Abstract
Leishmaniasis is a zoonotic infection acquired through the bite of a female sandfly, which introduces the amastigotes of Leishmania into the bloodstream. Cutaneous leishmaniasis is rare after solid organ transplantation. Its diagnosis is difficult in immunosuppressed patients. We report a case of isolated cutaneous leishmaniasis in a renal transplant patient resident in an endemic area. The patient was successfully treated with allopurinol and fluconazole and has remained relapse-free for 44 months. The diagnosis of cutaneous leishmaniasis must be considered in immunosuppressed patients living in endemic areas. Our report shows that cutaneous leishmaniasis may complicate the clinical course of kidney transplant recipients and its presentation can be atypical. Conventional treatment with pentavalent antimonial agents can cause many side effects; of particular concern in renal transplant patients are pancreatitis and nephrotoxicity. These latter may be avoided by using a combination of allopurinol and fluconazole.
Leishmaniasis is a parasitic infection and a zoonotic disease that is highly prevalent in the Mediterranean Basin and in areas such as India, China, East Africa, Southern Europe, and South America.1 Cutaneous leishmaniasis is a common disease in Tunisia, the predominant form being Leishmania major. The disease is acquired through the bite of a female sandfly, which introduces the promastigotes of Leishmania into the bloodstream. These are carried by the circulating macrophages where they reproduce as amastigotes. The infected macrophages rupture, releasing the amastigotes, which then infect other macrophages.2
Leishmaniasis has been described classically in immunocompetent patients, but several cases of leishmaniasis have also been reported in immunosuppressed patients, mainly HIV-infected patients and solid organ transplant recipients.1 These patients have deficient cell-mediated immunity, which increases their susceptibility to this infection.3 The clinical course of the disease in these patients is very different, and some classical clinical or laboratory findings such as splenomegaly or pancytopenia may be absent. The response to therapy is often not satisfactory and relapses are frequent.4 Pentavalent antimonial agents are usually the first-line treatment in visceral and cutaneous leishmaniasis. However, these drugs have many side effects, among which of particular concern are pancreatitis and nephrotoxicity.4 We report the successful treatment of cutaneous leishmaniasis with fluconazole plus allopurinol in a renal transplant patient.
CASE
A 34-year-old female underwent renal transplantation in November 2006 from a living-related donor. She was managed with hemodialysis for 6 months. Her only other medical problem was medically treated hypertension. Her physical examination at transplantation was unremarkable. Her panel-reactive antibody was 0%, and she had no HLA mismatch with her donor. Her kidney transplantation was successful, with a serum creatinine on day 10 of 105 μmol/L. She was on immunosuppressive treatment with tacrolimus, azathioprine, and prednisone. The patient came from Bir Ali, a rural area in the center west of Tunisia that was endemic for Leishmania major. In May of 2007, 8 months after renal transplantation, she developed a single ulcerated, purple, painless nodule measuring 1 cm on the right leg (Figure 1). The diagnoses considered were atypical cutaneous mycobacteriosis, bacillary angiomatosis, and pyogenic abscess. She was treated with oxacillin after a bacterial examination that returned negative later. Ten days later, the lesion became painful, warty, and scabby (Figure 2). Cutaneous Kaposi sarcoma or cutaneous leishmaniasis was suspected. The skin smear showed nonflagellated amastigote forms of Leishmania. Biopsy of the lesion demonstrated marked epithelial hyperplasia with subcutaneous lymphohistiocytic inflammation and intracellular nonflagellated amastigote forms (Figure 3a, b). An extensive clinical workup, including complete blood count, protein electrophoresis, liver enzymes, abdominal ultrasound, echocardiography, and bone marrow aspiration, found no evidence of visceral involvement by the disease; Leishmania serology was also negative.
