Abstract
Epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) are the standard first-line treatment for EGFR-mutant nonsmall cell lung cancer (NSCLC) patients. However, studies have reported that not all NSCLC patients harboring kinase domain mutations in epidermal growth factor receptor (EGFR) show significant clinical benefits from EGFR-targeted tyrosine kinase inhibitors (TKIs). Therefore, it is necessary to establish feasible biomarkers to predict the prognosis of EGFR-mutant NSCLC patients treated with EGFR-TKIs. This study aimed to determine biomarkers using inflammatory parameters from complete blood counts to predict the prognosis of EGFR-mutant NSCLC patients treated with EGFR-TKIs.
We retrospectively investigated 127 stage IIIB/IV NSCLC patients with activating EGFR mutations who were treated with EGFR-TKIs. We used receiver operating characteristic (ROC) curves to determine the optimal cut-off for the inflammatory markers as prognostic factors. Additionally, univariate and multivariate analyses were used to identify prognostic factors for progression-free survival (PFS) and overall survival (OS) of EGFR-mutant NSCLC patients treated with EGFR-TKIs.
The receiver operating characteristic analysis indicated that the lymphocyte-to-monocyte ratio (LMR) and neutrophil-to-lymphocyte ratio (NLR) cut-off values were 3.37 and 2.90, respectively. The univariate analysis showed that a high LMR (>3.37) and low NLR (≤2.90) were significantly correlated with long-term PFS and OS (LMR, P = .007; NLR, P < .001). The multivariate Cox regression analysis revealed that only low NLR was an independent prognostic factor for long-term PFS and OS (PFS, HR = 0.573, 95% CI: 0.340–0.964, P = .036; OS, HR = 0.491, 95% CI: 0.262–0.920, P = .026).
The data show that a low NLR was a good prognostic factor in EGFR-mutant NSCLC patients receiving EGFR-TKIs treatment. Moreover, the NLR measurement has better prognostic value than LMR.
Keywords: epidermal growth factor receptor, lymphocyte-to-monocyte ratio, neutrophil-to-lymphocyte ratio, nonsmall cell lung cancer, tyrosine kinase inhibitors
1. Introduction
Lung cancer is one of most aggressive tumors and is a leading cause of cancer death worldwide.[1] Nonsmall cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancer cases, and approximately 70% of patients with NSCLC are initially diagnosed with advanced stage disease, which results in poor prognosis.[2] The epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) gefitinib and erlotinib are part of a new treatment strategy for NSCLC patients with EGFR mutations and have improved the progression-free survival (PFS), overall survival (OS), and quality of life in patients compared to traditional platinum-based combination chemotherapy.[3–6] Epidermal growth factor receptor (EGFR) gene-activating mutations are strong predictive factors of response and survival to EGFR-TKIs.[7,8] It is generally accepted that 2 classical mutations (exon 19-del and exon 21-L858R) can enhance the sensitivity of tumor cells to EGFR-TKIs and are considered to be an effective predictor of the efficacy of EGFR-TKIs.[9] However, not all EGFR-mutated patients with nonsmall cell lung cancer show benefits from EGFR-TKIs. Although some patients benefit from EGFR-TKIs for more than 2 years, 20% to 30% of NSCLC cases have intrinsic or primary resistance to EGFR-TKIs despite harboring an activating EGFR mutation.[10] Therefore, it is critical to elucidate the factors influencing EGFR-TKIs response and establish feasible biomarkers to predict the efficacy of EGFR-TKIs.
Previous studies have investigated response biomarkers that can predict the prognosis of EGFR-TKIs efficacy using the next generation sequencing and other molecular analyses. However, these tests are expensive and difficult to perform and are impractical as routine exams. Thus, finding an effective way to evaluate the efficacy of EGFR-TKIs using routine clinical laboratory tests during tumor therapy will benefit advanced NSCLC patients.
Several recent studies evaluating the relationship between the immune system and tumors showed that the immune system plays important roles in killing tumor cells and preventing tumor growth while also providing an inflammatory microenvironment that fosters tumor growth via a process called immuno-editing.[11,12] It has been reported that the immune response profile and inflammatory signature in several cancers may provide useful information on patient prognosis and treatment.[13,14]
Complete blood count (CBC) is one of the most common laboratory tests performed in the clinic. The absolute count of neutrophils, lymphocytes, and monocytes reflects the inflammatory response and overall immune status of the body. Peripheral blood prognostic inflammatory markers including the neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), platelet-to-lymphocyte ratio (PLR), and red cell distribution width (RDW) are associated with patient prognosis and treatment outcome.[15–18] However, there are a limited number of reports about the relationship between these inflammatory markers and the efficacy of EGFR-TKIs in advanced NSCLC patients with EGFR mutations.
In this study, we conducted a retrospective analysis to assess the value of the inflammatory parameters obtained from CBCs in predicting the prognosis in EGFR-mutant NSCLC patients treated with EGFR-TKIs. Our results indicated NLR as an independent prognostic biomarker for PFS and OS in NSCLC patients with EGFR mutations following EGFR-TKIs treatment.
