Abstract
17p13.3 microduplication syndrome is a newly identified genetic disorder characterized by duplications in the 17p13.3 chromosome locus, resulting in a variety of disorders including autism spectrum disorder (ASD). Importantly, a minimum duplication region has been defined, and this region exclusively contains the gene encoding 14-3-3ε. Furthermore, duplication of this minimum region is strongly associated with the appearance of ASD in human patients, thus implicating the overexpression of 14-3-3ε in ASD. Using in vitro and in vivo techniques, we have found that 14-3-3ε binds to the microtubule binding protein doublecortin preventing its degradation. We also found that 14-3-3ε overexpression disrupts neurite formation by preventing the invasion of microtubules into primitive neurites, which can be rescued by the knockdown of doublecortin. To analyse the function of 14-3-3ε in neurite formation, we used 14-3-3ε flox mice and found that 14-3-3ε deficiency results in an increase in neurite formation. Our findings provide the first evidence of cellular pathology in 17p13.3 microduplication syndrome.
Introduction
Recently, a new genetic syndrome known as 17p13.3 microduplication syndrome has been identified and is characterized by gene duplications of various sizes in the 17p13.3 chromosome locus. These duplications result in severe neural developmental disorders, including autism spectrum disorder (ASD), epilepsy and mental retardation (1–8). Importantly, previous studies were able to define a microduplication minimal region that is strongly associated with the ASD phenotype (2). This 72kb microduplication minimal region within the 17p13.3 chromosome locus exclusively contains the YWHAE gene, encoding the 14-3-3ε protein. This strongly implicates the involvement of 14-3-3ε in abnormal neural development and as a causative gene for ASD, however the cellular and molecular mechanisms involved are entirely unknown.
14-3-3 proteins are a family of highly conserved proteins with a multitude of functions and are highly expressed in the brain, making up approximately 1% of total soluble brain protein (9,10). These proteins are known to interact with over 200 proteins through phosphoserine and phosphothreonine binding motifs. 14-3-3 proteins regulate a variety of cellular processes including signal transduction and cell cycle control (10,11). 14-3-3 proteins are known to have a number of effects on their binding partners, including providing protection from phosphatases and the regulation of binding partner stability (9,12).
The functions of 14-3-3 proteins in neural development have been analysed using mouse genetic approaches and their importance in neurogenesis and neuronal migration has been previously shown (12–14). However, their functions in later stages of cortical development have yet to be elucidated. In particular, 14-3-3 proteins have not been previously shown to be involved in cellular morphogenesis and the roles of 14-3-3 proteins in neuronal morphogenesis, to our knowledge, are completely unknown. In this work, we analyse the novel functions of 14-3-3ε in neuronal development, in particular its regulation of cortical neuromorphogenesis.
Here, we report a novel function of 14-3-3ε in the regulation of neurite formation during cortical development. We found that increased expression of 14-3-3ε in vitro and in vivo results in a decrease in neurite formation. Additionally, we found that 14-3-3ε binds to the microtubule binding protein, doublecortin (Dcx) in a phosphorylation dependent manner and that this binding increases the Dcx protein levels in the cell by interrupting ubiquitin based degradation. Furthermore, we found that the increased levels of Dcx disrupt microtubule invasion into lamellipodia, thus disrupting an initial stage of neurite formation, and that this can be rescued by the knockdown of Dcx in 14-3-3ε overexpressing cells. In addition, with the use of 14-3-3ε flox mice, we found that spatiotemporal 14-3-3ε deficiency results in an increase in neurite formation. This suggests that 14-3-3ε functions as a negative regulator of neurite formation. Overall, our findings elucidate novel functions of 14-3-3ε and Dcx in neuronal morphogenesis and implicate their role in 17p13.3 microduplication syndrome. To date, no pathological analyses have been performed in patients with 17p13.3 microduplication syndrome. Furthermore, ASD patients are known to show defects in neuronal morphogenesis (15–17). Therefore, our findings in this work may indicate that the defects we found when 14-3-3ε is overexpressed may contribute to the ASD phenotype seen in patients with the 17p.13.3 microduplication syndrome.
Results
Overexpression of 14-3-3ε in vitro and in vivo disrupts neurite formation
To analyse the effects of 14-3-3ε overexpression on the morphology of neuronal-like cells, we first overexpressed 14-3-3ε in mouse neuroblastoma cells (Neuro-2A) in vitro, using the pCAGIG-Ywhae plasmid. The pCAGIG-Ywhae plasmid contains an internal ribosomal entry site (IRES) allowing for the independent expression of 6xHis-14-3-3ε and GFP. This allows us to use GFP fluorescence to analyse cellular morphology. For control experiments we used the pCAGIG plasmid alone, which only expresses GFP. Neuro-2A cells have been extensively used to study neurite growth and neuronal differentiation (18,19). We have found that within minutes of removing fetal bovine serum (FBS) from the culture media, Neuro-2A cells will begin to extend neurites, and within 2 h multiple long dendritic like processes can be seen in control cells (Fig. 1A, left panels). We have found that overexpressing 14-3-3ε in Neuro-2A cells causes dramatic morphological changes, including a severe decrease in the percent of cells extending neurites (Fig. 1B, top graph), a decrease in the mean number of neurites extending from the soma (Fig. 1B, bottom left graph) as well as a decrease in mean neurite length (Fig. 1B, bottom right graph). Furthermore, this phenotype does not recover after 6 h of serum deprivation indicating that 14-3-3ε overexpression does not temporarily delay neurite formation, but does in fact produce lasting morphological deficits (Fig. 1C and D).
Figure 1.
14-3-3ε negatively regulates neurite formation in vitro and in vivo. (A–B) Overexpression of Ywhae (Encoding 14-3-3ε) in Neuro-2A cells for 36 h followed by 2 h or (C–D) 6 h of serum deprivation. 14-3-3ε overexpression resulted in a severe decrease in the percent of cells with neurites protruding from the soma, a decrease in the mean number of neurites protruding from the soma, and a significant decrease in the mean neurite length (n = 100 cells per analysis from three independent experiments per condition, Scale: a, top, 50 µm; bottom, 20 µm; c, 20 µm). (E) Images of P15 coronal brain sections following In utero overexpression of Ywhae at E16.5 via in utero electroporation (IUE). Bottom panels show high magnification images of the CP (Scale: 100 µm). (F) Sholl analysis of neuronal morphology in the CP (n = 25 neurons from three independent experiments per condition). (G) Number of primary neurites protruding from the soma of neurons in the CP at P15 after IUE. Note the severe decrease in the number of neurites indicating a deficit in neurite initiation (n = 25 neurons from 3 independent experiments per condition). (H–J) Inducible 14-3-3ε knockout via IUE of pCAG-ERT2Cre-GFPERT2 and mRFP in Control or Ywhae flox/flox mice at E16.5 followed by IP injection of Tamoxifen (75 mg/kg) at P0 and P1. Brain samples were collected and analysed at P15 (Scale: 50 µm). (I) Mean length of neurites and (J) mean number of neurites protruding from the soma of neurons in the CP at P15 significantly increases when Ywhae is abolished (n = 25 neurons from 3 independent experiments per condition).
