Abstract
Lymphovascular invasion (LVI) has been a predictor of worse survival outcomes in breast cancer. However, the role of LVI compared than pathologic complete response (pCR) following neoadjuvant chemotherapy (NAC) remains unclear. The aim of this study was to examine the association between LVI and survival outcomes and clinicopathological features in patients with breast cancer treated with NAC. We retrospectively analyzed 187 patients with breast cancer treated with NAC and surgery between 2005 and 2013 in our institution. Kaplan–Meier analyses were used to assess recurrence-free survival (RFS) and overall survival (OS). Median follow-up was 57.9 months. Mastectomy (vs breast conserving surgery [BCS]; hazard ratio [HR], 1.791; 95% confidence interval [CI], 1.022–3.139; P = .042), ypN1-3 stage (vs ypN0 stage; HR, 2.561; 95% CI, 1.247–5.261; P = .010), and LVI (vs no LVI; HR, 2.041; 95% CI, 1.170–3.562; P = .012) were associated with worse RFS. Mastectomy (vs BCS; HR, 2.768; 95% CI, 1.173–6.535; P = .020), LVI (vs no LVI; HR, 3.474; 95% CI, 1.646–7.332, P = .001), and human epidermal growth factor receptor 2 overexpression type (vs luminal A type; HR, 11.360; 95% CI, 1.501–85.972; P = .019) were associated with worse OS. Patients with LVI and hormone receptor-negative cancer had the worst RFS (P < .001) and OS (P < .001). LVI more than pCR in surgical breast cancer specimens obtained after NAC was a significant independent prognostic factor. Patients with hormonal receptor-negative cancer and LVI had unfavorable survival outcomes. We suggest that patients with hormone receptor-negative cancer and LVI should receive short-term follow-up and appropriate management.
Keywords: breast cancer, lymphovascular invasion, neoadjuvant chemotherapy
1. Introduction
Neoadjuvant chemotherapy (NAC) is the standard treatment for locally advanced breast cancer and inflammatory breast cancer. NAC not only results in sufficient reduction of tumor size to make surgery feasible, but may also lead to the possibility of breast conserving surgery (BCS) in women requiring a mastectomy. In addition, assessment of chemo-sensitivity could be conducted in advance of adjuvant treatment.[1] Breast cancer is a heterogeneous disease with diverse phenotypes, so responses to NAC differ and prognosis varies. Patient’ age, tumor size, the presence of lymph node involvement, histologic grade, hormone receptor status, and pathologic complete response (pCR) following NAC are well known as prognostic factors in breast cancer.[2–6]
Lymphovascular invasion (LVI) is defined as the presence of tumor cells within a definite endothelial-lined space (lymphatics or blood vessels) in the breast surrounding invasive carcinoma. The presence of LVI is associated with an increased risk of axillary lymph node and distant metastases.[7] Although the mechanism of LVI has not been completely elucidated, LVI is considered as a prognostic factor in patients with operable breast cancer with or without metastatic axillary lymph nodes who are undergoing adjuvant treatment.[8,9] However, few studies have reported on the significance of LVI in patients with breast cancer treated with NAC. The purpose of this study was to evaluate the importance of LVI and analyze clinical outcomes of patients with breast cancer treated with NAC and surgery.
2. Methods
2.1. Patients
We retrospectively reviewed medical records of patients that received NAC and surgery in Chonnam National Hwasun Hospital between 2005 and 2013. Of 269 patients analyzed, 31 had distant organ metastasis at initial diagnosis, 6 patients had bilateral breast cancer, and 8 patients had metastatic contralateral axillary or mediastinal lymph nodes at initial diagnosis, while other secondary malignancies except for papillary thyroid cancer were detected in 5 patients during the follow-up period. Differentiation between luminal A and luminal B subtypes was not possible in 32 patients with luminal breast cancer (hormone receptor-positive, negative for human epidermal growth factor receptor 2 [HER2]) owing to lack of information regarding Ki-67 in pathologic reports. Thus, 187 patients treated with NAC and surgery were included in the study. This study was approved by the institutional review board of our hospital. We analyzed menopausal status; underlying disease; surgical method utilized in breast and axilla; simultaneous neck dissection; histologic type and grade; clinical stage; pathological T and N stage after NAC (ypT and ypN stage); coexistence of ductal carcinoma in situ (DCIS); tumor size; LVI; status of estrogen receptor (ER), progesterone receptor (PR), and HER2; adjuvant treatment; recurrence; and survival.