2. Materials and methods
2.1. Patient and clinical characteristics
This study was approved by the institutional research ethics board. We retrospectively analyzed the clinical data of NSCLC patients at the Affiliated Tumor Hospital of Xinjiang Medical University between January 2013 and December 2015. The patients were followed-up until July 2017. The following inclusion criteria were used: adult patient aged 18 years or older; histologically or cytologically confirmed NSCLC; clinical stage IIIB or IV; harbor activating EGFR mutation (exon 19-del and exon 21 L858R); at least one evaluation of lesions according to the response evaluation criteria in solid tumors (RECIST); Eastern Cooperative Oncology Group (ECOG) performance status between 0 to 4; and treatment with EGFR-TKI as a first-line cancer therapy. The study exclusion criteria were the following: patients with other malignancies, infection, or hematological or autoimmune diseases; patients who are allergic and/or intolerant to EGFR-TKIs.
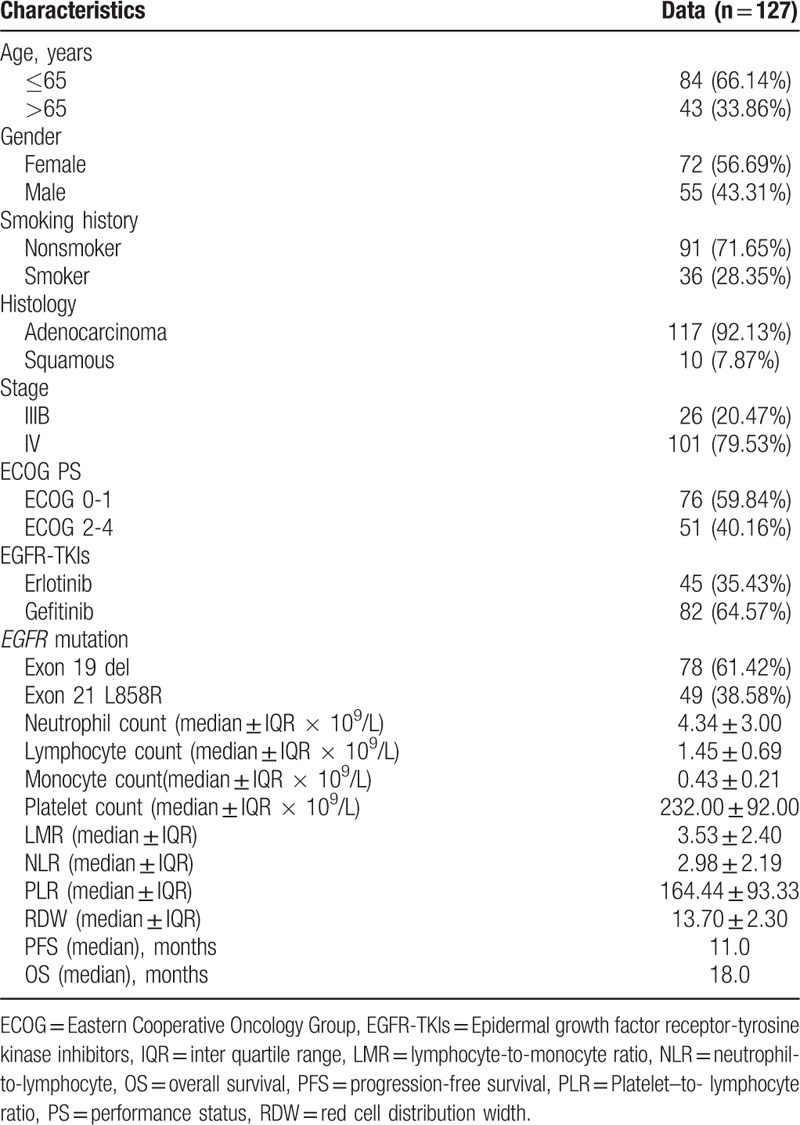
The following patient clinical characteristics were obtained: general condition, medical history, tumor pathology, ECOG performance status, EGFR mutation type, treatment history, laboratory values, and imaging data.
2.2. Treatment and monitoring methods
Patients received gefitinib (250 mg/day) or erlotinib (150 mg/day) until detection of progressive disease or intolerable toxicity. We obtained informed consent from all patients prior to treatment.
The patient disease baseline status was assessed 2 weeks prior to the initiation of EGFR-TKIs treatment. The disease assessments including clinical parameters, hematological parameters, biochemistry, tumor markers and chest radiography were performed every 4 weeks. The chest computed tomography (CT) or position emission tomography computed tomography (PET-CT) was performed every 2 to 3 months. Disease progression was assessed according to Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1).[19] The survival indicators for progression-free survival (PFS) are defined as the time from the initiation of EGFR-TKIs to disease progression, death before documented progression, or the last follow-up time. The patient overall survival (OS) is defined as the time from the initiation of EGFR-TKIs to death or last follow-up.
2.3. Sample collection
Venous blood samples were collected in ethylene diamine tetraacetic acid (EDTA) anticoagulant tubes. The tumor tissues were mainly obtained from CT-guided biopsies and were then fixed with 10% buffered formalin and embedded in paraffin. The blood samples and tissues were collected no >7 days before initiating EGFR-TKIs treatment.
