Abstract
Mutations in the ORF15 exon of the RPGR gene cause a common form of X-linked retinitis pigmentosa, which often results in severe loss of vision. In dogs and mice, gene augmentation therapy has been shown to arrest the progressive degeneration of rod and cone photoreceptors. However, the distribution of potentially treatable photoreceptors across the human retinas and the rate of degeneration are not known. Here, we have defined structural and functional features of the disease in 70 individuals with ORF15 mutations. We also correlated the features observed in patients with those of three Rpgr-mutant (Rpgr-ko, Rd9, and Rpgr-cko) mice. In patients, there was pronounced macular disease. Across the retina, rod and cone dysfunction showed a range of patterns and a spectrum of severity between individuals, but a high symmetry was observed between eyes of each individual. Genotype was not related to disease expression. In the Rpgr-ko mice, there were intra-retinal differences in rhodopsin and cone opsin trafficking. In Rd9 and Rpgr-cko mice, retinal degeneration showed inter-ocular symmetry. Longitudinal results in patients revealed localized rod and cone dysfunction with progression rates of 1.3 to 2.5 log per decade in sensitivity loss. Relatively retained rod and cone photoreceptors in mid- and far-peripheral temporal-inferior and nasal-inferior visual field regions should be good targets for future localized gene therapies in patients.
Introduction
Inherited retinal degenerations (IRDs) refer to a heterogeneous group of Mendelian disorders caused by mutations in over 200 genes (1,2). IRDs result in visual dysfunction due to loss of structure and function of rod and/or cone photoreceptors. Among the most common genetic causes of IRDs are mutations in the RPGR gene located on the X-chromosome; by far the majority of the RPGR mutations are located in the ORF15 exon of the gene (3–9). Naturally-occurring mutations in ORF15 exon are also found in dogs and mice with retinal degeneration (10,11). RPGR is alternatively spliced, and the transcript that includes the ORF15 exon is detected both in rod and cone photoreceptors of the retina (12). The exact function of RPGR in the rod and cone photoreceptors remains poorly understood but it is suggested to be involved in regulating ciliary transport (12–15). Currently, there are no approved treatments for IRDs caused by RPGR, except for the electronic chip implants for the very late stages of IRDs independent of genotype with nearly no vision remaining (16,17). However, successful experiments in gene augmentation therapy at different disease stages of dogs with naturally occurring RPGR-ORF15 mutations (18,19) taken together with Rpgr knockout mouse results (20,21) have set a clear path for clinical trials of gene augmentation therapy in patients.
However, do we know enough about the spatial topography of rod and cone disease in patients with RPGR-ORF15-associated retinal degenerations (RPGR-ORF15-RD) to select candidates, relevant outcome measures, and treatment locations of gene therapy, and evaluate the safety and efficacy of the intervention? There are reports of wide phenotypic variation in RPGR-ORF15-RD but these descriptions are based on traditional diagnostic nomenclature predating the molecular era. A majority (60–80%) of RPGR-ORF15-RD is clinically diagnosed as X-linked retinitis pigmentosa (XLRP) (3–6,22). Less common are diagnoses with XL forms of cone-rod dystrophy (CRD), cone dystrophy and even macular degeneration (3,5,6,18,23–31). Notably, this very limited diagnostic nomenclature encompasses a wide phenotypic range across a spectrum of clinical severities including variable involvement of the fovea, parafovea, midperiphery and periphery, and variable preservation of remnant rod and cone function throughout the retina or in specific regions (32–39). Accordingly, it is not surprising that RPGR-ORF15-RD also exhibits a wide phenotypic range.
Could some of the phenotypic variation be explained with different ORF15 genotypes? Mutations towards the 3’ end of the ORF15 exon are hypothesized to spare the rod photoreceptors and thus tend to be associated with CRD and related diagnoses considered relatively less severe (5,6,24,26–28,31,40,41). Numerous counter-examples to this hypothesis include families showing both RP and CRD (29,30,40,42–44), patients with RP having 3’ ORF15 mutations (45), and patients with CRD having 5’ ORF15 mutations (3,24,30,40). Thus, accurate description of the effect of ORF15 mutations on rods and cones is not possible within the limitations of the current diagnostic nomenclature. Here, we define the precise spatial distribution of rod and cone dysfunction across a large group of RPGR-ORF15-RD patients, demonstrating a wide spectrum of disease severity. Better understanding of the complexities of this phenotypic spectrum sets the stage for selecting appropriate stages of disease with retained rods and cones that can be targeted by subretinal gene therapy in future clinical trials.
Results
Molecular and clinical characteristics of patients
Patients with RPGR-ORF15 mutations (n = 70, ages 8–71 years at first visit, all hemizygous males) were from 45 families (Supplementary Material, Table S1). There were 30 distinct mutations consisting of 7 nonsense mutations and 23 frameshift mutations; there were no missense mutations, in frame indels, or splice site mutations in our cohort. The nonsense mutations are predicted to truncate the protein between amino acid residues 743 and 915. The frameshift mutations would have an aberrant protein tail of various lengths and lead to a downstream truncation between amino acid residues of 692 and 1088. Thus, all 30 mutations should lack the majority of the C-terminal basic (C2) domain of the RPGR-ORF15 isoform. Mutations were classified into early and late truncations (<915 vs >1065 residues, respectively; there were no patients between 915 and 1065), and categorized by aberrant tail lengths of none, short (<29 residues) and long (>29 residues) (Supplementary Material, Table S1). All but five mutations (c.2405_2406delAG, c.2236_2237delGA, c.2442_2445delAGAG, c.3092delA, and c.2257_2260delGGAG) were present in one family each.
The patient population tended to be myopic (spherical equivalent range −14D to +4D, median -3D). The best corrected visual acuities at the first visit ranged from 20/20 to light perception. A combination of cross-sectional and longitudinal data across ages showed a decline with increasing age (Supplementary Material, Fig. S1). Notably, only 30% of the patients had a visual acuity of better than 20/32 recorded at first visit; across the whole data set, the proportion of eyes retaining better than 20/32 acuity at any visit was 22% (42/193), which is consistent with the relatively severe natural history of visual acuity and foveal sensitivity loss described in XLRP (34,45,46).
Spatiotemporal distribution of macular disease
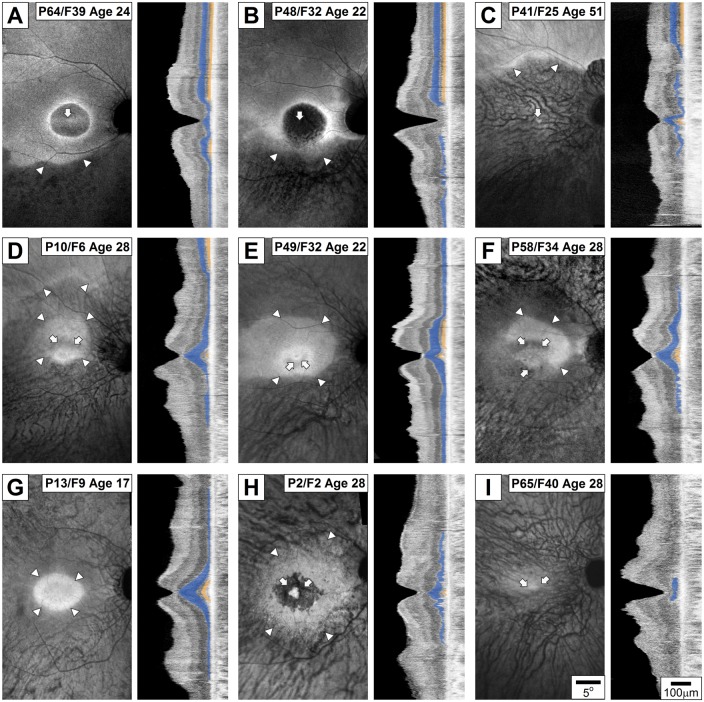
The structure of the RPE and photoreceptors was evaluated in the macula and surrounding paramacular areas to better understand the basis of the relatively severe visual acuity loss in patients. Both eyes of all patients with available imaging data (n = 49 for en face imaging, n = 46 for cross-sectional imaging) showed abnormal structure of the macula unlike the normal macular appearance in many RP patients (47). However, there were a range of severities, and abnormalities were distributed across a range of spatial patterns. One of the mildest disease stages was parafoveal maculopathy surrounded by near-normal retinal structure extending isotropically across the retina and with retained foveal vision (P36/F22 previously published in ref. 18). Figure 1 shows nine representative patients with various stages and patterns of disease. A relatively mild stage of disease showed an altitudinal pattern with greater superior retinal preservation compared to inferior retina such as P64/F39 (VA 20/80) and P48/F32 (VA 20/125) where a distinct line along the nasal-temporal direction demarcated the transition from inferior retinal disease to superior retinal health (Fig. 1A and B). P41/F25 (VA 20/70) demonstrated a greater severity of disease with degeneration of nearly all of the macula and with localization of the transition line beyond the superior aspect of the macula (Fig. 1C).
Figure 1.
Spectrum of spatial patterns of disease in RPGR-ORF15 evaluated with melanin autofluorescence (left panels) and OCT (right panels) across the fovea, macula and superior and inferior para-macular regions. Specific patients exemplify the altitudinal pattern with central maculopathy (A–C), anisotropic pattern with parafoveal defects (D–F), isotropic patterns with or without parafoveal defects (G–H), and end stage disease with indeterminate pattern (I). Arrowheads indicate the transition between diseased (darker) and healthier (brighter) retina near the boundaries of the macula region while arrows mark diseased regions near the fovea. On OCT scans, ONL layer is painted blue and IS/OS line is painted yellow for visibility. All eyes are shown as equivalent right eyes and images are individually contrast stretched for visibility of features. P, patient number, F, family number from Supplementary Material, Table S1. Age in years.
Further along in the severity scale was an anisotropic elliptical shape of preservation around the fovea with or without detectable altitudinal pattern beyond the macula. A representative of this pattern was P10/F6 (Fig. 1D, VA 20/63) demonstrating relative preservation of the foveal structure surrounded by a parafoveal defect, further surrounded by a perifoveal penumbra of preservation that extended more into the superior retina than inferior retina. Beyond the macula, the preservation was apparent in the superior but not in the inferior retina. P49/F32 (VA 20/25) had a similar pattern with better structural preservation of the fovea (Fig. 1E). Perifoveally preserved penumbra of P49 was larger than P10, but the anisotropic extension into the superior retina was similar. There was evidence of better RPE preservation in the superior paramacular region compared to the inferior, but extramacular photoreceptor degeneration was symmetric (Fig. 1E). P58/F34 (VA 20/32) had a macular structure similar to P10 and P49 but demonstrated complete degeneration of both PR and RPE concentrically in the extramacular area (Fig. 1F).
A subset of patients demonstrated evidence of retained macular structure in an isotropic spatial pattern. P13/F9 (VA 20/20) exemplified a well-retained foveal structure that transitioned to severe degeneration by the parafoveal region; a thin layer of photoreceptors and partially demelanized RPE was observed across the rest of the macula (Fig. 1G). P2/F2 (VA 20/50) retained a small foveal area with photoreceptors but lacked detectable outer segment structure (Fig. 1H). A parafoveal annulus of degeneration was circumscribed by a penumbra of retained perifoveal RPE; beyond the macula, there was a minimal outer nuclear layer observed. And lastly, P65/F40 (VA 20/60) showed a remnant fovea surrounded by complete degeneration (Fig. 1I). In summary, macular disease patterns in 5/49 were similar to those shown in Fig. 1A–C, in 5/49 were similar to that shown in Fig. 1D–F, in 14/49 were similar to that shown in Fig. 1G, and in 12/49 were similar to that shown in Fig. 1H; in 13/49, en face imaging was consistent with severe end-stage degeneration similar to Fig. 1I precluding assessment of the macular pattern of degeneration that may have preceded.
