Supplemental Digital Content is available in the text
Keywords: body mass index, breast cancer, metabolic syndrome, neutrophil-to-lymphocyte ratio, risk
Abstract
Breast cancer (BC), obesity, and metabolic syndrome (MetS) shared a common mechanism of dysregulated metabolism and inflammatory response in disease initiation. Neutrophil-to-lymphocyte ratio (NLR) is associated with adverse survival of BC patients. The aim of this study is to identify risk effect between NLR and BC in Chinese population with or without obesity and MetS. BC and age-matched breast benign disease (BBD) patients were retrospectively analyzed from Comprehensive Breast Health Center, Shanghai Ruijin Hospital. MetS was defined using AHA/NHLBI criteria. Individuals were classified into very low (0–1.30), low (1.31–1.67), intermediate (1.68–2.20), and high (>2.20) NLR subsets by each NLR quartile. In all, 1540 BC and 1540 BBD patients were included. Univariate and multivariate analysis found that NLR (OR: 1.27, 95% CI: 1.16–1.39, P < .001) and obesity (OR: 1.19, 95% CI: 1.00–1.42, P = .046) but not MetS (P = .060) were significantly associated with increased BC risk. Intermediate or high NLR substantially increased BC risk compared to very low NLR group (OR: 1.57, 95% CI: 1.29–1.92, P < .001; OR: 1.84, 95% CI: 1.50–2.25, P < .001; respectively) in whole population. Subgroup analysis found that the impact of higher NLR on BC risk was more obvious in patients without obesity (intermediate NLR, OR: 1.72, 95% CI: 1.37–2.16, P < .001; high NLR, OR: 1.92, 95% CI: 1.53–2.41, P < .001) or without MetS (intermediate NLR, OR: 1.70, 95% CI: 1.35–2.14, P < .001; high NLR, OR: 1.98, 95% CI: 1.57–2.51, P < .001). Higher preoperative NLR was found in BC patients compared with BBD patients. Intermediate to high NLR level substantially increased BC risk, which was more relevant for those without obesity or MetS.
1. Introduction
Breast cancer (BC) is the most common malignancy among women with an estimated 1.67 million newly diagnosed cancer cases in 2012, accounting for a quarter of all cancers.[1,2] During the past decade, BC incidence in China has been increasing rapidly with more than twice the rate of global growth.[3] Various independent variables have been identified for BC initiation, including lifestyle factors like pregnancy and breastfeeding history, menstrual history, alcohol consumption, lack of physical exercise, and being obese.
Obesity is a common BC risk factor that has been broadly accepted.[4–9] A meta-analysis including 282,137 incident cases found a relative risk of 1.16 (95% confidence interval [CI]: 1.01–1.32, P = .009) for premenopausal BC in Asia-Pacific women and a relative risk of 1.12 (95% CI: 1.08–1.16, P < .001) for postmenopausal BC with every 5 kg/m2 increase in BMI.[5] Meanwhile, metabolic syndrome (MetS), a group of pathophysiological disturbances and metabolic risk factors featuring central obesity, insulin resistance, dyslipidemia, and elevated blood pressure, has also been recognized for its increasing risk of BC. A recent meta-analysis confirmed a statistically significant increase of 47% BC risk for adult females with MetS (risk ratio [RR]: 1.47, 95% CI: 1.15–1.87, P < .002), demonstrating a modest positive association between MetS and BC.[10] Moreover, MetS has also shown to be associated with worse disease outcome in BC patients.[11] Among several potential pathways underlying between obesity, MetS, and BC, dysregulated systemic metabolism and inflammatory response play decisive roles. It is therefore of interest to develop the application of hematological parameters of systemic inflammatory response, for example, preoperative C-reactive protein (CRP) level, neutrophils, and lymphocytes, for their diagnostic and prognostic value.[12,13] Compared to absolute counts of leukocytes, which could be altered facing different physiological or pathological conditions, neutrophil-to-lymphocyte ratio (NLR) showed superiority for its relative stability,[12,14] and has been increasingly recognized for its prognostic value.[15,16]
Previous studies found that higher NLR was related with more advanced or aggressive disease regarding tumor stage, nodal stage, and metastasis.[13,17,18] Furthermore, we previously reported that higher NLR was associated with an adverse survival in BC patients,[19] which is consistent with other studies.[12,14,15,18,20] These works have proposed that cytokines and chemokines secreted by both tumor cells and leucocytes would accelerate tumor progression.[21] Nevertheless, there is currently limited evidence reporting the risk factor of NLR on BC development. The correlation of NLR, obesity, and MetS has not been fully elucidated yet. Hence, in this study, we aimed to evaluate the role of NLR on BC development and to analyze the association between NLR and obesity or MetS for BC risk.