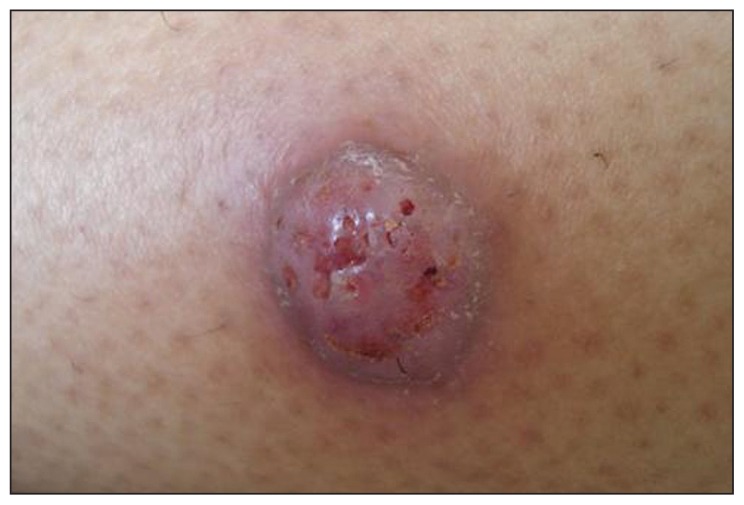
Figure 1.
A 1-cm sized ulcerated purple nodule on the right leg.
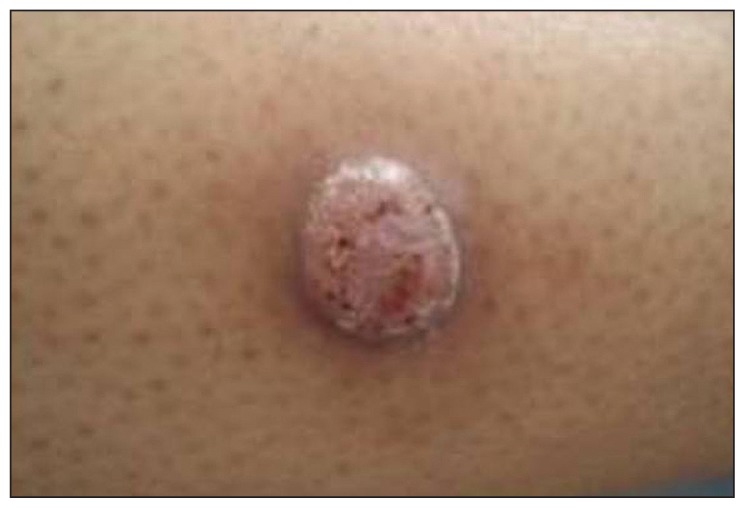
Figure 2.
Ten days after appearance the lesion has become warty and scabby.
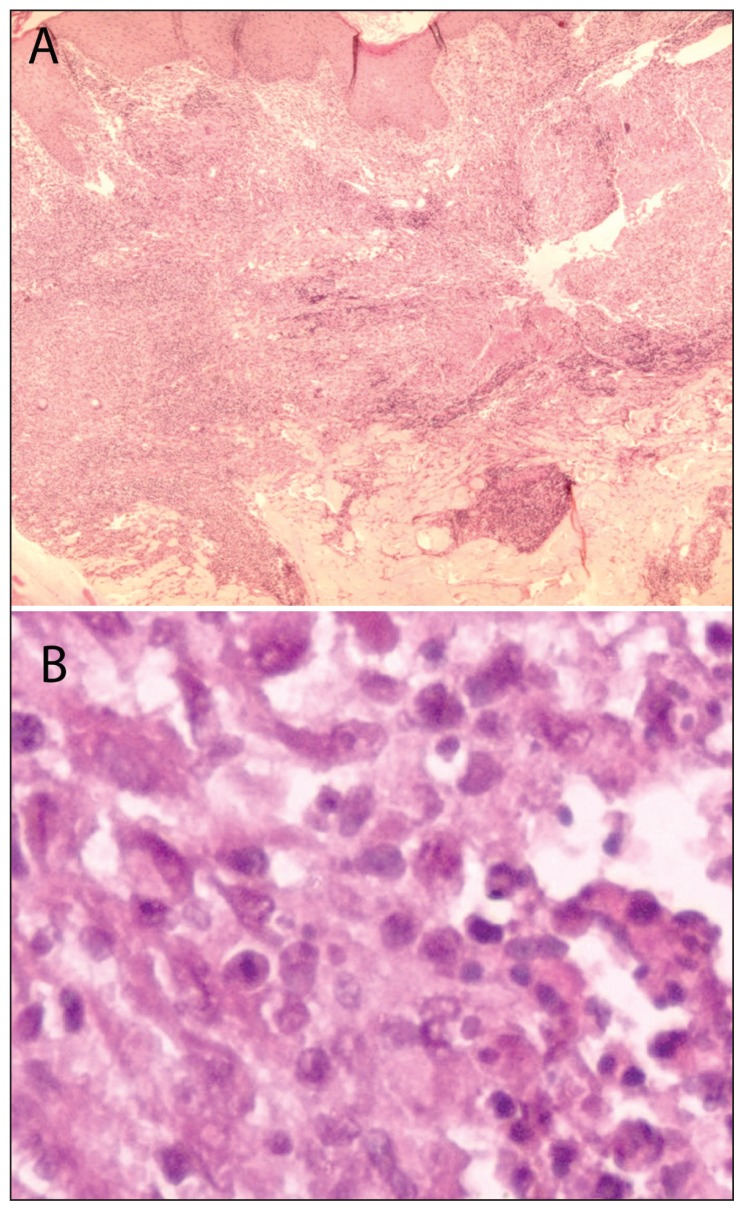
Figure 3.
A) Microscopic examination shows epithelial hyperplasia with subcutaneous lymphohistiocytic inflammation (HE stain). B) Round amastigotes within the cytoplasm of the histiocytes (HE stain; ×100).
The patient was treated with two courses of intradermal meglumine antimoniate (Glucantime, Aventis). The first course of treatment comprised 300 mg of meglumine antimoniate given intradermally once a week for 3 weeks. Two weeks later, she received a second course of meglumine antimoniate; this time the drug was given once a week for 2 weeks. She showed no clinical improvement with this treatment; on the contrary the lesion actually worsened, taking on a pseudotumoral appearance (Figure 4).