2.4. EGFR mutation testing
Genomic DNA was extracted from 5 sections of 10 μm thickness using the QIAamp DNA FFPE Tissue kit (Qiagen, Hilden, Germany). EGFR mutations were tested by an amplification refractory mutation system (ARMS) using the ADx-ARMS EGFR mutation test kit (Amoy Diagnostics, Xiamen, China) according to manufacturer's instructions.
2.5. Complete blood count (CBC) testing
The CBC with differential was tested by the automated hematology analyzer Sysmex XE-5000. The laboratory information collected from the CBC included the white blood cell count, absolute neutrophil, monocyte and lymphocyte counts, platelet counts and red cell distribution width (RDW). The peripheral LMR was calculated as the ratio of peripheral lymphocytes to monocytes. The peripheral NLR and PLR were calculated as the ratio of the neutrophils and platelets to lymphocytes.
2.6. Statistical analysis
The clinical characteristics of the patients were analyzed by descriptive statistics. Pearson's chi-square test, Fisher's exact test, or Student's t test was used to compare the baseline clinical characteristics between different groups as applicable. The receiver operating characteristic (ROC) curves and Youden's index were utilized to determine the optimal cut-off for the inflammatory markers (white blood cell count, absolute neutrophil, monocyte and lymphocyte counts, platelet counts, and red cell distribution width) as prognostic factors. The univariate analysis of PFS and OS outcomes in the study groups was performed using the Kaplan–Meier method and the log-rank test. A multivariate Cox proportional hazard model was used to further identify independent prognostic factors for PFS and OS. All statistical assessments were 2-sided. All results with P-values <0.05 were considered statistically significant. All analyses were performed using SPSS software (version 19.0., SPSS Inc., Chicago).
3. Results
3.1. Patient characteristics
There were 1371 stage IIIB/IV NSCLC patients with EGFR mutation testing results. Among these patients, 575 patients harbored activating EGFR mutations of either an exon 19 microdeletion or exon 21 point mutation (L858R). There were 227 patients treated with first-line EGFR-TKIs (gefitinib or erlotinib). There were 40 excluded patients who stopped EGFR-TKIs treatment before disease progression and 54 patients who were lost to follow-up. There were 6 patients who had no laboratory data before EGFR-TKI treatment. Thus, there were 127 patients enrolled in the final analysis (Fig. 1). The patient demographic and baseline characteristics are shown in Table 1. The mean age of the study population was 61.9 years. After a mean follow-up time of 28.12 months (range, 3–49), there were 108 patients who experienced disease progression, and 69 patients died. The median PFS was 11.0 months, and the median OS was 18.0 months. The median counts of neutrophils, lymphocytes, monocytes, and platelets were 4.34 × 109 cells/L (range, 1.17–11.02 × 109 cells/L), 1.45 × 109 cells/L (range, 0.27–3.85 × 109 cells/L), 0.43 × 109 cells/L (range, 0.03–1.28 × 109 cells/L), and 232.00 × 109 cells/L (range, 68–518 × 109 cells/L), respectively. The median NLR, LMR, PLR, and RDW were 2.98 (range, 0.62–29.53), 3.53 (range, 0.63–79.00), 164.44 (range, 48.76–618.52) and 13.7 (range, 11.40–19.90), respectively.
Figure 1.
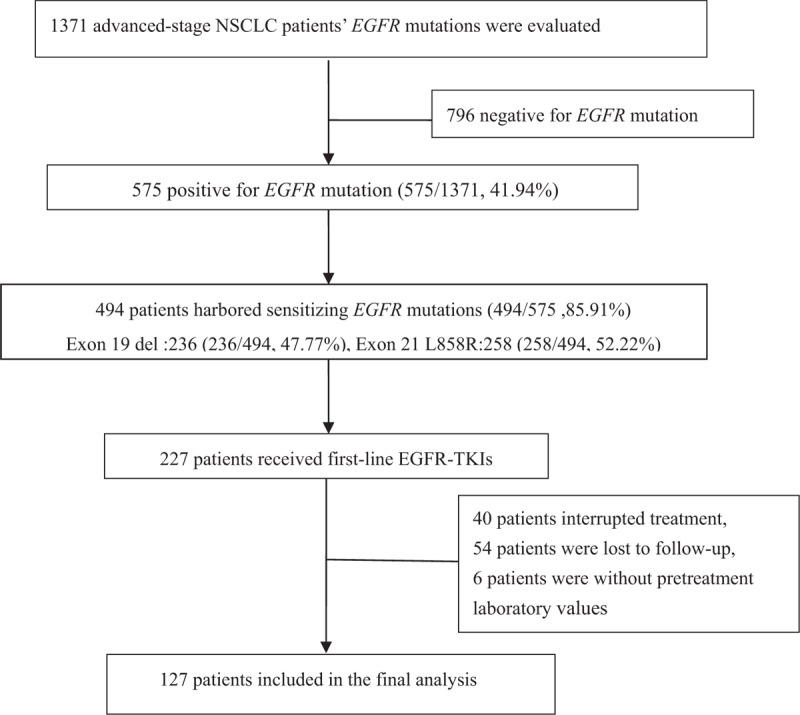
Flowchart for selecting patients.