2. Materials and methods
2.1. Patient population
We retrospectively analyzed patients who underwent surgical procedure from December 2012 to January 2016 in Comprehensive Breast Health Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China. Patients who met the following criteria were enrolled in the BC subgroup: female gender, BC proven by open excision biopsy or core needle biopsy, and complete clinical and follow-up data. The exclusion criteria included patients treated with neo-adjuvant therapy, de novo stage IV BC or inflammatory BC, and neutrophil count at diagnosis >10 × 109 L−1. Those who received surgery during the same period of time for pathologically proven breast benign disease (BBD) were matched 1:1 by patient age as control group. Clinical details for patients with BC or BBD were achieved from Shanghai Jiao Tong University Breast Cancer Database (SJTU-BCDB) and Ruijin Hospital inpatient medical records. This approach was approved by the independent Ethical Committees of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine.
2.2. Clinical and laboratory data
Patient preoperative peripheral hematological parameters and C-reaction protein (CRP) level were collected by nurses and analyzed using XN-series blood analyzer (Sysmex Corporation, Chuo-ku, Kobe, Hyogo, Japan) in the Department of Clinical Laboratory, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. Malignant and benign tumor diagnoses were performed in the Department of Pathology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
2.3. Definition of metabolic syndrome
The MetS was defined in accordance with the current guidelines, revised in 2005 by the American Heart Association/National Heart, Lung, and Blood Institute (AHA/NHLBI). We chose the AHA/NHLBI criteria over the other widely used International Diabetes Federation (IDF) definition, since the former diagnosed 4% more people with MetS in the Chinese population, as shown in a previous study.[22] Body mass index (BMI) was employed to evaluate central obesity with a cut-off value of no less than 25.0 kg/m2, according to the Chinese Medical Association criteria for MetS.[23] The criteria for clinical diagnosis of MetS adjusted to the study population are shown in Supplementary Table S1.
2.4. Statistical analysis
NLR was calculated by dividing absolute neutrophil count by number of lymphocytes. Different cut-off values were applied to classify NLR, including the median value of 1.67 for bipartition, as well as the first, second, and third quartiles of 1.30, 1.67, and 2.20 for quadripartition. χ2 test or Fisher exact test were applied for descriptive characteristics of categorical variables. Baseline clinicopathologic features were compared using multinomial logistic regression analysis between BC and BBD subgroups. Subgroup analyses for risk factors were conducted using stratified Mantel-Hænszel test to estimate odds ratio (OR) with 95% CI among subsets. IBM SPSS statistics software version 23 (SPSS, Inc, Chicago, IL) was used for data analysis. Two-sided P values <.05 were considered significant.
3. Results
3.1. Patients baseline characteristics
In all, 1540 BC patients and matched 1540 BBD patients were enrolled in this study with a mean age of 50.75 ± 10.36 years (range 24–87 years). Among them, 33.90%, 10.00%, and 27.11% individuals were diagnosed with hypertension, hyperglycemia and hyperlipidemia, respectively. A total of 22.05% patients were obese, and 26.36% met the criteria of MetS. The mean NLR of all population was 1.84 ± 0.82. The first, second, and third quartiles of NLR classified individuals into very low (0–1.30), low (1.31–1.67), intermediate (1.68–2.20), and high (>2.20) NLR subsets.
In univariate analysis, obesity was more frequent in BC patients than the BBD group (23.83% vs 20.26%, P = .017; Table 1). Three hundred and eighty-three patients (24.87%) in the BC group and 429 (27.86%) patients in the BBD group were diagnosed with MetS according to AHA/NHLBI criteria (P = .060). Compared with the BBD group, BC patients had a higher absolute neutrophil count (3.36 ± 1.19 × 109 L−1 vs 3.18 ± 1.10 × 109 L−1, P < .001), lower lymphocyte count (1.87 ± 0.55 × 109 L vs 1.92 ± 0.56 × 109 L−1, P = .009), and a subsequent higher NLR (1.92 ± 0.81 vs 1.76 ± 0.82, P < .001; Table 1). Besides, surgical history of BBD was also different between the BC and BBD subgroups (P < .001). Multinomial logistic regression found NLR was much higher in the BC group compared with patients in the BBD group (OR: 1.27, 95% CI: 1.16–1.39, P < .001; Supplementary Table S2). In addition, surgical history of BBD was an independent protective factor for BC (OR: 0.65, 95% CI: 0.53–0.78, P < .001), while obesity significantly increased BC risk (OR: 1.19, 95% CI: 1.00–1.42, P = .046). No significant difference was observed regarding other components of MetS including hypertension, hyperglycemia, or hyperlipidemia (P > .05; Table 1).
Table 1.
Patients characteristics and factors associated with breast cancer.