Next, to determine if this morphological phenotype seen in 14-3-3ε overexpressing Neuro-2A cells persists in vivo, we performed in utero electroporation where embryonic mice were injected with pCAGIG-Ywhae overexpression plasmid at E16.5. We then analysed neuronal morphology at P15 (Fig. 1E). In utero electroporation is a technique that allows for the transfection of neuronal progenitor cells in the ventricular zone of embryonic mouse brains (see the Methods section for details). These transfected neurons then migrate from the ventricular zone to the cortical plate to form the cortical layers of the brain during development. After arriving in the cortical plate, these neurons then grow neurites and form appropriate connections. Sholl analysis of 14-3-3ε overexpressing neurons in the cortical plate (CP) revealed that these neurons have severe deficits in neurite formation in comparison to control cells expressing GFP alone, similar to what was seen in the Neuro-2A cells. The Sholl profile shows that cells overexpressing 14-3-3ε have a significantly lower number of neurites protruding from the soma (Fig. 1F and G). Furthermore, the Sholl profile indicates that control cells have longer neurites compared to the 14-3-3ε overexpressing cells.
14-3-3 proteins share a high level of homology among them and can function as heterodimers as well as homodimers, suggesting functional redundancy. In order to examine if the morphological deficits are 14-3-3ε specific, we overexpressed each of the seven 14-3-3 isoforms in vitro in Neuro-2A cells and in vivo via in utero electroporation and analysed neurite formation (Supplementary Materials, Figs. S1 and S2). Both the in vitro and in vivo analysis indicated that only the overexpression of 14-3-3ε produces deficits in neurite formation compared to control cells expressing GFP alone. These data indicate that the deficits in neurite formation are specific to the overexpression of the 14-3-3ε isoform.
In the previous experiments (Fig. 1E–G), we overexpressed 14-3-3ε because the gene encoding this protein is duplicated in patients with the 17p13.3 microduplication syndrome. To further analyse the functions of 14-3-3ε in neurite formation in normal physiological conditions in vivo, we next performed an inducible 14-3-3ε knockout via in utero electroporation of tamoxifen inducible Cre-GFP for deleting the Ywhae gene and mRFP for visualizing cell morphology in WT or Ywhae flox/flox mice at E16.5 followed by the intraperitoneal injection of tamoxifen at P0 and P1 and the collection and analysis of neurons in the cortical plate at P15 (Fig. 1H). We have previously found that neurons electroporated at E16.5 reach the cortical plate beginning at P1 to P2 and begin to form neurites following their arrival. Thus, in this experimental setup 14-3-3ε is knocked out immediately following the arrival of neurons to the cortical plate prior to neurite formation. This methodology is essential to avoid potential deficits prior to neurite formation because we have previously found that the knockout of 14-3-3ε results in defects in neurogenesis and neuronal migration (12,13). We found that the knockout of 14-3-3ε in vivo produces the opposite results of its overexpression and results in a significant increase in neurite length and the number of neurites protruding from the soma indicating that 14-3-3ε is a negative regulator of neurite formation (Fig. 1I and J).
Time-lapse live imaging indicates that the overexpression of 14-3-3ε disrupts neurite initiation in vitro and in vivo
To further characterize how the overexpression of 14-3-3ε disrupts neurite formation we utilized time-lapse live imaging with Neuro-2A cells (Fig. 2A, Supplementary Materials, Movies S1 and S2). We were able to observe the rapid formation and retraction of filopodia and lamellipodia type structures in both control and 14-3-3ε overexpressing cells following serum deprivation. In the control cells, these structures would rapidly condense and extend into distinct neurites (Fig. 2A, top panel and Supplementary Material, Movie S1); however, in the 14-3-3ε overexpressing cells (Fig. 2A, bottom panel and Supplementary Material, Movie S2) these lamellipodia type structures rapidly formed and retracted over the entire experimental time course and neurites were never formed. This pattern is clearly illustrated in Fig. 2B where neurite length is graphed over time. These data indicate that the overexpression of 14-3-3ε disrupts neurite initiation and formation in Neuro-2A cells.
Figure 2.
In vitro and ex vivo time-lapse live imaging shows severe neurite initiation deficits in 14-3-3ε overexpressing neurons. (A) Time-lapse live imaging montage of control (top) and 14-3-3ε overexpressing (bottom) Neuro-2A cells following serum deprivation. Red arrows denote Ywhae overexpressing cells, the black arrow denotes a WT cell (non-transfected cell). See Supplementary Movies 1 and 2. (B) Mean neurite length over time showing how 14-3-3ε overexpressing cells fail to initiate and extend neurites with bouts of short extensions and rapid retractions over the entire experiment (n = 20 neurites from 10 cells in 3 independent experiments per condition). (C) Examples of E18.5 brain sections after IUE was performed at E14.5. Note that the electroporated neurons are arriving at the cortical plate where they were imaged in panel D, as designated by the white squares. (D) Ex vivo time-lapse live imaging of neurons in live brain slices harvested at E18.5 following IUE of control or 14-3-3ε expression plasmids at E14.5 (Scale: 10 µm). See Supplementary Movies 3 and 4. (E) Mean neurite length over time. Note the rapid formation and retraction of very short neurites in 14-3-3ε overexpressing neurons over the 18 h time lapse (n = 20 neurites from 10 different cells in 3 independent experiments per condition).
To verify the in vitro live imaging results, we performed time-lapse live imaging using mouse brain slices following in utero electroporation. E14.5 embryos were injected with either pCAGIG-Control or pCAGIG-Ywhae plasmids and brains were harvested and imaged at E18.5 for >18hrs as migrating neurons reached the CP and began neurite formation (Fig. 2C). In this experimental window, we were able to observe control cells begin to extend and form neurites (Fig. 2D, top panel and Supplementary Material, Movie S3). As seen in the Neuro-2A cells, the neurons overexpressing 14-3-3ε showed severe deficits in neurite formation with continual bouts of neurite retraction (Fig. 2D, bottom panel and Supplementary Material, Movie S4). This can clearly be seen in the graph when mean individual neurite length is plotted over time for control and 14-3-3ε overexpressing neurons (Fig. 2E).
14-3-3ε binds to the microtubule binding protein, doublecortin
We previously performed a shotgun proteomics analysis using PC12 cells, analogous to Neuro-2A cells, and GST tagged 14-3-3ε and found that Dcx strongly associates with 14-3-3ε (data not shown). To verify this interaction we performed a series of immunoprecipitation (IP) assays (Fig. 3). First, we overexpressed HAHA-14-3-3ε or HAHA alone as well as FLAG-Dcx in COS1 cells and found that 14-3-3ε strongly associates with Dcx (Fig. 3A). FLAG-Ndel1 was used as a positive control as previous work has shown it is a strong binding partner of 14-3-3ε (13). To verify this interaction with endogenous Dcx and 14-3-3ε in the developing brain, we next performed an IP against Dcx using mouse brain lysates and blotted against 14-3-3ε and confirmed the interaction between endogenous Dcx and 14-3-3ε (Fig. 3B). Next, to determine where the binding region for 14-3-3ε on Dcx is located, we performed a pull-down assay with a number of FLAG-Dcx truncation mutations (Fig. 3C). As schematically portrayed in Fig. 3D, we found that 14-3-3ε binds to the N-terminal region of Dcx (amino acids 1-171). More interestingly, another Dcx mutant (amino acids 47-171) was not able to bind to 14-3-3ε, suggesting that 14-3-3ε binds to the N-terminal of Dcx between the amino acids 1-47. We next used web-based software to search for potential 14-3-3ε binding locations within this region because 14-3-3ε binds to phosphorylated serine or threonine motifs. We found two potential 14-3-3ε binding sites on Dcx, which are threonine 42 and serine 47. Therefore, we created and utilized plasmids expressing FLAG-Dcx with point mutations in these two sites (Fig. 3E and F). We created glutamic acid and alanine point mutations at T42 and S47 to produce phosphorylated and un-phosphorylated mimics, respectively. As shown in Fig. 3F, we found that when a pull-down assay was performed using HA-14-3-3ε, the only time Dcx is not pulled down is when Dcx contains a T42A point mutation (Fig. 3E, the second lane from the left). In contrast, the phosphorylation mimic Dcx (T42E) mutant tended to show stronger binding to 14-3-3ε (Fig. 3E, the second lane from the right). Together, these experiments show that 14-3-3ε binds the N-terminal region of Dcx at T42 in a phosphorylation-dependent manner.