Table 1.
Clinical characteristics and therapy responses of 127 EGFR mutant NSCLC patients.
3.2. Determination of the best immunologic parameter cut-off values
We used PFS longer or shorter than 10 months as the binary variable for receiver operating characteristic (ROC) curves. According to the highest Youden index (specificity+sensitivity–1), the optimal cut-off value chosen for the LMR was 3.37, with an area under the curve (AUC) value of 0.652 [95% confidence interval (CI), 0.557–0.747, P = .003] (Fig. 2A). The most discriminating cut-off value of NLR was 2.90, with an area under the curve (AUC) value of 0.668 [95% confidence interval (CI), 0.572–0.764, P = .001] (Fig. 2B). However, we did not identify significant cut-off values for the PLR and RDW. In a subsequent analysis all patients’ LMR and NLR were divided into high-level and low-level groups according to the optimal cutoff values.
Figure 2.
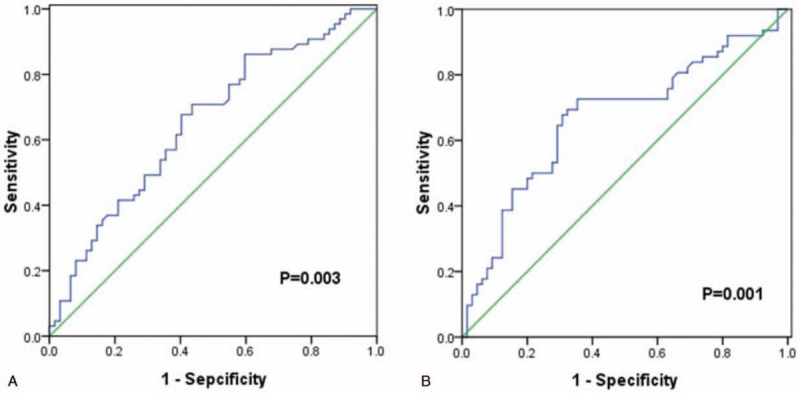
Receiver operating characteristic (ROC) curves analysis for lymphocyte-to-monocyte ratio (LMR) and neutrophil-to-lymphocyte ratio (NLR) in enrolled non-small cell lung cancer patients. (A) ROC curve analysis for LMR in enrolled nonsmall cell lung cancer patients. (B) ROC curves analysis for neutrophil-to-lymphocyte ratio (NLR) in enrolled nonsmall cell lung cancer patients. LMR = lymphocyte-to-monocyte ratio, NLR = neutrophil-to-lymphocyte, ROC = receiver operating characteristic.
3.3. Correlation of NSCLC patients’ characteristics with the LMR and NLR The univariate analysis between clinical factors
The baseline characteristics of the NSCLC patients according to the NLR and the LMR are listed in Table 2. We analyzed the correlation of the baseline characteristics for the NSCLC patients and the LMR and NLR. The results showed that there was no significant difference regarding the patient's age, smoking history, histology, stage, ECOG performance status, EGFR-TKIs, or EGFR mutations between 2 groups of high-level and low-level LMR. However, female NSCLC patients (P = .013), decreased neutrophil count (P = .002), increased lymphocyte count (P < .001), and decreased monocyte count (P < .001) were significantly associated with high-level LMR.
Table 2.
The associations between inflammatory marks and clinical features.
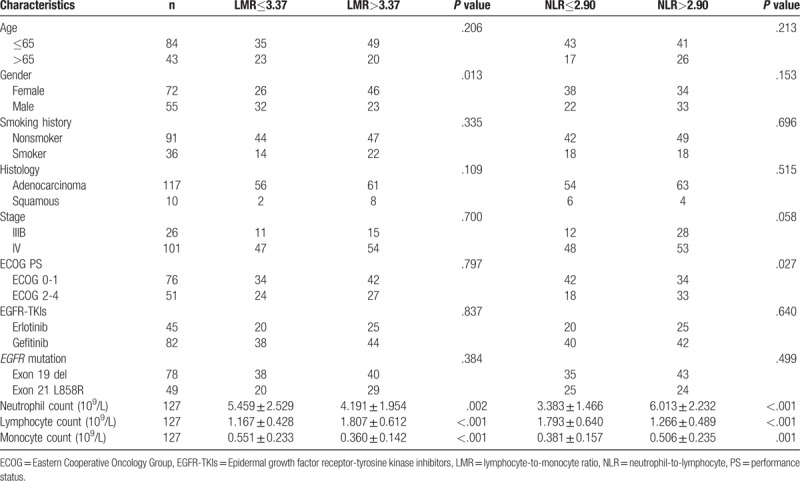
There was no significant difference identified regarding patient's age, gender, smoking history, histology, stage, ECOG performance status, EGFR-TKIs, or EGFR mutation between the high-level and low-level NLR groups. However, increased neutrophil count (P < .001), decreased lymphocyte count (P < .001) and increased monocyte count (P = .001) were significantly associated with high-level NLR.