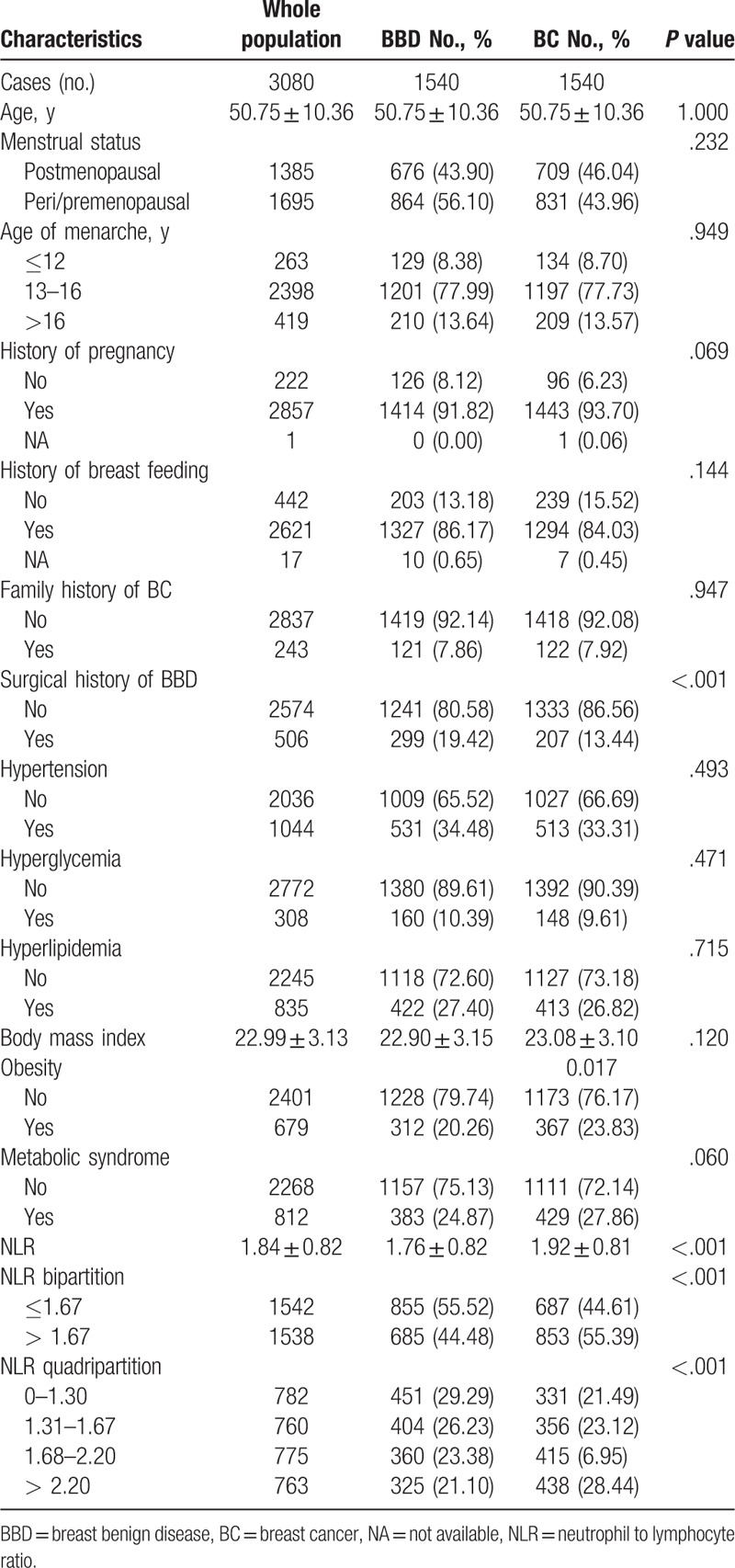
3.2. NLR and BC risk stratified by menopausal status
Overall, intermediate (1.68–2.20) and high (> 2.20) NLR significantly increased BC risk compared with very low (0–1.30) NLR group (OR: 1.57, 95% CI: 1.29–1.92, P < .001; OR: 1.84, 95% CI: 1.50–2.25, P < .001; respectively, Table 2). The mean NLR was 1.72 ± 0.76 for postmenopausal women, and 1.93 ± 0.85 for pre/perimenopausal women (P < .001). As shown in Table 2, the effect of intermediate to high NLR on increasing BC risk remained robust in both postmenopausal (OR: 1.62, 95% CI: 1.22–2.16, P = .001; OR: 1.59, 95% CI: 1.17–2.17, P = .003; respectively) and pre/perimenopausal population (OR: 1.61, 95% CI: 1.21–2.14, P = .001; OR: 2.13, 95% CI: 1.61–2.81, P < .001; respectively).
Table 2.
Risk of NLR on breast cancer stratified by menopausal status.