Figure 3.
14-3-3ε binds to the N-terminal region of Dcx at T42 in a phosphorylation dependent manner. (A) COS1 cells were transfected with indicated plasmids and a pull-down was performed against the HA tag and a western blot was performed using a FLAG tag specific antibody showing an interaction between 14-3-3ε and Dcx. Ndel1 is a known binding partner of 14-3-3ε and was used as a positive control. (B) Immunoprecipitation (IP) against Dcx using E15.5 brain lysates followed by a blot using a 14-3-3ε specific antibody verified the interaction between endogenous 14-3-3ε and Dcx. (C) COS1 cells were transfected with a series of FLAG-Dcx truncation mutants and HAHA-14-3-3ε and a pull-down was performed against the HA tag and blotted against the FLAG tag. (D) Summary of the interactions between 14-3-3ε and Dcx truncation mutants from panel C. Note that 14-3-3ε binds Dcx at the N-terminal. (E) COS1 cells were transfected with HAHA-14-3-3ε and FLAG tagged Dcx alanine or glutamic acid point mutations to mimic unphosphorylated and phosphorylated Dcx and a pull-down was performed against the HA tag and a blot against the FLAG tag. (F) Summary of the interaction between 14-3-3ε and Dcx point mutants from panel E.
The interaction between 14-3-3ε and Dcx prevents the ubiquitination and subsequent degradation of Dcx
It is known that 14-3-3 proteins can alter the protein level of their binding partners by regulating their degradation (10,12,20). To determine if 14-3-3ε overexpression alters Dcx protein levels during neurite formation we transfected Neuro-2A cells with a 14-3-3ε expression plasmid (pCAGIG-Ywhae) and cultured the cells for 36 h. We then induced neurite formation via serum deprivation, lysed the cells 0, 15, 60 and 120 min later, and performed a Western blot (WB) analysis (Fig. 4A). We found that 14-3-3ε overexpressing cells have significantly higher Dcx protein levels at all time points relative to control (Fig. 4B).
Figure 4.
14-3-3ε binding to Dcx prevents the ubiquitination and subsequent degradation of Dcx resulting in increased Dcx protein levels. (A) Western blot analysis of Dcx protein levels in Neuro-2A cells transfected with pCAGIG-Ywhae or a control pCAGIG plasmid 36 h prior to serum deprivation. (B) Quantitative analysis of relative Dcx protein levels following serum deprivation. (C) Western blot analysis of FLAG-Dcx levels in COS1 cells following the application of cycloheximide protein synthesis inhibitor. (D) FLAG-Dcx protein levels over time following addition of protein synthesis inhibitor to culture media. Note that Dcx is rapidly degraded in control cells yet remains at a high level in 14-3-3ε overexpressing cells indicating decreased degradation. Values normalized to GAPDH with control time zero value set to one. (E) Western blot of endogenous Dcx in Neuro-2A cells following the application of cycloheximide. (F) Relative levels of endogenous Dcx over time following cycloheximide treatment showing the rapid degradation of Dcx in control cells. (G) Western blot analysis of FLAG-Dcx with a T42-alanine point mutation to create an unphosphorylated 14-3-3ε binding site. (H) Relative FLAG-Dcx(42A) levels over time after cycloheximide treatment. Note that FLAG-Dcx(42A) levels match control levels when 14-3-3ε can no longer bind to Dcx. (I) Western blot analysis of FLAG-Dcx with a T42-glutamic acid mutation to mimic phosphorylated Dcx after cycloheximide treatment. (J) Relative FLAG-Dcx(42E) levels over time after cycloheximide treatment. Note the FLAG-Dcx(42E) levels in control cells were maintained, suggesting the prevention of FLAG-Dcx(42E) degradation. (K) Direct ubiquitination of Dcx was analysed by using HA-tagged ubiquitin. FLAG-Dcx was pulled down with anti-FLAG antibody and ubiquitination was visualized by a blot with anti-HA antibody.
To analyse the turnover rate of Dcx and to determine if the increase in Dcx protein levels in 14-3-3ε overexpressing cells is the result of decreased Dcx degradation, we next analysed Dcx protein levels by WB following cycloheximide treatment in order to inhibit protein synthesis (Fig. 4C). WB analysis showed that in control cells, by 18 h after cycloheximide treatment nearly all Dcx had been degraded; however, 14-3-3ε overexpressing cells showed nearly an identical level of Dcx at time zero as they did 30 h after cycloheximide treatment (Fig. 4D). We next verified these results by analysing endogenous Dcx in Neuro-2A cells (Fig. 4E and F). These indicate that the turnover rate of Dcx is greatly decreased when 14-3-3ε is overexpressed.
To determine if the phosphorylation dependent association between Dcx and 14-3-3ε is important for regulating the turnover rate of Dcx, we used Dcx point mutants. From our previous experiments, we know that 14-3-3ε can only bind to Dcx when T42 is phosphorylated (Fig. 3). We found that the turnover rate of the FLAG-Dcx (T42A) mutant, which cannot be bound by 14-3-3ε, remained at control levels and was degraded within the same time period as control cells when 14-3-3ε was overexpressed (Fig. 4G and H). In contrast, we found that the turnover rate of the FLAG-Dcx (T42E) mutant, which can be bound by 14-3-3ε to a higher degree, decreased in control cells to a level closer to that of 14-3-3ε overexpressing cells (Fig. 4I and J). Together, these results indicate that the turnover rate of Dcx is dependent upon the phosphorylation of the 14-3-3ε binding site on Dcx.
Together thus far our results demonstrate that 14-3-3ε strongly binds to Dcx and that the overexpression of 14-3-3ε results in an increase in Dcx protein levels and a decrease in Dcx turnover rate. A known function of 14-3-3 proteins is their ability to alter the turnover rate of their binding partners by altering ubiquitin based protein degradation (10,12,20,21). To determine if the overexpression of 14-3-3ε prevents the ubiquitination of Dcx, we transfected COS1 cells with EGFP-14-3-3ε, HA-ubiquitin and FLAG-Dcx. 36 h later cells were lysed and a pull-down was performed using an anti-FLAG antibody. The ubiquitination pattern on Dcx was then analysed by WB using an anti-HA antibody (Fig. 4K). In 14-3-3ε overexpressing cells, we found almost no highly ubiquitinated Dcx and low levels of minimally ubiquitinated Dcx (Fig. 4K). This indicates that the overexpression of 14-3-3ε causes a large decrease in the amount of ubiquitinated Dcx, potentially stabilizing Dcx by preventing its degradation and suggesting that 14-3-3ε binding to Dcx can block its ubiquitination.
