Abstract
The role of advanced glycation end products (AGEs) and its C-terminal truncated receptor (soluble receptor for advanced glycation end products, sRAGE) in ST-segment elevation myocardial infarction (STEMI) patients with or without diabetes is unknown. We compared their levels in patients with and without STEMI, as well as with and without diabetes. A prospective observational study was performed between December 2014 and December 2015. Study group included STEMI patients with coronary artery disease; control group included patients without coronary artery disease. Levels of AGEs and sRAGE were tested on Days 0, 2, and 5 after STEMI. Levels of creatine kinase-MB (CK-MB), cardiac troponin I, and N-terminal pro-brain natriuretic peptide (NT-proBNP) were tested on Days 0, 1, 2, and 3. Patient's diabetic status was determined by medical history or oral glucose tolerance test. Compared to patients in the control group, STEMI patients showed elevated levels of AGEs and sRAGE. In the STEMI group, diabetic patients had higher levels of AGEs and sRAGE compared to nondiabetic patients. The level of AGEs correlated with peak level of CK-MB in the overall population of patients with STEMI and with peak level of NT-proBNP in diabetic patients with STEMI. Levels of AGEs and sRAGE were elevated after STEMI, especially among patients with diabetes. These markers could serve to indicate the severity of myocardial injury and cardiac insufficiency, and play a potential role in predicting the prognosis of patients with STEMI.
Keywords: advanced glycation end products, diabetes, soluble receptor for advanced glycation end products, ST-segment elevation myocardial infarction
1. Introduction
Coronary artery disease is a common disorder that often results from atherosclerotic changes to coronary vessels.[1] When atherosclerotic changes leads to thrombus formation and blockage in the coronary artery, the blood supply to the myocardial cells is severed, resulting in myocardial infarction. If the electrocardiogram shows ST-segment elevation, an ST-segment elevation myocardial infarction (STEMI) can be diagnosed. The initial step in the management of patients with STEMI is expected to recognize these patients and to identify those at high risk for worse outcomes.
Diabetes is a common risk factor for coronary artery disease.[2] STEMI patients with diabetes also have worse outcomes compared to patients with normal glucose metabolism.[3] Advanced glycation end products (AGEs) are substances resulting from the nonenzymatic saccharification of proteins, lipids, and nucleic acids.[4] Hyperglycemic status and oxidative stress can stimulate the productions of AGEs. Advanced glycation end product receptor (RAGE) is an important receptor for AGEs.[5] Previous studies have established that the AGEs/RAGE system contributes significantly to the development of cardiovascular disease in patients with diabetes.[6,7] One isomer of human RAGE is soluble RAGE (sRAGE), which lacks a C-terminal transmembrane region and intracellular domain. sRAGE can compete with RAGE to bind to AGEs but cannot induce subsequent intracellular reactions.[8] One recent study reported a correlation between sRAGE level and coronary artery disease.[9–11] However, few studies have investigated levels of AGEs and sRAGE after STEMI. In the present study, we investigated dynamic changes in AGEs and sRAGE after STEMI. We also investigated how these changes manifest differently in STEMI patients with or without diabetes.
2. Materials and methods
2.1. Study design and participants
The present study was a prospective observational study performed in the cardiology department of an urban tertiary hospital between December 2014 and December 2015. The study protocol was approved by the Hospital Ethics Committee of Beijing Friendship Hospital, Capital Medical University. All participants in the study provided their informed consent.
Patients in the study group met the following criteria: age between 18 and 80 years; new clinical diagnosis of STEMI based on published diagnostic criteria[12]; symptoms onset within the last 12 hours or more than 12 hours but with persistent ischemic symptoms or hemodynamic instability; coronary artery disease confirmed by coronary angiography. The exclusion criteria included: history of congestive heart failure, myocardial disease, or cardiac valvular disease; congenital heart disease; severe renal insufficiency; severe infectious or rheumatic disease; hematologic disease; cancer.
The criteria for inclusion in the control group were: age between 18 and 80 years; clinical suspicion of coronary artery disease with normal cardiac enzyme tests; normal results on coronary angiography or coronary computed tomography. Exclusion criteria were the same as for the study group.