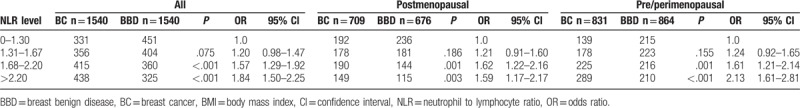
3.3. Association between NLR and BC risk stratified by obesity status
NLR was similarly distributed in patients with different BMI statuses (P = .957; Table 3). BC risk in patients with BMI <25.0 kg/m2 were more influenced by higher NLR, with an OR of 1.72 for intermediate NLR group (95% CI: 1.37–2.16, P < .001; Table 3; Fig. 1A) and an OR of 1.92 for high NLR group (95% CI: 1.53–2.41, P < .001). In contrast, the impact of higher NLR on BC risk was not statistically significant in patients with BMI ≥25.0 kg/m2 (P > .05). Subgroup analysis stratified by BMI status was also conducted to show the influence of bipartite NLR on BC risk (Fig. 2). Higher NLR level was related with increased BC risk among overall population, which was more obvious in the nonobese group (P < .001), but not in the obese patients (P = .182, Supplementary Table S3).
Table 3.
Risk of NLR on breast cancer stratified by BMI.
Figure 1.
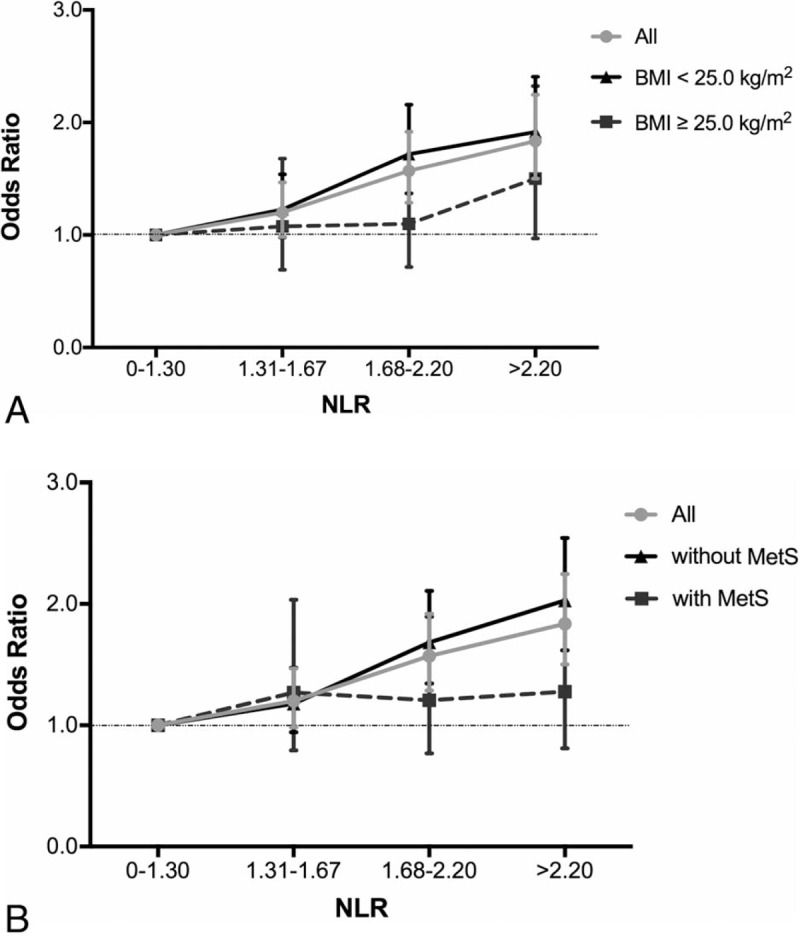
Association between NLR and odds ratio of BC by BMI status and MetS status: (A) Association between NLR and odds ratio of BC by BMI status. Overall odds ratios of different NLR ranges between BC versus BBD subgroups are shown as gray dots, which are then stratified into BMI < 25.0 kg/m2 (black triangles) and BMI ≥ 25.0 kg/m2 (black squares). I-shaped bars represent 95% CIs. Higher NLR levels are significantly associated with increasing BC risk in all population and in patients with BMI <25.0 kg/m2 (P < .001), but not in patients with BMI ≥25.0 kg/m2 (P > .05). (B) Association between NLR and odds ratio of BC by MetS status. Overall odds ratios of different NLR ranges between BC versus BBD subgroups are shown as gray dots, which are then stratified into subgroup without MetS (black triangles) and with MetS (black squares). Higher NLR levels are significantly associated with increasing BC risk in all population and in patients without MetS (P < .001), but not in patients with MetS (P > .05). BBD = breast benign disease, BC = breast cancer, BMI = body mass index, MetS = metabolic syndrome, NLR = Neutrophil-to-lymphocyte ratio.
Figure 2.