In summary, our results suggest that 14-3-3ε binds to Dcx in a phosphorylation dependent manner and that this binding prevents the ubiquitination of Dcx and its subsequent degradation, which can be seen as a large increase in Dcx protein levels when 14-3-3ε is overexpressed.
Dcx overexpression replicates the morphological phenotypes seen when 14-3-3ε is overexpressed and miRNA mediated knockdown of Dcx in vitro and in vivo rescues neurite initiation defects
The overexpression of 14-3-3ε results in an increase of Dcx protein levels. Therefore, in order to determine if the overexpression of Dcx can replicate the phenotype seen when 14-3-3ε is overexpressed, we overexpressed Dcx in Neuro-2A cells and analysed their morphology. We found that the overexpression of Dcx results in a decrease in the percent of cells extending neurites, a decrease in the mean number of neurites protruding from the soma and a decrease in mean neurite length matching what is seen when 14-3-3ε is overexpressed (Fig. 5A, middle panel and Fig .5B). These results indicate that the overexpression of Dcx can mimic what is seen when 14-3-3ε is overexpressed further supporting our hypothesis that 14-3-3ε is disrupting neurite formation by increase Dcx protein levels.
Figure 5.
In vitro and in vivo rescue of neurite initiation via Dcx knockdown in 14-3-3ε overexpressing neurons. (A) Images of control pCAGIG (left panel), 14-3-3ε overexpression (panel second from left), control + Dcx (middle panel), 14-3-3ε + scramble miRNA (panel second from right) or 14-3-3ε + Dcx-miRNA (right panel) expressing Neuro-2A cells 36 h after transfection followed by 2 h of serum deprivation to induce neurite outgrowth. (Scale: 20 µm) (B) Analysis of the percent of cells with neurites, the mean number of neurites protruding from the soma and mean neurite length. Note that the overexpression of Dcx mimics the morphological deficits seen in 14-3-3ε overexpressing cells. Also note that the knockdown of Dcx in 14-3-3ε overexpressing cells rescues neurite initiation as seen by the percent of cells with neurites and the mean number of neurites protruding from the soma. Scale: 20 µm, n = 100 cells per condition per analysis from three independent experiments. (C) Images of P15 coronal brain sections following IUE at E16.5 of control mCherry + Scramble miRNA, mCherry-14-3-3ε + scramble miRNA or mCherry-14-3-3ε + Dcx miRNA expression plasmids. Bottom panels show magnified images of the cortical plate. (Scale: 100 µm) (D) Mean number of primary neurites protruding from the soma indicating that the knockdown of Dcx in 14-3-3ε overexpressing neurons rescues neurite initiation. (E) Analysis of mean neurite length. n = 25 neurons from three independent experiments per condition.
In order to determine if the increased Dcx protein levels when 14-3-3ε is overexpressed is producing the deficits in neurite formation, we knocked down Dcx in vitro and in vivo in 14-3-3ε overexpressing cells and analysed neuronal morphology. In order to knockdown Dcx we utilized a Dcx-miRNA expressing plasmid because a previous report indicated off target effects of Dcx shRNA (22). WB analysis of Neuro-2A cells indicates that our Dcx miRNA has an approximate 75% Dcx knockdown efficiency (Supplementary Material, Fig. S3). To determine if the knockdown of Dcx in 14-3-3ε overexpressing cells in vitro can rescue neuronal morphology, we overexpressed 14-3-3ε as well as Dcx miRNA in Neuro-2A cells and analysed neuronal morphology (Fig. 5A, right panel and the second panel from the right). Importantly, the Dcx-miRNA and Scramble-miRNA constructs independently express EmGFP which is used for morphological analysis. We found that when Dcx is knocked down in 14-3-3ε overexpressing cells, the percent of cells having grown neurites and the mean number of neurites protruding from the soma of the cells is rescued (Fig. 5B, left and middle panels). The mean length of the neurites protruding from the soma when Dcx is knocked down does not fully recover to control levels (Fig. 5B, Right panel). This indicates that the knockdown of Dcx in 14-3-3ε overexpressing Neuro-2A cells is able to rescue the initiation of neurite formation but not the subsequent extension of these neurites to control lengths.
To verify our in vitro rescue results, we next knocked down Dcx in 14-3-3ε overexpressing cells in vivo using in utero electroporation. We performed in utero electroporation at E16.5 injecting plasmids to overexpress 14-3-3ε and to express Dcx-miRNA and harvested the brains at P15 (Fig. 5C). Analysis of 14-3-3ε/Dcx-miRNA double positive neurons in the CP revealed similar results as seen in Neuro-2A cells. We found that the knockdown of Dcx in 14-3-3ε overexpressing neurons rescued the mean number of neurites projecting from the soma; however, the mean neurite length is not rescued (Fig. 5D,E). These data indicate that the knockdown of Dcx in 14-3-3ε overexpressing neurons in vivo rescues neurite initiation.
14-3-3ε overexpression disrupts microtubule penetration into lamellipodia and filopodia during neurite initiation
During the process of neurite initiation neurons start extending actin based lamellipodia and filopodia around the soma. Following the formation of these structures, in typical neurons, microtubules will invade these actin based structures and stabilize them, which is a necessary step for neurite initiation. The failure of microtubules to penetrate lamellipodia and filopodia results in the rapid collapse of these structures and neurites will fail to form (23,24). Furthermore, previous research has shown that the ability of microtubules to invade lamellipodia and reach and edge of forming neurites is essential for neurite initiation (24–26). Using phase contrast time-lapse live imaging of Neuro-2A cells overexpressing 14-3-3ε we observed the rapid formation and retraction of large lamellipodia and filopodia structures protruding from the soma, however these structures failed to form neurites (Fig. 2A, Supplementary Material, Movie S2). Therefore, we hypothesized that the overexpression of 14-3-3ε and the subsequent increase in Dcx protein levels will interrupt proper microtubule dynamics and prevent the penetration of microtubules into lamellipodia thus disrupting neurite initiation.
In order to examine this hypothesis, because actin is essential for neurite initiation, we first verified that F-actin formation is not disrupted by the overexpression of 14-3-3ε and found that the formation of F-actin was not disrupted when 14-3-3ε was overexpressed (Supplementary Material, Fig. S4). We next analysed the ability of microtubules to penetrate lamellipodia during neurite initiation. To achieve this, we overexpressed 14-3-3ε and End Binding Protein 3 (EB3) in Neuro-2A cells and performed time lapse live imaging (Fig. 6A, Supplementary Materials, Movies S5–7). EB3 is a protein that specifically binds to the growing plus end of microtubules and can be visualized as comets (27). We found that significantly fewer EB3 comets were able to invade and reach the leading edge of forming neurites when 14-3-3ε was overexpressed (Fig. 6B and C). Furthermore, we found that the total number of comets in the soma of 14-3-3ε overexpressing cells was greatly decreased, indicating an overall decrease in microtubule activity (Fig. 6D). Next, we found that the knockdown of Dcx in 14-3-3ε overexpressing cells rescued the number of comets able to invade and reach the edge of forming neurites and the total number of comets present in the cell (Fig. 6A, right panels and Fig. 6B-D). Together, these data indicate that the overexpression of 14-3-3ε, and subsequent increase in Dcx, inhibits the entry of microtubules into lamellipodia during neurite initiation and the knockdown of Dcx can rescue microtubule invasion.