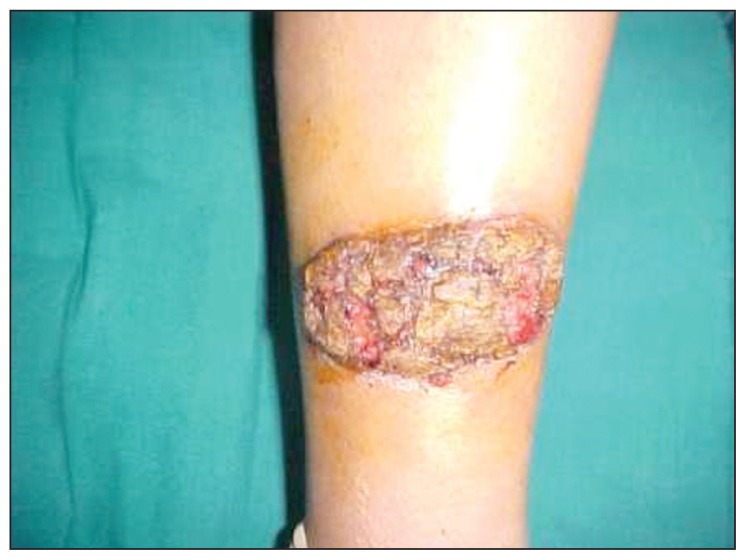
Figure 4.
Worsening of the skin lesion after intradermal meglumine antimoniate injection.
We then started her on allopurinol (300 mg daily) and fluconazole (400 mg daily) for 3 weeks. At the introduction of allopurinol, azathioprine was switched to mycophenolate mofetil (2 g/day for 15 days, followed by 1 g/day). Tacrolimus monitoring was carried out twice a week. There was an increase to 30 ng/mL after 2 weeks of treatment, with a slight increase in the serum creatinine level. Renal function improved after the tacrolimus dose was reduced. Two months after initiating treatment with allopurinol and flucanazole, the skin lesion had completely resolved, leaving only an indelible scar (Figure 5). The patient was on regular follow-up since her discharge from hospital, and there was no relapse even after 44 months; her serum creatinine was stable at 92 μmol/L, and she was pregnant.