2.2. Treatment
All patients were diagnosed with carcinoma through core needle biopsy or excisional biopsy in breast lesions, and fine-needle aspiration cytology (FNAC) was conducted in cases of lymph nodes suspicious for metastasis. Breast magnetic resonance image (MRI) and positron emission tomography–computed tomography (PET-CT) were conducted to identify distant metastasis and contralateral breast lesions prior to NAC. After 3 to 6 cycles of NAC, breast MRI and chest CT were conducted to evaluate the tumor response. All patients received NAC consisting of an anthracycline (adriamycin or epirubicin), taxane, or both. Five patients received trastuzumab in combination with NAC. BCS was considered in all patients. However, mastectomy was performed in patients with multicentric lesions, nipple invasion, expectation of cosmetic problems, and lack of negative frozen resection margin status, as well as for patients who refused BCS. No patients received immediate reconstruction. Axillary lymph node dissection (ALND) was performed in cases in which FNAC confirmed metastatic lymph nodes. However, patients with clinical N0 stage underwent sentinel lymph node biopsy prior to ALND. Patients with metastatic ipsilateral supraclavicular lymph nodes before NAC received neck ultrasonography after NAC. If supraclavicular lymph nodes were visible before surgery, neck dissection was performed. Adjuvant chemotherapy was administered in 3 to 6 cycles of the same regimen of NAC. Omission of adjuvant chemotherapy was considered according to achievement of pCR, severe chemotherapy-induced side effects, or poor performance status. Patients with hormone receptor-positive cancer were treated with hormonal therapy after adjuvant chemotherapy or surgery. Radiation therapy was considered for adjuvant treatment, except in cases of patients who underwent mastectomy and achieved pCR. Trastuzumab therapy was available from 2010 and was administered to patients who were positive for HER2.
2.3. Pathology
Histologic type was confirmed based on a diagnosis before NAC because of pCR. According to American Society of Clinical Oncology/College of American Pathologists guideline recommendations, tumors were ER or PR positive if staining was positive in cell nuclei in at least 1% of tumor cells examined.[10] Immunohistochemical analysis for HER2 status was scored 0, 1+, 2+, and 3+. Scores of 0 and 1+ were negative, and a score of 3+ was positive for HER2. When a score of 2+ was found, additional fluorescence in situ hybridization (FISH) or silver in situ hybridization (SISH) was conducted to evaluate HER2 gene amplification status. Our hospital used FISH before 2008 and SISH after 2009. Positive HER2 status was defined as an HER2 gene/chromosome 17 ratio of ≥2.0.[11] Molecular subtype was classified according to ER, PR, HER2 status, and value of Ki-67: luminal A (ER+ or PR+, HER2−, Ki-67 < 14%); luminal B (ER+ or PR+, HER2−, Ki-67 ≥ 14%; ER+ or PR+, HER2+); HER overexpression (ER−, PR−, HER2+); and triple negative (ER−, PR−, HER2−).[12] pCR was defined as lack of histopathologic evidence of residual invasive cancer cells in the breast or axillary lymph nodes, only residual in situ cancer.[13]
LVI was defined as the presence of carcinoma cells within a definite endothelial-lined space. LVI was assessed on the basis of the boundaries of the surgical specimens obtained after NAC on hematoxylin and eosin (H&E) stained slides. When the results were uncertain, specific markers, D2-40 for lymphatic endothelium and CD34 for endothelium of all vessels, were used to improve detection of LVI.
2.4. Statistical analysis
Chi-squared, Fisher exact, and t tests were used to compare relevant clinicopathological variables according to the presence or absence of LVI. Events were defined as local/regional recurrence, distant metastasis, contralateral invasive breast cancer, or death. Times of recurrence-free survival (RFS) and overall survival (OS) were calculated from date of initial surgery to date of first event or death or last follow-up (in cases without events). The Kaplan–Meier method and log-rank test were used to compare survival differences among groups according to RFS and OS in univariate analysis. For multivariate analysis, the Cox proportional hazards model was used for variables showing statistical significance in univariate analysis. All statistical analyses were performed using SPSS version 23.0 (IBM Inc, Armonk, NY). Statistical significance defined as a P value <.05.
