Abstract
Intraoperative cholangiography involving the excretion of fluorescent indocyanine green (ICG) into the bile is used to determine biliary anatomy in laparoscopic cholecystectomy (LC). This study aimed to evaluate the features of intraoperative ICG cholangiography, in LC with cholecystitis, and compared the delineation of the cystic duct (CD) between ICG cholangiography and magnetic resonance cholangiopancreatography (MRCP).
Participants comprised 65 patients undergoing LC using ICG cholangiography.
Fifty-eight patients (89.2%) were diagnosed with gallbladder stones and 32 (49.2%) with acute cholecystitis. ICG cholangiography identified CD in 54 patients (83.1%) and did not identify CD in 11 patients (16.9%). The mean value of the fluorescence intensity in the identified CD group by ICG cholangiography was 87.6 ± 31.5 arbitrary unit and that in the not identified CD group by ICG cholangiography was 24.4 ± 10.1 arbitrary unit (P < .001). Compared with the patients in the identified CD group, those in the not identified CD group had higher incidence of acute cholecystitis (P < .001), and higher conversion rates (P = .003). A correlation between the delineation of CD by ICG cholangiography and MRCP was analyzed, and it revealed a correlation between each other (P = .002)
Inflammation had harmful effects with regard to the passing of CD. If we can identify CD or common bile duct with ICG cholangiography, we may be able to perform LC with confidence, even in the presence of severe inflammation.
Keywords: acute cholecystitis, indocyanine green cholangiography, laparoscopic cholecystectomy, magnetic resonance cholangiopancreatography
1. Introduction
Laparoscopic cholecystectomy (LC) is one of the most common operations in the surgical field, with more than 60,000 operations performed in Japan and approximately 750,000 in the United States every year.[1] Bile duct injury is rare, with an incidence of 0.3% to 0.7%,[1] but it can lead to serious consequences. Surgery for cholecystitis tends to be difficult for even experienced doctors and has a high risk of complication.
Intraoperative fluorescent imaging with indocyanine green (ICG) has been employed for confirming the patency of vascular reconstruction surgery, liver transplantation,[2] anastomosis of the gastrointestinal tract,[3] brain aneurysms,[4] identification of sentinel lymph node navigation,[5] and hepatocellular carcinoma detection.[6] Recently, an intraoperative cholangiography technique in LC involving the excretion of fluorescent ICG in the bile after intravenous injection has been used to determine the bile duct anatomy.[1,7–9] Currently, some detailed reports[10,11] have been published on LC using intraoperative ICG cholangiography and suggested its safety and feasibility. In this study, we evaluated the features of intraoperative ICG cholangiography, including LC for cholecystitis, and compared the delineation of the cystic duct (CD) between ICG cholangiography and magnetic resonance cholangiopancreatography (MRCP).
2. Patients and methods
2.1. Patient characteristics
The study population comprised 65 patients undergoing LC using ICG cholangiography for gallbladder stones, gallbladder polyps, and acute cholecystitis between March 2015 and March 2017. Basically, all patients underwent preoperative MRCP or endoscopic retrograde cholangiopancreatography (ERCP) to estimate whether aberrant bile duct exists and especially, ERCP was performed for patients who were suspected common bile duct (CBD) stones. In this series, 8 other patients were excluded from the study because 5 of them had iodine allergy (as ICG contains iodine) and 3 had undergone open surgery from the start (Fig. 1).
Figure 1.

Patient's enrolment.
2.2. Informed consent
This study was approved by the ethics committees of Kagoshima Kouseiren Hospital (registration number 2017042601) and was conducted according to the ethical guidelines of the Declaration of Helsinki.
Written informed consent was obtained from each patient.