To better understand the progression of macular disease severity through different patterns, we extended the cross-sectional studies by evaluating data from a subset of 10 patients with available longitudinal en face imaging (follow-up interval, mean = 7.7 years, range = 5–11 years). P6/F4 had a circular region of approximately 18 deg diameter with relative retinal preservation at age 10; 8 years later at age 18, there was centripetal progression of RPE and photoreceptor disease from the edges of the macula constricting the melanized area to approximately 9° diameter (Supplementary Material, Fig. S2A). P35/F22 at age 23 had continuous RPE across the macula and retained outer nuclear layer only in the central fovea; 8 years later at age 31, a large region of atrophy had developed at the central macula with no detectable photoreceptors (Supplementary Material, Fig. S2B). P58/F34 showed progression from an anisotropically retained macular region to parafoveal degeneration to more severe macular degeneration over 11 years (Supplementary Material, Fig. S2C). P2/F2 and P47/F31 showed examples of parafoveal RPE defects extending substantially over 8–10 years (Supplementary Material, Fig. S2D and E). Taken together, cross-sectional and longitudinal results suggested a progressive spatio-temporal time course that could often be described with the sum of central and peripheral components, each with a different natural history (Supplementary Results and Supplementary Material, Fig. 3).
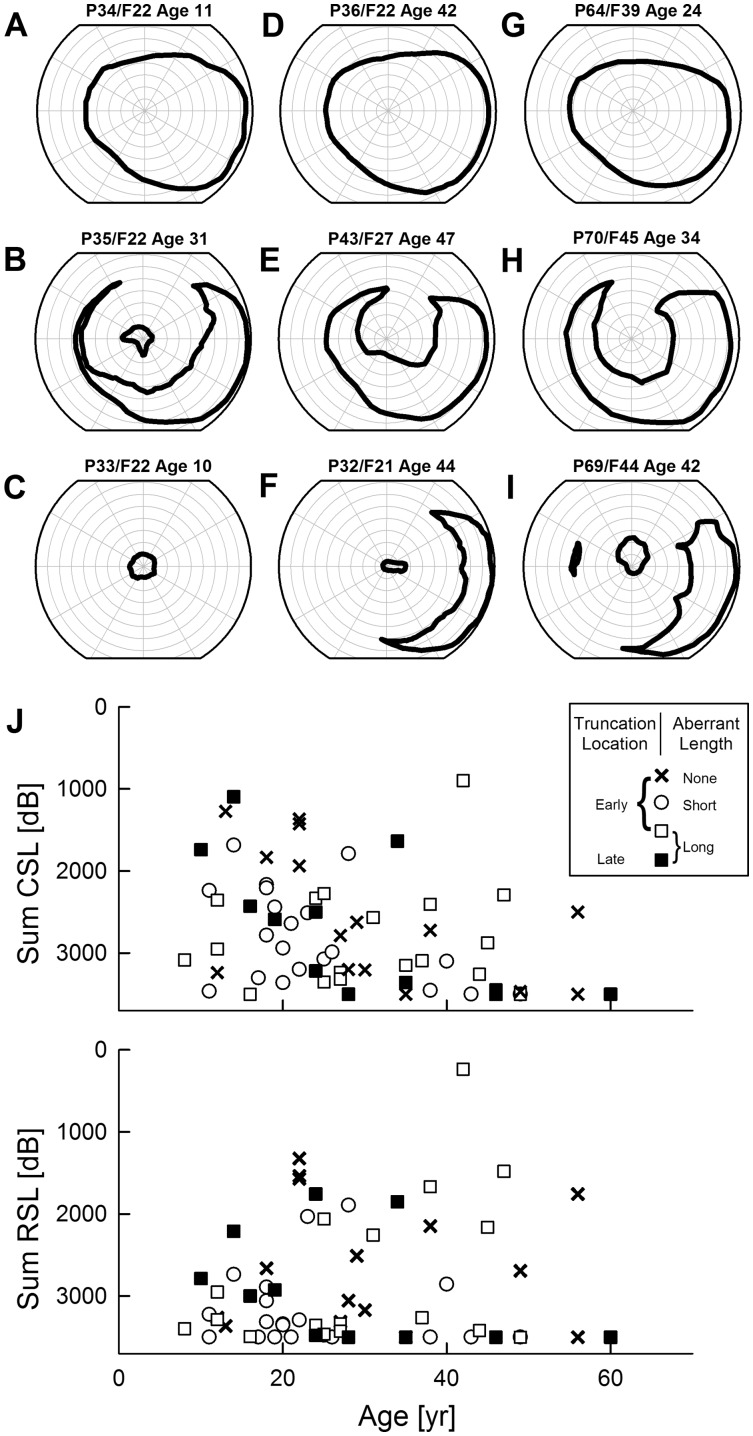
Figure 3.
Lack of relationship between severity of rod- or cone-mediated vision loss and age and RPGR-ORF15 genotype. (A–I) Extents of kinetic visual fields (V-4e) demonstrating examples of severity in younger and older patients with early (A–F) or late truncations (G–I). Note that patients shown in A–D are from the same family. (J) Retina wide severity of cone (upper) and rod (lower) photoreceptor mediated function plotted against age. Symbols define genotype categorized as early or late truncations and predicted aberrant protein tails of none, short or long. At any given age group, a range of severity is observed independent of genotype. CSL, cone sensitivity loss; RSL, rod sensitivity loss.
Photoreceptor structure at fovea and surrounding central retina
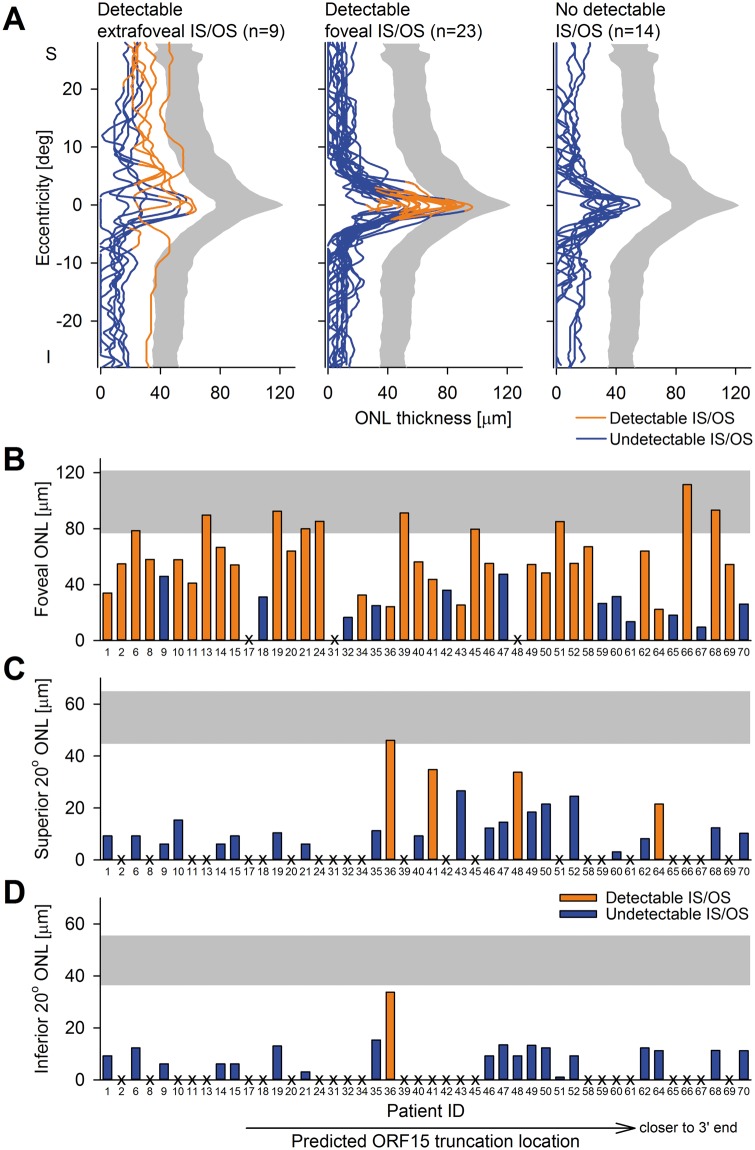
Next, we evaluated the consequences of RPGR-ORF15 mutations on photoreceptor degeneration. Ultra-wide extent OCT scans were quantified along the vertical meridian for the thickness of the ONL (where the photoreceptor nuclei are located) and for detecting the signal thought to be originating near the junction between inner and outer segments (IS/OS). Patients could be categorized into three groups according to their IS/OS line appearance. In 9/46 (20%) patients, the IS/OS line was detectable beyond the fovea and mostly in the superior retina (Fig. 2A, left). Retinal regions with detectable IS/OS tended to have a greater ONL thickness, which did not reach normal values in most patients. Most patients in this group tended to show an altitudinal pattern. In half (23/46 = 50%) of the patients, the IS/OS line was detectable only at the fovea, rarely extending beyond 2.5 deg eccentricity (Fig. 2A, middle). The foveal region with detectable IS/OS line showed the peak ONL thickness, which was mostly abnormally reduced. Outside the fovea, there was detectable but abnormally thinned ONL layer. In 14/46 (30%) patients, the IS/OS line was not detectable across the retina, and the ONL thickness was reduced throughout (Fig. 2A, right). There was no obvious genotype-phenotype correlation; all three groups included patients with nonsense and frame-shift mutations, predicted to truncate the protein early or late, and with shorter or longer aberrant tails.
Figure 2.
Photoreceptor degeneration across the central retina in RPGR-ORF15. (A) Central ONL thickness along the vertical meridian in RPGR-ORF15 is divided into three groups (Left: subjects with detectable IS/OS line outside the fovea; centre: subjects with detectable IS/OS line in the fovea only; right: subjects with no detectable IS/OS line). Blue lines indicate regions with no detectable IS/OS line and orange lines denote regions with detectable IS/OS line. Grey area indicates 95% confidence interval of ONL thickness from normal data. S, superior; I, inferior retina. (B-D) ONL thicknesses at fovea (B), 20° superior (C) and 20° inferior (D) retina show a wide spectrum among the patient population. Abscissae represent patient IDs which are ordered in terms of predicted truncation location; truncations closer to the 3’ end are to the right. Orange bars show subjects with detectable IS/OS line, blue bars show patients with no detectable IS/OS line. X denotes undetectable ONL.
We then asked whether the foveal photoreceptor retention was related to ORF15 genotype. Foveal ONL thickness ranged from normal to below the resolution of the imaging system (range = 0 to 111.5 µm; mean ± sd 48.9 ± 27.9 µm; normal 100.1 ± 11.1 µm). A relationship between foveal ONL retention and the location of the predicted truncation was not evident, either graphically (Fig. 2B) or statistically (2-way ANCOVA controlling for age, P = 0.08 for the locus of truncation, P = 0.55 for length of aberrant protein tail). There was a very limited ONL structure and even more limited IS/OS line beyond the fovea. Mean ONL thickness was 8.3(±11.1) µm (range = 0 to 46 µm; normal 54.8 ± 5.1 µm) at 20° superior retina (Fig. 2C), whereas mean ONL thickness was 4.8(±6.9) µm (range = 0 to 33.7 µm; normal 55.5 ± 4.8 µm) at 20° inferior retina (Fig. 2D). IS/OS at these locations were only rarely detectable (superior: 4/46; inferior: 1/46). These features at superior and inferior perifoveal locations showed no evident relation to predicted truncation location (Fig. 2C and D). In summary, both functional and structural measures (Figs. 1 and 2; Supplementary Material, Figs. S1–S3) strongly suggested that the central macula of a majority of RPGR-ORF15-RD tended to be very fragile independent of the genotype and would not be likely targets of interventions involving foveal detachments considering the potential consequences of such interventions (48–51).
