 MHC KO, MeCP2 ChAT rescue, and their controls were tested at 3–4 months of age.
Spontaneous arrhythmia quantification
Two hours of telemetry data were screened and counted for manually. The same time point from 6am-8am was analysed for all animals. Sinus pauses were classified as a temporary cessation of sinus node activity that was at least twice as long as the previous RR interval. AV block was considered when there was no sign of a QRS complex following a P wave. Premature contractions were considered as a single R wave overlapping a QRS complex. Non-sustained VT was classified as more than three continuous R waves. Data from each mouse was pooled and bouts were averaged to calculate bouts per hour.
Heart rate analysis
Software provided by DSI (Data Sciences International, St. Paul, MN) was utilized to detect beat calling and a noise filter setting was used to eliminate poor signal. Parser segments were created to analyse light and dark cycle differences. Twenty-four hour instant heart rate data were exported on to excel sheets and Python script was written to calculate the hourly coefficient of variation and bradycardic events below 200 bpm. Mice were in a 14 h light and 10 h dark cycle. The light and dark cycles were binned in 5 min segments and the standard deviation of the NN (SDNN) was calculated from the average heart rate in 5 min bins. The script calculates instantaneous CV as |RR[N]-RR[N + 1]|/RR[N + 1]. The hour-based summaries are average values for instantaneous CV, RR, HR, and Activity for measurements obtained with timestamps indicating the appropriate hour. Histograms were calculated by determining the total number of heartbeats contained within the indicated bin over the course of the measurement, and normalized histograms were generated by dividing the bin counts by the overall total of heartbeats within the full recording. Overall CV value was calculated as the mean CV of all heartbeats in the recording and is presented in the pdf output of the script. The Python script is available upon request.
Drug treatments
Mice were treated with 0.9% NaCl saline solution for two days then injected with either 1 mg/kg atropine (Sigma Aldrich), 4 mg/kg propranolol (Sigma Aldrich), atropine/propranolol, 3.3 mg/kg isoproterenol (Sigma Aldrich), or 0.5 mg/kg of charbacol (Sigma Aldrich),. All drugs were prepared in saline solution and sterilized by filtration. After the mice were injected, 1 h recordings were then taken to assess the drug response. To allow for the drug to wash out, drugs were administered at a 48-h interval.
Post drug treatment AVB/sinus pause quantification
Thirty minutes of telemetry data acquired post drug treatments were screened for AVB/sinus pauses and bouts were manually counted. Any pause whether AV block or sinus pause was pooled for the analysis and bouts were averaged to calculate bouts per 30 min.
Surface ECG
Mice were placed under anaesthesia using a continuous flow of 1.5% isoflurane in 95% O2 during the procedure. After sedation, mice were placed on surface pad electrodes to obtain six-lead surface ECG, as described previously (40). The ECG parameters (PR, QRS, QT, QTc, and RR) were calculated from the mean of ten measurements per mouse over a 10-min period, as previously described (22). The QTc was calculated using the formula QTc = QT + 0.3173 x (170-RR) (41).
Programmed electrical stimulation
Programmed electrical stimulation was performed, after collecting the ECG parameters, to assess the inducibility to ventricular tachycardia. A 1.1-F octapolar catheter was inserted through the jugular vein and carefully placed in the right atrial and ventricle to collect intra-cardiac recordings, as previously described (40). Overdrive pacing and extra stimulus protocols were used to assess susceptibility to VT. Sustained VT was classified to be VT lasting for 1 s or more, as previously described (9,22).
Plethysmography
Plethysmography was performed as previously reported (14). Briefly, mice were placed in an unrestrained whole-body plethysmography chamber. The mice were allowed to habituate for 20 min followed by a 30-min baseline recording. Soon after, the chamber was flushed with hypoxic gas (10% O2, balanced N2) for 20 min. Data were acquired using the Ponemah Software (Data Sciences International, St. Paul, MN) which was then exported for analysis using Matlab and filtered for movement artefacts. Artefacts from excessive movement and sniffing behaviour were filtered out by applying the following criteria as, previously reported (42). Breaths were only accepted if they demonstrated an inspiratory time greater than 0.03 s, an expiratory time less than 10 s, and a calculated exhaled tidal volume within ±50% of calculated inhaled tidal volume; the recordings were then split into 1-min intervals and only those intervals during which the animal spent less than 10% of its breaths above 500 breaths per minute were included in the analysis (42).