2.3. LC and ICG cholangiography
In our hospital, LC has been performed by young surgeons who have worked for several years under a supervisory doctor. For fluorescence, 2.5-mg ICG (2.5 mg/mL; Diagnogreen, Daiichi Sankyo, Tokyo, Japan) was intravenously injected approximately 2 hours before surgery. Laparoscopic imaging was performed using the D-LIGHT P System (Karl Storz Endoscopes, Tuttlingen, Germany) through a standard 12-mm umbilical trocar port. This imaging system comprises 2 wavelength-isolated light sources: a white light source and a near-infrared light source that produce light of 805-nm wavelength, and this imaging system detects infrared light of 835 nm. During operation, we have repeatedly performed ICG cholangiography before or after dissection of the Calot triangle. After dissecting the Calot triangle possibly, ICG cholangiography was performed to identify the anatomy of the CD and CBD and the picture was taken for the evaluation (Fig. 2). After that we made sure of critical view of safety and removed gallbladder. Then, we retrospectively evaluated the delineation of CD and CBD in each intraoperative picture of ICG cholangiography using Image J software (National Institutes of Health, Rockville, MD) and compared the fluorescence intensity between the identified CD or CBD group and the not identified CD or CBD group. Additionally, a correlation between the delineation of CD by ICG cholangiography and MRCP was analyzed.
Figure 2.
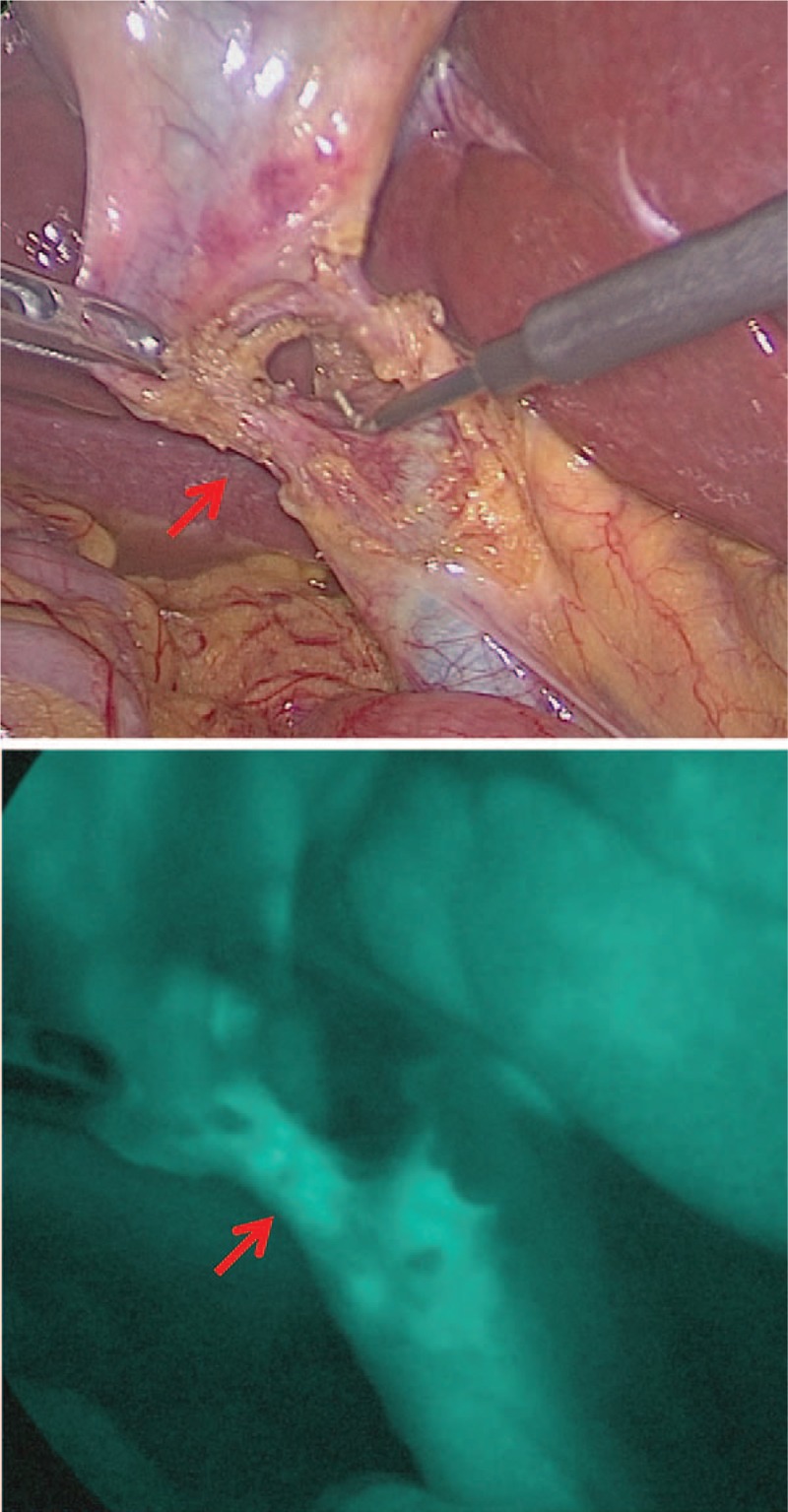
Upper panel was detected by a “white” light source and cystic duct was detected in lower panel by a “near infrared” light source after dissecting Calot triangle.