3. Results
3.1. Patients demographics
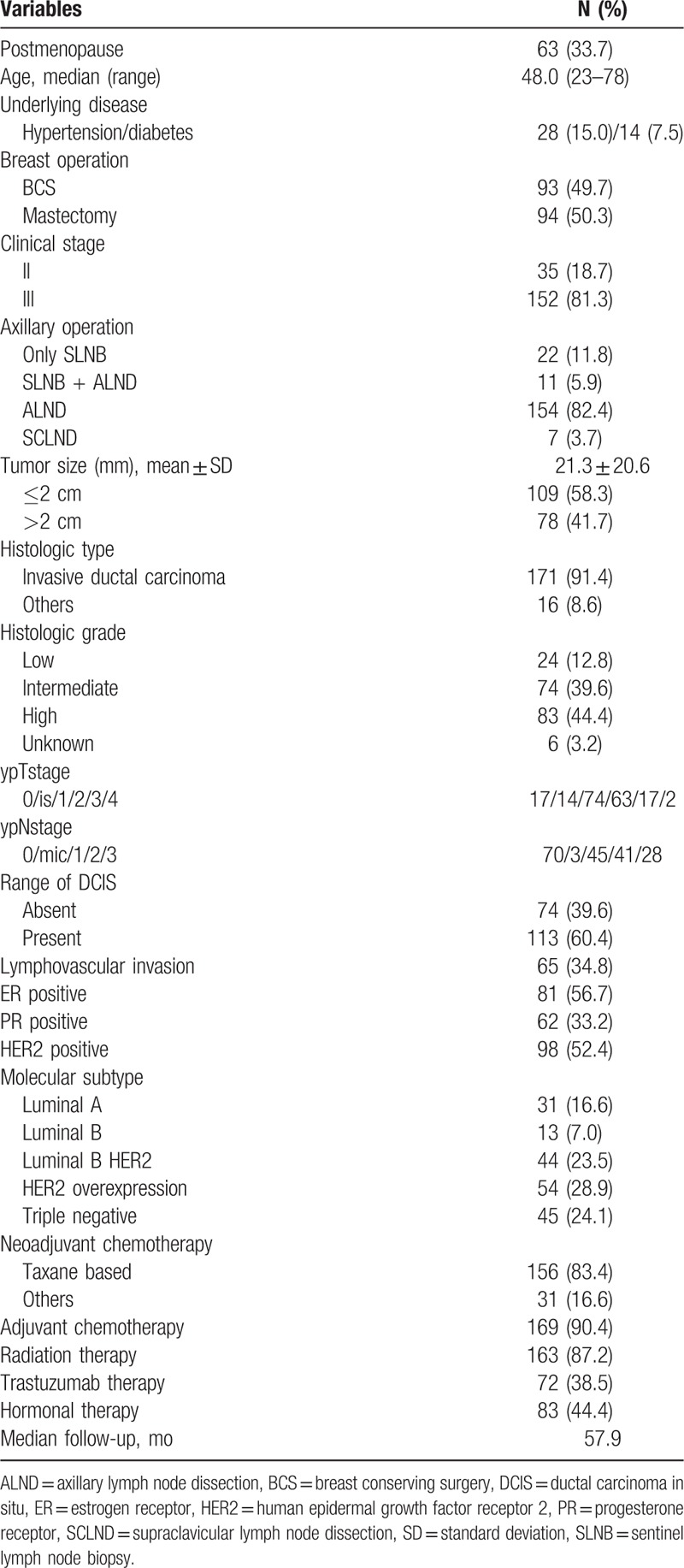
All 187 patients were female and 63 (33.7%) were of postmenopausal status. Median age (range) was 48 (23–78). Among the patients, 93 (49.7%) underwent BCS and 94 (50.3%) underwent mastectomy. Only sentinel lymph node biopsy for axillary lymph nodes staging was performed in 22 (11.8%) patients, while the remaining patients underwent ALND. The mean tumor size was 21.3 mm (standard deviation ± 20.6), and 79 (41.7%) patients had primary tumors >2 cm in size. The histologic type in 171 (91.4%) patients was invasive ductal carcinoma. High histologic grade was found in 83 (44.4%) patients, followed by intermediate grade (74 patients, 39.6%) and low grade (24 patients, 12.8%). Findings for ypT stage were as follows: ypT0, 17 patients (9.1%); ypTis, 14 (7.5%); ypT1, 74 (39.5%); ypT2, 63 (33.7%); ypT3, 17 (9.1%); and ypT4, 2 (1.1%). Findings for ypN stage were as follows: ypN0, 70 patients (37.4%); ypN1mic, 3 (1.6%); ypN1, 45 (24.1%); ypN2, 41 (21.9%); and ypN3, 28 (15.0%); 74 (39.6%) patients had no in situ lesions around invasive component in specimens, and 64 (34.8%) patients showed LVI. The patient distribution according to molecular subtype 31 (16.6%) with luminal A, 13 (7.0%) with luminal B HER2 negative, 44 (23.5%) with luminal B HER2 positive, 54 (28.9%) with HER2 overexpression, and 45 (24.1%) with triple negative. Among the study patients, 156 (83.4%) were administered anthracycline- and taxane-based NAC and 31 (16.6%) patients received anthracycline-based NAC. Adjuvant chemotherapy was administered to 160 (90.4%) patients after surgery. The median follow-up period was 57.9 months (range, 2–147 months) (Table 1).
Table 1.
Patients demographics.