2.2. Study protocol
All patients with STEMI received primary percutaneous coronary intervention and pharmacologic treatments. After informed consent was obtained, venous blood was drawn at the time of hospitalization (Day 0), 48 hours later (Day 2), and 5 days later (Day 5). Levels of cardiac troponin I, creatine kinase-MB (CK-MB), and N-terminal pro-brain natriuretic peptide (NT-proBNP) were tested on Days 0, 1, 2, and 3 at the hospital laboratory. Levels of AGEs and sRAGE were tested on Days 0, 2, and 5 by enzyme-linked immunosorbent assay, according to the manufacturer's instruction manual (Blue Gene for Life Science, Shanghai, China).
In addition, on Days 5 to 7 after hospitalization, all study participants without previous medical history of diabetes took an oral glucose tolerance test (OGTT). Based on the results of testing and medical history, patients were assigned to the normal glucose regulation group (NGR, fasting glucose < 5.6 mmol/L and 2 hours OGTT glucose < 7.8 mmol/L), impaired glucose regulation group (IGR, fasting glucose 5.6–6.9 mmol/L, or 2-hour OGTT glucose 7.8–11.0 mmol/L), or the newly diabetic group (fasting glucose ≥7.0 mmol/L or 2-hour OGTT glucose ≥11.1 mmol/L).
2.3. Statistical analysis
Descriptive data are presented as mean ± standard deviation. Categorical data are presented as relative frequency. After confirming the data distribution and equal variance, demographic data, and biochemical characteristics were compared between STEMI and control, as well as among patients with varying diabetic status (analysis of variance [ANOVA] for continuous variables and chi-square for categorical variables). Repeated-measures ANOVA was used to study dynamic changes in AGEs and sRAGE in patients with STEMI. Spearman correlation analysis was used for linear correlation analysis, because an uneven distribution was identified. P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 17.0 for Windows (SPSS Inc., Chicago, IL).
3. Results
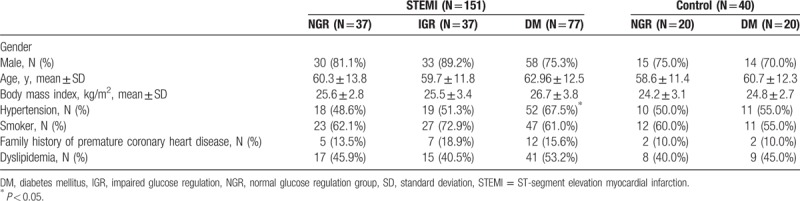
There were 151 patients in the study group and 40 patients in the control group. The study group included 37 NGR, 37 IGR, and 77 diabetes mellitus (DM) (35 newly diagnosed cases of DM and 42 patients with history of DM). The control group included 20 NGR and 20 DM patients. Patient baseline characteristics are presented in Table 1.
Table 1.
Baseline characteristics in STEMI and control groups.
3.1. AGEs and sRAGE levels at Day 0
As shown in Fig. 1, AGEs and sRAGE levels were higher in patients in the STEMI group when compared to patients in the control group at Day 0. This trend held true in both diabetic and nondiabetic patients.
Figure 1.
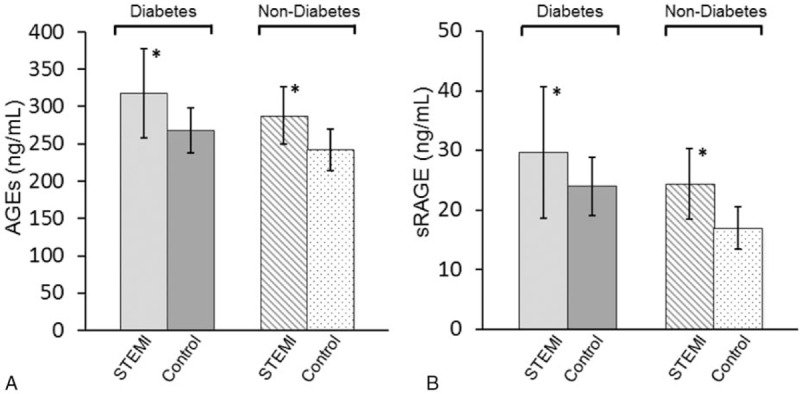
Comparisons of advanced glycation end products and soluble advanced glycation end product receptor in ST-segment elevation myocardial infarction and controls with or without diabetes (∗P < 0.05 compared to the corresponding control group).