Surgical complications were evaluated by the Clavien–Dindo classification,[12] and a complication with a score higher than grade III was defined as surgical complication in this study.
2.4. Diagnosis for acute cholecystitis
We adopted the 2013 Tokyo Guidelines (TG13) as the criteria for acute cholecystitis.[13]
2.5. Clinical factors
Clinical factors selected for evaluation were age, gender, surgical complication, bleeding, operation time, hospital stay, with or without stone, with or without acute cholecystitis, with or without conversion, visualization of CBD and preoperative laboratory values (white blood cell [WBC], serum aspartate aminotransferase [AST], serum alanine aminotransferase [ALT], serum total bilirubin [T-Bil], serum alkaline phosphatase [ALP], serum albumin, and serum C-reactive protein [CRP]).
2.6. Statistical analyses
The Chi-squared test was used to evaluate categorical variables, and the unpaired t test was used to evaluate continuous variables. Data are presented as mean ± standard deviation. A probability (P) value of <.05 was considered statistically significant. Statistical analyses were performed using the SPSS statistical software package (version 24; SPSS, Chicago, IL).
3. Results
3.1. Baseline characteristics
Patients comprised 31 men and 34 women, with a median age of 61.34 (range, 32–90) years. Fifty-eight patients (89.2%) were preoperatively diagnosed with gallbladder stones and 7 patients (10.8%) with gallbladder polyp or adenomyomatosis. Concomitant diagnosis of CBD stones was made in 16 patients (24.6%). In the patients with gallbladder polyps, 1 (1.5%) was diagnosed with gallbladder cancer through postoperative pathologic examination. Thirty-two patients (49.2%) were diagnosed with acute cholecystitis according to TG13.[13] Seven patients (10.7%) were converted to open surgery. The median operation time was 132.82 (range, 30–255) minutes. There were no surgical complications with scores higher than grade III as per the Clavien–Dindo classification (Table 1). All patients underwent preoperative cholangiography, including MRCP (n = 56), and/or endoscopic retrograde cholangiography (n = 20).
Table 1.
Patients’ characteristics.

3.2. Delineation of the CD using laparoscopic fluorescence imaging systems
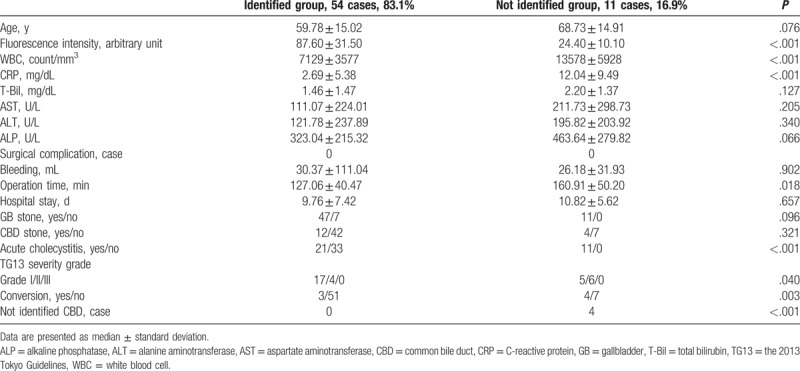
The ICG cholangiography using laparoscopic fluorescence imaging systems showed 54 patients (83.1%) in the identified CD group (Fig. 2) and 11 patients (16.9%) in the not identified CD group (Fig. 3). The mean value of the fluorescence intensity in the identified CD group was 87.6 ± 31.5 arbitrary unit (maximum value 156.2, minimum value 50.2) and that in the not identified CD group was 24.4 ± 10.1 arbitrary unit (maximum value 35.9, minimum value 10.4). Significant difference was found between 2 groups (P < .001). Compared with patients in the identified CD group, those in the not identified CD group had higher WBC counts (P < .001), higher CRP levels (P < .001), longer operation times (P = .018), higher incidence of acute cholecystitis (P < .001), and higher conversion rates (P = .003). Meanwhile, no significant difference was found in the transaminase value and existence of gallbladder stones between the 2 groups. CBD was not identified in 4 patients (6.2%) (Table 2).