3.2. Variables associated with LVI
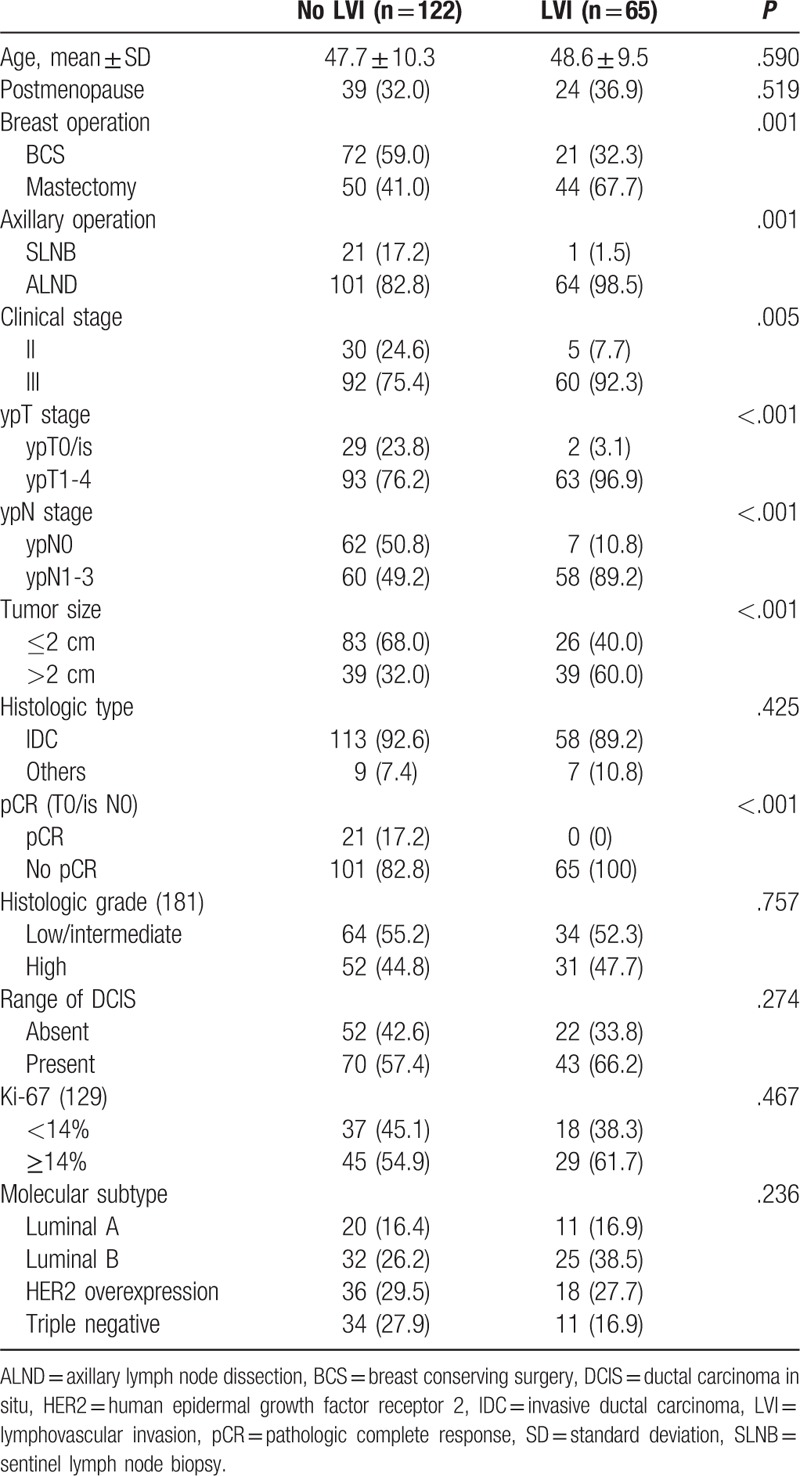
The mean patient age was 47.7 in the no LVI group and 48.6 in the LVI group. Patients in the LVI group had higher rates of mastectomy (P = .001) and ALND (P = .001). The LVI group tended to have advanced status in terms of disease burden; therefore, clinical III stage (vs clinical II, P = .005), ypT1-4 stage (vs ypT0/is stage, P < .001), ypN1-3 stage (vs ypN0 stage, P < .001), and primary tumors larger than 2 cm in size (vs ≤2 cm primary tumor, P < .001) had statistical significance in the LVI group. There was no association of histologic type, histologic grade, range of DCIS, value of Ki-67, or molecular subtype according to presence or absence of LVI. There were no patients who achieved pCR in the LVI group, as assumed in accordance with the definition of pCR, while 21 patients in the no LVI group achieved pCR (Table 2).
Table 2.
Characteristic of patients according to LVI status.
3.3. Survival outcomes
Of the total 187 patients, recurrence was observed in 59 (31.6%) patients. Locoregional recurrence was found in 20 (10.7%) patients; 21 (11.2%) patients had distant metastases; and 13 (7.0%) had both types of recurrence during the follow-up period. Five (2.7%) patients experienced invasive contralateral breast cancer. Among all study patients, 34 (18.2%) died from cancer progression during the follow-up (Table 3).
Table 3.
Type of events.

In univariate analysis of association with recurrence, mastectomy (P = .003), ALND (P = .028), ypN1-3 stage (P < .001), pCR (P = .040), and LVI (P < .001) showed statistically significant differences. However, no differences were observed in RFS with menopausal status, histologic type and grade, ypT stage, range of DCIS, tumor size, and value of Ki-67. In the univariate analysis examining the association with cancer-related death, mastectomy (P = .001), ypN1-3 stage (P = .006), LVI (P < .001), and other molecular type (vs luminal A type, P = .043) had statistical significance. However, there was no association of cancer-related death with menopausal status, method of axillary operation, histologic type and grade, ypT stage, pCR, range of DCIS, tumor size, and value of Ki-67 (Table 4).