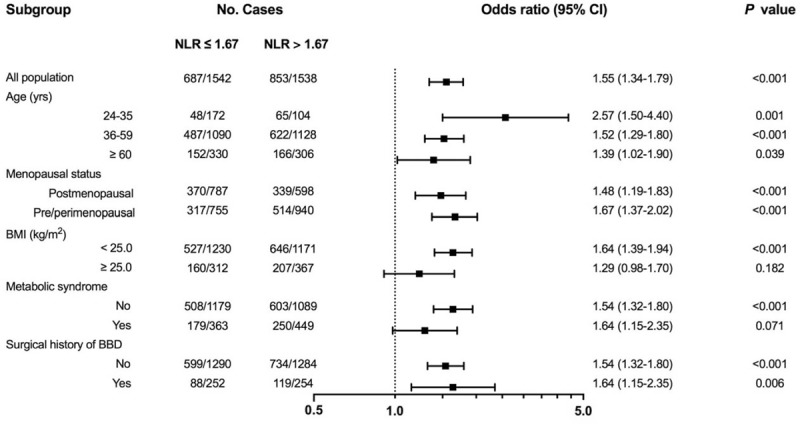
Subgroup analysis of NLR effect on BC risk. Prespecified subgroup analyses for risk factors were conducted to estimate OR with 95% CI among subgroups with the use of 2-sided P values. Higher preoperative NLR was an independent risk factor in all subgroups except for obese patients and MetS patients. BC = breast cancer, CI = confidence interval, MetS = metabolic syndrome, NLR = Neutrophil-to-lymphocyte ratio, OR = odds ratio.
3.4. Association between NLR and BC risk stratified by MetS
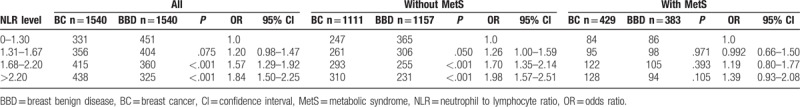
No difference of NLR distribution was observed across different MetS statuses (P = .541; Table 4). In patients without MetS, intermediate NLR caused a 70.00% BC risk increase compared with very low NLR group (95% CI: 1.35–2.14, P < .001; Table 4; Fig. 1B). Meanwhile, high NLR almost doubled BC risk with an OR of 1.98 (95% CI: 1.57–2.51, P < .001). However, in patients with MetS, NLR level was no longer associated with increased BC risk (P > .05). Using bipartite NLR classification (NLR ≤ 1.67 vs NLR > 1.67), we also found that MetS status might influence the association between NLR level and BC risk (Fig. 2). Higher NLR level increased BC risk in patients without MetS (P < .001), but not in those with MetS (P = .071, Supplementary Table S3).
Table 4.
Risk of NLR on BC development stratified by metabolic syndrome.
4. Discussion
To our knowledge, the present study, enrolling a total of 3080 patients, is the largest study to evaluate the association between NLR and BC risk, and to explore its relationship with obesity and MetS in Chinese population. In this study, we found that higher preoperative NLR was associated with increased BC risk in the overall population, regardless of menopausal status. The risk-carrying effect of NLR was more evident in individuals with BMI < 25.0 kg/m2, as well as in those without MetS.
Various modifiable lifestyle risk factors have been identified for BC initiation in previous studies. Obesity, defined as having a BMI ≥25 kg/m2 for Chinese population,[23] is known to affect BC risk and survival.[4–9] Meanwhile, studies on MetS, another potential risk factor with an increasing prevalence worldwide, produced inconsistent results, especially in Asians.[24–28] In the present study, we found a much higher NLR in pre/perimenopausal women, compared with postmenopausal women (1.93 ± 0.85 vs 1.72 ± 0.76, P < .001). Similar conclusion was drawn in a study of Chen et al that women <50 years old had significantly higher NLR than women of 51 to 70 years of age.[29] Despite this different distribution, the impact of NLR on BC risk is consistent regardless of menopausal status. In addition, we showed that BMI ≥25 kg/m2 at diagnosis is an independent risk factor for BC in Chinese population. MetS occurs slightly more frequently in BC patients compared with BBD carriers, but this difference is not statistically significant (P = .060). The effect of obesity for BC risk may be realized through numbers of overlapping pathways, altering adiposity-related host factors that result in high circulating insulin/insulin-like growth factor I, chronic inflammation, high serum leptin concentration, and low adiponectin. Furthermore, obesity has potential impact in clinical management of BC patients with regard to chemotherapy dosing decision, surgical treatment, and related comorbidities.[30,31]
Previous studies have confirmed the role of inflammation in the initiation, promotion, and progression of BC.[11–13] It is therefore of interest to establish the role of hematological parameters for systemic inflammatory response. NLR is an ideal factor to evaluate the balance between the innate and adaptive immunity. There is much evidence for the prognostic value of NLR, stating that higher preoperative NLR was associated with an adverse survival outcome in BC patients.[12,14,15,18–20] A recent meta-analysis found that NLR greater than the cut-off value was associated with worse disease-free survival (hazard ratio [HR]: 1.74, 95% CI: 1.47–2.07, P < .001) and overall survival (HR: 2.56, 95% CI: 1.96–3.35, P < .001), either in early-stage BC setting or metastatic setting.[15] On the other hand, the association between NLR and BC risk has not been completely understood. In our study, we found that NLR was significantly higher in the BC group compared with the BBD group, and patients with NLR >1.67 were related to a substantial increase risk factor for BC. Our finding was consistent with that of Ozyalvacli et al[18] and Okuturlar et al,[32] indicating NLR as an independent risk factor for BC development. Potential mechanism of the association between NLR and BC remained under-elucidated. Queen et al found that when cocultured with BC cells, neutrophils were stimulated to produce oncostatin M, a pleiotropic cytokine of interleukin-6 family, which promoted tumor progression by inducing vascular endothelial growth factor expression and enhancing metastasis.