3.4. Association between clinical factors of NSCLC patients and survival
We subsequently investigated the association between clinical factors of NSCLC patients and survival in the univariate analysis and multivariate Cox regression analysis.
The univariate analysis between clinical factors, inflammatory markers, and PFS showed that a longer PFS duration was significantly correlated with low ECOG PS (ECOG 0-1) (P < .001), high LMR (P = .007) (Fig. 3A) and low NLR (P < .001) (Fig. 3B). However, age, gender, history of smoking, type of histology, EGFR-TKIs, and EGFR mutation had no significant influence on PFS (Table 3). The multivariate Cox regression analysis revealed that the independent predictive factors for longer PFS were low ECOG PS (hazard ratio 0.467, 95% confidence interval: 0.299–0.731, P = .001), exon 19 del (hazard ratio 0.507, 95% confidence interval: 0.327–0.786, P = .002) and low NLR (hazard ratio 0.573, 95% confidence interval: 0.340–0.964, P = .036) (Table 4).
Figure 3.
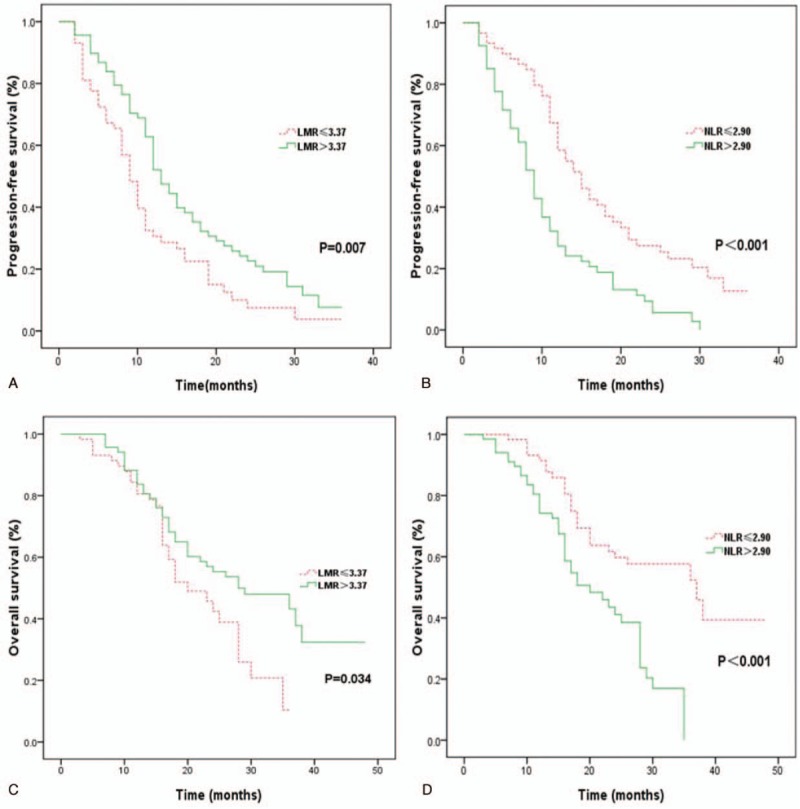
Kaplan–Meier curves for EGFR mutant non-small cell lung cancer patients treated with epidermal growth factor receptor-tyrosine kinase inhibitors. (A) PFS between high and low baseline lymphocyte-to-monocyte ratio (LMR) patients; (B) PFS between high and low neutrophil-to-lymphocyte ratio (NLR) patients; (C) OS between high and low baseline LMR patients; (D) OS between high and low baseline NLR patients. LMR = lymphocyte-to-monocyte ratio, NLR = neutrophil-to-lymphocyte, OS = overall survival, PFS = progression-free survival.
Table 3.
Univariate analysis of prognostic factors for progression-free survival and overall survival in 127 NSCLC patients with EGFR mutations.
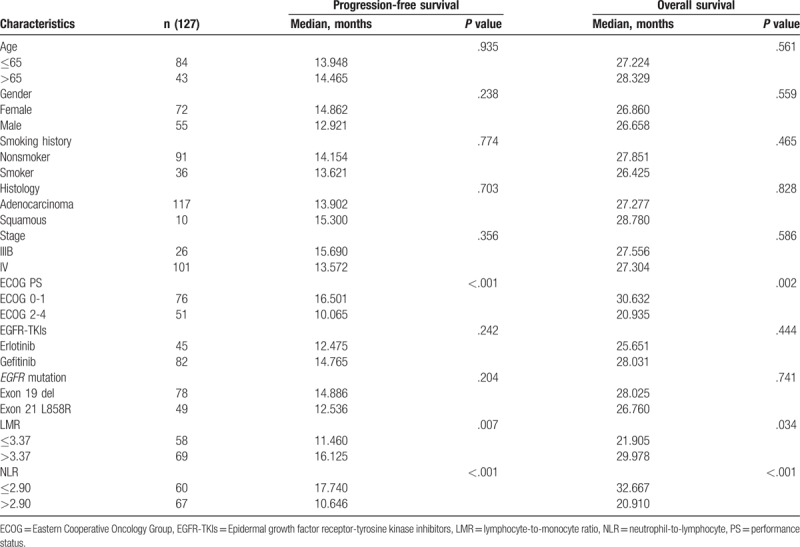
Table 4.