Figure 5.
Regression of the lesion, leaving an indelible scar.
DISCUSSION
In the last 2 decades, the prevalence of leishmaniasis has increased significantly in transplant patients because of the increasing frequency of organ transplantation. 1 In immunosuppressed subjects, the presentation of cutaneous leishmaniasis is usually uncommon and may present in various ways.4 Atypical presentations reported in published studies include cases with purple or erythematous papules and violaceous plaques.4,5 Our patient presented with a purple nodule on the right leg and the diagnosis was made only after histopathological examination revealed intracytoplasmic amastigotes of Leishmania. In fact, demonstration of Leishmania in a cutaneous lesion is a diagnostic of cutaneous leishmaniasis in renal transplant recipients.5,6
Leishmaniasis is common in organ transplant recipients and AIDS patients.2 Leishmaniasis usually occurs late after transplantation, with the median delay being about 18 months. However, this delay varies depending on the transplant organ; after liver transplantation, for example, the median delay is 6 months, as opposed to 19 months after kidney transplantation.6,7 Our patient developed her infection relatively early—8 months after transplantation, which may be because she had received antilymphocyte antibodies and corticosteroid pulses and also because of her geographic area of origin. In fact, immunosuppressive treatment alters T-cell activation and proliferation, thereby predisposing recipients to the infection of intracellular microorganisms.6
Eighty-five cases have been reported in published studies.6 The majority of these cases were either isolated visceral or simultaneous cutaneous and visceral leishmaniasis. Sixty-two cases of leishmaniasis have been described in kidney transplantation patients. To our knowledge, only 5 cases of isolated cutaneous leishmaniasis have been reported in transplant patients. In immunosuppressed patients cutaneous leishmaniasis may be misdiagnosed because of its atypical presentation and the delay between the onset of symptoms and diagnosis of the disease. Cutaneous leishmaniasis can be confused with a neoplasia or with a pyogenic abcess. 6 Demonstration of Leishmania amastigotes in the skin lesion can help clinch the diagnosis. Leishmania PCR is another means for establishing the diagnosis with high sensitivity, as has been demonstrated in both immunocompetent and HIV-infected patients with visceral leishmaniasis. Furthermore, PCR is a noninvasive tool for the diagnosis of the disease, a useful surrogate marker of disease activity and the response to specific therapy, and can be used for monitoring of disease recurrence.6
In immunocompetent patients, cutaneous leishmaniasis is usually treated by intralesional injection of antimonial drugs. The parenteral route is recommended if there is no improvement with intralesional therapy. However, the toxicity of the standard antileishmanial drugs for transplant organs poses therapeutic problems. In fact, antimonial drugs have established toxic effects on the heart, liver, pancreas, and kidney.8 Moreover, these drugs are mainly excreted by the kidney, and dose adjustments are recommended when the glomerular filtration rate is less than 15 mL/min.6,9 In addition, primary failures as well as relapses are more frequent in immunocompromised patients treated with antimonial drugs. Rescue therapy with the liposomal form of amphotericin B has been reported to be useful for the treatment of visceral leishmaniasis. 6,7,10,11 Liposomal amphotericin B can also be prescribed in cutaneous leishmaniasis. This drug, despite its nephrotoxicity and other adverse effects (fever, hypokalemia, anemia, renal tubular damage, hepatitis), seems to have lower toxicity than pentavalent antimonials. 6 Ketoconazole, both as monotherapy as well as in combination with allopurinol, has been used successfully in some cases of visceral leishmaniasis, as has itraconazole.9,12,13