Figure 6.
14-3-3ε overexpression prevents the invasion of EB3 comets into initiating primitive neurites and the invasion defect is rescued by Dcx knockdown. (A) Time-lapse live imaging of EB3 dynamics in Neuro-2A cells during neurite initiation. Left panels demonstrate a representative time-lapse montage of a control cell expressing pCAG-CFP + EB3-mEmerald where microtubule comets can be seen invading primitive neurite. The middle panels depict a cell overexpressing 14-3-3ε + EB3-mEmerald where comets are not seen entering primitive neurite. The right panels show a cell overexpressing 14-3-3ε + EB3-mCherry (shown in green) + Dcx-miRNA where EB3 comet invasion into the initiating neurite is rescued. The top panel in each column shows the entire cell morphology with the white squares indicating the region shown in the panels below. Edge determined by phase contrast imaging and outlined with a white dotted line. Scale: 5 µm. See Supplementary Movies 5-7. (B) Mean number of comets to reach the edge of forming neurites every 30 s showing a significant decrease in 14-3-3ε overexpressing cells. (C) Mean comet distance from the forming primitive neurite edge indicating that microtubule comets are unable to reach the edge of forming neurites in 14-3-3ε overexpressing cells. (D) Mean number of comets in the entire soma indicating a decrease in microtubule activity in 14-3-3ε overexpressing cells.
Discussion
In this work we found that the overexpression of 14-3-3ε in vitro and in vivo inhibits the degradation of Dcx resulting in an increase in Dcx protein levels which disrupts microtubule dynamics. This disruption prevents the invasion of microtubules into lamellipodia during the initial stages of neurite growth, resulting in severe neuronal morphological defects. Furthermore, we found that the spatiotemporal knockout of 14-3-3ε produces opposite effects on neuronal morphology. In addition, the knockdown of Dcx in 14-3-3ε overexpressing cells can rescue defects in neurite initiation and microtubule dynamics. These results indicate that 14-3-3ε is a negative regulator of neurite formation during development and potentially provides the first pathological findings for 17p13.3 microduplication syndrome.
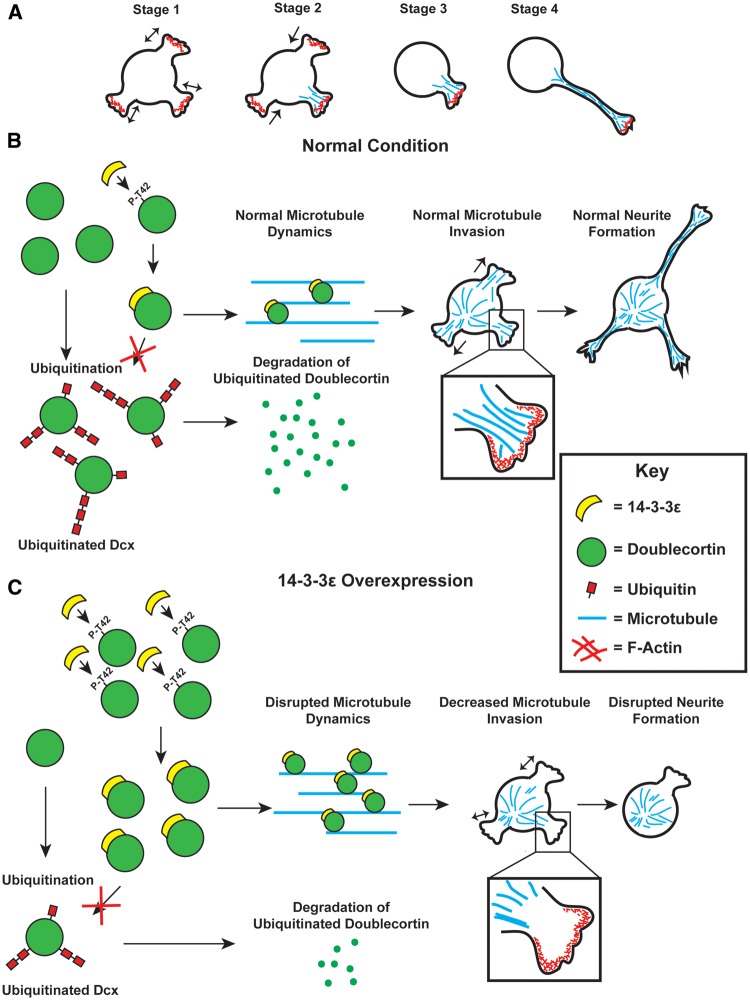
In typical neurons, neurite initiation is broken into multiple stages (Fig. 7A). In the first stage, neurons form actin based lamellipodia type structures that rapidly form and collapse. The next and key stage in neurite initiation is where microtubules invade these lamellipodia structures and stabilize them preventing their collapse, followed by the rapid condensation and extension of these stabilized protrusions (Fig. 7A) (23,24). Previous research has also shown that the microtubules may be important for pushing the leading edge of forming neurites outward during initiation (25,26). Our in vitro and in vivo morphological analysis shows that the overexpression of 14-3-3ε produces severe deficits in the initial formation of neurites. Furthermore, our in vitro and ex vivo time lapse live imaging shows that the initial formation of neurites in 14-3-3ε overexpressing cells is severely interrupted with these cells rapidly forming and retracting short lamellipodia type structures. However, in control cells, these lamellipodia are quickly stabilized and extend (Fig. 2). In addition, we found through live imaging of EB3 comets that in 14-3-3ε overexpressing cell microtubules were unable to invade lamellipodia type formations during neurite initiation and that this was able to be rescued by knocking down Dcx (Fig. 6). Interestingly, both the control and 14-3-3ε overexpressing cells rapidly form these lamellipodia structures (Fig. 2) suggesting that actin dynamics are not disrupted in these cells. In addition, the percent of cells that form lamellipodia type actin formations is not disrupted when 14-3-3ε is overexpressed further indicating that actin is unaffected (Supplementary Material, Fig. S4).
Figure 7.
Schematic illustration of proposed mechanism (A) Schematic illustration of the stages of neurite initiation. In stage 1, lamellipodia type structures rapidly form and retract. In stage 2, microtubules begin to invade and stabilize lamellipodia structures preventing their collapse. In stage 3, neurites that have been invaded by microtubules become stable structures and next begin to extend in stage 4. (B) Under normal condition, 14-3-3ε binds to Dcx at phosphorylated threonine-42 (P-T42). Remaining Dcx that is not bound by 14-3-3ε is rapidly ubiquitinated and subsequently degraded. Dcx stabilized by 14-3-3ε, preventing its ubiquitination and subsequent degradation, binds to microtubules allowing for normal microtubule dynamics. During initial neurite formation microtubules are able to enter and stabilize lamellipodia type structures allowing for normal neurite initiation. (C) When 14-3-3ε is overexpressed, there is increased binding of 14-3-3ε to Dcx, preventing its ubiquitination and subsequent degradation resulting in an increase in Dcx protein levels. The increased Dcx binds to microtubules in excess, and this disrupts microtubule dynamics. This prevents microtubules from invading lamellipodia type structures during neurite initiation thus inhibiting normal neurite formation.
