Abstract
Background and aim:
Several studies have identified that obesity and being overweight can increase the risk of developing myocardial infarction (MI). However, the predictive value of the central obesity index, that is, the waist-hip ratio (WHR), regarding MI risk remains unclear. This study aimed to provide a systematic review and meta-analysis of WHR as a predictor of MI incidence.
Methods:
This study used relevant keywords and MeSH terms to identify studies of MI risk and WHR from PubMed, Web of Science, Embase, and Cochrane databases in November 2017.
Results:
We conducted a meta-analysis of 12 case-control studies in 14 eligible trials and further explored whether the predictive value of WHR on MI risk varies according to sex. The results showed that a high WHR increased MI risk (pooled odds ratio [OR] 2.62, 95% confidence interval [CI] 2.02–3.39, P < 0.00001) and that elevated WHR is more strongly predictive of MI in women than in men (pooled OR 4.63, 95% CI 3.28–6.53 in women; pooled OR 2.71, 95% CI 2.15–3.41 in men).
Conclusions:
MI is significantly associated with increased WHR, with a stronger association among women.
Keywords: meta-analysis, myocardial infarction, obesity, systematic review, waist-hip ratio
1. Introduction
Overweight and obesity have been recognized as a major risk factor for coronary heart disease (CHD) with increasing prevalence.[1–3] Experts have predicted that the current growth rate of obesity (an estimated 7% increase in men and 10% increase in women by 2020) will lead to an increase in the number of CHD events by 14% in 2035.[4] Among the various types of CHD, myocardial infarction (MI) is associated with a high incidence rate, acute onset, and increased lethality, thereby posing a serious threat to the life of the patient.
Body mass index (BMI) can serve as a marker indicating the general obesity, and the relationship between MI and BMI has been intensively investigated, but there are still certain limitations requiring our concern.[5,6] In relation with BMI, there seem to be closer correlations of the anthropometric measures of abdominal obesity (such as the waist circumference [WC], waist-to-height ratio [WHtR], waist-hip ratio (WHR), and the sagittal abdominal diameter) with the metabolic risk factors, MI events, and death events.[7] Moreover, excessive fat mass in the body, rather than excessive body weight, accounts for the leading cause of the increased risk of MI among the obese population.[8] Consequently, some studies indicate that central obesity index, WC, WHtR, and WHR are the risk factors for predicting MI, which can also overcome the limitations of BMI.[9,10]
Among them, WHR may serve as a preferred risk factor for predicting MI, which can be attributed to its inclusion of measuring the hip circumference. Typically, WHR is negatively correlated with the cardiometabolic risk and MI.[11] Increase in hip circumstance will lead to increases in subcutaneous fat mass in hip, gluteal muscle, and total muscle mass in leg. Specifically, the muscle mass in leg, which can serve as an indirect measurement of physical activity, is negatively correlated with the cardiometabolic risk. Besides, it is also suggested in the INTERHEART study[12] that, among the extensively applied anthropometric measures, WHR is the most closely correlated with the risk of MI globally.
Therefore, the current meta-analysis was conducted to systemically review all related literature enrolling MI patients with available WHR measurements, so as to examine the correlation of WHR with MI and to explore whether the association intensity varied between different sexes.
2. Materials and methods
2.1. Study design
This review and meta-analysis was conducted according to the general guidelines recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.[13]
2.2. Information sources
In November 2017, we systematically searched the PubMed, EMBASE, Cochrane, and Web of Science databases for articles that reported the association between WHR and MI risk.
2.3. Search
Keywords and medical subject headings (MeSH) were used for specific searches. In each database, the MeSH terms “myocardial infarction” or “percutaneous coronary intervention” or “coronary artery bypass surgery” were combined with the MeSH term “waist-hip ratio.” The keyword terms corresponding to each of these MeSH terms were also mapped in a similar manner. To obtain relatively complete data, the search strategy had no language, study type, or publication date restrictions. In addition, the reference sections of relevant reviews or systematic reviews were also searched.