Figure 3.
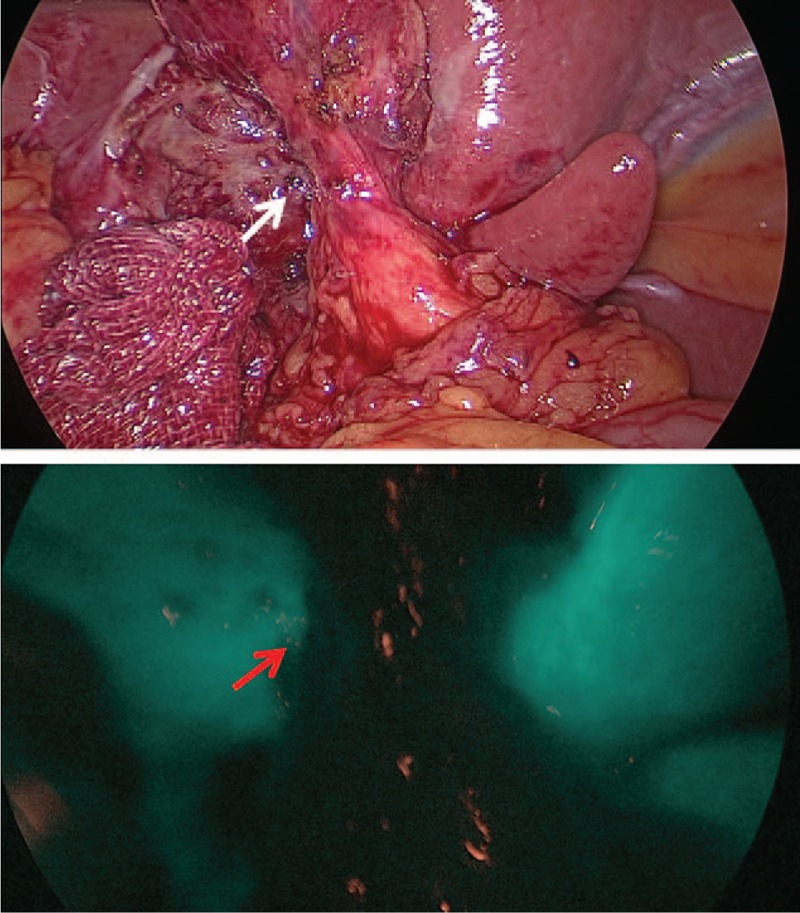
Cystic duct was not detected in lower panel by a “near infrared” light source after dissecting Calot triangle.
Table 2.
The delineation of cystic duct by indocyanine green cholangiography.
3.3. Delineation of the CBD using laparoscopic fluorescence imaging systems
The ICG cholangiography using laparoscopic fluorescence imaging systems showed 61 patients (93.8%) in the identified CBD group and 4 patients (6.2%) in the not identified CBD group. The all cases of the not identified CBD group were included in the not identified CD group. The mean value of the fluorescence intensity in the identified CBD group was 81.99 ± 34.56 arbitrary unit (maximum value 163.3, minimum value 32.4) and that in the not identified CBD group was 15.57 ± 8.18 arbitrary unit (maximum value 24.0, minimum value 9.4). Significant difference was found between the 2 groups (P = .002). Compared with patients in the identified CBD group, those in the not identified CBD group had higher WBC counts (P = .035), higher CRP levels (P = .037), higher incidence of acute cholecystitis (P = .036), and higher conversion rates (P = .009). Meanwhile, no significant difference was found in operation times between the 2 groups (Table 3).