[33] In addition, neutrophil-derived leukotrienes were shown to aid the colonization of distant tissue by selectively expanding highly tumorigenic BC cells.[34] Meanwhile, it has been broadly accepted that lymphocytes play a role in antitumor activity in the adaptive immune system. Lymphocytopenia was established as independent prognostic factor for BC patient survival in both early and metastatic BC settings.[35,36] Therefore, BC patient prognosis could be influenced by either peripheral neutrophils or lymphocytes counts, and these studies revealed the potential ability of NLR to reflect situations of different immune and inflammatory pathways.
Chronic systematic inflammation was also a critical mechanism underlying pathophysiology of obesity[37,38] and MetS.[39,40] Obesity and MetS produce a long-term low-grade activation of the immune system that affects metabolic homeostasis over time. In previous studies, NLR was found to be associated with the presence and severity of MetS in Turkish population,[41] and to increase linearly with increasing number of MetS components (P for trend <.001) in Asian Indians.[42] However, in this study, we found that NLR was similarly distributed in patients obese or nonobese, with or without MetS; but the effect of NLR on BC risk varied across different BMI or MetS statuses. In nonobese patients or those without MetS, NLR may be a better indicator for systematic inflammation caused by carcinogenesis, mostly due to the effect of neutrophils in creating a microenvironment conducive for tumor development. In contrast, NLR was not a good indicator for inflammatory status in patients with obesity and MetS, possibly because neutrophils and lymphocytes increased simultaneously with obesity degree and severity of MetS, thus the increase of NLR was not significant.[43] Besides, it is hypothesized that other inflammatory factors and cytokines may also play important roles in intermediating the relationship between NLR and BC. In our study, additional test concerning CRP, an inflammation-related parameter, was conducted to assess its potential interaction between NLR, obesity, MetS, and BC. We found the distribution of CRP was similar between BC (N = 708) and BBD (N = 700) groups (0.28 ± 0.02, 0.26 ± 0.01 for BC and BBD, respectively; P = .220; Supplementary Figure S1A). Furthermore, CRP level was significantly lower in BBD group compared with BC group in patients with lower NLR (0.30 ± 0.03, 0.24 ± 0.01 for BC and BBD, respectively; P = .031), but not in patients with higher NLR (0.26 ± 0.01, 0.30 ± 0.02 for BC and BBD, respectively; P = .178). Both obesity and MetS were found to have no interaction with CRP on BC risk (Supplementary Figure S1B, S1C). Further studies are needed to evaluate other potential inflammation-related factors in consideration for assessing the causal relationship between NLR and BC.
There are several potential limitations in this study. First, we retrospectively included patients into this age-matched cohort study, so there might be a selection bias. In addition, waist circumference and waist–hip ratio were usually applied in previous studies to define MetS, but it was demonstrated that BMI ≥25.0 kg/m2 could also be used as surrogate to reflect central obesity, especially in Chinese population.[23] Moreover, due to the limited data about clinical and hematological factors from normal individuals, the control group in our study was BBD patients instead of normal individuals, thus our findings need to be further explored and validated in independent normal individuals.
5. Conclusions
In conclusion, preoperative NLR was much higher in BC patients compared with BBD carriers. NLR was an independent risk factor for BC, regardless of menopausal status. Intermediate-to-high NLR level was associated with increased BC risk for the overall population, especially in nonobese or MetS-free patients. Future studies should be performed with additional normal patients to evaluate the relationship and interaction between NLR, obese, MetS, and BC risk in Chinese population.
Author contributions
Conceptualization: Qiong FANG, Kunwei SHEN, Beiwen WU, Xiaosong CHEN.
Data curation: Qiong FANG, Yiwei TONG, Gen WANG, Nan ZHANG, Beiwen WU, Xiaosong CHEN.
Formal analysis: Gen WANG.