2.4. Study eligibility criteria
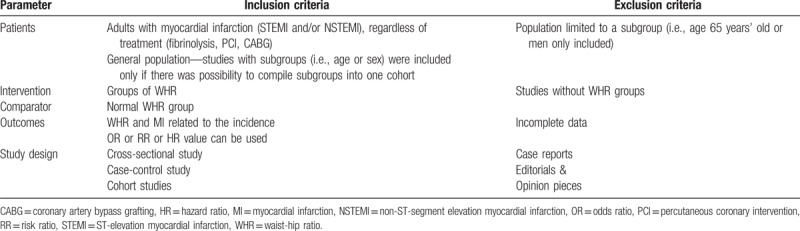
The study inclusion and exclusion criteria are presented in Table 1. Only studies that met the eligibility criteria were included in the analysis.
Table 1.
PICOS criteria for inclusion and exclusion of studies into quantitative (meta-analysis) analyses.
2.5. Study selection
Independent dual-selection of the eligible articles was conducted by the first 2 investigators (C.Q.Q. and Y.S.). Any discrepancies between reviewers in the determination of study eligibility were resolved by consensus and consultation with the third author if necessary.
2.6. Data abstraction
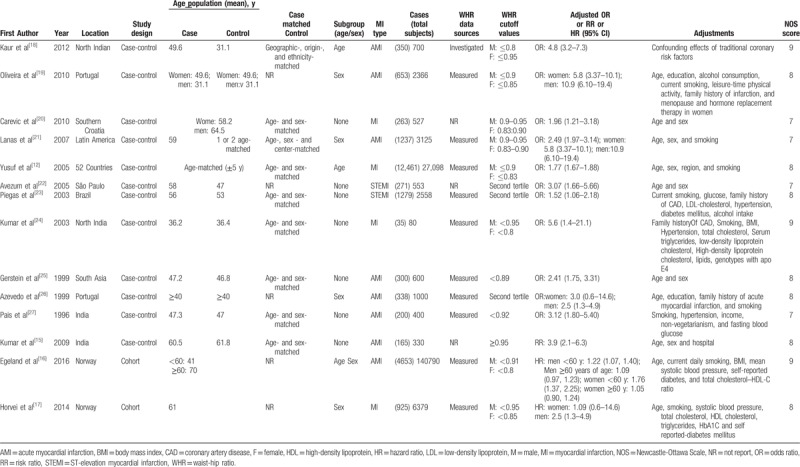
Data extraction for the included studies was independently performed by the first 2 authors, and a third researcher was consulted for input in the event of uncertainties. All sources of data were derived from the original literature, and data on the basic characteristics of the eligible articles were collected as follows: first author, year of publication, location, study design, age population, case matched control, subgroup (age/sex), MI type, MI cases (total subjects), WHR sources, WHR cutoff values, adjusted odd ratios (ORs), relative risks (RRs), or hazard ratios (HRs) (95% confidence interval [CI]), adjustments, and Newcastle-Ottawa Scale (NOS) scores.
2.7. Study appraisal
The modified NOS was used to appraise the quality of the included studies.[14] Study quality was evaluated independently by 2 researchers. Any disagreements were resolved by discussion with the third researcher.
2.8. Statistical analysis
2.8.1. Synthesis of results
The meta-analysis was assessed using Review Manager 5.3 software from the Cochrane Collaboration (London, United Kingdom). Random-effects models with the generic inverse variance method were used to calculate pooled odds risks (ORs). All analyses were calculated at the 95% CI level. Statistical heterogeneity was assessed using Cochran Q statistics and quantified using I2 statistics. The I2 statistic denotes the percentage of total variation attributable to between-study heterogeneity rather than chance. The presence of publication bias was determined by examining the funnel plot symmetry and whether the included studies fell within the boundaries. Sensitivity analysis was performed by deleting individual studies while observing any changes in the I2 statistic.
2.8.2. Summary measures
Summary effect measures were reported as ORs and 95% CIs in this study. The RR for 1 included case-control study[15] was also reported as the OR. As 2 cohort studies[16,17] used survival analysis as opposed to logistic regression to determine the effects of the HR, they were not included in the final meta-analysis.