Table 4.
Univariate analysis according to recurrence and cancer-related death.

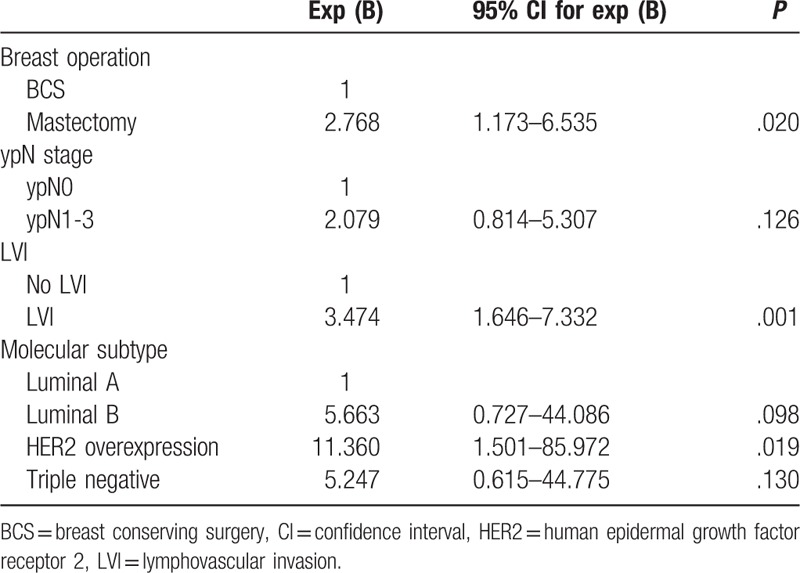
In multivariate analysis of association with recurrence, mastectomy (vs BCS; HR, 1.791; 95% CI, 1.022–3.139; P = .042), ypN1-3 stage (vs ypN0 stage; HR, 2.561; 95% CI, 1.247–5.261; P = .010), and LVI (vs no LVI; HR, 2.041; 95% CI, 1.170–3.562; P = .012) were associated with worse RFS (Table 5). Mastectomy (vs BCS; HR, 2.768; 95% CI, 1.173–6.535; P = .020), LVI (vs no LVI; HR, 3.474; 95% CI, 1.646–7.332, P = .001), and HER2 overexpression type (vs luminal A type; HR, 11.360; 95% CI, 1.501–85.972; P = .019) were associated with worse OS (Table 6).
Table 5.
Multivariate analyses according to recurrence.
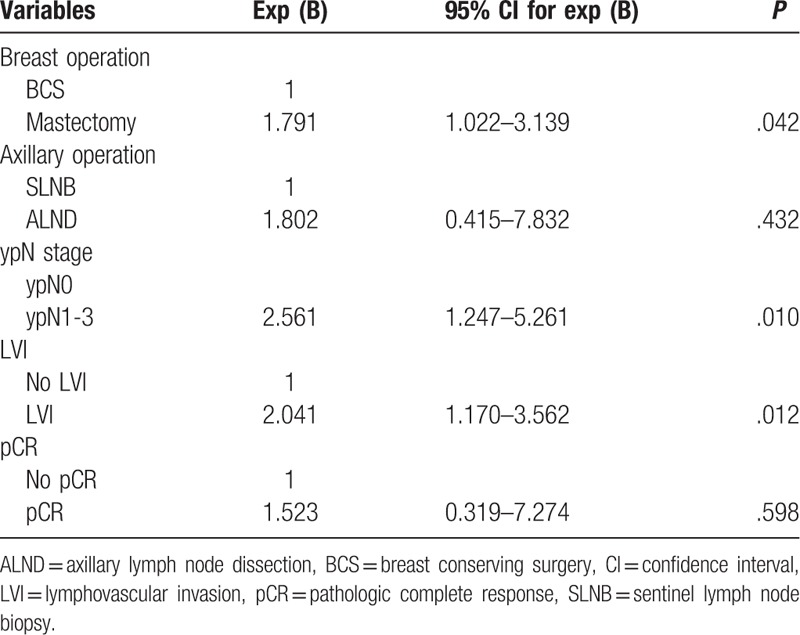
Table 6.
Multivariate analyses according to overall survival.
Hormone receptor status had no impact on RFS and OS. However, when stratified by hormone receptor status and LVI, the patients with LVI and negative hormone receptor status had the worst RFS and OS (Fig. 1).
Figure 1.