3.2. Dynamic changes in AGEs and sRAGE post-STEMI
Figure 2 showed that both AGEs and sRAGE levels were highest at Day 0. Levels of AGEs declined at Day 2, and then increased again at Day 5.
Figure 2.
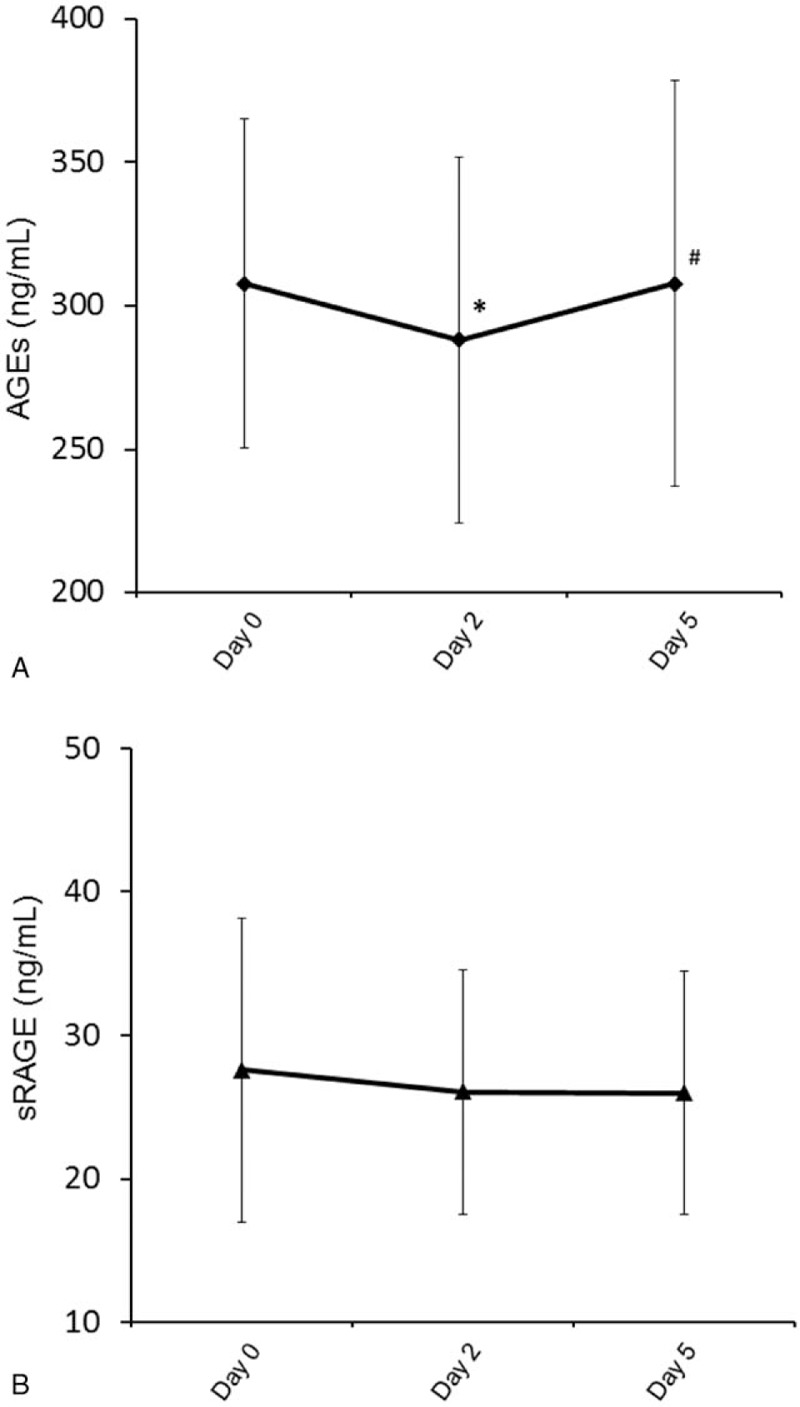
Dynamic changes in advanced glycation end products (A) and soluble advanced glycation end product receptor (B) in patients with ST-segment elevation myocardial infarction (∗P < 0.05 compared to Day 0; #P < 0.05 compared to Day 2).