The information presented in this study was collected from the previous research, so there was no need for approval by an ethical review board or informed consent.
3. Results
3.1. Literature search
A total of 1723 potentially relevant articles (325 articles from PubMed, 304 articles from EMBASE, 37 articles from Cochrane, and 1057 articles from Web of Science) were obtained after the published data search. First, using EndNote X6, we deleted 377 duplicate records, filtered out 1004 unrelated studies, and deleted 214 other duplicate records by reading the title and abstract. After browsing the remaining 128 studies, 61 were deleted. Then, 67 review articles/commentaries were screened, of which 45 studies identified as eligible for further full-text (or abstracts as necessary) review. Finally, after reading the literature, reviews, and references, a total of 14 studies,[12,15–27] including 12 case-control studies,[12,15,18–27] met the inclusion criteria and were included in the meta-analysis. Specific details as to the screening and article deletion processes are shown in Figure 1.
Figure 1.
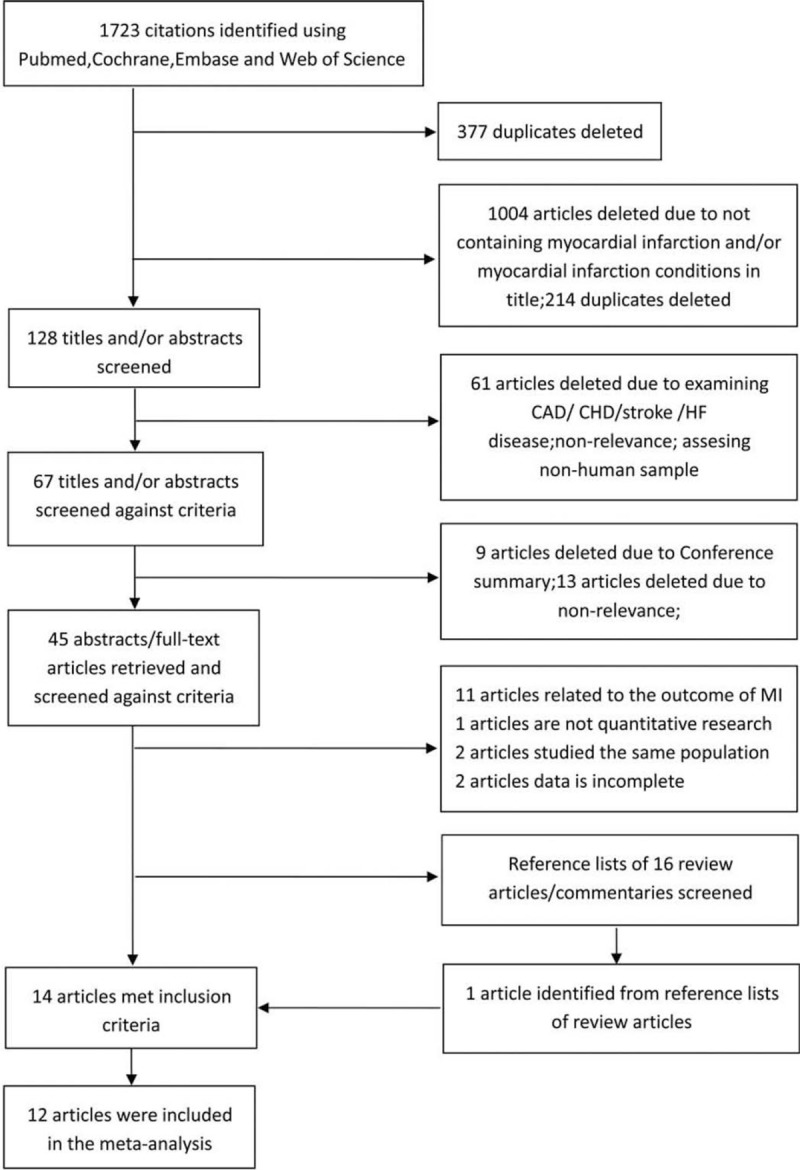
Flow diagram of the study (according to PRISMA statement). CAD = coronary artery disease, CHD = coronary heart disease, HF = heart failure.