Multivariate Cox proportional regression analyses of prognostic factors for progression-free survival and overall survival.
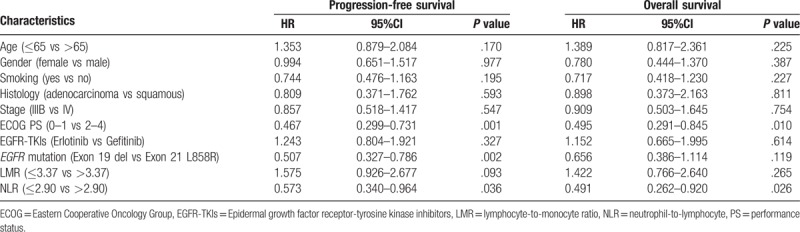
In the univariate analysis, there were significant correlations between longer OS and low ECOG PS (P = .002), high LMR (P = .034) (Fig. 3C), and low NLR (P < .001) (Fig. 3D). OS duration had no significant correlation with age, gender, history of smoking, type of histology, EGFR-TKIs, or EGFR mutation (Table 3). However, the Cox proportional multivariate hazard model revealed that low ECOG PS (hazard ratio 0.495, 95% confidence interval: 0.291–0.845, P = .010) and low NLR (hazard ratio 0.491, 95% confidence interval: 0.262–0.920, P = .026) were independent prognostic factors correlated with longer OS (Table 4).
4. Discussion
The analysis of prognostic factors affecting tumor therapy can facilitate the use of an optimal management strategy. In this retrospective study, we investigated the prognostic values of inflammatory parameters from CBC and other clinical factors in NSCLC patients with EGFR mutations treated with EGFR-TKIs. Our results demonstrated that decreased NLR and increased LMR were significantly associated with longer survival, PFS and OS in EGFR-mutant NSCLC patients following treatment with EGFR-TKIs. However, the multivariate analysis Cox model indicated that only the NLR remained an independent significant predictor of PFS and OS. The LMR had no prognostic significance for NSCLC patient survival. It is possible that the strong predictive ability of NLR affected the predicative function of LMR in our multivariate analysis. Thus, NLR was superior to LMR in the prognosis of EGFR-mutant NSCLC patients treated with EGFR-TKIs.
It has been shown that the tumor microenvironment (TME) orchestrates tumorigenesis and malignant progression. The TME significantly influences both tumor therapeutic response and its efficacy.[20] Additionally, tumor-associated neutrophils (TANs), tumor infiltrating lymphocytes (TILs), and tumor associated macrophages (TAMs) are important components of the TME and regulate the inflammatory response. These cells have also been identified as prognostic factors in malignant tumors including NSCLC.[21,22] The TANs in pretreatment peripheral blood are correlated with high-grade invasive histologic subtypes of lung adenocarcinomas and were identified as an independent prognostic factor using large cohorts of patients with advanced NSCLC.[23,24] NSCLC patients with high levels of CD3+ TILs in tumor lesions and IL-2-expressing tumors had significantly better 5-year OS rates.[25] It has also been shown that low PD-L1 expression in CD8+ TILs is associated with longer PFS and OS in NSCLC patients.[22] High TAM infiltration is closely related to drug resistance and poor prognosis in various cancers including NSCLC.[26,27] In addition, high TAMs were significantly related to poor progression-free survival and overall survival in EGFR-mutant NSCLC patients.[28,29]
Several recent clinical studies indicated the lymphocyte-to-monocyte ratio (LMR) and neutrophil-to-lymphocyte ratio (NLR) in pretreatment peripheral blood had significant prognostic values in patients with malignant tumors.[30–33] Jia et al[34] showed the LMR and NLR were significantly associated with survival of triple-negative breast cancer patients, but the neutrophil, lymphocyte and monocyte counts alone were not associated. Our results also indicate low LMR and high NLR were significantly associated with short-term PFS and OS in EGFR-mutant NSCLC patients treated with EGFR-TKIs. Recently, Chen et al[35] reported that baseline and LMR trends were prognostic factors in EGFR-mutant NSCLC patients treated with EGFR-TKIs. However, other studies reported that low NLR values were strongly correlated with better PFS and OS in EGFR-mutated NSCLC patients receiving EGFR-TKIs.[36] In our studies, we found that both LMR and NLR were significantly correlated with the survival of EGFR-mutant NSCLC patients in the univariate analysis. Moreover, the multivariate Cox regression analysis found that high NLR was superior to low LMR as an independent significant predictor of long-term PFS and OS in EGFR-mutant NSCLC patients treated with EGFR-TKIs.
Our study results suggest that EGFR-mutant NSCLC patients with high NLR levels at baseline might have poor survival outcomes if simply treated with EGFR-TKIs. For these NSCLC patients, alternating or combination chemotherapy strategies with EGFR-TKIs may achieve ideal therapeutic effects. However, further clinical studies are required. The major limitation of our research is that it is a retrospective study. Therefore, we could not control for underlying positive or negative biases during the treatment or selection of patients in our analysis. There are also limitations due to its single-institute retrospective design. Thus, we believe that it is important to validate these data in future multicenter prospective studies.