3.3. Comparison of AGEs and sRAGE in STEMI patients with or without diabetes
When compared to STEMI patients without diabetes, STEMI patients with diabetes had higher AGEs and sRAGE levels at Day 0, 2, and 5 (Fig. 3). In addition, sRAGE level had statistically significant decrease at Day 2 and 5 when compared to Day 0 in the diabetic STEMI patients.
Figure 3.
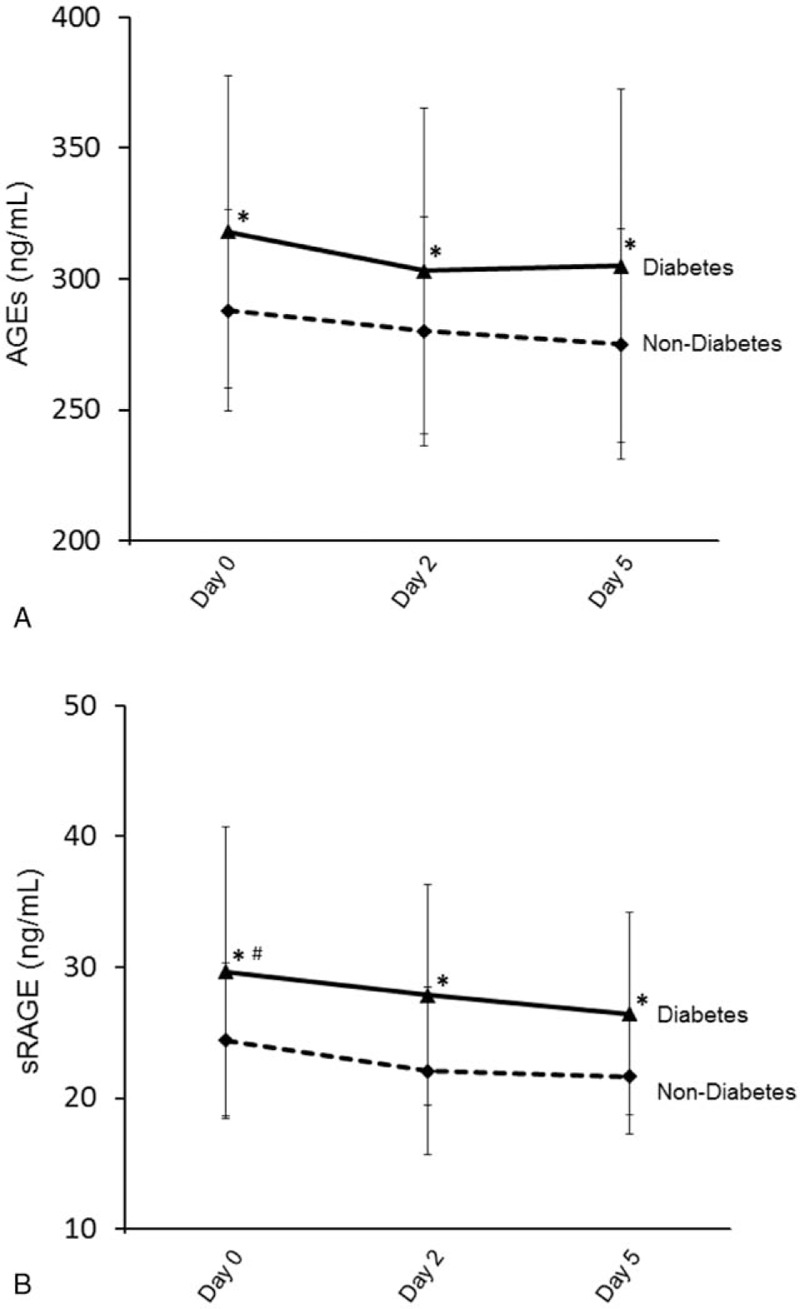
Dynamic changes in advanced glycation end products (A) and soluble advanced glycation end product receptor (B) in ST-segment elevation myocardial infarction patients with or without diabetes (∗P < 0.05 compared to nondiabetic group; #P < 0.05 compared to Days 2 and 5).
3.4. Correlation analysis
In STEMI patients, statistical significant correlations were identified between AGEs and sRAGE, as well as between peak levels of CK-MB and AGEs, at Day 0. In STEMI patients with diabetes, a statistically significant correlation was also found between peak levels of NT-proBNP and AGEs (at Day 0). There was no statistically significant correlation between CK-MB peak and AGEs or sRAGE in diabetic STEMI patients (Fig. 4).