3.2. Study characteristics and quality
For the included studies, 12 studies[12,15,18–27] were case-controls and 2[16,17] were cohort studies. These articles enrolled 23,163 patients with MI between 1996 and 2016. The majority of included studies were conducted in Europe and Africa,[15–20,24,26,27] whereas 2 studies were conducted in South America,[22,23] 1 study was conducted in Latin America,[21] 1 study was conducted in South Asia,[25] and a large sample study was carried out in 52 countries.[12]
For the quality assessment using the Newcastle-Ottawa Scale criteria, all of the included studies scored ≥7 of 9, and 10 studies[12,15–19,23–26] were high-quality with scores of 8 or 9. Of the 12 case-control studies[12,15,18–27] that were included, there were 5 control group studies[12,20–23] with population biases, as they included hospitalizations or lacked descriptions. The exposure factor for this meta-analysis was high WHR. Among the total studies, WHR was measured in 10,[12,16,17,19,21,23–27] 1 study[18] used recorded WHR data and WHR was not specifically described in the other 3.[15,20,22] The characteristics and quality assessment information for all included studies are provided in Table 2.
Table 2.
Characteristics of the included Studies.
Among the 12 case-control studies,[12,15,18–27] 3[19,21,26] investigated whether there were sex differences in the prediction of MI by WHR measurement. Based on the OR values yielded by these findings, we performed a meta-analysis to evaluate the association between sex and the predictive value of WHR on MI. Three studies[22,23,26] used tertiles for the cutoff values of WHR rather than categorizing the WHR as normal or abnormal; therefore, to ensure the consistency of the research, we adopted the second tertile versus the first tertile as a reference. Finally, we conducted a meta-analysis of 10 articles[12,15,18,20–25,27]with a general population along with a meta-analysis of three articles[19,21,26] that provided separate OR values for men and women.
3.3. Association between WHR and MI
3.3.1. General population
Our findings from the meta-analyses support previous systematic and narrative reviews.[28,29] The pooled OR for WHR as a predictor of MI was 2.62 (95% CI 2.02–3.39, P < .00001) with high and significant heterogeneity (I2 = 95%) (Fig. 2). Of the 10 studies included in the meta-analysis, 7 of the studies reported ORs that adjusted for age and sex. This further increases the credibility of the results of this analysis.
Figure 2.
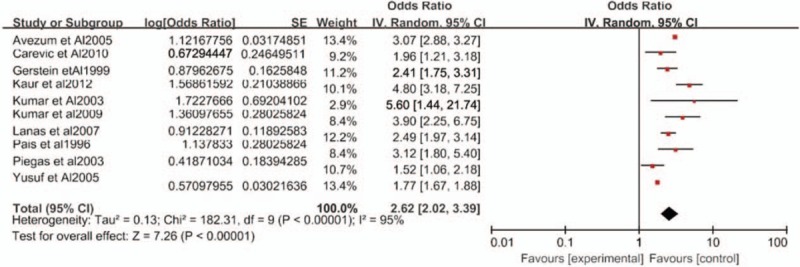
Forest plot of the odds ratio for waist-hip ratio as a predictor of myocardial infarction risk. CI = confidence interval, SE = standard error.
To minimize between-study heterogeneity, we conducted a sensitivity analysis. In the influence study, correlations were statistically affected with significance when 1 study was excluded at a time, and we found that Yusuf et al's study[12] had a higher heterogeneity. After both Yusuf et al's[12] and Piegas et al's studies[23] were eliminated, the heterogeneity between studies was significantly reduced (pooled OR, 2.95, 95% CI 2.49–3.49, I2 = 51%) (Fig. 3).
Figure 3.