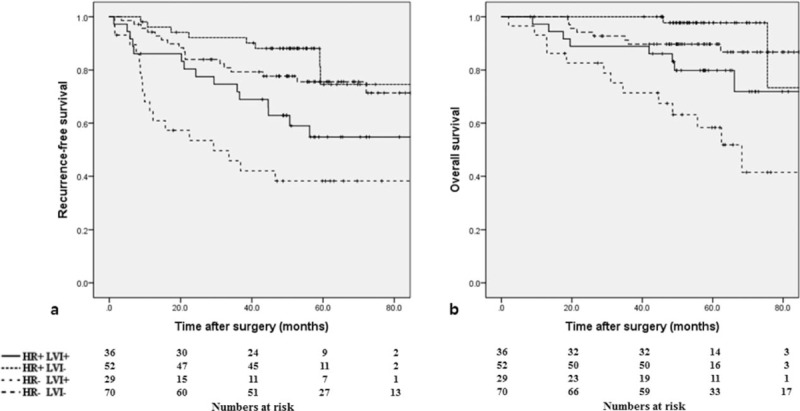
(A) Recurrence-free survival curve and (B) overall survival curve according to hormone receptor (HR) and lymphovascular invasion (LVI) (P < .001 and P < .001, respectively).
4. Discussion
NAC is the generally accepted standard treatment for locally advanced and inflammatory breast cancer. It offers the possibility of resection for patients with unresectable breast cancer and makes BCS feasible for those with no option but mastectomy. The result of a large phase III study (NSABP B-18) including 1523 patients with operable breast cancer revealed that there were no survival differences between preoperative or postoperative administration of doxorubicin and cyclophosphamide.[14] The addition of preoperative or postoperative taxane after preoperative administration of anthracycline and cyclophosphamide (NSABP B-27) resulted in slight improvement of RFS due to a lower level of local recurrence; however, there was no significant impact on the OS.[15] Based on these 2 studies, anthracycline and taxane have been widely used not only adjuvant setting, but as NAC. Adding taxane to anthracycline-based chemotherapy has achieved rates of pCR as high as 30%.[16] A meta-analysis suggested that NAC was equivalent to adjuvant chemotherapy in terms of disease progression and survival.[17] In this study, 186 patients were treated with anthracycline- or taxane-based chemotherapy. One patient was administered cyclophosphamide, methotrexate, and 5-fluorouracil (known as CMF) because of poor performance status.
Heterogeneous characteristics of breast cancer are related to various responses to NAC. Hormone receptor status, HER2 status, histologic grade, tumor size, and nodal involvement are well known for predictive factors of response to NAC. In addition, some studies have suggested that patients who achieved pCR had better survival outcomes than those with residual tumor burden.[18,19] The definition of pCR is varies according to the research groups proposing it: No invasive or noninvasive residual cancer in breast or lymph nodes allowed (ypT0 ypN0) by the German Breast Group; no invasive residual cancer in breast or lymph nodes/noninvasive breast residual cancer allowed (ypT0/is ypN0) by the MD Anderson Cancer Center (MDACC); no invasive residual cancer in the breast/noninvasive breast residual cancer and infiltrated lymph nodes allowed (ypT0/is ypN0/+) by National Surgical Adjuvant Breast and Bowel Project (NSABP).[20] This study followed the MDACC criteria, based on the finding that the presence of only residual DCIS in the breast following NAC did not adversely affect survival or recurrence rate.[21]
Previous studies have reported that biological markers are associated with pCR after NAC. Patients treated with NAC were more likely to achieve pCR when negative for ER or positive for HER2 and treated with trastuzamab.[22,23] Among 21 (11.2%) patients who attained pCR in this study, 5 were hormone receptor-positive and 16 were hormone receptor-negative. In accordance with aforementioned studies, this study showed that 1 patient with luminal A molecular type achieved pCR, while 7 patients with triple negative breast cancer attained pCR. One metaregression analysis revealed that pCR is not a surrogate end point for clinical outcome in breast cancer.[24] Interestingly, the patients who achieved pCR in this study had relatively prolonged RFS in univariate analysis; however, no significant differences were found in the multivariate analysis between the patients who did not achieve pCR, and RFS and OS. Although patients with luminal A molecular type had a lower rate of pCR, they experienced better OS compared with other molecular subtypes. Thus, pCR may be a good predictive factor for patients with ER-negative breast cancer, in contrast to those with ER-positive cancer. These results were consistent with the finding that pCR is a more applicable marker in tumors with HER2 overexpression or in triple negative breast cancer than in luminal type breast cancer.[20] The frequency of pCR among patients with hormone receptor-positive cancer was statistically lower than in those with hormone receptor-negative cancer. Interestingly, this study revealed that only complete remission of axillary lymph node spread had statistical significance in OS irrespective of residual primary tumor in breast. Axillary lymph node response following NAC may be a key early surrogate marker of long-term outcome in breast cancer.[25]
LVI refers to invasion to lymphatic spaces, blood vessels, or both in the peritumoral area by tumor emboli. Although the mechanism of LVI has not been clearly proven, LVI could reflect a surrounding tumor microenvironment that predicts underlying aggressive tumor and worse prognosis. One study reported that LVI is not an independent predictor of locoregional control or survival in early-stage breast cancer.[26] However, LVI is an independent prognostic factor in lymph node negative breast cancer treated with adjuvant chemotherapy and in operable breast cancer with positive axillary lymph nodes.[8,9] Uematsu et al[27] classified the degrees of LVI as no, minimal, moderate, and marked. They suggested that the degree of LVI is an important factor for prediction of NAC efficacy in breast cancer. Liu et al[28] reported that patients with no LVI with tumors positive for hormone receptor or HER2 overexpression had the most favorable RFS and OS when stratified by molecular subtype. Additionally, the authors showed that the patients with LVI and triple negative subtype had the worst RFS and OS. The population-based study showed that the presence of LVI in patients with operable breast cancer was associated with shorter RFS and OS in high-risk patients (positive lymph nodes, tumor size >2 cm, high histologic grade, negative hormone receptor status, or age younger than 35 years).[7]
In this study, LVI was observed in 65 (34.8%) patients. Patients with LVI had high rates of mastectomy and ALND because these patients had more advanced tumor characteristics. Therefore, the patients with LVI had greater severity of tumor burden and lymph node involvement after NAC. Among the patients with LVI, there were none who achieved pCR, as we had expected. However, this study did not show that pCR indicated better survival outcomes. To the contrary, LVI showed a significant association with recurrence and death. When LVI was stratified by hormone receptor status, the patients with hormone receptor-positive cancer and no LVI had the most favorable survival outcomes. Hormone receptor-negative status with LVI was associated with higher risk of recurrence and death.