5. Conclusions
The NLR and LMR are reliable and convenient biomarkers for predicting the outcomes of NSCLC patients with EGFR mutations who were treated with EGFR-TKIs. The high LMR and low NLR were significantly associated with better PFS and OS in the univariate analysis. The multivariate analysis indicated that low NLR is an independent prognostic biomarker for long-term PFS and OS and is superior to high LMR in advanced NSCLC patients after EGFR-TKI treatment. The NLR was directly derived from CBC data and can be easily applied in clinical practice.
Author contributions
Data curation: Yuan Zhang, Yang-Chun Feng, Hong-Ge Zhu, Jia Song.
Formal analysis: Yuan Zhang, Yang-Chun Feng.
Investigation: Yang-Chun Feng, Hong-Ge Zhu.
Methodology: Ting-Chuan Xiong.
Project administration: Yuan Zhang, Yan-Shen Hou.
Resources: Yan-Shen Hou.
Supervision: Wei Jiang.
Writing – original draft: Yuan Zhang, Chang-Jun Zhu.
Writing – review & editing: Wei Jiang.
Footnotes
Abbreviations: ARMS = amplification refractory mutation system, CBC = complete blood count, CT = computed tomography, ECOG = Eastern Cooperative Oncology Group, EDTA = ethylene diamine tetraacetic acid, EGFR = epidermal growth factor receptor, EGFR-TKIs = epidermal growth factor receptor-tyrosine kinase inhibitors, LMR = lymphocyte-to-monocyte ratio, NLR = neutrophil-to-lymphocyte ratio, NSCLC = nonsmall cell lung cancer, OS = overall survival, PET-CT = position emission tomography computed tomography, PFS = progression-free survival, PLR = platelet-to-lymphocyte ratio, PS = performance status, RDW = red cell distribution width, RECIST = response evaluation criteria in solid tumors, ROC = receiver operating characteristic, TAMs = tumor associated macrophages, TANs = tumor-associated neutrophils, TILs = tumor infiltrating lymphocytes, TKIs = tyrosine kinase inhibitors, TME = tumor microenvironment.
Funding: This work was supported by the Joint Funds of the Xinjiang Uygur Autonomous Region Natural Science Foundation (2016D01C375 to YZ).
The authors have no conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin 2009;59:225–49. [DOI] [PubMed] [Google Scholar]
- [3].An N, Zhang Y, Niu H, et al. EGFR-TKIs versus taxanes agents in therapy for nonsmall-cell lung cancer patients: a PRISMA-compliant systematic review with meta-analysis and meta-regression. Medicine (Baltimore) 2016;95:e5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fan L, Yang H, Yao F, et al. Clinical outcomes of epidermal growth factor receptor tyrosine kinase inhibitors in recurrent adenosquamous carcinoma of the lung after resection. Onco Targets Ther 2017;10:239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kuykendall A, Chiappori A. Advanced EGFR mutation-positive non-small-cell lung cancer: case report, literature review, and treatment recommendations. Cancer Control 2014;21:67–73. [DOI] [PubMed] [Google Scholar]
- [6].Zhou C, Wu YL, Chen G, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol 2015;26:1877–83. [DOI] [PubMed] [Google Scholar]
- [7].Kanthala S, Pallerla S, Jois S. Current and future targeted therapies for non-small-cell lung cancers with aberrant EGF receptors. Future Oncol 2015;11:865–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Khalil FK, Altiok S. Advances in EGFR as a predictive marker in lung adenocarcinoma. Cancer Control 2015;22:193–9. [DOI] [PubMed] [Google Scholar]
- [9].Yang Y, Zhang B, Li R, et al. EGFR-tyrosine kinase inhibitor treatment in a patient with advanced non-small cell lung cancer and concurrent exon 19 and 21 EGFR mutations: a case report and review of the literature. Oncol Lett 2016;11:3546–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang J, Wang B, Chu H, et al. Intrinsic resistance to EGFR tyrosine kinase inhibitors in advanced non-small-cell lung cancer with activating EGFR mutations. Onco Targets Ther 2016;9:3711–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011;331:1565–70. [DOI] [PubMed] [Google Scholar]
- [12].Vesely MD, Kershaw MH, Schreiber RD, et al. Natural innate and adaptive immunity to cancer. Annu Rev Immunol 2011;29:235–71. [DOI] [PubMed] [Google Scholar]
- [13].Kristensen VN, Vaske CJ, Ursini-Siegel J, et al. Integrated molecular profiles of invasive breast tumors and ductal carcinoma in situ (DCIS) reveal differential vascular and interleukin signaling. Proc Natl Acad Sci U S A 2012;109:2802–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Meniawy TM, Lake RA, McDonnell AM, et al. PD-L1 on peripheral blood T lymphocytes is prognostic in patients with non-small cell lung cancer (NSCLC) treated with EGFR inhibitors. Lung Cancer 2016;93:9–16. [DOI] [PubMed] [Google Scholar]
- [15].Guo D, Han A, Jing W, et al. Preoperative to postoperative change in neutrophil-to-lymphocyte ratio predict survival in colorectal cancer patients. Future Oncol 2018;14:1187–96. [DOI] [PubMed] [Google Scholar]