Dcx is a microtubule binding protein and it is well known that its overexpression can disrupt the organization and polymerization of microtubules (28,29). Our data show that 14-3-3ε binds to phosphorylated Dcx and this binding prevents Dcx ubiquitination and subsequent degradation. This results in an increase in Dcx protein levels, which subsequently prevents the invasion of microtubules into lamellipodia type structure during neurite initiation, thus disrupting the proper neurite formation (Fig. 7). Interestingly, our time-lapse live imaging data shows that 14-3-3ε overexpressing cells, and thus increased Dcx expressing cells, have a significant decrease in the number of EB3 comets in the cell body (Fig. 6). This is interesting because EB3 is known to only bind to the plus-tip of growing microtubules and a decrease in EB3 comets indicates a decrease in microtubule polymerization in 14-3-3ε overexpressing cells (27). This disruption in microtubule polymerization may then produce the decrease of microtubules invading primitive neurites during initiation as seen in our data here.
Our in vitro and in vivo data revealed that the knockdown of Dcx via miRNA in 14-3-3ε overexpressing cells rescued initial neurite formation recovering the number of neurites protruding from the soma. However, our rescue experiments were unable to completely rescue the neurite elongation resulting in mature neurites shorter than their counterparts in control neurons (Fig. 5). These data indicate that the increased Dcx protein levels resulting from 14-3-3ε overexpression prevents the initiation of neurites but is not entirely responsible for the deficits in neurite length. This suggests that 14-3-3ε is acting through a separate mechanism through another binding partner in neurite elongation following initiation. 14-3-3 proteins are known to bind to a number of microtubules associated proteins and it stands to reason that the increased 14-3-3ε expression may be altering the function of one of these binding partners during neurite elongation following initial neurite formation. One potential microtubule associated protein that 14-3-3ε may be interacting with is Map1b. Map1b has been previously shown to be extensively involved in neurite extension following initiation (30–34). Furthermore, our shotgun proteomics analysis previously mentioned indicated a strong association between Map1b and 14-3-3ε. However, further analysis is required to determine the role of 14-3-3ε and Map1b in neurite elongation.
17p13.3 microduplication syndrome results in a single extra copy of the YWHAE gene encoding 14-3-3ε in human patients. This suggests that there is an approximate 1.5 fold increase in 14-3-3ε expression. However, this may not necessarily be the case as previous work indicates that it is possible that the duplication of a gene may actually interrupt the expression of the gene by disrupting the exons or the promoter/enhancer, resulting in a possible mechanism of haploinsufficiency or the disruption of topologically associating domains (TADs) (35,36).
Our findings in this work provide the first cellular pathological findings for the overexpression of 14-3-3ε as implicated in 17p13.3 microduplication syndrome as well as elucidate the molecular mechanism producing the cellular deficits in neurite formation. Our evidence will bring clinical attention to 14-3-3ε and the cellular pathologies involved in these developmental disorders and the associated ASD phenotype.
Materials and Methods
Mice
All animal experiments were performed following protocols approved by the Drexel University Animal Care and Use Committees and the guidelines provided by the US National Institutes of Health. ICR mice were purchased from Taconic Inc. The 14-3-3ε flox mice were produced and maintained as previously described (12) (JAX Stock No. 029055). The morning of the appearance of a vaginal plug was defined as embryonic day (E) 0.5.
Plasmids
pHAHA-14-3-3ε, pEGFP-14-3-3ε, HA-tagged Ubiquitin plasmids were obtained as previously described (12). Mouse Dcx and Ndel1 were cloned from mRNA prepared from mouse embryonic brains by RT-PCR into pcDNA3.1(+) plasmid, and then Dcx truncation mutants were also amplified from pcDNA3.1(+)-Dcx with specific primers and cloned into pcDNA3.1(+). Dcx point mutation mutants (T42A, T42E, S47A and S47E) were produced using QuickChange II site-directed mutagenesis kit (Agilent Technologies. Inc.). pCAGIG plasmid was obtained from Addgene (pCAGIG was a gift from Connie Cepko), and 6XHis-tagged 14-3-3 proteins were cloned into pCAGIG as previously described (37). To produce the pCAG-CFP-14-3-3ε plasmid, we performed PCR and cloned the products into the pCAG-CFP plasmid (pCAG-CFP was a gift from Connie Cepko (Addgene plasmid # 11179)) (38). EB3-mEmerald was amplified from the mEmerald-EB3-7 plasmid (mEmerald-EB3-7 was a gift from Michael Davidson (Addgene plasmid # 54075)) (39) by PCR and sub-cloned into a pCAGEN plasmid (pCAGEN was a gift from Connie Cepko (Addgene plasmid # 11160)) (38) to produce pCAGEN-EB3-mEmerald. To produce pCAG-ERT2Cre-GFPERT2 plasmid, Cre in pCAG-ERT2CreERT2 (pCAG-ERT2CreERT2 was a gift from Connie Cepko (Addgene plasmid # 13777)) (40) was replaced by Cre-GFP amplified from pCAG-Cre:GFP (pCAG-Cre:GFP was a gift from Connie Cepko (Addgene plasmid # 13776)) (40) by PCR. To produce pCAG-EmGFP-Scramble-miRNA and pCAG-EmGFP-Dcx-miRNA, pcDNA6.2-GW/EmGFP-miR-SUMO23 plasmid was obtained from Addgene and the CMV promoter was replaced with a CAG promoter using PCR (pcDNA6.2-GW/EmGFP-miR-SUMO23 was a gift from Wulf Paschen (Addgene plasmid # 31073)) (41). Then, the miRNAs for SUMO 2 and 3 in this plasmid were replaced by scramble or Dcx miRNA by PCR. The sequences of all plasmids we created were confirmed by sequencing. We purified all plasmids by NucleoBond Xtra purification kit (MACHEREY-NAGEL).
Immunoprecipitation and pull-down analysis
Immunoprecipitation and pull-down analyses were performed as previously described (12). Briefly, transfected-cells were lysed by lysis buffer. After being precleared with pre-cleared Protein-G Plus-Agarose, supernatant was immunoprecipitated by anti-HA or -FLAG antibody conjugated Agarose. After being thoroughly washed by washing buffer, immunoprecipitated proteins were separated by SDS-PAGE and blotted with an appropriate antibody. For the analysis of the Dcx turnover rate, transfected cells were treated with cycloheximide (200 μg/ml) 36 h after transfecting the cells before preparing the protein lysates.
In u tero electroporation
In utero electroporation was performed as previously described (37,42). In brief, pregnant dams were anaesthetized and the uterine horn containing the mouse embryos was surgically exposed. Plasmids (1 µg) were then injected into the lateral ventricle of E14.5 or E16.5 embryo brains. The embryo heads were then placed between electrodes with the positive anode angled toward the cortex. Then electric pulses (three pulses of 30 mV for E14.5 or 35 mV for E16.5) were applied with 50 ms intervals by a CUY21 electroporator (Nepa GENE). Embryos were then placed back into the uterus and allowed to develop naturally until they were harvested at E18.5 or P15.