One study reported that the grade of LVI in surgical specimens obtained after NAC was significantly associated with increasing HRs for tumor recurrence and tumor-related death.[29] Another study showed that absence of LVI in surgical specimens following NAC correlated with pathologic response.[30] Therefore, assessment of LVI in surgical specimens acquired after NAC is important for prediction of survival outcomes.
This study had limitations. The first was that this retrospective study included only a small cohort of patients from in a single institution, some of whom underwent relatively short-term follow-up. Further validation by prospective studies using larger cohort is therefore needed. The second limitation was that not all patients with HER2 overexpression were treated with trastuzumab. The third is that LVI was not routinely assessed with selective endothelial cell markers such as CD34 and D2-40 that could potentially improve the accuracy of LVI detection, even though LVI was assessed by H&E staining.
In conclusion, even though pCR did not have meaningful significance, pathologic remission of axillary lymph nodes was an influential prognostic factor in patients with breast cancer treated with NAC and surgery. LVI in surgical breast cancer specimens obtained after NAC was a significant independent prognostic factor. Patients with hormone receptor-positive cancer did not have a survival benefit compared to those with hormone receptor-negative cancer. However, when LVI was stratified according to presence or absence of hormone receptor, the patients with hormone receptor-negative status and LVI had unfavorable rates of recurrence and cancer-related death. We suggest that patients with hormone receptor-negative cancer and LVI should receive short-term follow-up and appropriate management.
Author contributions
Conceptualization: Young Jae Ryu.
Data curation: Young Jae Ryu, Jin Seong Cho.
Formal analysis: Jin Seong Cho.
Funding acquisition: Young Jae Ryu.
Investigation: Min Ho Park.
Methodology: Min Ho Park.
Resources: Shin Jae Kang.
Software: Shin Jae Kang.
Supervision: Jung Han Yoon.
Validation: Jung Han Yoon.
Writing – original draft: Young Jae Ryu.
Writing – review and editing: Young Jae Ryu, Min Ho Park.
Footnotes
Abbreviations: ALND = axillary lymph node dissection, BCS = breast conserving surgery, DCIS = ductal carcinoma in situ, ER = estrogen receptor, FISH = fluorescence in situ hybridization, FNAC = fine-needle aspiration cytology, H&E = hematoxylin and eosin, HER2 = human epidermal growth factor receptor 2, LVI = lymphovascular invasion, MDACC = MD Anderson Cancer Center, MRI = magnetic resonance image, NAC = neoadjuvant chemotherapy, NSABP = National Surgical Adjuvant Breast and Bowel Project, OS = overall survival, pCR = pathologic complete response, PET-CT = positron emission tomography-computed tomography, PR = progesterone receptor, RFS = recurrence-free survival, SISH = silver in situ hybridization.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Sachelarie I, Grossbard ML, Chadha M, et al. Primary systemic therapy of breast cancer. Oncologist 2006;11:574–89. [DOI] [PubMed] [Google Scholar]
- [2].Fisher CS, Ma CX, Gillanders WE, et al. Neoadjuvant chemotherapy is associated with improved survival compared with adjuvant chemotherapy in patients with triple-negative breast cancer only after complete pathologic response. Ann Surg Oncol 2012;19:253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tsuji W, Teramukai S, Ueno M, et al. Prognostic factors for survival after first recurrence in breast cancer: a retrospective analysis of 252 recurrent cases at a single institution. Breast Cancer 2014;21:86–95. [DOI] [PubMed] [Google Scholar]
- [4].Love RR, Duc NB, Dinh NV, et al. Young age as an adverse prognostic factor in premenopausal women with operable breast cancer. Clin Breast Cancer 2002;2:294–8. [DOI] [PubMed] [Google Scholar]
- [5].Schwartz AM, Henson DE, Chen D, et al. Histologic grade remains a prognostic factor for breast cancer regardless of the number of positive lymph nodes and tumor size: a study of 161 708 cases of breast cancer from the SEER Program. Arch Pathol Lab Med 2014;138:1048–52. [DOI] [PubMed] [Google Scholar]
- [6].Lai HW, Kuo SJ, Chen LS, et al. Prognostic significance of triple negative breast cancer at tumor size 1 cm and smaller. Eur J Surg Oncol 2011;37:18–24. [DOI] [PubMed] [Google Scholar]