Table 3.
The delineation of common bile duct by indocyanine green cholangiography.

3.4. Delineation of CD by MRCP
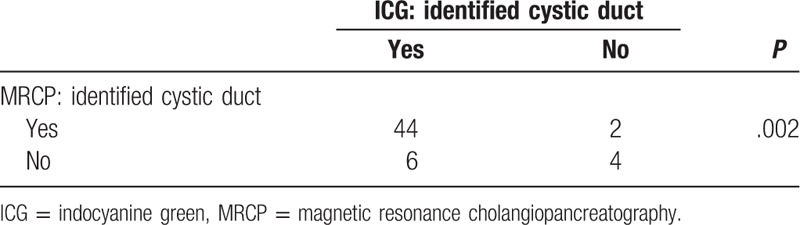
Among 65 patients, 56 (86.2%) were preoperatively examined using MRCP and analyzed as previously described. Compared with patients in the identified CD group of MRCP, those in the not identified CD group of MRCP had higher WBC counts (P = .005), higher CRP levels (P = .001), higher incidence of gallbladder (GB) stones (P = .037), and higher incidence of acute cholecystitis (P = .009). CBD was detected in all patients (Table 4). A correlation between the delineation of CD by ICG cholangiography and MRCP was analyzed, and it revealed a correlation between each other (P = .002) (Table 5).
Table 4.
The delineation of cystic duct by magnetic resonance cholangiopancreatography.
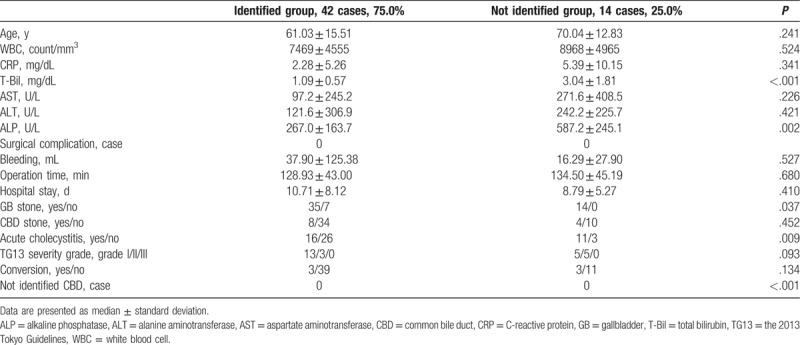
Table 5.
The comparison of the delineation of cystic duct between ICG cholangiography and MRCP.
4. Discussion
The ICG is a tricarbocyanine dye. Following intravenous injection, it rapidly and completely binds to albumin and is selectively taken up by hepatocytes and excreted into the bile.[14] Therefore, ICG has been widely used in liver function tests. After binding to proteins, such as albumin, ICG normally emits light in the infrared region of the electromagnetic spectrum at approximately 835 nm.[15] An infrared camera detects infrared light identified as strong fluorescence in response to ICG.[7] The advantages of this method are no special requirements with regard to the arrangement of X-ray equipment and no radioactivity exposure. Moreover, this method is very useful in the verification of biliary anatomy without the risk of bile duct and blood vessel injury.[7] The disadvantage of this method is the difficulty in identifying CBD stones. However, this problem can be resolved with preoperative MRCP, computed tomography, or ultrasonography examination.
In this study, all 11 patients in the not identified CD group for ICG cholangiography were included in the group of 32 patients with severe inflammation diagnosed with acute cholecystitis according to the TG13 (Table 2). Patients in the not identified CD group had a significantly longer operation time, reflecting the difficulty of the operation. Four patients with the not identified CBD group with ICG cholangiography were also included in the 11 patients in the not identified CD group and were converted to open surgery reflecting the inflammation and the difficulty of the operation.