Retina-wide disease severity is unrelated to genotype
Considering the pattern and severity of central retinal disease was not related to the locus of the altered codon within ORF15, we next considered whether genotype was related to retina-wide measures of disease. The extent and shape of the remaining visual fields in IRDs contribute to a patient’s difficulties with orientation and mobility. Smaller visual fields imply greater retinal areas of photoreceptor degeneration and thus greater severity of disease. In a given individual, there is usually age-related increase in severity corresponding to constriction of visual fields but data become complex across individuals (5,43,45,52). In RPGR-ORF15, individuals from a single family can show a very wide spectrum of severity (Fig. 3A–D): young patients can have normal (Fig. 3A) or severely constricted (Fig. 3C) visual fields, whereas older patients can show normal extents (Fig. 3D) or abnormal constriction (Fig. 3B). Relatively mild disease corresponding to retention of normal visual fields in the third decade of life or later can be observed with mutations predicted to truncate the RPGR-ORF15 early (Fig. 3D) or late (Fig. 3G). Similarly, loss of mid-peripheral vision can occur with early truncations (Fig. 3B and E) as well as late truncations (Fig. 3H). Most commonly, a pattern of retained far-temporal islands of vision is observed with or without a small central island, and this pattern is also found with early (Fig. 3F) and late (Fig. 3I) truncations across different ages.
Severity of retina-wide disease can be better quantified by the light sensitivity within the retained visual field. Therefore, we investigated whether the genotypes were correlated to light sensitivities mediated by rod and cone cells within the retained visual fields. For this purpose, we used the sum of sensitivity losses sampled on a grid across the retina similar to previous studies (53,54). Both cone- as well as rod-photoreceptor-mediated sensitivity losses (sum CSL and sum RSL, respectively) showed a wide spectrum cross-sectionally across decades of life (Fig. 3J). Controlling for age, severity parameters did not reveal a statistically significant relationship to the location of the predicted truncation (2-way ANCOVA, P = 0.62 and 0.76 for RSL and CSL, respectively) or to the length of the aberrant protein extent (same analysis, P = 0.11 and 0.25 for RSL and CSL, respectively).
Variegated spatial patterns of rod and cone disease across the retina
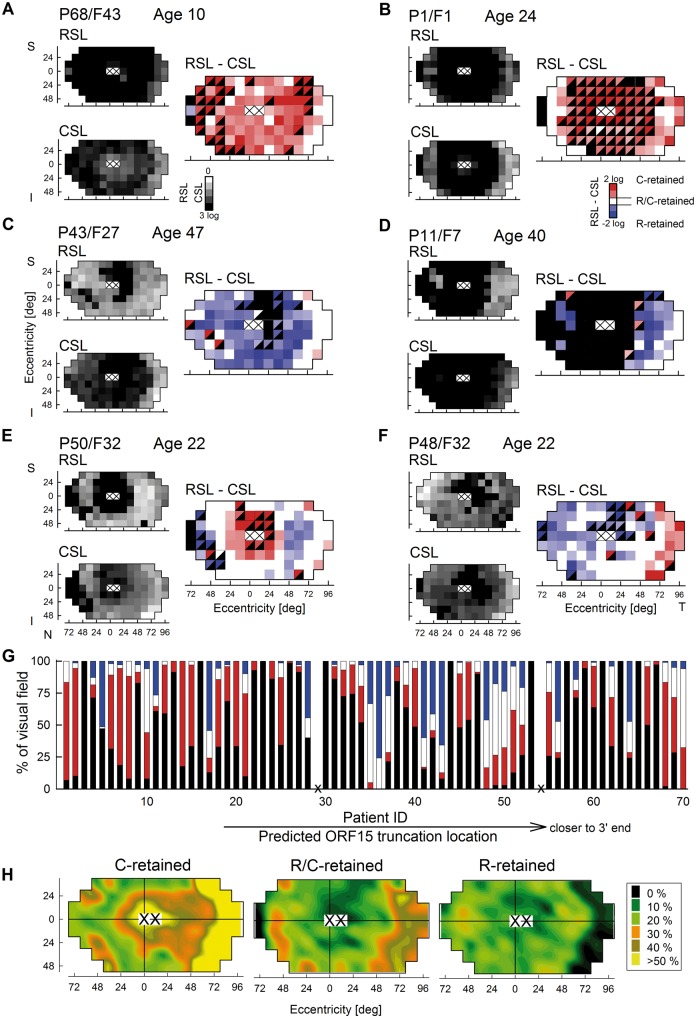
The spectrum of retina-wide disease observed in this large cohort of RPGR-ORF15-RD patients (Fig. 3) was further investigated by considering the detailed spatial distribution of rod versus cone disease across the retina. Some patients showed a prevalence of relatively cone-photoreceptor retained (C-retained, coded red) regions across the retina (Fig. 4A and B), whilst others exhibited a prevalence of relatively rod-photoreceptor retained (R-retained, coded blue) regions across the retina (Fig. 4C and D). Additionally, there could be regions with equal rod and cone retention (R/C-retained, coded white). In most patients, however, the spatial distribution of rod and cone disease was not homogeneous. Central C-retained regions could be surrounded by an annulus of R/C-region in the mid-periphery, further surrounded by R-regions (Fig. 4E). A more complex distribution of rod and cone disease was also evident (Fig. 4F). Far-periphery could demonstrate nasal-temporal differences regarding relative involvement of rod and cone disease (Fig. 4E and F).
Figure 4.
Retina-wide maps of retained rod and cone function show complex spatial patterns in RPGR-ORF15-RD. (A–F) Topographic representation of rod- and cone-mediated sensitivity losses (RSL and CSL, smaller greyscale maps) and classification of whether rod (R, blue), cone (C, red) or both photoreceptors (R/C, white) are retained across the full extent of the retina (larger colour maps) in representative patients showing substantially different patterns and severities. Black squares represent no detectable vision. Half-filled colour squares represent loci where one of the photoreceptor function was not measurable but one was measurable. Loci corresponding to the fovea and locus of the optic nerve (white squares with cross) are not included in the analyses. S, superior; I, inferior; N, nasal; T, temporal visual field. (G) Percentage of the retina-wide loci showing R-, C-, R/C-retention or no vision in all 70 patients. Abscissa represents patient IDs which are ordered in terms of predicted truncation location; truncations closer to the 3’ end are to the right. (H) Spatial distribution of retinal loci showing R-, C- and R/C-retention across all patients. Pseudocolour coding describes the percentage of patients at a given retinal locus that show a specific type of dysfunction.
The proportion of the visual field demonstrating C-, R-, R/C-retained regions and blindspots are provided for all patients (Fig. 4G, note that patients are ordered by predicted location of the truncation from early truncations on the left to late truncations on the right). The proportion of the retina covered by C, R or R/C retention was not significantly related to the location of the predicted truncation (early or late) or to the length of the aberrant protein extent (none, short or long) (2-way ANCOVA, all P > 0.05).
Across all patients with detectable vision (defined as rod or cone vision detectable at three or more retinal locations, n = 49 patients), C-retained regions tended to be located centrally extending to the temporal mid-peripheral field and across a wide swath of temporal far peripheral field (Fig. 4H, left). C-retained regions did not localize to a mid-peripheral incomplete annulus covering superior, nasal and inferior fields; peripheral nasal and infero-nasal fields demonstrated C-retained regions in about a third of the patients. R-retained regions tended to localize in a circular mid-peripheral annulus extending from 25 to 70 degrees (Fig. 4H, right). R-regions were distinctly absent from the far peripheral temporal field. R/C-retained regions tended to be located on a circular mid-peripheral annulus similar to R-retained regions but also included the far temporal field similar to C-retained regions (Fig. 4H, centre).
Transitions between regions of vision and neighbouring regions of visual dysfunction in the mid- and far-peripheral retina could often correspond to detectable structural changes. Specifically on ultra-wide angle imaging with near-infrared autofluorescence, transitions were detectable between lower signal implying demelanization of the RPE to higher signal corresponding to melanized RPE. Two examples (P49/F32 and P1/F1) demonstrate the transitions in the mid- (Supplementary Material, Fig. S4A) and far-peripheral (Supplementary Material, Fig. S4B) retinal regions. Across all patients with such ultra-wide angle imaging available (n = 18), the disease/health transition occurred across a wide annulus (Supplementary Material, Fig. S4C), which corresponded closely to the relative distribution of regions with a detectable vision across all patients (Supplementary Material, Fig. S4D).
Inter-ocular symmetry supports deterministic gene expression patterns
Considering the lack of ORF15 genotype contributions to the phenotypic rod and cone features, alternative explanations for substantial differences in spatial distribution of rod and cone dysfunction and overall severity of disease include individual-specific retinal gene expression patterns that are deterministic or stochastic. Intra-retinal stochastic events would predict lack of symmetry between the eyes, such as that observed in carriers of XLRP (55), likely due to stochastic X-inactivation. Underlying deterministic, but individualized, gene expression patterns on the other hand would predict inter-ocular symmetry.
In a subset of patients (n = 29), complete retina-wide rod and cone function measurements were performed in both eyes. P45 and P49 represented two patients where the spatial pattern of rod and cone disease is very different. P45 showed an annular mid-peripheral scotoma neighboured by a peripheral ring and a centre of mostly retained cones (Supplementary Material, Fig. S5A). P49, on the other hand, revealed a milder disease stage of mid-peripheral rod retention and central cone retention; temporal periphery was mostly cone-retained whereas the nasal periphery mainly rod-retained (Supplementary Material, Fig. S5B). The spatial patterns of R and C retention and disease severity in contralateral eyes of the two patients were near perfect duplicates, such that inter-ocular variations were minor compared to inter-individual variations (Supplementary Material, Fig. S5A and B). Next, we considered all available inter-ocular comparisons. For RSL (Supplementary Material, Fig. S5C) and CSL (Supplementary Material, Fig. S5D), inter-ocular limits of agreement were estimated from calculable differences at loci with sensitivity losses or scotoma in both eyes (2520/2862 = 88.1% for RSL and 2465/2862 = 86.1% for CSL). A minority of points (342/2862 = 11.9% for RSL and 397/2862 = 13.9% for CSL) with scotoma in one eye and measurable sensitivity loss in the other eye were not included since a difference could not be calculated for these points. Estimates of limits of agreement were ±8.4 dB and ±8.1 dB for RSL and CSL, respectively. As a reference, our previously reported intra-visit variability value for DA static perimetry would predict limits of agreement for the difference between the same eye measured twice to be ±7.5 dB (56). Thus inter-ocular differences in RSL and CSL were similar to that expected from test-retest variability.