- [7].Ejlertsen B, Jensen MB, Rank F, et al. Population-based study of peritumoral lymphovascular invasion and outcome among patients with operable breast cancer. J Natl Cancer Inst 2009;101:729–35. [DOI] [PubMed] [Google Scholar]
- [8].Lee AH, Pinder SE, Macmillan RD, et al. Prognostic value of lymphovascular invasion in women with lymph node negative invasive breast carcinoma. Eur J Cancer 2006;42:357–62. [DOI] [PubMed] [Google Scholar]
- [9].Song YJ, Shin SH, Cho JS, et al. The role of lymphovascular invasion as a prognostic factor in patients with lymph node-positive operable invasive breast cancer. J Breast Cancer 2011;14:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med 2010;134:907–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997–4013. [DOI] [PubMed] [Google Scholar]
- [12].Goldstein NS, Decker D, Severson D, et al. Molecular classification system identifies invasive breast carcinoma patients who are most likely and those who are least likely to achieve a complete pathologic response after neoadjuvant chemotherapy. Cancer 2007;110:1687–96. [DOI] [PubMed] [Google Scholar]
- [13].Green MC, Buzdar AU, Smith T, et al. Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol 2005;23:5983–92. [DOI] [PubMed] [Google Scholar]
- [14].Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 1998;16:2672–85. [DOI] [PubMed] [Google Scholar]
- [15].Bear HD, Anderson S, Smith RE, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 2006;24:2019–27. [DOI] [PubMed] [Google Scholar]
- [16].Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 2008;26:778–85. [DOI] [PubMed] [Google Scholar]
- [17].Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst 2005;97:188–94. [DOI] [PubMed] [Google Scholar]
- [18].Kim H, Park W, Huh SJ, et al. Clinical outcomes according to molecular subtypes in stage II-III breast cancer patients treated with neoadjuvant chemotherapy followed by surgery and radiotherapy. Asia Pac J Clin Oncol 2017;13:329–36. [DOI] [PubMed] [Google Scholar]
- [19].Luangdilok S, Samarnthai N, Korphaisarn K. Association between pathological complete response and outcome following neoadjuvant chemotherapy in locally advanced breast cancer patients. J Breast Cancer 2014;17:376–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 2012;30:1796–804. [DOI] [PubMed] [Google Scholar]
- [21].Mazouni C, Peintinger F, Wan-Kau S, et al. Residual ductal carcinoma in situ in patients with complete eradication of invasive breast cancer after neoadjuvant chemotherapy does not adversely affect patient outcome. J Clin Oncol 2007;25:2650–5. [DOI] [PubMed] [Google Scholar]
- [22].Ring AE, Smith IE, Ashley S, et al. Oestrogen receptor status, pathological complete response and prognosis in patients receiving neoadjuvant chemotherapy for early breast cancer. Br J Cancer 2004;91:2012–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 2010;375:377–84. [DOI] [PubMed] [Google Scholar]
- [24].Berruti A, Amoroso V, Gallo F, et al. Pathologic complete response as a potential surrogate for the clinical outcome in patients with breast cancer after neoadjuvant therapy: a meta-regression of 29 randomized prospective studies. J Clin Oncol 2014;32:3883–91. [DOI] [PubMed] [Google Scholar]
- [25].Hennessy BT, Hortobagyi GN, Rouzier R, et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol 2005;23:9304–11. [DOI] [PubMed] [Google Scholar]
- [26].Freedman GM, Li T, Polli LV, et al. Lymphatic space invasion is not an independent predictor of outcomes in early stage breast cancer treated by breast-conserving surgery and radiation. Breast J 2012;18:415–9. [DOI] [PubMed] [Google Scholar]
- [27].Uematsu T, Kasami M, Watanabe J, et al. Is lymphovascular invasion degree one of the important factors to predict neoadjuvant chemotherapy efficacy in breast cancer? Breast Cancer 2011;18:309–13. [DOI] [PubMed] [Google Scholar]
- [28].Liu YL, Saraf A, Lee SM, et al. Lymphovascular invasion is an independent predictor of survival in breast cancer after neoadjuvant chemotherapy. Breast Cancer Res Treat 2016;157:555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tamura N, Hasebe T, Okada N, et al. Tumor histology in lymph vessels and lymph nodes for the accurate prediction of outcome among breast cancer patients treated with neoadjuvant chemotherapy. Cancer Sci 2009;100:1823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sullivan PS, Apple SK. Should histologic type be taken into account when considering neoadjuvant chemotherapy in breast carcinoma? Breast J 2009;15:146–54. [DOI] [PubMed] [Google Scholar]