Statistics
All analyses were used using SPSS version 20 (SPSS, Chicago IL, USA).
One way ANOVA for multiple comparisons was used to determine statistical significances. Pearson Chi-Square followed by Fisher’s exact test for comparisons between pairs of data was used for categorical variables. Paired t-test was used were appropriate. P value < 0.05 was considered significant. Survival was analysed using the Kaplan-Meier survival analysis with Tarone-Ware method applied to detect differences in survival between genotypes.
Study approval
All experiments were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee.
Supplementary Material
Supplementary Material is available at HMG online.
Supplementary Material
Acknowledgements
We thank IDDRC Neurobehavioral Core (Directors Richard Paylor and Corinne Spencer) for use of the facility and advice in behavioural experiments, and Diana Parra for technical assistance.
Conflict of Interest statement. None declared.
Funding
This work was supported by the U.S. National Institutes of Health Grants R01HD062553 (JLN), U54HD083092 and P30HD024064 (BCM IDDRC Neurobehavioral Core), R01HL089598, R01HL091947, and R01HL117641 (XHTW), R25GM56929 and T32GM088129 (JAH), and the Cynthia and Anthony Petrello Scholar fund at the Jan and Dan Duncan Neurological Research Institute, Texas Children's Hospital (JLN), the International Rett Syndrome Foundation Grant #2813 (JLN), American Heart Association grant 13EIA14560061 (XHTW) and 14PRE18710063 (JAH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Eunice Kennedy Shriver Child Health and Human Development Institute (NICHD).
References
- 1. Hagberg B. (1985) Rett’s syndrome: prevalence and impact on progressive severe mental retardation in girls. Acta Paediatr. Scand., 74, 405–408. [DOI] [PubMed] [Google Scholar]
- 2. Amir R.E., Van den Veyver I.B., Wan M., Tran C.Q., Francke U., Zoghbi H.Y. (1999) Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet., 23, 185–188. [DOI] [PubMed] [Google Scholar]
- 3. Chahrour M., Jung S.Y., Shaw C., Zhou X., Wong S.T.C., Qin J., Zoghbi H.Y. (2008) MeCP2, a Key Contributor to Neurological Disease, Activates and Represses Transcription. Science, 320, 1224–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Glaze D.G., Percy A.K., Skinner S., Motil K.J., Neul J.L., Barrish J.O., Lane J.B., Geerts S.P., Annese F., Graham J., et al. (2010) Epilepsy and the natural history of Rett syndrome. Neurology, 74, 909–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Neul J.L., Kaufmann W.E., Glaze D.G., Christodoulou J., Clarke A.J., Bahi-Buisson N., Leonard H., Bailey M.E.S., Schanen N.C., Zappella M., et al. (2010) Rett syndrome: Revised diagnostic criteria and nomenclature. Ann. Neurol., 68, 944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Julu P.O.O. (2001) Characterisation of breathing and associated central autonomic dysfunction in the Rett disorder. Arch. Dis. Child., 85, 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ellaway C., Sholler G., Leonard H., Christodoulou J. (1999) Prolonged QT interval in Rett syndrome. Arch. Dis. Child, 80, 470.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sekul E.A., Moak J.P., Schultz R.J., Glaze D.G., Dunn J.K., Percy A.K. (1994) Electrocardiographic findings in Rett syndrome: An explanation for sudden death?. J. Pediatr., 125, 80–82. [DOI] [PubMed] [Google Scholar]
- 9. McCauley M.D., Wang T., Mike E., Herrera J., Beavers D.L., Huang T.W., Ward C.S., Skinner S., Percy A.K., Glaze D.G., et al. (2011) Pathogenesis of Lethal Cardiac Arrhythmias in Mecp2 Mutant Mice: Implication for Therapy in Rett Syndrome. Sci. Transl. Med., 3, 113ra125-113ra125.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Madan N., Levine M., Pourmoghadam K., Sokoloski M. (2004) Severe Sinus Bradycardia in a Patient with Rett Syndrome: A New Cause for a Pause?. Pediatr. Cardiol., 25, 53–55. [DOI] [PubMed] [Google Scholar]