Brain slice preparation and time lapse live imaging on brain slices
Brain slice preparation and time lapse live imaging on brain slices were performed as previously described (37). E18.5 brains were removed and placed in ice-cold artificial cerebrospinal fluid (CSF) for slicing. Brains were then embedded in 3% low-melting agarose and 300 µm thick slices were cut using a VTS1000 vibratome (Leica) in ice cold artificial CSF. Slices were then incubated in D-MEM/F-12 imaging media without phenol red supplemented with N-2 supplement (Invitrogen) and 10% FBS for 1h at 37 °C, 5% CO2 for recovery. Slices were then carefully transferred to a 35mm dish and immersed in neutralized rat tail collagen I (Life Technologies). After collagen solidification for 30 min at 37 °C, 5%CO2, slices were covered with imaging media. Time-lapse live imaging was then performed using an upright confocal laser-scanning microscope (TCS SP2 VIS/405, Leica) with a 20X HCX APO L water-dipping objective (NA 0.5). During imaging, slices were cultured in the imaging media and kept at 37 °C with 95% air/5% CO2 in a stage top chamber incubator (DH-40iL, Warner Instruments). Confocal Z-stack images were taken every 10 min for more than 18 h.
In vitro time lapse live imaging
Neuro-2A cells were cultured at 37 °C with 5% CO2 on 35 mm glass bottom dishes (MatTek) in DMEM culture media (Caisson) supplemented with 10% FBS. Cells were then transfected using PolyJet (SignaGen Laboratories) transfection reagent following the manufacturer specifications. Transfected cells were cultured for 36 h, and then the medium was removed, and the cells were washed with PBS. The medium was replaced with Leibovitz’s media (Life Technologies) supplemented with 10% FBS and cultured at 37 °C for 1 h. Cells were then washed with PBS three times and medium was replaced with Leibovitz’s media without FBS to induce neurite outgrowth and cells were immediately imaged. Time-lapse live imaging was performed using an inverted fluorescent microscope (Zeiss, Axio Observer Z1) with a 20X (Zeiss) objective for Movies 1-2 and a 100x (Zeiss) objective for Supplementary Materials, Movies S5–7. For Supplementary Materials, Movies S1 and 2 (Fig. 2) images were captured every 3 min for up to 2 h while cells were maintained at 37 °C with a stage top incubator. For Supplementary Materials, Movies S5–7 (Fig. 6), images were captured every 3 s for up to 10 min while maintained at 37 °C with a stage top incubator (Zeiss).
Analysis of neuronal morphology
Neurites were classified as protrusions from the soma with a length greater than 5 µm. Neurite lengths were measured using ImageJ software. Sholl analysis was performed using the Sholl Analysis Plugin (Gosh Lab, UCSD) for ImageJ following the developer instructions.
Statistical analysis
Quantitative data were subjected to statistical analysis using SPSS (IBM Analytics) and Prism (GraphPad Software). The data were analysed by two-tailed, unpaired Student t-tests and one-way ANOVA with Bonferroni post hoc tests when appropriate. Values represented as mean ± S.E.M. Results were deemed statistically significant if the P value was <0.05. *, ** and *** indicate P <0.05, P <0.01 and P <0.001, respectively.
Supplementary Material
Supplementary Material is available at HMG online.
Supplementary Material
Acknowledgements
The authors are grateful to Christina M. Stinger and Dr. Richard B. Huneke for their assistance with animal maintenance.
Conflict of interest statement. None declared.
Funding
This work was supported by startup funds from the Department of Neurobiology and Anatomy at Drexel University College of Medicine and a research grant from the NIH (NS096098) (to K.T.).
References
- 1. Roos L., Jonch A.E., Kjaergaard S., Taudorf K., Simonsen H., Hamborg-Petersen B., Brondum-Nielsen K., Kirchhoff M. (2009) A new microduplication syndrome encompassing the region of the Miller-Dieker (17p13 deletion) syndrome. J. Med. Genet., 46, 703–710. [DOI] [PubMed] [Google Scholar]
- 2. Bruno D.L., Anderlid B.M., Lindstrand A., van Ravenswaaij-Arts C., Ganesamoorthy D., Lundin J., Martin C.L., Douglas J., Nowak C., Adam M.P., et al. (2010) Further molecular and clinical delineation of co-locating 17p13.3 microdeletions and microduplications that show distinctive phenotypes. J. Med. Genet., 47, 299–311. [DOI] [PubMed] [Google Scholar]
- 3. Avela K., Aktan-Collan K., Horelli-Kuitunen N., Knuutila S., Somer M. (2011) A microduplication on chromosome 17p13.1p13.3 including the PAFAH1B1 (LIS1) gene. Am. J. Med. Genet. A, 155A, 875–879. [DOI] [PubMed] [Google Scholar]
- 4. Hyon C., Marlin S., Chantot-Bastaraud S., Mabboux P., Beaujard M.P., Al Ageeli E., Vazquez M.P., Picard A., Siffroi J.P., Portnoi M.F. (2011) A new 17p13.3 microduplication including the PAFAH1B1 and YWHAE genes resulting from an unbalanced X;17 translocation. Eur. J. Med. Genet., 54, 287–291. [DOI] [PubMed] [Google Scholar]
- 5. Curry C.J., Rosenfeld J.A., Grant E., Gripp K.W., Anderson C., Aylsworth A.S., Saad T.B., Chizhikov V.V., Dybose G., Fagerberg C., et al. (2013) The duplication 17p13.3 phenotype: analysis of 21 families delineates developmental, behavioral and brain abnormalities, and rare variant phenotypes. Am. J. Med. Genet. A, 161A, 1833–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Przybylska-Kruszewska A., Kutkowska-Kazmierczak A., Krzywdzinska A., Smyk M., Nowakowska B., Gryglicka H., Obersztyn E., Hozyasz K.K. (2016) 17p13.3 duplication as a cause of psychomotor developmental delay in an infant - a further case of a new syndrome. Pol. Merkur. Lekarski, 40, 255–259. [PubMed] [Google Scholar]
- 7. Ibitoye R.M., Roberts J., Goodacre T., Kini U. (2015) 17p13.3 microduplication, a potential novel genetic locus for nonsyndromic bilateral cleft lip and palate. Cleft Palate Craniofac. J., 52, 359–362., [DOI] [PubMed] [Google Scholar]
- 8. Eriksson M.A., Lieden A., Westerlund J., Bremer A., Wincent J., Sahlin E., Gillberg C., Fernell E., Anderlid B.M. (2015) Rare copy number variants are common in young children with autism spectrum disorder. Acta Paediatr., 104, 610–618. [DOI] [PubMed] [Google Scholar]
- 9. Berg D., Holzmann C., Riess O. (2003) 14-3-3 proteins in the nervous system. Nat. Rev. Neurosci., 4, 752–762. [DOI] [PubMed] [Google Scholar]
- 10. Foote M., Zhou Y. (2012) 14-3-3 proteins in neurological disorders. Int. J. Biochem. Mol. Biol., 3, 152–164. [PMC free article] [PubMed] [Google Scholar]
- 11. Mhawech P. (2005) 14-3-3 proteins–an update. Cell Res., 15, 228–236. [DOI] [PubMed] [Google Scholar]
- 12. Toyo-oka K., Wachi T., Hunt R.F., Baraban S.C., Taya S., Ramshaw H., Kaibuchi K., Schwarz Q.P., Lopez A.F., Wynshaw-Boris A. (2014) 14-3-3epsilon and zeta regulate neurogenesis and differentiation of neuronal progenitor cells in the developing brain. J. Neurosci., 34, 12168–12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Toyo-oka K., Shionoya A., Gambello M.J., Cardoso C., Leventer R., Ward H.L., Ayala R., Tsai L.H., Dobyns W., Ledbetter D., et al. (2003) 14-3-3epsilon is important for neuronal migration by binding to NUDEL: a molecular explanation for Miller-Dieker syndrome. Nat. Genet., 34, 274–285. [DOI] [PubMed] [Google Scholar]