Funding acquisition: Kunwei SHEN.
Investigation: Nan ZHANG.
Methodology: Yiwei TONG, Xiaosong CHEN.
Resources: Nan ZHANG, Weiguo CHEN, Yafen LI, Kunwei SHEN, Xiaosong CHEN.
Supervision: Weiguo CHEN, Yafen LI, Kunwei SHEN, Beiwen WU.
Validation: Gen WANG.
Writing – original draft: Qiong FANG, Yiwei TONG.
Writing – review & editing: Qiong FANG, Yiwei TONG, Kunwei SHEN, Xiaosong CHEN.
Supplementary Material
Footnotes
Abbreviations: AHA/NHLBI = American Heart Association/National Heart, Lung, and Blood Institute, BBD = breast benign disease, BC = breast cancer, BMI = body mass index, CI = confidence interval, CRP = C-reactive protein, ER = estrogen receptor, HER2 = human epidermal growth factor receptor-2, HR = hazard ratio, IDF = International Diabetes Federation, MetS = metabolic syndrome, NLR = neutrophil-to-lymphocyte ratio, OR = odds ratio, PR = progesterone receptor, RR = risk ratio.
This study was financially supported by grants from National Natural Science Foundation of China (Grant Number: 81472462, 81772797), Technology Innovation Act Plan of Shanghai Municipal Science and Technology Commission (15411952500 and 15411952501), Medical Guidance Foundation of Shanghai Municipal Science and Technology Commission (15411966400), Shanghai Municipal Education Commission-Gaoyuan Nursing Grant Support (Hlgy15001yjx) and Joint Research Project of the Emerging Cutting-edge Technology of Shanghai Shen-kang Hospital Development Center (SHDC12014103). All these financial sponsors had no role in the study design, in the collection, analysis and interpretation of data.
The authors declare that they have no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- [2].Youlden DR, Cramb SM, Dunn NA, et al. The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol 2012;36:237–48. [DOI] [PubMed] [Google Scholar]
- [3].Fan L, Strasser-Weippl K, Li J-J, et al. Breast cancer in China. Lancet Oncol 2014;15:e279–89. [DOI] [PubMed] [Google Scholar]
- [4].Allott EH, Hursting SD. Obesity and cancer: mechanistic insights from transdisciplinary studies. Endocr Relat Cancer 2015;22:R365–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Colditz GA, Peterson LL. Obesity and cancer: evidence, impact, and future directions. Clin Chem 2017;64:154–62. [DOI] [PubMed] [Google Scholar]
- [6].Feola A, Ricci S, Kouidhi S, et al. Multifaceted breast cancer: the molecular connection with obesity. J Cell Physiol 2017;232:69–77. [DOI] [PubMed] [Google Scholar]
- [7].Ligibel JA, Cirrincione CT, Liu M, et al. Body mass index, PAM50 Subtype, and outcomes in node-positive breast cancer: CALGB 9741 (Alliance). J Natl Cancer Inst 2015;107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat 2010;123:627–35. [DOI] [PubMed] [Google Scholar]
- [9].Wang X, Li L, Gao J, et al. The association between body size and breast cancer in Han women in Northern and Eastern China. Oncologist 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bhandari R, Kelley GA, Hartley TA, et al. Metabolic syndrome is associated with increased breast cancer risk: a systematic review with meta-analysis. Int J Breast Cancer 2014;2014:189384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Berrino F, Villarini A, Traina A, et al. Metabolic syndrome and breast cancer prognosis. Breast Cancer Res Treat 2014;147:159–65. [DOI] [PubMed] [Google Scholar]
- [12].Azab B, Bhatt VR, Phookan J, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol 2012;19:217–24. [DOI] [PubMed] [Google Scholar]
- [13].Guthrie GJ, Charles KA, Roxburgh CS, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 2013;88:218–30. [DOI] [PubMed] [Google Scholar]
- [14].Proctor MJ, McMillan DC, Morrison DS, et al. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br J Cancer 2012;107:695–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ethier JL, Desautels D, Templeton A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res 2017;19:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Faria SS, Fernandes PC, Jr, Silva MJ, et al. The neutrophil-to-lymphocyte ratio: a narrative review. Ecancermedicalscience 2016;10:702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Koh CH, Bhoo-Pathy N, Ng KL, et al. Utility of pre-treatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer 2015;113:150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ozyalvacli G, Yesil C, Kargi E, et al. Diagnostic and prognostic importance of the neutrophil lymphocyte ratio in breast cancer. Asian Pac J Cancer Prev 2014;15:10363–6. [DOI] [PubMed] [Google Scholar]
- [19].Hong J, Mao Y, Chen X, et al. Elevated preoperative neutrophil-to-lymphocyte ratio predicts poor disease-free survival in Chinese women with breast cancer. Tumour Biol 2016;37:4135–42. [DOI] [PubMed] [Google Scholar]