The ICG shows a bile juice passage, and the inflammatory thickness of the tissue around the gallbladder is directly associated with the difficulty in dissecting the tissue. Meanwhile, MRCP mainly indicates bile juice passage. Therefore, significant correlation was found in the delineation of CD between ICG cholangiography and MRCP (Table 4). The results indicated that inflammation had harmful effects with regard to the passing of CD or CBD and increased the difficulty of the operation. During operation, we have repeatedly performed ICG cholangiography before or after dissection of the Calot triangle. Our presented data showed the strongest intensity of ICG cholangiography during operation. Actually, most of data were obtained after dissection of Calot triangle, but the data included the cases whose Calot triangle was difficult to dissect due to severe inflammation.
For ICG administration, the dose was standardized at 2.5 mg and the timing at approximately 2 hours before surgery. Ishizawa et al and Kono et al have reported an injection of 2.5 mg of ICG 30 minutes before entering the operating room.[1,16] Boogerd et al performed clinical trial to optimize the dose of ICG and dosing time in ICG cholangiography. They showed the highest bile duct-to-liver ratio which was the indication of bile duct identification was achieved 3 to 7 hours after administration of 5 mg and 5 to 25 hours after administration of 10 mg ICG.[17] Zarrinpar et al showed a dose of 0.25 mg/kg administered at least 45 minutes prior to visualization facilitates intraoperative anatomical identification.[18] Taken together these reports, 2.5 to 10 mg ICG administration just before operation or 10 to 12.5 mg ICG administration on the day before operation were both acceptable dose of ICG and dosing time in ICG.
Thus, if we could identify CD or CBD with ICG cholangiography before dissection of Calot triangle, we might have confidently performed LC even in the presence of severe inflammation. In our experience, ICG cholangiography is useful for LC.
Because this study was not a randomized control trial, there was a limitation and we could not demonstrate the safety of LC combined with intraoperative ICG cholangiography compared with normal LC.
5. Conclusion
We might have been able to perform LC with more confidence, and we had been able to identify CD or CBD with ICG cholangiography.
Inflammation had harmful effects with regard to the passing of CD. If we can identify CD or CBD with ICG cholangiography, we may be able to perform LC with confidence, even in the presence of severe inflammation.
Acknowledgment
The authors thank Dr M. Shimonosono, Dr H. Shimomura, Dr M. Wada, Dr K. Minamimagari, and Dr Y. Tsuruta as LC operators.
Author contributions
Conceptualization: Kiyokazu Hiwatashi, Hiroshi Okumura, Tetsuro Setoyama, Yoshito Ogura, Kuniaki Aridome, Shigeho Maenohara, Shoji Natsugoe.
Formal analysis: Kiyokazu Hiwatashi.
Investigation: Kiyokazu Hiwatashi, Tetsuro Setoyama, Kei Ando.
Methodology: Kiyokazu Hiwatashi, Hiroshi Okumura.
Project administration: Shigeho Maenohara.
Supervision: Hiroshi Okumura.
Writing – original draft: Kiyokazu Hiwatashi.
Writing – review & editing: Kiyokazu Hiwatashi, Hiroshi Okumura.