- [16].Hirahara N, Matsubara T, Mizota Y, et al. Prognostic value of preoperative inflammatory response biomarkers in patients with esophageal cancer who undergo a curative thoracoscopic esophagectomy. BMC Surg 2016;16:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kang KH, Efird JT, Sharma N, et al. Prognostic potential of neutrophil-to-lymphocyte ratio and lymphocyte nadir in stage III non-small-cell lung cancer. Future Oncol 2017;13:1405–14. [DOI] [PubMed] [Google Scholar]
- [18].Tangthongkum M, Tiyanuchit S, Kirtsreesakul V, et al. Platelet to lymphocyte ratio and red cell distribution width as prognostic factors for survival and recurrence in patients with oral cancer. Eur Arch Otorhinolaryngol 2017;274:3985–92. [DOI] [PubMed] [Google Scholar]
- [19].Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- [20].Klemm F, Joyce JA. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol 2015;25:198–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mantovani A. Wandering pathways in the regulation of innate immunity and inflammation. J Autoimmun 2017;85:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mazzaschi G, Madeddu D, Falco A, et al. Low PD-1 expression in cytotoxic CD8+ tumor infiltrating lymphocytes confers an immune privileged tissue microenvironment in NSCLC with a prognostic and predictive value. Clin Cancer Res 2017;24:407–19. [DOI] [PubMed] [Google Scholar]
- [23].Rakaee M, Busund LT, Paulsen EE, et al. Prognostic effect of intratumoral neutrophils across histological subtypes of non-small cell lung cancer. Oncotarget 2016;7:72184–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Teramukai S, Kitano T, Kishida Y, et al. Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: an analysis of Japan Multinational Trial Organisation LC00-03. Eur J Cancer 2009;45:1950–8. [DOI] [PubMed] [Google Scholar]
- [25].Tian C, Lu S, Fan Q, et al. Prognostic significance of tumor-infiltrating CD8(+) or CD3(+) T lymphocytes and interleukin-2 expression in radically resected non-small cell lung cancer. Chin Med J (Engl) 2015;128:105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Heusinkveld M, van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med 2011;9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pei BX, Sun BS, Zhang ZF, et al. Interstitial tumor-associated macrophages combined with tumor-derived colony-stimulating factor-1 and interleukin-6, a novel prognostic biomarker in non-small cell lung cancer. J Thorac Cardiovasc Surg 2014;148:1208–16. [DOI] [PubMed] [Google Scholar]
- [28].Chung FT, Lee KY, Wang CW, et al. Tumor-associated macrophages correlate with response to epidermal growth factor receptor-tyrosine kinase inhibitors in advanced non-small cell lung cancer. Int J Cancer 2012;131:E227–235. [DOI] [PubMed] [Google Scholar]
- [29].Zhang B, Zhang Y, Zhao J, et al. M2-polarized macrophages contribute to the decreased sensitivity of EGFR-TKIs treatment in patients with advanced lung adenocarcinoma. Med Oncol 2014;31:127. [DOI] [PubMed] [Google Scholar]
- [30].Deng YX, Lin JZ, Peng JH, et al. Lymphocyte-to-monocyte ratio before chemoradiotherapy represents a prognostic predictor for locally advanced rectal cancer. Onco Targets Ther 2017;10:5575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jin F, Han A, Shi F, et al. The postoperative neutrophil-to-lymphocyte ratio and changes in this ratio predict survival after the complete resection of stage I non-small cell lung cancer. Onco Targets Ther 2016;9:6529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lee SF, Luque-Fernandez MA. Prognostic value of lymphocyte-to-monocyte ratio and neutrophil-to-lymphocyte ratio in follicular lymphoma: a retrospective cohort study. BMJ Open 2017;7:e017904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Song Y, Yang Y, Gao P, et al. The preoperative neutrophil to lymphocyte ratio is a superior indicator of prognosis compared with other inflammatory biomarkers in resectable colorectal cancer. BMC Cancer 2017;17:744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jia W, Wu J, Jia H, et al. The peripheral blood neutrophil-to-lymphocyte ratio is superior to the lymphocyte-to-monocyte ratio for predicting the long-term survival of triple-negative breast cancer patients. PLoS One 2015;10:e0143061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chen YM, Lai CH, Chang HC, et al. Baseline and trend of lymphocyte-to-monocyte ratio as prognostic factors in epidermal growth factor receptor mutant non-small cell lung cancer patients treated with first-line epidermal growth factor receptor tyrosine kinase inhibitors. PLoS One 2015;10:e0136252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ding PN, Roberts TL, Chua W, et al. Clinical outcomes in patients with advanced EGFR-mutated non-small cell lung cancer in South Western Sydney Local Health District. Intern Med J 2017;47:1405–11. [DOI] [PubMed] [Google Scholar]