Progression of rod and cone disease
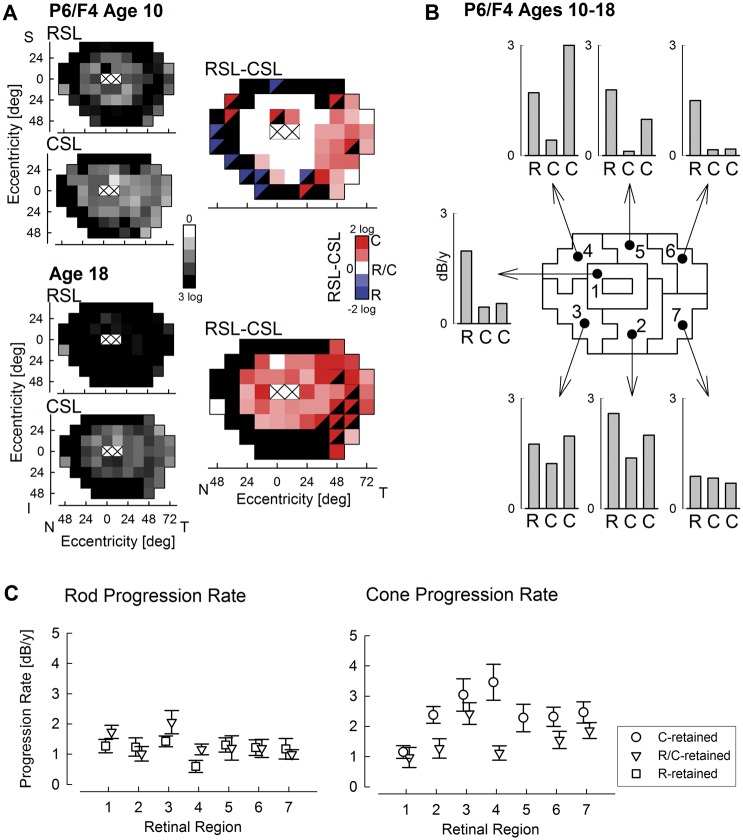
IRDs are slowly progressive, and thus serial recordings of visual function over short periods of time are complicated by the interaction of test variability with disease progression. Recordings performed over long periods of time, on the other hand, are expected to show large changes due to progression and thus can provide more reliable progression rate estimates. A representative example is P6, who was followed over 8 years (Fig. 5A). There were substantial losses of rod and cone function at his visit at age 10; by age 18, the visual dysfunction had become greater (Fig. 5A, left column). At age 10, there was a mid-peripheral region of R/C-retention, and a large temporal field region of C-retention; however, most of the retina was converted to C-retention type by age 18 (Fig. 5A, right column). But, what is the rate of rod and cone loss, and how does it vary across the retina? For this purpose, we tiled the retina into seven non-overlapping regions with 8–12 samples each and calculated the rate of rod and cone function loss at loci that showed R/C-retention at the first visit as well as the rate of cone function loss at loci showing C-retention (Fig. 5B). In the para-macular ring (region 1), the rod progression rate was greater than the cone progression rate, explaining the conversion of this region to C-retention type. Indeed, the rod progression rate was greater than the cone progression rate at R/C-retention loci in all regions. Notably, the cone progression rate of C-retention loci was greater than the cone progression rate of R/C-retention loci in most regions. Overall, the highest cone progression rates were in regions 3 and 2.
Figure 5.
Long-term progression of rod and cone dysfunction. (A) Rod- and cone-mediated sensitivity losses (RSL and CSL, smaller greyscale maps) and classification of whether rod, cone or both photoreceptors are retained (same as Fig. 4) in a representative patient recorded at ages 10 and 18 show substantial and non-homogeneous progression. (B) Calculated progression rates in dB per year across the retina tiled into seven neighbouring regions. Progression rates for rods and cones are specified for those loci that were R/C-retained at first visit (R and centre C, respectively), and for those loci that were C-retained at first visit (right C). Progression rates for loci with R-retention at first visit were not calculated since rod function at most of these points was undetectable at the second visit. (C) Rod and cone function progression rates across all patients with long-term followup as a function of retinal region described in Panel B. Rod progression is specified at loci with R- and R/C-retention at first visit, and cone progression at loci with C- and R/C-retention at the first visit.
To better understand the progression of RPGR disease, available longitudinal data from a subset of patients (n = 14) obtained over the long term (average 13 years; range 5 to 27 years) were analyzed (Fig. 5C). The progression rate of rod dysfunction averaged 1.3 dB/year, as estimated from 325 loci with R-retention or R/C-retention (Fig. 5C, left). Loci with C-retention tended to have very large rod dysfunction (>3 log units) at the first visit and resulted in floor effects in rate calculations. Rod progression rate appeared to be independent of retinal region. The progression rate of cone dysfunction for 140 loci with R/C-retention averaged 1.5 dB/year with inferior retinal regions 3 and 7 showing greater rates than other regions (Fig. 5C, right). For C-retained loci (n = 221), cone progression rate was higher and averaged 2.4 dB/year with nasal regions 3 and 4 showing greater rates than other regions. Loci with R-retention tended to have very large cone dysfunction (>3 log units) at the first visit and resulted in floor effects in rate calculations.
Retinotopic distribution of disease and degeneration in Rpgr-mutant mice
Similar to the patient results presented above, Rpgr-mutant mice have been shown to demonstrate a range of retina-wide severity as well as intraretinal variation of photoreceptor degeneration (43). To determine if the mouse models of the disease also show retinotopic variegation of rod and cone disease, we first evaluated the effect of loss of RPGR in Rpgr-ko mice which carry a deletion within exons 4 and 6 of the Rpgr gene (57). Mis-localization of OS proteins rhodopsin and cone opsins to the IS, ONL, and outer plexiform layer (OPL) is a hallmark of photoreceptor dysfunction and degeneration. Our hypothesis was that if the RPGR disease observed in patients is also recapitulated in mouse models, then Rpgr-ko mice will exhibit different effects of rhodopsin and cone opsin mistrafficking across different regions of the retina. To test this, we performed immunofluorescence analysis of retinal cryosections from 8 months old Rpgr-ko mice along the dorsal and ventral regions, and quantified the relative intensity of anti-rhodopsin (red) or anti-M-opsin (green) –specific staining in the OS, IS, ONL, and OPL. While ∼82% of the total rhodopsin staining was detected in the ONL of the ventral retina of the Rpgr-ko mice, ∼69% rhodopsin was detected in the ONL of the dorsal retina. Increased anti-rhodopsin-specific staining was detected in the IS and OPL as compared to the OS in the ventral retina than in the dorsal retina (Supplementary Material, Fig. S6A and B). On the other hand, M-opsin mislocalization to the IS, ONL, and OPL was relatively more severe in the dorsal retina as compared to the ventral retina of the Rpgr-ko mice (Supplementary Material, Fig. S6C and D). Although there was intra-retinal variation, the extent of rhodopsin and M-opsin mislocalization was consistent between the left and the right eyes.
We then evaluated the effects of greater disease severity in two other Rpgr-mutant mice. For this purpose, we determined whether the mutant mice show interocular symmetry of retinal degeneration as estimated in vivo from the ONL thickness measurements performed along the dorsal-ventral axis through the optic nerve (Supplementary Material, Fig. S6E, inset). Rd9 mice on a B6 background with a naturally-occurring frameshift mutation within ORF15 of the Rpgr gene (11) showed relatively mild disease with ONL thickness ranging from 65 to 109% of the mean control (C57BL/6) value over the ages of 4 to 10 months (Supplementary Material, Fig. S6E, unfilled symbols). There was variation across and within eyes; however, there was high inter-ocular symmetry with co-localized retinal regions showing an average difference of 0% and inter-ocular limits of agreement of 10% which was within the measurement uncertainty. Genetically engineered conditional-knockout mice (Rpgr-cko) (43) on BALB/c background demonstrated on average greater degeneration compared to Rd9 at equivalent ages (Supplementary Material, Fig. S6E, filled symbols). Over the ages of 2 to 15 months, there was a wide range of ONL thickness loss measurable in Rpgr-cko mice ranging from 0 to 108% of mean control (BALB/c). Similar to Rd9 results, Rpgr-cko ONL thickness showed variation across and within eyes; however, there was high inter-ocular symmetry with co-localized retinal regions showing an average difference of 0% and interocular limits of agreement of 10%, which was within the measurement uncertainty. In summary, inter-individual and intra-retinal variation yet inter-ocular symmetry of retinal degeneration observed in human RPGR-ORF15-RD appeared to also be present in Rpgr-mutant mice.
Discussion
Mutations in the ORF15 exon of RPGR result in one of the most common molecular forms of inherited retinal degeneration accounting for 6–20% of all cases of RP (3–8,58–60). Recent pre-clinical success in gene augmentation therapy (18–21,61,62) has raised the likelihood of translation of this potential treatment modality to patients. Here, we evaluated the retinal structure and function in a large cohort of patients with RPGR-ORF15 mutations in preparation for Phase I/II trials of gene augmentation therapy.
Unexpectedly common macular degeneration
The human macula is a circular region of the central retina surrounding (and including) the fovea which normally provides highest spatial resolution and best colour vision due to the peak of the cone photoreceptor density (63). Macular degeneration refers to loss of photoreceptor cells of the macula that may often start in a parafoveal ring and expand over time sometimes sparing the fovea. Typical forms of RP do not cause macular degeneration; indeed there is often relative retention of macular structure and function well into the later stages of disease (64,65). Retained macular structure and/or function has been described in autosomal dominant and recessive RP, and Usher syndrome patients (47,66–74). Even though XLRP is often simply thought of as a particularly severe form of RP, it has been known since the pre-molecular era that macular degeneration can occur in up to 72% of XLRP patients (33,34). More recently, however, analyses of retinal cross-section in XLRP maculas (including some with RPGR-ORF15 mutations) implied relatively retained photoreceptor structure (75–79). Our results in a large cohort of RPGR-ORF15 patients provide some further clarity. Specifically 100% of our patients including those as young as 10 years showed substantial macular degeneration either with or without foveal preservation. None of our patients showed a contiguous IS/OS line extending beyond the fovea and thus would not be candidates for measuring outer segment disease progression as previously proposed (76). The genotype of cohorts could potentially explain some of the apparent differences between our results and the literature. Our large and genotypically homogeneous cohort was exclusively RPGR-ORF15-RD patients with nonsense and frameshift mutations only (no missense mutations). Some of the previous work was in ungenotyped XLRP (76), contained cohorts combining results of RPGR mutations with other genotypes (75,79), or combining results of exon 1–14 and ORF15 mutations in RPGR (76–78).
Retinotopic distribution of rod and cone photoreceptor disease across the retina
The RPGR isoform that includes exon ORF15 is thought to be expressed in both rod and cone photoreceptors (15,57,80–84) and thus it is not surprising that both photoreceptor types are affected in most patients with ORF15 mutations. Rarely, however, there have been reports of disease phenotypes demonstrating either exclusive or preferential involvement of cone photoreceptors, typically diagnosed with so-called cone- or cone-rod-dystrophies or atrophic macular degeneration (3,5,6,23–27, 29–31,85,86). Retinotopic distribution of rod and cone disease has not been known to date. Our large cohort of RPGR-ORF15 patients included rare examples of retina-wide loss of rod function with homogeneously retained cone function as well as retina-wide loss of cone function with homogeneously retained rod function. However, in the great majority of the patients, there was a variegated patchwork of retinal regions showing stark variation of rod versus cone disease: regions of rod retention neighboured regions of cone retention or equal loss. Severity and relative rod or cone retention were unrelated to the location of the truncation within ORF15 or to the length of the predicted aberrant protein. Thus, our results did not conform to the hypothesis raised previously (5,6,24,26,27,31) that mutations occurring towards the 3’ end of ORF15 were milder and showed greater rod retention. Our results were consistent with many previous case reports or case series showing a wide spectrum of disease in patients with the same ORF15 mutation or among patients with ORF15 mutations near the 3’ or 5’ ends (3,24,29,30,44).
Spatial distribution and severity of rod and cone losses varied substantially between patients. Considering the lack of phenotypic correlation with ORF15 mutations in this population, the variation could be speculated to result from modifier genes or differences in retinotopic gene expression patterns. Modifier genes have been hypothesized to contribute to disease in patients with RPGR mutations and their animal models (42,87,88), however no strong candidates have been found thus far. Stochastic gene expression ‘noise’ could also provide a basis for the variation observed. Cell to cell variation (variegation) in gene expression of post-mitotic photoreceptor cells (89,90) would not be consistent with the significant interocular symmetry found between the eyes of patients in this cohort. Inter-individual gene expression differences occurring very early in development could interact with RPGR-ORF15 mutations to define the retinotopic pattern of rod and cone photoreceptor losses in each individual. Such expression differences would have to be evident early enough during development to result in interocular symmetry. Recent results suggest there are many genes that are differentially expressed between the macula and periphery of the human retina (91–93); however, retinotopic patterns of inter-individual expression differences, especially of the relevant genes, are currently not known.