Figure 4.
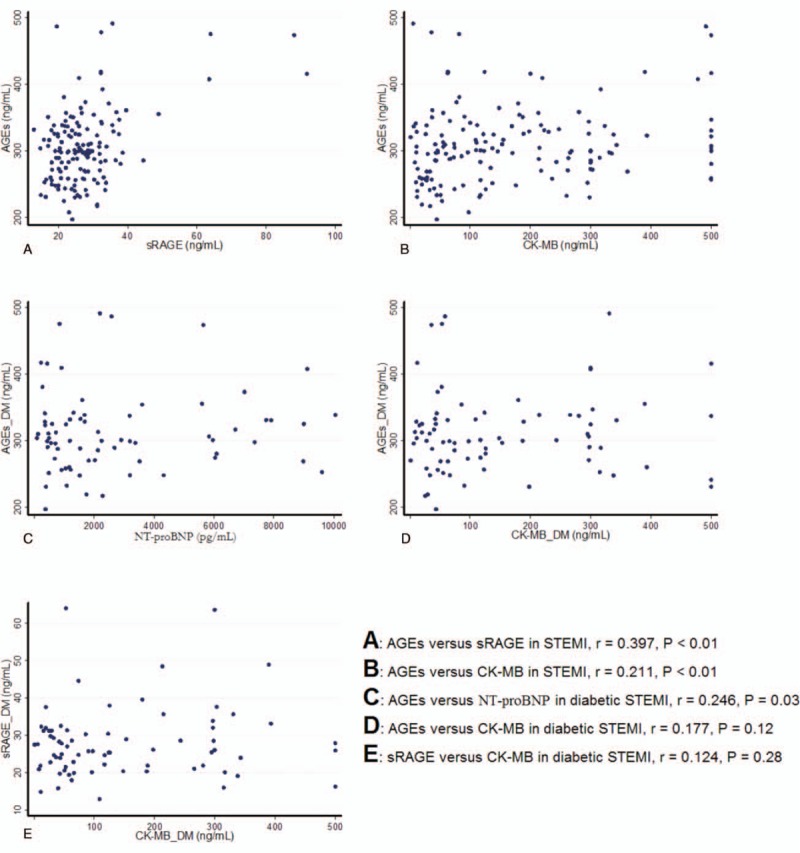
Scatterplots in correlation analysis. (A) Advanced glycation end products (AGEs) vs. soluble advanced glycation end product receptor (sRAGE) in ST-segment elevation myocardial infarction (STEMI) patients. (B), AGEs versus creatine kinase–myocardial band (CK-MB) peak in STEMI patients. (C) AGEs versus N-terminal pro-brain natriuretic peptide peak in STEMI patients with diabetes. (D) AGEs versus CK-MB peak in STEMI patients with diabetes. (E) sRAGE versus CK-MB peak in STEMI patients with diabetes.
4. Discussion
Our present study showed that, compared to the control group, STEMI patients with or without diabetes had elevated levels of both AGEs and sRAGE. Levels peaked at Day 0 after STEMI. In the STEMI group, patients with diabetes had higher levels of AGEs and sRAGE than did nondiabetic patients. There was a correlation between AGE level and with peak concentrations of CK-MB and NT-proBNP in STEMI patients.