- 14. Cheah P.S., Ramshaw H.S., Thomas P.Q., Toyo-Oka K., Xu X., Martin S., Coyle P., Guthridge M.A., Stomski F., van den Buuse M., et al. (2012) Neurodevelopmental and neuropsychiatric behaviour defects arise from 14-3-3zeta deficiency. Mol. Psychiat., 17, 451–466. [DOI] [PubMed] [Google Scholar]
- 15. Hashimoto R., Nakazawa T., Tsurusaki Y., Yasuda Y., Nagayasu K., Matsumura K., Kawashima H., Yamamori H., Fujimoto M., Ohi K., et al. (2015) Whole-exome sequencing and neurite outgrowth analysis in autism spectrum disorder. J. Hum. Genet., 61, 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doers M.E., Musser M.T., Nichol R., Berndt E.R., Baker M., Gomez T.M., Zhang S.C., Abbeduto L., Bhattacharyya A. (2014) iPSC-derived forebrain neurons from FXS individuals show defects in initial neurite outgrowth. Stem Cells Dev., 23, 1777–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Phillips M., Pozzo-Miller L. (2015) Dendritic spine dysgenesis in autism related disorders. Neurosci. Lett., 601, 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schubert D., Humphreys S., Baroni C., Cohn M. (1969) In vitro differentiation of a mouse neuroblastoma. Proc. Natl. Acad. Sci. U. S. A, 64, 316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tremblay R.G., Sikorska M., Sandhu J.K., Lanthier P., Ribecco-Lutkiewicz M., Bani-Yaghoub M. (2010) Differentiation of mouse Neuro 2A cells into dopamine neurons. J. Neurosci. Methods, 186, 60–67. [DOI] [PubMed] [Google Scholar]
- 20. Dar A., Wu D., Lee N., Shibata E., Dutta A. (2014) 14-3-3 proteins play a role in the cell cycle by shielding cdt2 from ubiquitin-mediated degradation. Mol. Cell. Biol., 34, 4049–4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mizuno E., Kitamura N., Komada M. (2007) 14-3-3-dependent inhibition of the deubiquitinating activity of UBPY and its cancellation in the M phase. Exp. Cell Res., 313, 3624–3634. [DOI] [PubMed] [Google Scholar]
- 22. Baek S.T., Kerjan G., Bielas S.L., Lee J.E., Fenstermaker A.G., Novarino G., Gleeson J.G. (2014) Off-target effect of doublecortin family shRNA on neuronal migration associated with endogenous microRNA dysregulation. Neuron, 82, 1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dehmelt L., Halpain S. (2004) Actin and microtubules in neurite initiation: are MAPs the missing link? J. Neurobiol., 58, 18–33. [DOI] [PubMed] [Google Scholar]
- 24. Flynn K.C. (2013) The cytoskeleton and neurite initiation. Bioarchitecture, 3, 86–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dehmelt L., Nalbant P., Steffen W., Halpain S. (2006) A microtubule-based, dynein-dependent force induces local cell protrusions: Implications for neurite initiation. Brain Cell Biol., 35, 39–56. [DOI] [PubMed] [Google Scholar]
- 26. Lu W., Fox P., Lakonishok M., Davidson M.W., Gelfand V.I. (2013) Initial neurite outgrowth in Drosophila neurons is driven by kinesin-powered microtubule sliding. Curr. Biol., 23, 1018–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Akhmanova A., Steinmetz M.O. (2008) Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat. Rev. Mol. Cell Biol., 9, 309–322. [DOI] [PubMed] [Google Scholar]
- 28. Horesh D., Sapir T., Francis F., Wolf S.G., Caspi M., Elbaum M., Chelly J., Reiner O. (1999) Doublecortin, a stabilizer of microtubules. Hum. Mol. Genet., 8, 1599–1610. [DOI] [PubMed] [Google Scholar]
- 29. Gleeson J.G., Lin P.T., Flanagan L.A., Walsh C.A. (1999) Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron, 23, 257–271. [DOI] [PubMed] [Google Scholar]
- 30. Dajas-Bailador F., Bonev B., Garcez P., Stanley P., Guillemot F., Papalopulu N. (2012) microRNA-9 regulates axon extension and branching by targeting Map1b in mouse cortical neurons. Nat. Neurosci., 15, 697–699. [DOI] [PubMed] [Google Scholar]
- 31. Feltrin D., Fusco L., Witte H., Moretti F., Martin K., Letzelter M., Fluri E., Scheiffele P., Pertz O. (2012) Growth cone MKK7 mRNA targeting regulates MAP1b-dependent microtubule bundling to control neurite elongation. PLoS Biol., 10, e1001439.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takei Y., Teng J., Harada A., Hirokawa N. (2000) Defects in axonal elongation and neuronal migration in mice with disrupted tau and map1b genes. J. Cell Biol., 150, 989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Teng J., Takei Y., Harada A., Nakata T., Chen J., Hirokawa N. (2001) Synergistic effects of MAP2 and MAP1B knockout in neuronal migration, dendritic outgrowth, and microtubule organization. J. Cell Biol., 155, 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tymanskyj S.R., Scales T.M., Gordon-Weeks P.R. (2012) MAP1B enhances microtubule assembly rates and axon extension rates in developing neurons. Mol. Cell. Neurosci., 49, 110–119. [DOI] [PubMed] [Google Scholar]
- 35. Lupianez D.G., Spielmann M., Mundlos S. (2016) Breaking TADs: How Alterations of Chromatin Domains Result in Disease. Trends Genet., 32, 225–237. [DOI] [PubMed] [Google Scholar]
- 36. Prasad A., Merico D., Thiruvahindrapuram B., Wei J., Lionel A.C., Sato D., Rickaby J., Lu C., Szatmari P., Roberts W., et al. (2012) A discovery resource of rare copy number variations in individuals with autism spectrum disorder. G3 (Bethesda), 2, 1665–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wachi T., Cornell B., Marshall C., Zhukarev V., Baas P.W., Toyo-Oka K. (2016) Ablation of the 14-3-3gamma Protein Results in Neuronal Migration Delay and Morphological Defects in the Developing Cerebral Cortex. Dev. Neurobiol., 76, 600–614. [DOI] [PubMed] [Google Scholar]
- 38. Matsuda T., Cepko C.L. (2004) Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc. Natl. Acad. Sci. U. S. A, 101, 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang F., Hartwich T.M., Rivera-Molina F.E., Lin Y., Duim W.C., Long J.J., Uchil P.D., Myers J.R., Baird M.A., Mothes W., et al. (2013) Video-rate nanoscopy using sCMOS camera-specific single-molecule localization algorithms. Nat. Methods, 10, 653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Matsuda T., Cepko C.L. (2007) Controlled expression of transgenes introduced by in vivo electroporation. Proc. Natl. Acad. Sci. U. S. A, 104, 1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang W., Paschen W. (2009) Gene expression and cell growth are modified by silencing SUMO2 and SUMO3 expression. Biochem. Biophys. Res. Commun., 382, 215–218. [DOI] [PubMed] [Google Scholar]
- 42. Tabata H., Nakajima K. (2001) Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neuroscience, 103, 865–872. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.