Retained far peripheral photoreceptors
One of the common visual field patterns of RP is a limited extent of central field separated from a far peripheral island of vision (94). Many RPGR-ORF15 patients also show this pattern (43). Here we extended our rod and cone functional measures to the far periphery (96° eccentric from fovea) in order to evaluate the photoreceptor contents of these remaining peripheral islands of vision. We found the islands to show either cone-retention or rod/cone-retention (but not rod-retention). Unexpectedly, the light sensitivity of cones and/or rods within the remaining far peripheral islands could be normal or near normal in the eyes that otherwise displayed very severe vision loss across the rest of the retina. Why are these far-peripheral photoreceptors retained in RPGR-ORF15, or in other forms of RP in general? One hypothesis is that the environmental light load is an important contributor to disease progression (95,96) and the far peripheral retina accumulates less light exposure (97) over the lifetime of the individual. To test this hypothesis, we also examined the far nasal field which is normally obscured by the nose and cheeks, and thus would be expected to receive even less light exposure as compared to the far temporal field. The results showed that retained rod or cone function was possible in the far nasal field but it was not nearly as common as retained far temporal field function thus ruling out an exclusive contribution from environmental light.
An alternative hypothesis has been raised to explain the far-peripheral retention of photoreceptors in some models of retinal degeneration (98). This hypothesis is based on the cone-rich rim of the human retina that is most developed in the far nasal retina near the ora serrata (99,100). At or near this retinal locus, there is evidence from several species including primates that adult retinas have the potential for neurogenesis (101–104). Such neurogenetic potential may be activated in adults by certain diseases or growth factors at the peripheral retina (101,105,106) or in some cases even in the more central retina (107).
Ideal retinal regions of localized therapeutic intervention
What are the best retinal regions for early stage localized therapeutic interventions, such as Phase I gene augmentation therapy for RPGR-ORF15 disease based on strong preclinical proof-of-concept results (18–21,61,62)? The answers should include considerations of: 1) minimizing the risk of losing the most useful vision of patients in case of unexpected local toxicity; 2) availability of rod and cone photoreceptor based function to evaluate possible differences in efficacy; 3) direction of expansion of visual field to be useful for mobility in case of efficacy; and, 4) accessibility of the retinal region for safe subretinal surgery. Considering the extreme fragility of the central macular region found in all patients, and previous experience with foveal detachments (48–51), the central macula should not be a targeted region for early stage gene therapy. In the extramacular region, possible extension of the inferior visual field is more useful than the superior visual field. Within the inferior visual field (superior retina) of RPGR-ORF15 patients, structurally and functionally detectable transitions from diseased to healthier retina were observed at eccentricities ranging from about 30 to 50° in the supero-nasal and supero-temporal retinas. Thus localized subretinal treatment blebs could be starting near the inside boundary of disease/health transitions with the aim of extending the bleb peripherally into regions of better preserved function. Progression of cone dysfunction in these regions is expected to average between 20–30 dB (2–3 log units) per decade, and rod dysfunction between 10–25 dB (1–2.5 log units) per decade. Taking advantage of the strong interocular symmetry, significant treatment related effects should be detectable over a 3 year follow-up period using the fellow eye as a control.
Methods
Subjects
The study population consisted of 70 patients from 45 families with mutations in the ORF15 exon of the RPGR gene (see Supplementary Material, Table S1). Details of PCR analysis for RPGR-ORF15 genotyping for the majority of the patients has been previously reported (8). In a minority of patients, additional RPGR-ORF15 sequencing was performed in CLIA-approved and other laboratories by methods that overcome the difficulty in amplifying the 1.7 kb purine-rich repeat region within exon ORF15. All methods required prior high fidelity PCR amplification of the repetitive region using gene specific primers, followed by either Sanger sequencing with overlapping ORF15 primers or next generation sequencing, as discussed in detail (108).
A complete eye examination was performed in all subjects, including best-corrected ETDRS visual acuity and kinetic visual fields. A subset of 35 patients has been published previously (8,43,109) but all data shown here are either newly acquired or represent new analyses of previously acquired data. Institutional review board approval and informed consent were obtained, and the procedures adhered to the tenets of the Declaration of Helsinki.
Retinal imaging
En face imaging was performed with a confocal scanning laser ophthalmoscope (Spectralis HRA, Heidelberg Engineering, Heidelberg, Germany) to obtain near-infrared (NIR) reflectance, NIR excited reduced-illuminance autofluorescence (NIR-RAFI), and short-wavelength (SW) excited RAFI (SW-RAFI) (110,111). The ‘automatic real time’ (ART) feature of the acquisition software was used to average up to 40 frames with intensity normalization turned off. All images were acquired with the high speed mode wherein either 30ox30° square or 55° diameter circular fields were sampled onto 768x768 pixels. Images from left eyes are displayed as equivalent right eyes for ease of comparability. In a subset of patients, additional imaging with the 55° diameter lens was obtained to extend coverage to the far periphery. Ultrawide-field NIR-RAFI montages were assembled by manually specifying corresponding retinal landmark pairs in overlapping segments using custom-written software (MATLAB 6.5; MathWorks, Natick, MA, USA) as previously described (111). The images were evaluated for peripheral transitions from lower to higher signal in order to estimate the structural demarcations corresponding to transitions from scotoma to retained retinal function.
Cross-sectional imaging was performed with a spectral-domain (SD) OCT system (RTVue-100; Optovue Inc., Fremont, CA) along the vertical meridian crossing the fovea. Postacquisition processing of OCT data was performed with custom programs (MatLab 7.5, MathWorks, Natick, MA). Individual scans were digitally stitched together to form 60 deg long scans. Thickness of the outer nuclear layer (ONL) where the photoreceptor nuclei reside was segmented manually using both intensity and slope information on longitudinal reflectivity profiles (LRPs). In addition, the existence of the hyperreflective layer thought to originate near the boundary between inner and outer segments (IS/OS) (also known as ellipsoid zone, EZ) was demarcated along the length of each scan.
Rod and cone function measures
Static threshold perimetry under dark- and light-adapted conditions was used to quantify rod- and cone-mediated visual function across the visual field (43,112–115). In all patients, sensitivity measurements were made across the retina with a 12 deg grid sampling 70 loci across 120 deg width and 84 deg height. In a subset of 32 patients, nasal and temporal fields were extended by 24 deg each to cover a total retinal area of 168 deg width by 84 deg height sampled by 102 loci on a 12 deg grid. The far peripheral nasal field testing was performed by rotating the head of the individual by 20 deg nasally while placing the fixation target temporally, in order to circumvent blocking of the infero-nasal visual field by the nose of the subject. Rod mediation was determined by comparison of dark-adapted sensitivities to 500 and 650 nm stimuli. At loci where sensitivities to 500 nm were mediated by the rod system, sensitivity losses were obtained by subtraction from locus-specific mean normal value.
Light-adapted testing was performed with 600 nm stimuli in all patients. In addition, in a subset of 47 patients both 600 nm and achromatic (white) stimuli were used to measure cone-mediated light-adapted sensitivity across the retina. Sensitivity losses were obtained by subtraction from mean locus-specific normal value of each stimulus. In these RPGR-ORF15 XLRP patients sensitivity losses estimated from white and 600 nm stimuli were highly correlated (Supplementary Material, Fig. S7A). Test results with the achromatic stimulus had the advantage of providing 12.6 dB wider dynamic range thus allowing estimation of sensitivity losses at greater severity of disease compared to those available with the 600 nm stimulus (Supplementary Material, Fig. S7A). Thus, when available, results with the achromatic stimulus were used. For long-term progression analyses, the same stimulus (600 nm or achromatic) was used at both time points.
One of the most important goals of the current work was to directly compare rod and cone dysfunction. Ideally light sensitivity of each photoreceptor system should be compared under the same conditions. We first used limited data from RPGR-ORF15 XLRP (331 measurements in 30 patients) and RHO-ADRP (540 measurements in 25 patients) to evaluate the relationship between light-adapted and dark-adapted sensitivity losses at cone-photoreceptor mediated loci (Supplementary Material, Fig. S7B). Data were mathematically modeled by fitting a function of the form Y= B/[B + X], where Y is the linear light-adapted cone sensitivity loss (Y = 100.1*LA-CSL, when LA-CSL is specified in dB units), X is the linear dark-adapted cone sensitivity loss (X = 100.1*DA-CSL, when DA-CSL is specified in dB units), B corresponds to the X value that produces LA-CSL of -3 dB. All CSL values reported throughout are dark-adapted CSL values estimated from light-adapted CSL values using the model.
At each retinal locus, relative involvement of rod and cone disease was estimated from the difference of RSL and CSL. The extent of rod and cone disease was considered ‘equal’ (R/C-retained, white- coded colours) when the RSL-CSL difference was within ±7.5 dB [95% limits assuming each variable to have an equal and a Gaussian distribution with a standard deviation of 2.65 dB corresponding to the test-retest variability (56,116)]. Beyond these limits, differences were classified as preferentially retained rods (R-retained, blue-coded colours), or preferentially retained cones (C-retained, red-coded colours).
Statistics
Interocular symmetry was evaluated by the 95% limits of agreement analysis for repeated measures with linked replications (117–119). The relationship between mutant ORF15 characteristics and phenotypic parameters was assessed with 2-way ANCOVA using age at time of test as a covariate. Protein characteristics were represented by two factors: the predicted truncation location (early or late), and the length of the aberrant tail (none, short, or long). The phenotypic parameters were foveal ONL thickness, rod and cone disease severity, and rod and cone retained proportion of visual fields. Significance levels were set at α = 0.05. Analyses were performed using the R statistical software (120).
Animals
Three Rpgr-mutant mouse lines, Rpgr-ko (57), Rd9 (11) and Rpgr-cko (43), and their respective controls were used. All experiments were performed in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research with approval from the Institutional Animal Care and Use Committees of the University of Massachusetts Medical School and University of Pennsylvania. Histopathology and immunochemistry was performed in Rpgr-ko mice as previously described (121). In short, Rpgr-ko mouse eyes were enucleated and fixed in 4% paraformaldehyde. Cryosections were cut along the dorsal-ventral axis. The sections were stained with anti-rhodopsin or M-opsin (EMD Millipore, USA) antibodies. Staining with S-opsin was performed to validate the ventral region of the retina. To quantify opsin staining in the different regions of the photoreceptors, we first calculated the total intensity of staining by determining the product of the mean value of pixel intensity and sum pixel area of the OS, IS, ONL, and OPL. The percentage of the staining in each region was then determined by dividing the intensity of that region by the total intensity. In vivo OCT was performed in Rd9 and Rpgr-cko mice as previously described (43). In short, OCTs were acquired with a 3.2 µm resolution system (Bioptigen, Inc., Durham, NC) from animals anaesthetized by intraperitoneal injection (ketamine HCl, 65 mg/kg and xylazine, 5 mg/kg). Pupils were dilated topically (tropicamide, 1% and phenylephrine, 2.5%) and corneas were lubricated frequently during the imaging session (Systane Ultra ophthalmic lubricant; Alcon Ltd., Fort Worth, TX). Scans were obtained along the dorsal-ventral axis crossing the optic nerve head (ONH). Post-acquisition processing of OCT data was performed with commercial software (InVivoVue Clinic software; Bioptigen, Inc.) and custom programs (MATLAB 6.5; MathWorks, Natick, MA). The ONL thickness was quantified every 0.1 mm sampled between 0.2 and 1.1 mm from the ONH.
Supplementary Material
Supplementary Material is available at HMG online.
Conflict of Interest statement. AVC and SGJ are listed as inventors on the patent application (PCT/US2013/022628) titled ‘AAV-mediated gene therapy for RPGR X-linked retinal degeneration’ held by the Trustees of the University of Pennsylvania and licensed to AGTC. A. Swaroop is listed as a co-inventor on a patent application related to gene therapy of RPGR submitted by the National Eye Institute. Remaining authors declare no relevant conflicts.
Funding
This was supported by AGTC (Alachua, FL, USA), National Eye Institute (EY017549, EY001583, EY022372, EY022012), Foundation Fighting Blindness (Columbia, MD, USA), Macula Vision Research Foundation (West Conshohocken, PA, USA), Chatlos Foundation (Longwood, FL, USA), and Intramural Research Program of the National Eye Institute (EY000546).