Patients with diabetes have high risk for cardiovascular disease. AGEs are substances that result from the nonenzymatic saccharification of proteins, lipids, and nucleic acids. High glucose status and oxidative stress can induce the production of AGEs, which in turn increase the production of reactive oxygen species, impair antioxidant systems, and contribute to chronic stress situations.[13–15] Studies have also shown that AGEs can increase levels of oxidized low-density lipoproteins by decreasing nitric oxide, activating chemotactic mononuclear cells, upregulating inflammation, and impairing endothelial function.[16–18] AGEs can also deposit in the vascular wall and directly induce vascular endothelial cell apoptosis, increasing endothelial cell procoagulant activity and thrombosis formation.[19–22] All of these factors can contribute to the development and progression of atherosclerosis, which can lead to coronary artery diseases, including acute myocardial infarction. Previous studies have shown a correlation between AGEs and the severity of coronary artery disease, regardless of the presence of diabetes.[23,24] High serum levels of AGEs suggest instability of the coronary plaque and could be used to predict obstructive coronary artery disease.[25] This study shows that STEMI patients had higher levels of AGEs than control patients at Day 0. This trend held true in both diabetic and nondiabetic patients. Among patients with STEMI, diabetic patients also had higher levels of AGEs compared to nondiabetic patients (from Day 0 to 5). The elevation of AGE levels not only correlated with diabetes but also with acute coronary artery ischemia. These findings were consistent with previous reports on potential connections among diabetes, AGEs levels, and coronary artery disease. STEMI patients with diabetes usually had worse outcomes compared to patients without diabetes. AGEs might be used as a biomarker for acute myocardial injury and might also predict patient prognosis. AGEs levels peaked at Day 0, and then decreased at Day 2. At Day 5, levels increased again. It was unknown whether this fluctuation represented the recurrence of coronary artery blockage. Further research should study AGEs changes within hours after STEMI and investigate the clinical significance of prolonged elevation of AGEs.
Our study showed correlations between AGEs and the peak level of CK-MB in STEMI patients as well as correlations between AGEs and the peak levels of NT-proBNP in STEMI patients with diabetes. CK-MB is considered a biomarker for myocardial cell necrosis and its level reach the peak 24 to 36 hours after acute myocardial infarction. Studies have shown that CK-MB could predict infarct size, left ventricular function, and mortality.[26] NT-proBNP is a neurohormone secreted from the heart ventricles in response to increased wall stress. Increased NT-proBNP levels in patients with myocardial infarction suggest ventricular remodeling and dilation, which are closely associated with patient prognosis.[27–29] The correlation between AGEs and peak levels of CK-MB and NT-proBNP suggests that AGEs could also contribute to left ventricular insufficiency after STEMI. AGEs could therefore be used as a biomarker for prognosis after STEMI, especially in patients with diabetes.[13,30] Myocardial injury and ventricular insufficiency might be related to injury and related apoptotic effects in protein and DNA.
AGEs bind to their receptors to mediate intracellular effects. RAGE is an important receptor for AGEs. The level of RAGE can increase under several conditions, including stress, ischemia-reperfusion injury, and atherosclerosis.[31,32] A soluble form of RAGE, namely sRAGE, could bind AGEs but lacks the necessary intracellular activation. It is considered to antagonize the RAGE signaling pathway. Its production may be induced in response to decreased oxidative stress to counteract the effects of RAGE.[33] It was shown that sRAGE could delay the progression of atherosclerosis, protect against myocardial ischemia-reperfusion injury, and lower inflammatory responses.[34,35] Previous studies have shown that sRAGE levels were elevated in patients with acute coronary syndrome when compared to healthy controls; few studies have addressed the influence of diabetes on sRAGE. In the present study, we showed that sRAGE was higher in STEMI patients compared to control patients. The level of sRAGE was further increased in patients with STEMI and diabetes, suggesting that sRAGE could be induced after acute cardiac ischemia. The highest level of sRAGE was detected at Day 0, and then slowly decreased over several days. Unlike AGEs, its level did not increase at Day 5, suggesting its triggers might be different from those for AGEs. It was reported that sRAGE level elevation occurred earlier than did elevation of the traditional cardiac injury marker, troponin, which indicated that sRAGE could be used as an early marker for cardiac injury.[36] We did not find a statistically significant correlation with cardiac injury marker CK-MB, because sRAGE changes only happened during a short time period after STEMI.[11] Further studies should investigate whether sRAGE could be used as an early marker for cardiac injury.
Limitations of the present study included are conducted as a single-center study with a small sample size and short observation period. We detected dynamic changes in AGEs and sRAGE, but did not study correlations with clinical outcomes. Future studies should address these.
5. Conclusion
In summary, levels of AGEs and sRAGE were elevated in STEMI patients when compared to the control group. Levels were also higher in STEMI patients with diabetes compared to STEMI patients without diabetes. Elevated levels may correlate with the extent of cardiac injury and might be used as biomarkers for acute cardiac injury, as well as predictors of prognosis.
Author contributions
Authorship: HQ and HL designed and conducted the study, and drafted the manuscript. WL, XS, XG, and BH help conducting the study and analyzing the data.