Footnotes
Abbreviations: ALP = alkaline phosphatase, ALT = alanine aminotransferase, AST = aspartate aminotransferase, CBD = common bile duct, CD = cystic duct, CRP = C-reactive protein, ERCP = endoscopic retrograde cholangiopancreatography, GB = gallbladder, ICG = indocyanine green, LC = laparoscopic cholecystectomy, MRCP = magnetic resonance cholangiopancreatography, P-value = probability value, T-Bil = total bilirubin, TG13 = the 2013 Tokyo Guidelines, WBC = white blood cell.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Ishizawa T, Bandai Y, Ijichi M, et al. Fluorescent cholangiography illuminating the biliary tree during laparoscopic cholecystectomy. Br J Surg 2010;97:1369–77. [DOI] [PubMed] [Google Scholar]
- [2].Kubota K, Kita J, Shimoda M, et al. Intraoperative assessment of reconstructed vessels in living-donor liver transplantation, using a novel fluorescence imaging technique. J Hepatobiliary Pancreat Surg 2006;13:100–4. [DOI] [PubMed] [Google Scholar]
- [3].Saito T, Yano M, Motoori M, et al. Subtotal gastrectomy for gastric tube cancer after esophagectomy: a safe procedure preserving the proximal part of gastric tube based on intraoperative ICG blood flow evaluation. J Surg Oncol 2012;106:107–10. [DOI] [PubMed] [Google Scholar]
- [4].Raabe A, Beck J, Gerlach R, et al. Near-infrared indocyanine green video angiography: a new method for intraoperative assessment of vascular flow. Neurosurgery 2003;52:132–9. [DOI] [PubMed] [Google Scholar]
- [5].Ohdaira H, Nimura H, Mitsumori N, et al. Validity of modified gastrectomy combined with sentinel node navigation surgery for early gastric cancer. Gastric Cancer 2007;10:117–22. [DOI] [PubMed] [Google Scholar]
- [6].Gotoh K, Yamada T, Ishikawa O, et al. A novel image-guided surgery of hepatocellular carcinoma by indocyanine green fluorescence imaging navigation. J Surg Oncol 2009;100:75–9. [DOI] [PubMed] [Google Scholar]
- [7].Mitsuhashi N, Kimura F, Shimizu H, et al. Usefulness of intraoperative fluorescence imaging to evaluate local anatomy in hepatobiliary surgery. J Hepatobiliary Pancreat Surg 2008;15:508–14. [DOI] [PubMed] [Google Scholar]
- [8].Tagaya N, Shimoda M, Kato M, et al. Intraoperative exploration of biliary anatomy using fluorescence imaging of indocyanine green in experimental and clinical cholecystectomies. J Hepatobiliary Pancreat Sci 2010;17:595–600. [DOI] [PubMed] [Google Scholar]
- [9].Aoki T, Murakami M, Yasuda D, et al. Intraoperative fluorescent imaging using indocyanine green for liver mapping and cholangiography. J Hepatobiliary Pancreat Sci 2010;17:590–4. [DOI] [PubMed] [Google Scholar]
- [10].Schols RM, Connell NJ, Stassen LP. Near-infrared fluorescence imaging for real-time intraoperative anatomical guidance in minimally invasive surgery: a systematic review of the literature. World J Surg 2015;39:1069–79. [DOI] [PubMed] [Google Scholar]
- [11].Vlek SL, van Dam DA, Rubinstein SM, et al. Biliary tract visualization using near-infrared imaging with indocyanine green during laparoscopic cholecystectomy: results of a systematic review. Surg Endosc 2017;31:2731–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yokoe M, Takada T, Strasberg SM, et al. Tokyo Guidelines Revision Committee. New diagnostic criteria and severity assessment of acute cholecystitis in revised Tokyo Guidelines. J Hepatobiliary Pancreat Sci 2012;19:578–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cherrick GR, Stein SW, Leevy CM, et al. Indocyanine green: observations on its physical properties, plasma decay, and hepatic extraction. J Clin Invest 1960;39:592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Landsman ML, Kwant G, Mook GA, et al. Light-absorbing properties, stability, and spectral stabilization of indocyanine green. J Appl Physiol 1976;40:575–83. [DOI] [PubMed] [Google Scholar]
- [16].Kono Y, Ishizawa T, Tani K, et al. Techniques of fluorescence cholangiography during laparoscopic cholecystectomy for better delineation of the bile duct anatomy. Medicine (Baltimore) 2015;94:e1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Boogerd LSF, Handgraaf HJM, Huurman VAL, et al. The best approach for laparoscopic fluorescence cholangiography: overview of the literature and optimization of dose and dosing time. Surg Innov 2017;24:386–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zarrinpar A, Dutson EP, Mobley C, et al. Intraoperative laparoscopic near-infrared fluorescence cholangiography to facilitate anatomical identification: when to give indocyanine green and how much. Surg Innov 2016;23:360–5. [DOI] [PubMed] [Google Scholar]