- 11. Panossian S.I., Duro E.A. (2004) [Tachyarrhythmia as the first manifestation in a classic Rett syndrome]. Rev. Neurol., 39, 299–300. [PubMed] [Google Scholar]
- 12. Guideri F., Acampa M. (2005) Sudden death and cardiac arrhythmias in Rett syndrome. Pediatr. Cardiol., 26, 111.. [DOI] [PubMed] [Google Scholar]
- 13. Guideri F., Acampa M., DiPerri T., Zappella M., Hayek Y. (2001) Progressive Cardiac Dysautonomia Observed in Patients Affected by Classic Rett Syndrome and Not in the Preserved Speech Variant. J. Child Neurol., 16, 370–373. [DOI] [PubMed] [Google Scholar]
- 14. Ward C.S., Arvide E.M., Huang T.W., Yoo J., Noebels J.L., Neul J.L. (2011) MeCP2 Is Critical within HoxB1-Derived Tissues of Mice for Normal Lifespan. J. Neurosci., 31, 10359–10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wither R.G., Colic S., Wu C., Bardakjian B.L., Zhang L., Eubanks J.H. (2012) Daily rhythmic behaviors and thermoregulatory patterns are disrupted in adult female MeCP2-deficient mice. PloS One, 7, e35396.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guy J., Gan J., Selfridge J., Cobb S., Bird A. (2007) Reversal of Neurological Defects in a Mouse Model of Rett Syndrome. Science, 315, 1143–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen R.Z., Akbarian S., Tudor M., Jaenisch R. (2001) Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat. Genet., 27, 327–331. [DOI] [PubMed] [Google Scholar]
- 18. Lowell B. (2006) Development and phenotype of ChAT-IRES-Cre mice. MGI Direct Data Submiss, [Google Scholar]
- 19. Jeong J.H., Lee D.K., Blouet C., Ruiz H.H., Buettner C., Chua S., Jr, Schwartz G.J., Jo Y.H. (2015) Cholinergic neurons in the dorsomedial hypothalamus regulate mouse brown adipose tissue metabolism. Mol. Metab., 4, 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rossi J., Balthasar N., Olson D., Scott M., Berglund E., Lee C.E., Choi M.J., Lauzon D., Lowell B.B., Elmquist J.K. (2011) Melanocortin-4 Receptors Expressed by Cholinergic Neurons Regulate Energy Balance and Glucose Homeostasis. Cell Metab., 13, 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Agah R., Frenkel P.A., French B.A., Michael L.H., Overbeek P.A., Schneider M.D. (1997) Gene recombination in postmitotic cells. Targeted expression of Cre ecombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J. Clin. Invest., 100, 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Herrera J.A., Ward C.S., Pitcher M.R., Percy A.K., Skinner S., Kaufmann W.E., Glaze D.G., Wehrens X.H.T., Neul J.L. (2015) Treatment of cardiac arrhythmias in a mouse model of Rett syndrome with Na+-channel-blocking antiepileptic drugs. Dis. Model. Mech., 8, 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kron M., Howell C.J., Adams I.T., Ransbottom M., Christian D., Ogier M., Katz D.M. (2012) Brain activity mapping in Mecp2 mutant mice reveals functional deficits in forebrain circuits, including key nodes in the default mode network, that are reversed with ketamine treatment. J. Neurosci., 32, 13860–13872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aiba I., Noebels J.L. (2015) Spreading depolarization in the brainstem mediates sudden cardiorespiratory arrest in mouse SUDEP models. Sci. Transl. Med., 7, 282ra46-282ra46.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kalume F., Westenbroek R.E., Cheah C.S., Yu F.H., Oakley J.C., Scheuer T., Catterall W.A. (2013) Sudden unexpected death in a mouse model of Dravet syndrome. J. Clin. Invest., 123, 1798–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qi Y., Wang J., Bomben V.C., Li D.P., Chen S.R., Sun H., Xi Y., Reed J.G., Cheng J., Pan H.L., et al. (2014) Hyper-SUMOylation of the Kv7 Potassium Channel Diminishes the M-Current Leading to Seizures and Sudden Death. Neuron, 83, 1159–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goldman A.M., Glasscock E., Yoo J., Chen T.T., Klassen T.L., Noebels J.L. (2009) Arrhythmia in Heart and Brain: KCNQ1 Mutations Link Epilepsy and Sudden Unexplained Death. Sci. Transl. Med., 1, 2ra6-2ra6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baikie G., Ravikumara M., Downs J., Naseem N., Wong K., Percy A., Lane J., Weiss B., Ellaway C., Bathgate K., et al. (2014) Gastrointestinal dysmotility in Rett syndrome. J. Pediatr. Gastroenterol. Nutr., 58, 237–244. [DOI] [PubMed] [Google Scholar]