Supplementary Material
References
- 1. Bramall A.N., Wright A.F., Jacobson S.G., McInnes R.R. (2010) The genomic, biochemical, and cellular responses of the retina in inherited photoreceptor degenerations and prospects for the treatment of these disorders. Annu. Rev. Neurosci., 33, 441–472. [DOI] [PubMed] [Google Scholar]
- 2. Wright A.F., Chakarova C.F., Abd El-Aziz M.M., Bhattacharya S.S. (2010) Photoreceptor degeneration, genetic and mechanistic dissection of a complex trait. Nat. Rev. Genet., 11, 273–284. [DOI] [PubMed] [Google Scholar]
- 3. Vervoort R., Lennon A., Bird A.C., Tulloch B., Axton R., Miano M.G., Meindl A., Meitinger T., Ciccodicola A., Wright A.F. (2000) Mutational hot spot within a new RPGR exon in X-linked retinitis pigmentosa. Nat. Genet., 25, 462–466. [DOI] [PubMed] [Google Scholar]
- 4. Breuer D.K., Yashar B.M., Filippova E., Hiriyanna S., Lyons R.H., Mears A.J., Asaye B., Acar C., Vervoort R., Wright A.F., et al. (2002) A comprehensive mutation analysis of RP2 and RPGR in a North American cohort of families with X-linked retinitis pigmentosa. Am. J. Hum. Genet., 70, 1545–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sharon D., Sandberg M.A., Rabe V.W., Stillberger M., Dryja T.P., Berson E.L. (2003) RP2 and RPGR mutations and clinical correlations in patients with X-linked retinitis pigmentosa. Am. J. Hum. Genet., 73, 1131–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pelletier V., Jambou M., Delphin N., Zinovieva E., Stum M., Gigarel N., Dollfus H., Hamel C., Toutain A., Dufier J.L., et al. (2007) Comprehensive survey of mutations in RP2 and RPGR in patients affected with distinct retinal dystrophies, genotype-phenotype correlations and impact on genetic counseling. Hum. Mutat., 28, 81–91. [DOI] [PubMed] [Google Scholar]
- 7. Shu X., Black G.C., Rice J.M., Hart-Holden N., Jones A., O'Grady A., Ramsden S., Wright A.F. (2007) RPGR mutation analysis and disease, an update. Hum. Mutat., 28, 322–328. [DOI] [PubMed] [Google Scholar]
- 8. Branham K., Othman M., Brumm M., Karoukis A.J., Atmaca-Sonmez P., Yashar B.M., Schwartz S.B., Stover N.B., Trzupek K., Wheaton D., et al. (2012) Mutations in RPGR and RP2 account for 15% of males with simplex retinal degenerative disease. Invest. Ophthalmol. Vis. Sci., 53, 8232–8237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Churchill J.D., Bowne S.J., Sullivan L.S., Lewis R.A., Wheaton D.K., Birch D.G., Branham K.E., Heckenlively J.R., Daiger S.P. (2013) Mutations in the X-linked retinitis pigmentosa genes RPGR and RP2 found in 8.5% of families with a provisional diagnosis of autosomal dominant retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci., 54, 1411–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang Q., Acland G.M., Wu W.X., Johnson J.L., Pearce-Kelling S., Tulloch B., Vervoort R., Wright A.F., Aguirre G.D. (2002) Different RPGR exon ORF15 mutations in Canids provide insights into photoreceptor cell degeneration. Hum. Mol. Genet., 11, 993–1003. [DOI] [PubMed] [Google Scholar]
- 11. Thompson D.A., Khan N.W., Othman M.I., Chang B., Jia L., Grahek G., Wu Z., Hiriyanna S., Nellissery J., Li T., et al. (2012) Rd9 is a naturally occurring mouse model of a common form of retinitis pigmentosa caused by mutations in RPGR-ORF15. PloS. One, 7, e35865.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Megaw R.D., Soares D.C., Wright A.F. (2015) RPGR, Its role in photoreceptor physiology, human disease, and future therapies. Exp. Eye Res., 138, 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raghupathy R.K., Gautier P., Soares D.C., Wright A.F., Shu X. (2015) Evolutionary characterization of the Retinitis Pigmentosa GTPase Regulator gene. Invest. Ophthalmol. Vis. Sci., 56, 6255–6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khanna H. (2015) Photoreceptor sensory cilium, traversing the Ciliary Gate. Cells, 4, 674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rao K.N., Li L., Zhang W., Brush R.S., Rajala R.V., Khanna H. (2016) Loss of human disease protein retinitis pigmentosa GTPase regulator (RPGR) differentially affects rod or cone-enriched retina. Hum. Mol. Genet., 25, 1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jacobson S.G., Cideciyan A.V. (2010) Treatment possibilities for retinitis pigmentosa. N. Engl. J. Med., 363, 1669–1671. [DOI] [PubMed] [Google Scholar]
- 17. Ho A.C., Humayun M.S., Dorn J.D., da Cruz L., Dagnelie G., Handa J., Barale P.O., Sahel J.A., Stanga P.E., Hafezi F., Argus II Study Group., et al. (2015) Long-term results from an epiretinal prosthesis to restore sight to the blind. Ophthalmology, 122, 1547–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beltran W.A., Cideciyan A.V., Lewin A.S., Iwabe S., Khanna H., Sumaroka A., Chiodo V.A., Fajardo D.S., Román A.J., Deng W.T., et al. (2012) Gene therapy rescues photoreceptor blindness in dogs and paves the way for treating human X-linked retinitis pigmentosa. Proc. Natl Acad. Sci. U.S.A, 109, 2132–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beltran W.A., Cideciyan A.V., Iwabe S., Swider M., Kosyk M.S., McDaid K., Martynyuk I., Ying G.S., Shaffer J., Deng W.T., et al. (2015) Successful arrest of photoreceptor and vision loss expands the therapeutic window of retinal gene therapy to later stages of disease. Proc. Natl Acad. Sci. U.S.A, 112, E5844–E5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu Z., Hiriyanna S., Qian H., Mookherjee S., Campos M.M., Gao C., Fariss R., Sieving P.A., Li T., Colosi P., Swaroop A. (2015) A long-term efficacy study of gene replacement therapy for RPGR-associated retinal degeneration. Hum. Mol. Genet., 24, 3956–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pawlyk B.S., Bulgakov O.V., Sun X., Adamian M., Shu X., Smith A.J., Berson E.L., Ali R.R., Khani S., Wright A.F., et al. (2016) Photoreceptor rescue by an abbreviated human RPGR gene in a murine model of X-linked retinitis pigmentosa. Gene Ther., 23, 196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jin Z.B., Liu X.Q., Hayakawa M., Murakami A., Nao-i N. (2006) Mutational analysis of RPGR and RP2 genes in Japanese patients with retinitis pigmentosa, identification of four mutations. Mol. Vis., 12, 1167–1174. [PubMed] [Google Scholar]
- 23. Kirschner R., Rosenberg T., Schultz-Heienbrok R., Lenzner S., Feil S., Roepman R., Cremers F.P., Ropers H.H., Berger W. (1999) RPGR transcription studies in mouse and human tissues reveal a retina-specific isoform that is disrupted in a patient with X-linked retinitis pigmentosa. Hum. Mol. Genet., 8, 1571–1578. [DOI] [PubMed] [Google Scholar]
- 24. Demirci F.Y., Rigatti B.W., Wen G., Radak A.L., Mah T.S., Baic C.L., Traboulsi E.I., Alitalo T., Ramser J., Gorin M.B. (2002) X-linked cone-rod dystrophy (locus COD1), identification of mutations in RPGR exon ORF15. Am. J. Hum. Genet., 70, 1049–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ayyagari R., Demirci F.Y., Liu J., Bingham E.L., Stringham H., Kakuk L.E., Boehnke M., Gorin M.B., Richards J.E., Sieving P.A. (2002) X-linked recessive atrophic macular degeneration from RPGR mutation. Genomics, 80, 166–171. [DOI] [PubMed] [Google Scholar]
- 26. Yang Z., Peachey N.S., Moshfeghi D.M., Thirumalaichary S., Chorich L., Shugart Y.Y., Fan K., Zhang K. (2002) Mutations in the RPGR gene cause X-linked cone dystrophy. Hum. Mol. Genet., 11, 605–611. [DOI] [PubMed] [Google Scholar]
- 27. Ebenezer N.D., Michaelides M., Jenkins S.A., Audo I., Webster A.R., Cheetham M.E., Stockman A., Maher E.R., Ainsworth J.R., Yates J.R., et al. (2005) Identification of novel RPGR ORF15 mutations in X-linked progressive cone-rod dystrophy (XLCORD) families. Invest. Ophthalmol. Vis. Sci., 45, 1891–1898. [DOI] [PubMed] [Google Scholar]
- 28. Duncan J.L., Zhang Y., Gandhi J., Nakanishi C., Othman M., Branham K.E., Swaroop A., Roorda A. (2007) High-resolution imaging with adaptive optics in patients with inherited retinal degeneration. Invest. Ophthalmol. Vis. Sci., 48, 3283–3291. [DOI] [PubMed] [Google Scholar]
- 29. Walia S., Fishman G.A., Swaroop A., Branham K.E., Lindeman M., Othman M., Weleber R.G. (2008) Discordant phenotypes in fraternal twins having an identical mutation in exon ORF15 of the RPGR gene. Arch. Ophthalmol., 126, 379–384. [DOI] [PubMed] [Google Scholar]
- 30. Ruddle J.B., Ebenezer N.D., Kearns L.S., Mulhall L.E., Mackey D.A., Hardcastle A.J. (2009) RPGR ORF15 genotype and clinical variability of retinal degeneration in an Australian population. Br. J. Ophthalmol., 93, 1151–1154. [DOI] [PubMed] [Google Scholar]
- 31. Thiadens A.A., Soerjoesing G.G., Florijn R.J., Tjiam A.G., den Hollander A.I., van den Born L.I., Riemslag F.C., Bergen A.A., Klaver C.C. (2011) Clinical course of cone dystrophy caused by mutations in the RPGR gene. Graefes Arch. Clin. Exp. Ophthalmol., 249, 1527–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berson E.L., Gouras P., Gunkel R.D., Myrianthopoulos N.C. (1969) Rod and cone responses in sex-linked retinitis pigmentosa. Arch. Ophthalmol., 81, 215–225. [DOI] [PubMed] [Google Scholar]
- 33. Bird A.C. (1975) X-linked retinitis pigmentosa. Br. J. Ophthalmol., 59, 177–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fishman G.A., Farber M.D., Derlacki D.J. (1988) X-linked retinitis pigmentosa. Profile of clinical findings. Arch. Ophthalmol., 106, 369–375. [DOI] [PubMed] [Google Scholar]
- 35. Heckenlively J.R., Bird A.C. X-linked recessive retinitis pigmentosa In, Heckenlively JR. (ed), Retinitis Pigmentosa, p.162 Philadelphia, JB Lippincott; 1988. [Google Scholar]
- 36. Jacobson D.M., Thompson H.S., Bartley J.A. (1989) X-linked progressive cone dystrophy. Clinical characteristics of affected males and female carriers. Ophthalmology, 96, 885–895. [DOI] [PubMed] [Google Scholar]
- 37. Yagasaki K., Jacobson S.G. (1989) Cone-rod dystrophy. Phenotypic diversity by retinal function testing. Arch. Ophthalmol., 107, 701–708. [DOI] [PubMed] [Google Scholar]
- 38. Keith C.G., Denton M.J., Chen J.D. (1991) Clinical variability in a family with X-linked retinal dystrophy and the locus at the RP3 site. Ophthalmic Paediatr. Genet., 12, 91–98. [DOI] [PubMed] [Google Scholar]