- [20].Iwase T, Sangai T, Sakakibara M, et al. An increased neutrophil-to-lymphocyte ratio predicts poorer survival following recurrence for patients with breast cancer. Mol Clin Oncol 2017;6:266–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 2009;12:223–6. [DOI] [PubMed] [Google Scholar]
- [22].Liu J, Grundy SM, Wang W, et al. Ethnic-specific criteria for the metabolic syndrome: evidence from China. Diabetes Care 2006;29:1414–6. [DOI] [PubMed] [Google Scholar]
- [23].Chinese Diabetes Society Metabolic Syndrome Research Group. Metabolic syndrome: suggestion from Chinese Diabetes Society. Chin J Diab 2004;12:156–61. [in Chinese]. [Google Scholar]
- [24].Inoue M, Noda M, Kurahashi N, et al. Japan Public Health Center-based Prospective Study Group. Impact of metabolic factors on subsequent cancer risk: results from a large-scale population-based cohort study in Japan. Eur J Cancer Prev 2009;18:240–7. [DOI] [PubMed] [Google Scholar]
- [25].Noh HM, Song YM, Park JH, et al. Metabolic factors and breast cancer risk in Korean women. Cancer Causes Control 2013;24:1061–8. PMID: 23504150; DOI: 10.1007/s10552-013-0183-3. [DOI] [PubMed] [Google Scholar]
- [26].Osaki Y, Taniguchi S, Tahara A, et al. Metabolic syndrome and incidence of liver and breast cancers in Japan. Cancer Epidemiol 2012;36:141–7. [DOI] [PubMed] [Google Scholar]
- [27].Wang M, Cheng N, Zheng S, et al. Metabolic syndrome and the risk of breast cancer among postmenopausal women in north-west China. Climacteric 2015;18:852–8. [DOI] [PubMed] [Google Scholar]
- [28].Wu AH, Vigen C, Butler LM, et al. Metabolic conditions and breast cancer risk among Los Angeles County Filipina Americans compared with Chinese and Japanese Americans. Int J Cancer 2017;141:2450–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen Y, Zhang Y, Zhao G, et al. Difference in leukocyte composition between women before and after menopausal age, and distinct sexual dimorphism. PLoS One 2016;11:e0162953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Parekh N, Chandran U, Bandera EV. Obesity in cancer survival. Annu Rev Nutr 2012;32:311–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Schmitz KH, Neuhouser ML, Agurs-Collins T, et al. Impact of obesity on cancer survivorship and the potential relevance of race and ethnicity. J Natl Cancer Inst 2013;105:1344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Okuturlar Y, Gunaldi M, Tiken EE, et al. Utility of peripheral blood parameters in predicting breast cancer risk. Asian Pac J Cancer Prev 2015;16:2409–12. [DOI] [PubMed] [Google Scholar]
- [33].Queen MM, Ryan RE, Holzer RG, et al. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res 2005;65:8896–904. [DOI] [PubMed] [Google Scholar]
- [34].Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature 2015;528:413–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].DeMichele A, Yee D, Esserman L. Mechanisms of resistance to neoadjuvant chemotherapy in breast cancer. N Engl J Med 2017;377:2287–9. [DOI] [PubMed] [Google Scholar]
- [36].Wei B, Yao M, Xing C, et al. The neutrophil lymphocyte ratio is associated with breast cancer prognosis: an updated systematic review and meta-analysis. Onco Targets Ther 2016;9:5567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Aydin M, Yilmaz A, Donma MM, et al. Neutrophil/lymphocyte ratio in obese adolescents. North Clin Istanb 2015;2:87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest 2011;121:2111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Meng G, Zhu Q, Shao J, et al. Comparing the diagnostic ability of inflammatory markers in metabolic syndrome. Clin Chim Acta 2017;475:1–6. [DOI] [PubMed] [Google Scholar]
- [40].Welty FK, Alfaddagh A, Elajami TK. Targeting inflammation in metabolic syndrome. Transl Res 2016;167:257–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Buyukkaya E, Karakas MF, Karakas E, et al. Correlation of neutrophil to lymphocyte ratio with the presence and severity of metabolic syndrome. Clin Appl Thromb Hemost 2014;20:159–63. [DOI] [PubMed] [Google Scholar]
- [42].Surendar J, Indulekha K, Mohan V, et al. Association of neutrophil-lymphocyte ratio with metabolic syndrome and its components in Asian Indians (CURES-143). J Diabetes Complications 2016;30:1525–9. [DOI] [PubMed] [Google Scholar]
- [43].Bahadır A, Baltacı D, Türker Y, et al. Is the neutrophil-to-lymphocyte ratio indicative of inflammatory state in patients with obesity and metabolic syndrome? Anatol J Cardiol 2015;15:816–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