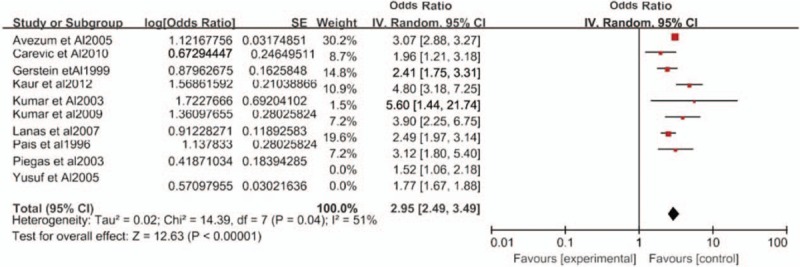
Sensitivity analysis of the odds ratio for waist-hip ratio as a predictor of myocardial infarction risk. CI = confidence interval, SE = standard error.
Additionally, our funnel plots for the meta-analysis were symmetrical above the average value, suggesting no publication bias.
3.3.2. Men and women
As previously mentioned, 3 case-control studies[19,21,26] provided data stratified by sex. The ORs for both sexes were computed, and the results showed that high WHR increased the MI risk in each sex (pooled OR 4.63, 95% CI 3.28–6.53 in women; pooled OR 2.71, 95% CI 2.15–3.41 in men) (Fig. 4). However, as seen in Figure 4, there was no heterogeneity in the female group, but high heterogeneity in the male group. Owing to the small number of included studies, we did not conduct further sensitivity analyses.
Figure 4.
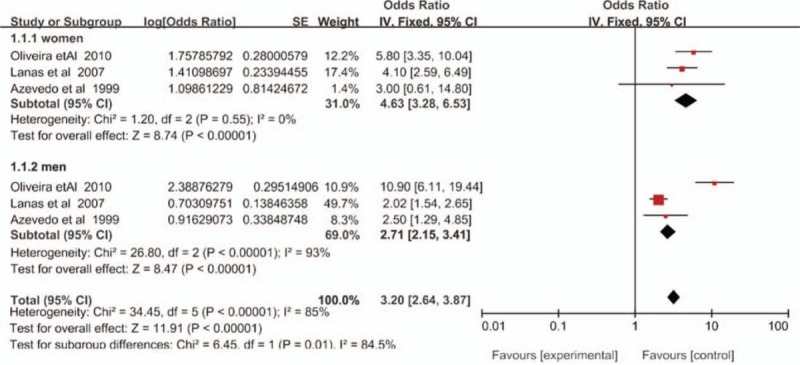
Forest plot of the odds ratio for waist-hip ratio as a predictor of myocardial infarction risk, stratified by sex. CI = confidence interval, SE = standard error.
4. Discussion
This systematic review and meta-analysis appraises and summarizes the currently available evidence over the past 20 years concerning the predictive value of WHR on MI. The present study is the first to investigate WHR and MI using a meta-analysis, and the current data add to the available evidence that WHR is a promising factor in the prediction of MI risk, with a strong predictive ability. This result is similar to the findings in patients with other cardiovascular diseases,[30] suggesting that the consideration of WHR may be critical in identifying at-risk individuals and for population projections of the burden of disease for young to middle-aged adults.
To explore whether MI risk differed between men and women, we stratified our analyses by sex. We found a sex difference in the moderately adjusted subgroup analysis, according to which elevated WHR is a stronger predictor of MI in women than in men. This is consistent with Wiklund et al's[31] study in which WHR and WC were found to be predictors of CVD according to sex differences. This may be related to the reduced psychological health and lower levels of physical activity among women compared to men.
Two larger cohort studies that were not included in the meta-analysis concluded that an increased WHR was a predictor of MI. To verify the rationality of deleting the 2 studies, we included 2 studies in the meta-analysis and found that the heterogeneity of the study increased significantly. Egeland et al[16] observed 140,790 subjects for 9 years and found that WHR was a significant predictor of MI among men and women without an enlarged WC (<102 cm for men and <88 cm for women) in adjusted analyses. The study also indicated that WHR far more accurately identifies individuals at risk for MI compared to conventional risk factors, BMI, or an enlarged WC in middle-aged adults. During an average of 15.7 years of follow-up of 6379 subjects, Horvei et al[17] found that WHR and WHtR, in comparison to WC and hip circumference, yielded the highest risk estimates for MI. The results have implications for improving the identification of high-risk individuals and for the prediction of the burden of obesity in populations.