- 39. Flaxel C.J., Jay M., Thiselton D.L., Nayudu M., Hardcastle A.J., Wright A., Bird A.C. (1999) Difference between RP2 and RP3 phenotypes in X linked retinitis pigmentosa. Br. J. Ophthalmol., 83, 1144–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zahid S., Khan N., Branham K., Othman M., Karoukis A.J., Sharma N., Moncrief A., Mahmood M.N., Sieving P.A., Swaroop A., et al. (2013) Phenotypic conservation in patients with X-linked retinitis pigmentosa caused by RPGR mutations. JAMA Ophthalmol., 131, 1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bassuk A.G., Sujirakul T., Tsang S.H., Mahajan V.B. (2014) A novel RPGR mutation masquerading as Stargardt disease. Br. J. Ophthalmol., 98, 709–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fahim A.T., Bowne S.J., Sullivan L.S., Webb K.D., Williams J.T., Wheaton D.K., Birch D.G., Daiger S.P. (2011) Allelic heterogeneity and genetic modifier loci contribute to clinical variation in males with X-linked retinitis pigmentosa due to RPGR mutations. PLoS. One, 6, e23021.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huang W.C., Wright A.F., Roman A.J., Cideciyan A.V., Manson F.D., Gewaily D.Y., Schwartz S.B., Sadigh S., Limberis M.P., Bell P., et al. (2012) RPGR-associated retinal degeneration in human X-linked RP and a murine model. Invest. Ophthalmol. Vis. Sci., 53, 5594–5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang L., Yin X., Feng L., You D., Wu L., Chen N., Li A., Li G., Ma Z. (2014) Novel mutations of RPGR in Chinese retinitis pigmentosa patients and the genotype-phenotype correlation. PLoS. One, 9, e85752.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sandberg M.A., Rosner B., Weigel-DiFranco C., Dryja T.P., Berson E.L. (2007) Disease course of patients with X-linked retinitis pigmentosa due to RPGR gene mutations. Invest. Ophthalmol. Vis. Sci., 48, 1298–1304. [DOI] [PubMed] [Google Scholar]
- 46. Massof R.W., Finkelstein D. (1979) Vision threshold profiles in X-linked retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci., 18, 426–429. [PubMed] [Google Scholar]
- 47. Jacobson S.G., Roman A.J., Aleman T.S., Sumaroka A., Herrera W., Windsor E.A., Atkinson L.A., Schwartz S.B., Steinberg J.D., Cideciyan A.V. (2010) Normal central retinal function and structure preserved in retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci., 51, 1079–1085. [DOI] [PubMed] [Google Scholar]
- 48. Maguire A.M., Simonelli F., Pierce E.A., Pugh E.N., Jr., Mingozzi F., Bennicelli J., Banfi S., Marshall K.A., Testa F., Surace E.M., et al. (2008) Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med., 358, 2240–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jacobson S.G., Cideciyan A.V., Ratnakaram R., Heon E., Schwartz S.B., Roman A.J., Peden M.C., Aleman T.S., Boye S.L., Sumaroka A., et al. (2012) Gene therapy for leber congenital amaurosis caused by RPE65 mutations, safety and efficacy in 15 children and adults followed up to 3 years. Arch. Ophthalmol., 130, 9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bainbridge J.W., Mehat M.S., Sundaram V., Robbie S.J., Barker S.E., Ripamonti C., Georgiadis A., Mowat F.M., Beattie S.G., Gardner P.J., et al. (2015) Long-term effect of gene therapy on Leber's congenital amaurosis. N. Engl. J. Med., 372, 1887–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bennett J., Wellman J., Marshall K.A., McCague S., Ashtari M., DiStefano-Pappas J., Elci O.U., Chung D.C., Sun J., Wright J.F., et al. (2016) Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations, a follow-on phase 1 trial. Lancet, 388, 661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Massof R.W., Finkelstein D. (1987) A two-stage hypothesis for the natural course of retinitis pigmentosa In Zrenner E., Krastel H., Goebel H.-H. (eds), Research in Retinitis Pigmentosa. Advances in the Biosciences. Pergamon Press, Oxford, Vol. 62, pp. 29–58. [Google Scholar]
- 53. Berson E.L., Rosner B., Sandberg M.A., Weigel-DiFranco C., Moser A., Brockhurst R.J., Hayes K.C., Johnson C.A., Anderson E.J., Gaudio A.R., et al. (2004) Clinical trial of docosahexaenoic acid in patients with retinitis pigmentosa receiving vitamin A treatment. Arch. Ophthalmol., 122, 1297–1305. [DOI] [PubMed] [Google Scholar]
- 54. Berson E.L., Rosner B., Sandberg M.A., Weigel-DiFranco C., Brockhurst R.J., Hayes K.C., Johnson E.J., Anderson E.J., Johnson C.A., Gaudio A.R., et al. (2010) Clinical trial of lutein in patients with retinitis pigmentosa receiving vitamin A. Arch. Ophthalmol., 128, 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jacobson S.G., Yagasaki K., Feuer W.J., Román A.J. (1989) Interocular asymmetry of visual function in heterozygotes of X-linked retinitis pigmentosa. Exp. Eye Res., 48, 679–691. [DOI] [PubMed] [Google Scholar]
- 56. Cideciyan A.V., Aleman T.S., Boye S.L., Schwartz S.B., Kaushal S., Roman A.J., Pang J.J., Sumaroka A., Windsor E.A., Wilson J.M., et al. (2008) Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc. Natl Acad. Sci. U.S.A, 105, 15112–15117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hong D.H., Pawlyk B.S., Shang J., Sandberg M.A., Berson E.L., Li T. (2000) A retinitis pigmentosa GTPase regulator (RPGR)-deficient mouse model for X-linked retinitis pigmentosa (RP3). Proc. Natl Acad. Sci. U.S.A, 97, 3649–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rozet J.M., Perrault I., Gigarel N., Souied E., Ghazi I., Gerber S., Dufier J.L., Munnich A., Kaplan J. (2002) Dominant X linked retinitis pigmentosa is frequently accounted for by truncating mutations in exon ORF15 of the RPGR gene. J. Med. Genet., 39, 284–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bader I., Brandau O., Achatz H., Apfelstedt-Sylla E., Hergersberg M., Lorenz B., Wissinger B., Wittwer B., Rudolph G., Meindl A., Meitinger T. (2003) X-linked retinitis pigmentosa, RPGR mutations in most families with definite X linkage and clustering of mutations in a short sequence stretch of exon ORF15. Invest. Ophthalmol. Vis. Sci., 44, 1458–1463. [DOI] [PubMed] [Google Scholar]
- 60. Wright A.F., Shu X. (2007) Focus on molecules, RPGR. Exp. Eye Res., 85, 1–2. [DOI] [PubMed] [Google Scholar]
- 61. Beltran W.A., Cideciyan A.V., Lewin A.S., Hauswirth W.W., Jacobson S.G., Aguirre G.D. (2014) Gene augmentation for X-linked retinitis pigmentosa caused by mutations in RPGR. Cold Spring Harb. Perspect. Med., 5, a017392.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Deng W.T., Dyka F.M., Dinculescu A., Li J., Zhu P., Chiodo V.A., Boye S.L., Conlon T.J., Erger K., Cossette T., Hauswirth W.W. (2015) Stability and safety of an AAV vector for treating RPGR-ORF15 X-linked retinitis pigmentosa. Hum. Gene Ther., 26, 593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stone E.M. (2007) Macular degeneration. Annu. Rev. Med., 58, 477–490. [DOI] [PubMed] [Google Scholar]
- 64. Hartong D.T., Berson E.L., Dryja T.P. (2006) Retinitis pigmentosa. Lancet, 368, 1795–1809. [DOI] [PubMed] [Google Scholar]
- 65. Bhatti M.T. (2006) Retinitis pigmentosa, pigmentary retinopathies, and neurologic diseases. Curr. Neurol. Neurosci. Rep., 6, 403–413. [DOI] [PubMed] [Google Scholar]
- 66. Cideciyan A.V., Hood D.C., Huang Y., Banin E., Li Z.Y., Stone E.M., Milam A.H., Jacobson S.G. (1998) Disease sequence from mutant rhodopsin allele to rod and cone photoreceptor degeneration in man. Proc. Natl Acad. Sci. U.S.A., 95, 7103–7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jacobson S.G., Cideciyan A.V., Iannaccone A., Weleber R.G., Fishman G.A., Maguire A.M., Affatigato L.M., Bennett J., Pierce E.A., Danciger M., et al. (2000) Disease expression of RP1 mutations causing autosomal dominant retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci., 41, 1898–1908. [PubMed] [Google Scholar]
- 68. Schwartz S.B., Aleman T.S., Cideciyan A.V., Swaroop A., Jacobson S.G., Stone E.M. (2003) De novo mutation in the RP1 gene (Arg677ter) associated with retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci., 44, 3593–3597. [DOI] [PubMed] [Google Scholar]
- 69. Jacobson S.G., Cideciyan A.V., Aleman T.S., Sumaroka A., Roman A.J., Gardner L.M., Prosser H.M., Mishra M., Bech-Hansen N.T., Herrera W., et al. (2008) Usher syndromes due to MYO7A, PCDH15, USH2A or GPR98 mutations share retinal disease mechanism. Hum. Mol. Genet., 17, 2405–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Aleman T.S., Cideciyan A.V., Sumaroka A., Windsor E.A., Herrera W., White D.A., Kaushal S., Naidu A., Roman A.J., Schwartz S.B., et al. (2008) Retinal laminar architecture in human retinitis pigmentosa caused by Rhodopsin gene mutations. Invest. Ophthalmol. Vis. Sci., 49, 1580–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gibbs D., Cideciyan A.V., Jacobson S.G., Williams D.S. (2009) Retinal pigment epithelium defects in humans and mice with mutations in MYO7A, imaging melanosome-specific autofluorescence. Invest. Ophthalmol. Vis. Sci., 50, 4386–4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jacobson S.G., Aleman T.S., Sumaroka A., Cideciyan A.V., Roman A.J., Windsor E.A., Schwartz S.B., Rehm H.L., Kimberling W.J. (2009) Disease boundaries in the retina of patients with Usher syndrome caused by MYO7A gene mutations. Invest. Ophthalmol. Vis. Sci., 50, 1886–1894. [DOI] [PubMed] [Google Scholar]
- 73. Stone E.M., Luo X., Héon E., Lam B.L., Weleber R.G., Halder J.A., Affatigato L.M., Goldberg J.B., Sumaroka A., Schwartz S.B., et al. (2011) Autosomal recessive retinitis pigmentosa caused by mutations in the MAK gene. Invest. Ophthalmol. Vis. Sci., 52, 9665–9673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jacobson S.G., Cideciyan A.V., Gibbs D., Sumaroka A., Roman A.J., Aleman T.S., Schwartz S.B., Olivares M.B., Russell R.C., Steinberg J.D., et al. (2011) Retinal disease course in Usher syndrome 1B due to MYO7A mutations. Invest. Ophthalmol. Vis. Sci., 52, 7924–7936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hood D.C., Lazow M.A., Locke K.G., Greenstein V.C., Birch D.G. (2011) The transition zone between healthy and diseased retina in patients with retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci., 52, 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Birch D.G., Locke K.G., Wen Y., Locke K.I., Hoffman D.R., Hood D.C. (2013) Spectral-domain optical coherence tomography measures of outer segment layer progression in patients with X-linked retinitis pigmentosa. JAMA Ophthalmol., 131, 1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cai C.X., Locke K.G., Ramachandran R., Birch D.G., Hood D.C. (2014) A comparison of progressive loss of the ellipsoid zone (EZ) band in autosomal dominant and X-linked retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci., 55, 7417–7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Birch D.G., Locke K.G., Felius J., Klein M., Wheaton D.K., Hoffman D.R., Hood D.C. (2015) Rates of decline in regions of the visual field defined by frequency-domain optical coherence tomography in patients with RPGR-mediated X-linked retinitis pigmentosa. Ophthalmology, 122, 833–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sujirakul T., Lin M.K., Duong J., Wei Y., Lopez-Pintado S., Tsang S.H. (2015) Multimodal imaging of central retinal disease progression in a 2-year mean follow-up of retinitis pigmentosa. Am. J. Ophthalmol., 160, 786–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Roepman R., Bernoud-Hubac N., Schick D.E., Maugeri A., Berger W., Ropers H.H., Cremers F.P., Ferreira P.A. (2000) The retinitis pigmentosa GTPase regulator (RPGR) interacts with novel transport-like proteins in the outer segments of rod photoreceptors. Hum. Mol. Genet., 9, 2095–2105. [DOI] [PubMed] [Google Scholar]