Data curation: Wei-Ping Li.
Formal analysis: Hui Qiu.
Investigation: Hui Qiu, Xu-Hua Shen, Bing Hua.
Methodology: Wei-Ping Li, Xu-Hua Shen, Xiang-Yu Guo, Bing Hua.
Resources: Xu-Hua Shen, Xiang-Yu Guo.
Supervision: Hong-Wei Li.
Writing – original draft: Hui Qiu.
Writing – review & editing: Hui Qiu, Hong-Wei Li.
Footnotes
Abbreviations: AGE = advanced glycation end product, CK-MB = creatine kinase-MB, NGR = normal glucose regulation group, NT-proBNP = N-terminal pro-brain natriuretic peptide, OGTT = oral glucose tolerance test, RAGE = advanced glycation end product receptor, sRAGE = soluble RAGE, STEMI = ST-segment elevation myocardial infarction.
Funding/support: The authors thank the National Natural Science Foundation of China (81670315) and Beijing Natural Science Foundation Program (7172059) for the support.
The authors have no conflicts of interest to disclose.
References
- [1].Leone A. Markers of atherosclerotic disease: what do they mean? Current opinion and future trends. Curr Pharm Des 2016;22:7–17. [DOI] [PubMed] [Google Scholar]
- [2].Chiha M, Njeim M, Chedrawy EG. Diabetes and coronary heart disease: a risk factor for the global epidemic. Int J Hypertens 2012;2012:697240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mazurek M, Kowalczyk J, Lenarczyk R, et al. The prognostic value of different glucose abnormalities in patients with acute myocardial infarction treated invasively. Cardiovasc Diabetol 2012;11:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wu JT. Advanced glycosylation end products: a new disease marker for diabetes and aging. J Clin Lab Anal 1993;7:252–5. [DOI] [PubMed] [Google Scholar]
- [5].Bucciarelli LG, Wendt T, Rong L, et al. RAGE is a multiligand receptor of the immunoglobulin superfamily: implications for homeostasis and chronic disease. Cell Mol Life Sci 2002;59:1117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wendt T, Bucciarelli L, Qu W, et al. Receptor for advanced glycation endproducts (RAGE) and vascular inflammation: insights into the pathogenesis of macrovascular complications in diabetes. Curr Atheroscler Rep 2002;4:228–37. [DOI] [PubMed] [Google Scholar]
- [7].Ramasamy R, Yan SF, Schmidt AM. Receptor for AGE (RAGE): signaling mechanisms in the pathogenesis of diabetes and its complications. Ann N Y Acad Sci 2011;1243:88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Raucci A, Cugusi S, Antonelli A, et al. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10). FASEB J 2008;22:3716–27. [DOI] [PubMed] [Google Scholar]
- [9].McNair ED, Wells CR, Mabood Qureshi A, et al. Soluble receptors for advanced glycation end products (sRAGE) as a predictor of restenosis following percutaneous coronary intervention. Clin Cardiol 2010;33:678–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].McNair ED, Wells CR, Qureshi AM, et al. Low levels of soluble receptor for advanced glycation end products in non-ST elevation myocardial infarction patients. Int J Angiol 2009;18:187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jensen LJ, Flyvbjerg A, Bjerre M. Soluble receptor for advanced glycation end product: a biomarker for acute coronary syndrome. Biomed Res Int 2015;2015:815942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].CCD Committee. Guidelines for acute ST segment elevation myocardial infarction diagnosis and treatment. J Chin Cardiovasc Dis 2010;38:15. [Google Scholar]
- [13].Kitano D, Takayama T, Nagashima K, et al. A comparative study of time-specific oxidative stress after acute myocardial infarction in patients with and without diabetes mellitus. BMC Cardiovasc Disord 2016;16:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part II: Animal and human studies. Circulation 2003;108:2034–40. [DOI] [PubMed] [Google Scholar]
- [15].Harrison D, Griendling KK, Landmesser U, et al. Role of oxidative stress in atherosclerosis. Am J Cardiol 2003;91:A7–11. [DOI] [PubMed] [Google Scholar]
- [16].Bucala R, Tracey KJ, Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Invest 1991;87:432–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bucala R, Makita Z, Vega G, et al. Modification of low density lipoprotein by advanced glycation end products contributes to the dyslipidemia of diabetes and renal insufficiency. Proc Natl Acad Sci U S A 1994;91:9441–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Santilli F, Lapenna D, La Barba S, et al. Oxidative stress-related mechanisms affecting response to aspirin in diabetes mellitus. Free Radic Biol Med 2015;80:101–10. [DOI] [PubMed] [Google Scholar]