Alrajhi et al conducted a randomized, double-blind, placebo-controlled trial to assess the efficacy of 6 weeks of oral fluconazole in a dose of 200 mg daily for the treatment of cutaneous leishmaniasis. In their study, 106 patients were assigned to receive fluconazole and 103 patients were assigned to receive placebo. The rates of healing were, respectively, 59% and 22%, with the time for healing being shorter in the fluconazole group (median 8.5 weeks vs 11.2 weeks in the placebo group; P<.001).14 Torrüs et al reported 3 cases of visceral leishmaniasis (1 renal transplant patient and 2 AIDS patients) successfully treated with fluconazole plus allopurinol. 15 Similarly, Harzallah et al described a case of visceral leishmaniasis that was successfully treated with allopurinol. The patient was initially treated with pentavalent antimonial drugs but that had to be discontinued because of side effects (pancreatitis and cytolysis).16
The inhibition of xanthine oxidase by allopurinol induces either an accumulation of oxypurines or a deficit of other metabolites that is eventually lethal for the parasite.9,12 Velez et al reported a controlled study in which allopurinol was ineffective as monotherapy against leishmaniasis.17 It seems that its combination with other drugs such as imidazoles may exhibit synergistic effects. The imidazoles are able to inhibit sterol synthesis, disrupting parasitic membranes.17 In the case that we have reported, the combination of allopurinol and fluconazole was successful in treating cutaneous leishmaniasis. Discontinuation of azathioprine and changing over to mycophenolate mofetil should be considered when introducing xanthine oxidase inhibitors so as to avoid pancytopenia.
The diagnosis of cutaneous leishmaniasis should be considered in transplant recipients in endemic areas. The treatment must be adequate to avoid relapses and to preserve organ function.11,18 The combination of allopurinol and fluconazole is a treatment option with relatively fewer side effects and affordable cost. In our patient, no relapse has occurred even 44 months after the treatment with allopurinol and fluconazole. In conclusion, leishmaniasis is a rare infection, but it can occur in immunosuppressed patients living in endemic areas and must be kept in mind. The demonstration of Leishmania in a cutaneous lesion can establish the diagnosis. Pentavalent antimonial drugs, classically used for treating leishmaniasis, have many adverse effects. An alternative treatment with allopurinol and fluconazole may be effective and safe in renal transplant patients with a functioning allograft.
REFERENCES
- 1.Basset D, Faraut F, Marty P, Dereure J, Rosenthal E, Mary C, et al. Visceral leishmaniasis in organ transplant recipients: 11 new cases and a review of the literature. Microbes Infect. 2005;7:1370–5. doi: 10.1016/j.micinf.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Barsoum RS. Parasitic infections in organ transplantation. Exp Clin Transplant. 2004;2:258–63. [PubMed] [Google Scholar]
- 3.Berenguer J, Gómez-Campderá F, Padilla B, Rodríguez-Ferrero M, Anaya F, Moreno S, et al. Visceral leishmaniasis (Kala-Azar) in transplant recipients: Case report and review. Transplantation. 1998;65:1401. doi: 10.1097/00007890-199805270-00022. [DOI] [PubMed] [Google Scholar]
- 4.Sabbatini M, Pisani A, Ragosta A, Gallo R, Borrelli F, Cianciaruso B. Visceral leishmaniasis in renal transplant recipients: Is it a challenge to the nephrologists? Transplantation. 2002;73:299–301. doi: 10.1097/00007890-200201270-00026. [DOI] [PubMed] [Google Scholar]