- 81. Hong D.H., Pawlyk B., Sokolov M., Strissel K.J., Yang J., Tulloch B., Wright A.F., Arshavsky V.Y., Li T. (2003) RPGR isoforms in photoreceptor connecting cilia and the transitional zone of motile cilia. Invest. Ophthalmol. Vis. Sci., 44, 2413–2421. [DOI] [PubMed] [Google Scholar]
- 82. Ferreira P.A. (2005) Insights into X-linked retinitis pigmentosa type 3, allied diseases and underlying pathomechanisms. Hum. Mol. Genet., 14, Spec No. R259–R267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Shu X., Fry A.M., Tulloch B., Manson F.D., Crabb J.W., Khanna H., Faragher A.J., Lennon A., He S., Trojan P., et al. (2005) RPGR ORF15 isoform co-localizes with RPGRIP1 at centrioles and basal bodies and interacts with nucleophosmin. Hum. Mol. Genet., 14, 1183–1197. [DOI] [PubMed] [Google Scholar]
- 84. Khanna H., Hurd T.W., Lillo C., Shu X., Parapuram S.K., He S., Akimoto M., Wright A.F., Margolis B., Williams D.S., et al. (2005) RPGR-ORF15, which is mutated in retinitis pigmentosa, associates with SMC1, SMC3, and microtubule transport proteins. J. Biol. Chem., 280, 33580–33587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mears A.J., Hiriyanna S., Vervoort R., Yashar B., Gieser L., Fahrner S., Daiger S.P., Heckenlively J.R., Sieving P.A., Wright A.F., et al. (2000) Remapping of the RP15 locus for X-linked cone-rod degeneration to Xp11.4-p21.1, and identification of a de novo insertion in the RPGR exon ORF15. Am. J. Hum. Genet., 67, 1000–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Demirci F.Y., Gupta N., Radak A.L., Rigatti B.W., Mah T.S., Milam A.H., Gorin M.B. (2005) Histopathologic study of X-linked cone-rod dystrophy (CORDX1) caused by a mutation in the RPGR exon ORF15. Am. J. Ophthalmol., 139, 386–388. [DOI] [PubMed] [Google Scholar]
- 87. Brunner S., Skosyrski S., Kirschner-Schwabe R., Knobeloch K.P., Neidhardt J., Feil S., Glaus E., Luhmann U.F., Rüther K., Berger W. (2010) Cone versus rod disease in a mutant Rpgr mouse caused by different genetic backgrounds. Invest. Ophthalmol. Vis. Sci., 51, 1106–1115. [DOI] [PubMed] [Google Scholar]
- 88. Appelbaum T., Becker D., Santana E., Aguirre G.D. (2016) Molecular studies of phenotype variation in canine RPGR-XLPRA1. Mol. Vis., 22, 319–331. [PMC free article] [PubMed] [Google Scholar]
- 89. Hjelmeland L.M., Fujikawa A., Oltjen S.L., Smit-McBride Z., Braunschweig D. (2010) Quantification of retinal pigment epithelial phenotypic variation using laser scanning cytometry. Mol. Vis., 16, 1108–1121. [PMC free article] [PubMed] [Google Scholar]
- 90. Munsky B., Neuert G., van Oudenaarden A. (2012) Using gene expression noise to understand gene regulation. Science, 336, 183–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bernstein S.L., Wong P. (1998) Regional expression of disease-related genes in human and monkey retina. Mol. Vis., 4, 24.. [PubMed] [Google Scholar]
- 92. Sharon D., Blackshaw S., Cepko C.L., Dryja T.P. (2002) Profile of the genes expressed in the human peripheral retina, macula, and retinal pigment epithelium determined through serial analysis of gene expression (SAGE). Proc. Natl Acad. Sci. U.S.A, 99, 315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Li M., Jia C., Kazmierkiewicz K.L., Bowman A.S., Tian L., Liu Y., Gupta N.A., Gudiseva H.V., Yee S.S., Kim M., et al. (2014) Comprehensive analysis of gene expression in human retina and supporting tissues. Hum. Mol. Genet., 23, 4001–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Grover S., Fishman G.A., Anderson R.J., Alexander K.R., Derlacki D.J. (1997) Rate of visual field loss in retinitis pigmentosa. Ophthalmology, 104, 460–465. [DOI] [PubMed] [Google Scholar]
- 95. Paskowitz D.M., LaVail M.M., Duncan J.L. (2006) Light and inherited retinal degeneration. Br. J. Ophthalmol., 90, 1060–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hunter J.J., Morgan J.I., Merigan W.H., Sliney D.H., Sparrow J.R., Williams D.R. (2012) The susceptibility of the retina to photochemical damage from visible light. Prog. Retin. Eye Res., 31, 28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Schwartz L., Boëlle P.Y., D'hermies F., Ledanois G., Virmont J. (2003) Blue light dose distribution and retinitis pigmentosa visual field defects, an hypothesis. Med. Hypotheses, 60, 644–649. [DOI] [PubMed] [Google Scholar]
- 98. García-Ayuso D., Ortín-Martínez A., Jiménez-López M., Galindo-Romero C., Cuenca N., Pinilla I., Vidal-Sanz M., Agudo-Barriuso M., Villegas-Pérez M.P. (2013) Changes in the photoreceptor mosaic of P23H-1 rats during retinal degeneration, implications for rod-cone dependent survival. Invest. Ophthalmol. Vis. Sci., 54, 5888–5900. [DOI] [PubMed] [Google Scholar]
- 99. Williams R.W. (1991) The human retina has a cone-enriched rim. Vis Neurosci., 6, 403–406. [DOI] [PubMed] [Google Scholar]
- 100. Mollon J.D., Regan B.C., Bowmaker J.K. (1998) What is the function of the cone-rich rim of the retina?. Eye (Lond), 12, 548–552. [DOI] [PubMed] [Google Scholar]
- 101. Fischer A.J., Reh T.A. (2003) Growth factors induce neurogenesis in the ciliary body. Dev. Biol., 259, 225–240. [DOI] [PubMed] [Google Scholar]
- 102. Mayer E.J., Hughes E.H., Carter D.A., Dick A.D. (2003) Nestin positive cells in adult human retina and in epiretinal membranes. Br. J. Ophthalmol., 87, 1154–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Martínez-Navarrete G.C., Angulo A., Martín-Nieto J., Cuenca N. (2008) Gradual morphogenesis of retinal neurons in the peripheral retinal margin of adult monkeys and humans. J. Comp. Neurol., 511, 557–580. [DOI] [PubMed] [Google Scholar]
- 104. Vugler A.A., Semo M., Joseph A., Jeffery G. (2008) Survival and remodeling of melanopsin cells during retinal dystrophy. Vis. Neurosci., 25, 125–138. [DOI] [PubMed] [Google Scholar]
- 105. Moshiri A., Reh T.A. (2004) Persistent progenitors at the retinal margin of ptc+/- mice. J. Neurosci., 24, 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Beltran W.A., Wen R., Acland G.M., Aguirre G.D. (2007) Intravitreal injection of ciliary neurotrophic factor (CNTF) causes peripheral remodeling and does not prevent photoreceptor loss in canine RPGR mutant retina. Exp. Eye. Res., 84, 753–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Gardiner K.L., Downs L., Berta-Antalics A.I., Santana E., Aguirre G.D., Genini S. (2016) Photoreceptor proliferation and dysregulation of cell cycle genes in early onset inherited retinal degenerations. BMC Genomics, 17, 221.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Li J., Tang J., Feng Y., Xu M., Chen R., Zou X., Sui R., Chang E.Y., Lewis R.A., Zhang V.W., et al. (2016) Improved diagnosis of inherited retinal dystrophies by high-fidelity PCR of ORF15 followed by next-generation sequencing. J. Mol. Diagn., 18, 817–824. [DOI] [PubMed] [Google Scholar]
- 109. Aleman T.S., Cideciyan A.V., Sumaroka A., Schwartz S.B., Roman A.J., Windsor E.A., Steinberg J.D., Branham K., Othman M., Swaroop A., et al. (2007) Inner retinal abnormalities in X-linked retinitis pigmentosa with RPGR mutations. Invest. Ophthalmol. Vis. Sci., 48, 4759–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cideciyan A.V., Swider M., Aleman T.S., Roman M.I., Sumaroka A., Schwartz S.B., Stone E.M., Jacobson S.G. (2007) Reduced-illuminance autofluorescence imaging in ABCA4-associated retinal degenerations. J. Opt. Soc. Am. A., 24, 1457–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Cideciyan A.V., Swider M., Schwartz S.B., Stone E.M., Jacobson S.G. (2015) Predicting progression of ABCA4-associated retinal degenerations based on longitudinal measurements of the leading disease front. Invest. Ophthalmol. Vis. Sci., 56, 5946–5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Jacobson S.G., Voigt W.J., Parel J.M., Apáthy P.P., Nghiem-Phu L., Myers S.W., Patella V.M. (1986) Automated light- and dark-adapted perimetry for evaluating retinitis pigmentosa. Ophthalmology, 93, 604–611. [DOI] [PubMed] [Google Scholar]
- 113. Jacobson S.G., Roman A.J., Cideciyan A.V., Robey M.G., Iwata T., Inana G. (1992) X-linked retinitis pigmentosa, functional phenotype of an RP2 genotype. Invest. Ophthalmol. Vis. Sci., 33, 3481–3492. [PubMed] [Google Scholar]
- 114. Jacobson S.G., Buraczynska M., Milam A.H., Chen C., Järvaläinen M., Fujita R., Wu W., Huang Y., Cideciyan A.V., Swaroop A. (1997) Disease expression in X-linked retinitis pigmentosa caused by a putative null mutation in the RPGR gene. Invest. Ophthalmol. Vis. Sci., 38, 1983–1997. [PubMed] [Google Scholar]
- 115. Jacobson S.G., Cideciyan A.V., Huang W.C., Sumaroka A., Roman A.J., Schwartz S.B., Luo X., Sheplock R., Dauber J.M., Swider M., et al. (2014) TULP1 mutations causing early-onset retinal degeneration, preserved but insensitive macular cones. Invest. Ophthalmol. Vis. Sci., 55, 5354–5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. McGuigan D.B., 3rd, Roman A.J., Cideciyan A.V., Matsui R., Gruzensky M.L., Sheplock R., Jacobson S.G. (2016) Automated light- and dark-adapted perimetry for evaluating retinitis pigmentosa: Filling a need to accommodate multicenter clinical trials. Invest. Ophthalmol. Vis. Sci., 57, 3118–3128. [DOI] [PubMed] [Google Scholar]
- 117. Bland J.M., Altman D.G. (2007) Agreement between methods of measurement with multiple observations per individual. J. Biopharm. Stat., 17, 571–582. [DOI] [PubMed] [Google Scholar]
- 118. Myles P.S., Cui J. (2007) Using the Bland-Altman method to measure agreement with repeated measures. Br. J. Anaesth., 99, 309–311. [DOI] [PubMed] [Google Scholar]
- 119. Carstensen B., Simpson J., Gurrin L.C. (2008) Statistical models for assessing agreement in method comparison studies with replicate measurements. Int. J. Biostat., 4, Article 16. [DOI] [PubMed] [Google Scholar]
- 120. R Core Team (2015). R, A language and environment for statistical computing, v. 3.2.2. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. Accessed June 28, 2016.
- 121. Li L., Rao K.N., Zheng-Le Y., Hurd T.W., Lillo C., Khanna H. (2015) Loss of retinitis pigmentosa 2 (RP2) protein affects cone photoreceptor sensory cilium elongation in mice. Cytoskeleton (Hoboken), 72, 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.