- [19].Kirstein M, Brett J, Radoff S, et al. Advanced protein glycosylation induces transendothelial human monocyte chemotaxis and secretion of platelet-derived growth factor: role in vascular disease of diabetes and aging. Proc Natl Acad Sci U S A 1990;87:9010–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Basta G. Receptor for advanced glycation endproducts and atherosclerosis: from basic mechanisms to clinical implications. Atherosclerosis 2008;196:9–21. [DOI] [PubMed] [Google Scholar]
- [21].Wautier MP, Chappey O, Corda S, et al. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab 2001;280:E685–94. [DOI] [PubMed] [Google Scholar]
- [22].Willemsen S, Hartog JW, Hummel YM, et al. Tissue advanced glycation end products are associated with diastolic function and aerobic exercise capacity in diabetic heart failure patients. Eur J Heart Fail 2011;13:76–82. [DOI] [PubMed] [Google Scholar]
- [23].Kiuchi K, Nejima J, Takano T, et al. Increased serum concentrations of advanced glycation end products: a marker of coronary artery disease activity in type 2 diabetic patients. Heart 2001;85:87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kanauchi M, Tsujimoto N, Hashimoto T. Advanced glycation end products in nondiabetic patients with coronary artery disease. Diabetes Care 2001;24:1620–3. [DOI] [PubMed] [Google Scholar]
- [25].Won KB, Chang HJ, Park SH, et al. High serum advanced glycation end-products predict coronary artery disease irrespective of arterial stiffness in diabetic patients. Korean Circ J 2012;42:335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bagai A, Schulte PJ, Granger CB, et al. Prognostic implications of creatine kinase-MB measurements in ST-segment elevation myocardial infarction patients treated with primary percutaneous coronary intervention. Am Heart J 2014;168:503–11.e2. [DOI] [PubMed] [Google Scholar]
- [27].Palazzuoli A, Gallotta M, Quatrini I, et al. Natriuretic peptides (BNP and NT-proBNP): measurement and relevance in heart failure. Vasc Health Risk Manag 2010;6:411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hirayama A, Kusuoka H, Yamamoto H, et al. Serial changes in plasma brain natriuretic peptide concentration at the infarct and non-infarct sites in patients with left ventricular remodelling after myocardial infarction. Heart 2005;91:1573–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hirayama A, Kusuoka H, Yamamoto H, et al. Usefulness of plasma brain natriuretic peptide concentration for predicting subsequent left ventricular remodeling after coronary angioplasty in patients with acute myocardial infarction. Am J Cardiol 2006;98:453–7. [DOI] [PubMed] [Google Scholar]
- [30].Liu Q, Wang S, Cai L. Diabetic cardiomyopathy and its mechanisms: role of oxidative stress and damage. J Diabetes Investig 2014;5:623–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Goldin A, Beckman JA, Schmidt AM, et al. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation 2006;114:597–605. [DOI] [PubMed] [Google Scholar]
- [32].Daffu G, del Pozo CH, O'Shea KM, et al. Radical roles for RAGE in the pathogenesis of oxidative stress in cardiovascular diseases and beyond. Int J Mol Sci 2013;14:19891–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Prasad K. Low levels of serum soluble receptors for advanced glycation end products, biomarkers for disease state: myth or reality. Int J Angiol 2014;23:11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Park L, Raman KG, Lee KJ, et al. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med 1998;4:1025–31. [DOI] [PubMed] [Google Scholar]
- [35].Bucciarelli LG, Wendt T, Qu W, et al. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null mice. Circulation 2002;106:2827–35. [DOI] [PubMed] [Google Scholar]
- [36].Jensen LJ, Lindberg S, Hoffmann S, et al. Dynamic changes in sRAGE levels and relationship with cardiac function in STEMI patients. Clin Biochem 2015;48:297–301. [DOI] [PubMed] [Google Scholar]