Central obesity, which refers to a high WHR, likely contributes to MI via multiple pathways involving oxidative stress and inflammation, steroid hormones, free fatty acids, and altered production and function of adipocyte-derived hormones.[32,33] Recent cardiac metabolism imaging studies conducted in large cohort studies (the Framingham Heart Study[34] and Jackson Heart Study[35]) have shown that visceral fat hyperplasia can exceed its storage capacity and become oversaturated, leading to a spillover of lipids that are then stored in normally lean tissues such as the heart, liver, and intrathoracic fat, contributing significantly to cardiac and metabolic abnormalities. In addition, adipose tissue fat cells are involved in the promotion of atherosclerotic regulation processes, and excessive visceral fat is associated with insulin resistance, hypertriglyceridemia, highly atherogenic small LDL particles, and low HDL levels, all proatherogenic factors.[36] Subsequently, endothelial vasomotor dysfunction, a hypercoagulable state, and dyslipidemia are triggered, eventually leading to MI.[37]
In light of these results, first, the ability of WHR to predict MI risk is evident, and healthcare professionals should consider the pivotal role of WHR in identifying populations at higher risk of MI, especially in women. Second, further work is needed to discern the best practice guidelines for capturing the various dimensions of WHR that contribute to MI risk. Third, the potential risk of high WHR should be included in the health education of patients so that patients understand that a normal body weight does not preclude the presence of abdominal obesity. Fourth, this study presents a new challenge to medical rehabilitation professionals involved in monitoring the physical activity of obese patients in that they should pay more attention to the patient's WHR and not just the BMI. Finally, as a central obesity index, WHR is more clinically relevant than BMI and merits increased attention, especially in terms of the acute onset of disease, to reduce the risk of morbidity.
The heterogeneity of the studies must be addressed because it may affect the justification for pooling the data into one analysis. In the present meta-analysis, statistical heterogeneity may have been caused by clinical heterogeneity, such as differences in the study population and different WHR cutoff points, as well as by different study quality characteristics. Among them, the high heterogeneity of Yusuf et al's research[12] may be attributed to the fact that it was a study with a large sample size involving 52 countries. Although the major influencing factors such as age, sex, and geographical location were adjusted for, there are still some differences in methodological quality compared with the other studies. However, the statistical tests of heterogeneity were within the acceptable range for the pooling of studies.
Our study has several strengths. First, the studies included in the meta-analysis are all case-control trials to minimize the potential deviations associated with research design. Second, the number of patients evaluated in the included studies was high with some very large samples such as those of the INTERHEART[12] and CONO[16] studies. Finally, most of the WHR data were measured, not reported, making the findings more objective and relevant.
Potential limitations should also be noted with our review. Although the process of a systematic literature review and meta-analysis is a robust way of generating a more powerful estimate of true effect size with less random error than individual studies, it does come with limitations. For example, the articles included in this study were case-control trials, and we lacked cohort studies with high levels of evidence. Clearly, more cohort studies should be conducted in the future to enhance the level of evidence of the findings. Another limitation was the use of different cutoff values for WHR between studies, thereby preventing the identification of a single threshold for WHR that is potentially predictive of MI risk. Finally, there are discrepancies between the various studies in terms of the measurement of WHR, which should be objectively measured using standard techniques in future studies.
5. Conclusions
Based on our pooled results, WHR can be deemed an excellent predictor of MI risk, as there is a significantly increased risk of MI among patients with a high WHR. The predictive effect of WHR on the risk of MI is even more significant in women than in men. Thus, the measurement of WHR may have clinical utility in MI risk assessments, particularly for those patients with elevated WHRs.
Author contributions
Conceptualization: Qinqin Cao.
Data curation: Qinqin Cao, Wenji Xiong, Jinwei Li.
Formal analysis: Qinqin Cao, Yuewei Li.
Investigation: Qinqin Cao.
Methodology: Qinqin Cao.
Software: Huimin Li.
Supervision: Shui Yu.