- 29. Fyffe S.L., Neul J.L., Samaco R.C., Chao H.T., Ben-Shachar S., Moretti P., McGill B.E., Goulding E.H., Sullivan E., Tecott L.H., et al. (2008) Deletion of Mecp2 in Sim1-Expressing neurons reveals a critical role for MeCP2 in feeding behavior, aggression, and the response to stress. Neuron, 59, 947–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Colic S., Wither R.G., Zhang L., Eubanks J.H., Bardakjian B.L. (2013) Characterization of seizure-like events recorded in vivo in a mouse model of Rett syndrome. Neural Netw. Off. J. Int. Neural Netw. Soc., 46, 109–115. [DOI] [PubMed] [Google Scholar]
- 31. Goffin D., Brodkin E.S., Blendy J.A., Siegel S.J., Zhou Z. (2014) Cellular origins of auditory event-related potential deficits in Rett syndrome. Nat. Neurosci., 17, 804–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Curia G., Longo D., Biagini G., Jones R.S.G., Avoli M. (2008) The pilocarpine model of temporal lobe epilepsy. J. Neurosci. Methods, 172, 143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jett D.A. (2012) Chemical toxins that cause seizures. Neurotoxicology, 33, 1473–1475. [DOI] [PubMed] [Google Scholar]
- 34. Boillot M., Baulac S. (2015) Genetic models of focal epilepsies. J. Neurosci. Methods, 10.1016/j.jneumeth.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 35. Chao H.T., Chen H., Samaco R.C., Xue M., Chahrour M., Yoo J., Neul J.L., Gong S., Lu H.C., Heintz N., et al. (2010) Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature, 468, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Meng X., Wang W., Lu H., He L.J., Chen W., Chao E.S., Fiorotto M.L., Tang B., Herrera J.A., Seymour M.L., et al. (2016) Manipulations of MeCP2 in glutamatergic neurons highlight their contributions to Rett and other neurological disorders. eLife, 5, e14199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Samaco R.C., McGraw C.M., Ward C.S., Sun Y., Neul J.L., Zoghbi H.Y. (2013) Female Mecp2+/− mice display robust behavioral deficits on two different genetic backgrounds providing a framework for pre-clinical studies. Hum. Mol. Genet., 22, 96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Buchovecky C.M., Turley S.D., Brown H.M., Kyle S.M., McDonald J.G., Liu B., Pieper A.A., Huang W., Katz D.M., Russell D.W., et al. (2013) A suppressor screen in Mecp2 mutant mice implicates cholesterol metabolism in Rett syndrome. Nat. Genet., 45, 1013–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McCauley M.D., Wehrens X.H.T. (2010) Ambulatory ECG recording in mice. J. Vis. Exp.,27, doi: 10.3791/1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li N., Wehrens X.H. (2010) Programmed electrical stimulation in mice. J. Vis. Exp.,26, 10.3791/1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nuyens D., Stengl M., Dugarmaa S., Rossenbacker T., Compernolle V., Rudy Y., Smits J.F., Flameng W., Clancy C.E., Moons L., et al. (2001) Abrupt rate accelerations or premature beats cause life-threatening arrhythmias in mice with long-QT3 syndrome. Nat. Med., 7, 1021–1027. [DOI] [PubMed] [Google Scholar]
- 42. Huang W.H., Tupal S., Huang T.W., Ward C.S., Neul J.L., Klisch T.J., Gray P.A., Zoghbi H.Y. (2012) Atoh1 governs the migration of postmitotic neurons that shape respiratory effectiveness at birth and chemoresponsiveness in adulthood. Neuron, 75, 799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.