- 5.Vinhal FA, Afonso-Cardoso SR, Silva AG, Pereira CG, Sousa W, Botelho SM, et al. Atypical presentation of cutaneous leishmaniasis after renal transplantation. Nephrol Dial Transpl. 2007;22:3674. doi: 10.1093/ndt/gfm520. [DOI] [PubMed] [Google Scholar]
- 6.Antinori S, Cascio A, Parravicini C, Bianchi R, Corbellino M. Leishmaniasis among organ transplant recipients. Lancet Infect Dis. 2008;8:191–9. doi: 10.1016/S1473-3099(08)70043-4. [DOI] [PubMed] [Google Scholar]
- 7.Veroux M, Corona D, Giuffrida G, Cacopardo B, Sinagra N, Tallarita T, et al. Visceral leishmaniasis in the early post transplant period after kidney transplantation: Clinical features and therapeutic management. Transpl Infect Dis. 2010;12:387–91. doi: 10.1111/j.1399-3062.2010.00520.x. [DOI] [PubMed] [Google Scholar]
- 8.Oliveira CM, Oliveira ML, Andrade SC, Girão ES, Ponte CN, Mota MU, et al. Visceral leishmaniasis in renal transplant recipients: Clinical aspects, diagnostic problems, and response to treatment. Transpl Proc. 2008;40:755–60. doi: 10.1016/j.transproceed.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 9.Hueso M, Bover J, Serón D, Gil-Vernet S, Rufí G, Alsina J, et al. The renal transplant patient with visceral leishmaniasis who could not tolerate meglumine antimoniate cure with ketoconazole and allopurinol. Nephrol Dial Transplant. 1999;14:2941–3. doi: 10.1093/ndt/14.12.2941. [DOI] [PubMed] [Google Scholar]
- 10.Bern C, Adler-Moore J, Berenguer J, Boelaert M, den Boer M, Davidson RN, et al. Liposomal amphotericin B for the treatment of visceral leishmaniasis. Clin Infect Dis. 2006;43:917–24. doi: 10.1086/507530. [DOI] [PubMed] [Google Scholar]
- 11.Campos-Varela I, Len O, Castells L, Tallada N, Ribera E, Dopazo C, et al. Visceral leishmaniasis among liver transplant recipients: An overview. Liver Transpl. 2008;14:1816–9. doi: 10.1002/lt.21538. [DOI] [PubMed] [Google Scholar]
- 12.Halim MA, Alfurayh O, Kalim ME, Dammas S, Al-eisa A, Damanhouri G. Successful treatment of visceral leishmaniasis with allopurinol plus ketokonazole in a renal transplant recipient after occurrence of pancreatitis due to stiboglyconate. Clin Infect Dis. 1993;16:397–9. doi: 10.1093/clind/16.3.397. [DOI] [PubMed] [Google Scholar]
- 13.Lafeuillade A, Chaffanjon P, Delbeke E, Quilichini R. Maintenance itraconazole for visceral leishmaniasis in HIV infection. Am J Med. 1992;92:449. doi: 10.1016/0002-9343(92)90281-f. [DOI] [PubMed] [Google Scholar]
- 14.Alrajhi AA, Ibrahim EA, De Vol EB, Khairat M, Faris RM, Maguire JH. Fluconazole for the treatment of cutaneous leishmaniasis caused by Leishmania major. N Engl J Med. 2002;346:891–5. doi: 10.1056/NEJMoa011882. [DOI] [PubMed] [Google Scholar]
- 15.Torrüs D, Boix V, Massa B, Portilla J, Pérez-Mateo M. Fluconazole plus allopurinol in treatment of visceral leishmaniasis. J Antimicrob Chemother. 1996;37:1042–3. doi: 10.1093/jac/37.5.1042. [DOI] [PubMed] [Google Scholar]
- 16.Harzallah K, Belhadj R, Jemli B, Haloues M, Berraies N, Gargouri S, et al. Visceral leishmaniasis in a renal transplant recipient treated with allopurinol. Saudi J Kidney Dis Transpl. 2010;21:105–8. [PubMed] [Google Scholar]
- 17.Velez I, Agudelo S, Hendrickx E, Puerta J, Grogl M, Modabber F, et al. Inefficacy of allopurinol as monotherapy for Colombian cutaneous leishmaniasis. A randomised controlled trial. Ann Intern Med. 1997;126:232–6. doi: 10.7326/0003-4819-126-3-199702010-00010. [DOI] [PubMed] [Google Scholar]
- 18.Aardema H, Sijpkens YW, Visser LG. Pancytopenia in a simultaneous pancreas and kidney transplant recipient: An unexpected cause. Clin Nephrol. 2009;71:460–2. doi: 10.5414/cnp71460. [DOI] [PubMed] [Google Scholar]