Writing – original draft: Qinqin Cao, Feng Li.
Writing – review & editing: Qinqin Cao, Feng Li.
Footnotes
Abbreviations: BMI = body mass index, CHD = coronary heart disease, CI = Confidence Interval, HRs = hazard ratios, MI = myocardial infarction, NOS = Newcastle-Ottawa Scale, ORs = odd ratios, RRs = relative risks, WC = waist circumference, WHR = waist-hip ratio, WHtR = waist-to-height ratio.
The author(s) report no conflicts of interest.
References
- [1].Ndumele CE, Matsushita K, Lazo M, et al. Obesity and subtypes of incident cardiovascular disease. J Am Heart Assoc 2016;5:334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kang MY, Hong YC. Inter-correlation between working hours, sleep duration, obesity, and 10-year risk for CHD. Am J Ind Med 2016;59:338–9. [DOI] [PubMed] [Google Scholar]
- [3].Kwagyan J, Retta TM, Ketete M, et al. Obesity and cardiovascular diseases in a high-risk population: evidence-based approach to CHD risk reduction. Ethn Dis 2015;25:208–13. [PMC free article] [PubMed] [Google Scholar]
- [4].Bibbins-Domingo K, Coxson P, Pletcher MJ, et al. Adolescent overweight and future adult coronary heart disease. N Engl J Med 2007;357:2371–9. [DOI] [PubMed] [Google Scholar]
- [5].O’Brien EC, Fosbol EL, Peng SA, et al. Association of body mass index and long-term outcomes in older patients with non-ST-segment-elevation myocardial infarction: results from the CRUSADE Registry. Circ Cardiovasc Qual Outcomes 2014;7:102–9. [DOI] [PubMed] [Google Scholar]
- [6].Thomsen M, Nordestgaard BG. Myocardial infarction and ischemic heart disease in overweight and obesity with and without metabolic syndrome. JAMA Intern Med 2014;174:15–22. [DOI] [PubMed] [Google Scholar]
- [7].Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med 2008;359:2105–20. [DOI] [PubMed] [Google Scholar]
- [8].Wormser D, Kaptoge S, Di Angelantonio E, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58prospective studies. Lancet 2011;377:1085–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ashwell M, Cole TJ, Dixon AK. Ratio of waist circumference to height is strong predictor of intra-abdominal fat. BMJ (Clinical research ed) 1996;313:559–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].de Koning L, Merchant AT, Pogue J, et al. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J 2007;28:850–6. [DOI] [PubMed] [Google Scholar]
- [11].Myint PK, Kwok CS, Luben RN, et al. Body fat percentage, body mass index and waist-to-hip ratio as predictors of mortality and cardiovascular disease. Heart 2014;100:1613–9. [DOI] [PubMed] [Google Scholar]
- [12].Yusuf S, Hawken S, Ounpuu S, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet 2005;366:1640–9. [DOI] [PubMed] [Google Scholar]
- [13].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- [14].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [15].Kumar A, Nagtilak S, Sivakanesan R, et al. Cardiovascular risk factors in elderly normolipidemic acute myocardial infarct patients—a case controlled study from India. Southeast Asian J Trop Med Public Health 2009;40:581–92. [PubMed] [Google Scholar]
- [16].Egeland GM, Igland J, Vollset SE, et al. High population attributable fractions of myocardial infarction associated with waist-hip ratio. Obesity 2016;24:1162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Horvei LD, Brækkan SK, Mathiesen EB, et al. Obesity measures and risk of venous thromboembolism and myocardial infarction. Eur J Epidemiol 2014;29:821–30. [DOI] [PubMed] [Google Scholar]
- [18].Kaur R, Das R, Ahluwalia J, et al. Synergistic effect of angiotensin II type-1 receptor 1166A/C with angiotensin-converting enzyme polymorphism on risk of acute myocardial infarction in north Indians. J Renin Angiotensin Aldosterone Syst 2012;13:440–5. [DOI] [PubMed] [Google Scholar]
- [19].Oliveira A, Rodriguez-Artalejo F, Severo M, et al. Indices of central and peripheral body fat: association with non-fatal acute myocardial infarction. Int J Obes 2010;34:733–41. [DOI] [PubMed] [Google Scholar]
- [20].Carevic V, Kuzmanic M, Rumboldt M, et al. Predictive impact of coronary risk factors in southern Croatia: a case control study. Coll Antropol 2010;34:1363–8. [PubMed] [Google Scholar]
- [21].Lanas F, Avezum A, Bautista LE, et al. Risk factors for acute myocardial infarction in Latin America—The INTERHEART Latin American study. Circulation 2007;115:1067–74. [DOI] [PubMed] [Google Scholar]
- [22].Avezum Á, Piegas LS, Pereira JCR. Risk factors associated with acute myocardial infarction in the São Paulo Metropolitan Region. A developed region in a developing country. Arq Bras Cardiol 2005;84:206–13. [DOI] [PubMed] [Google Scholar]
- [23].Piegas LS, Avezum Á, Pereira JCR, et al. Risk factors for myocardial infarction in Brazil. Am Heart J 2003;146:331–8. [DOI] [PubMed] [Google Scholar]
- [24].Kumar P, Luthra K, Dwivedi M, et al. Apolipoprotein E gene polymorphisms in patients with premature myocardial infarction: a case-controlled study in Asian Indians in North India. Ann Clin Biochem 2003;40:382–7. [DOI] [PubMed] [Google Scholar]
- [25].Gerstein HC, Pais P, Pogue J, et al. Relationship of glucose and insulin levels to the risk of myocardial infarction: A case-control study. J Am Coll Cardiol 1999;33:612–9. [DOI] [PubMed] [Google Scholar]
- [26].Azevedo A, Ramos E, von Hafe P, et al. Upper-body adiposity and risk of myocardial infarction. J Cardiovasc Risk 1999;6:321–5. [DOI] [PubMed] [Google Scholar]
- [27].Pais P, Pogue J, Gerstein H, et al. Risk factors for acute myocardial infarction in Indians: a case-control study. Lancet 1996;348:358–63. [DOI] [PubMed] [Google Scholar]
- [28].Bastien M, Poirier P, Lemieux I, et al. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis 2014;56:369–81. [DOI] [PubMed] [Google Scholar]
- [29].Chrysant SG, Chrysant GS. New insights into the true nature of the obesity paradox and the lower cardiovascular risk. J Am Soc Hypertens 2013;7:85–94. [DOI] [PubMed] [Google Scholar]
- [30].Sharma S, Batsis JA, Coutinho T, et al. Normal-weight central obesity and mortality risk in older adults with coronary artery disease. Mayo Clin Proc 2016;91:343–51. [DOI] [PubMed] [Google Scholar]
- [31].Wiklund P, Toss F, Jansson JH, et al. Abdominal and gynoid adipose distribution and incident myocardial infarction in women and men. Int J Obes 20052010;34:1752–8. [DOI] [PubMed] [Google Scholar]
- [32].Neeland IJ, Ayers CR, Rohatgi AK, et al. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity (Silver Spring, Md) 2013;21:E439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].McLaughlin T, Lamendola C, Liu A, et al. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab 2011;96:E1756–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wilson PW, D’Agostino RB, Sullivan L, et al. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med 2002;162:1867–72. [DOI] [PubMed] [Google Scholar]
- [35].Liu J, Hickson DA, Musani SK, et al. Dietary patterns, abdominal visceral adipose tissue, and cardiometabolic risk factors in African Americans: the Jackson heart study. Obesity (Silver Spring, Md) 2013;21:644–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chaudhary K, Buddineni JP, Nistala R, et al. Resistant hypertension in the high-risk metabolic patient. Curr Diab Rep 2011;11:41–6. [DOI] [PubMed] [Google Scholar]
- [37].Garcia-Garcia HM, Jang IK, Serruys PW, et al. Imaging plaques to predict and better manage patients with acute coronary events. Circ Res 2014;114:1904–17. [DOI] [PMC free article] [PubMed] [Google Scholar]


