Abstract
Background:
Hyperglycemia is associated with dismal outcomes in patients with traumatic brain injury (TBI), which is frequently treated with insulin therapy. In this study, a systematic review and meta-analysis of the published randomized controlled trials (RCTs) was performed to assess the safety and efficacy of intensive glycemic control (IGC) versus conventional glycemic control (CGC) for patients following TBI.
Methods:
Databases, including PubMed, Embase, and the Cochran database, were retrieved up to January 2018. The outcomes evaluated in this study included mortality, neurological outcome, infection rate, hypoglycemia episode, and length of stay (LOS) in intensive care unit (ICU). The enrolled trials were analyzed using the Review Manager 5.3 software.
Results:
A total of 7 randomized controlled trials (RCTs) involving 1013 cases were enrolled in this study, and the results indicated no significant difference in 6-month mortality (risk ratio [RR], 0.92; 95% confidence interval [CI] 0.76–1.10; P = .34). Subsequently, IGC was associated with a better neurological outcome (RR, 1.22; 95% CI 1.05–1.43; P = .01), lower infection rate (RR, 0.65; 95% CI 0.51–0.82; P = .0003) and shorter LOS in ICU (mean difference [MD] = –1.37; 95%CI = –2.11, –0.63; P = .0003). In addition, IGC would also increase the risk of hypoglycemia episode (RR, 4.53; 95% CI 2.18–9.42; P < .001).
Conclusions:
IGC plays a protective role in improving neurological outcome, decreasing infection rate and reducing the LOS in ICU. However, IGC therapy can also remarkably increase the risk of hypoglycemia, but it will not affect the mortality in TBI patients.
Keywords: conventional glycemic control, intensive glycemic control, meta-analysis, traumatic brain injury
1. Introduction
Hyperglycemia frequently occurs in critically ill patients, which is also linked with increased morbidity and mortality.[1] These observations can be found in general patients as well as those with traumatic brain injury (TBI).[2,3] Typically, TBI will lead to profoundly increased glucose utilization (also known as hyperglycolysis), which can persist for up to 1 week, finally altering the ability to use ketone bodies as energetic substrates.[4,5] It is suggested that hyperglycemia can exacerbate secondary brain injury and independently predict the dismal neurological outcome in severe TBI patients.[6,7]
Conventional glucose control (CGC), the traditional treatment for hyperglycemia, administers insulin at the glucose level of >200 to 220 mg/dL.[8] In the light of reports on worse outcomes of hyperglycemia, a new therapeutic approach, intensive glucose control (IGC), has been sought to maintain the glucose level within the range of 80 to 110 mg/dL.[9–11] Specifically, patients treated with IGC have distinctly lower morbidity and mortality compared with those undergoing CGC.[12,13] Additional studies also reveal the benefits of IGC in lowering mortality and incidence of infections among different groups of critically ill patients.[14,15] However, strict glycemic control with low target ranges will inevitably carries a risk of inadvertent hypoglycemic episodes. On the contrary, other studies show that IGC has no benefits and fails to achieve glycemic control; what's worse, patients under strict glycemic control suffer from a potentially higher incidence of hypoglycemia.[16,17]
Many studies and randomized controlled trials (RCTs) have addressed the question of whether IGC can result in better outcomes for TBI patients than CGC, but no consensus has been reached yet.[8–11,18–20] Therefore, the current meta-analysis of RCTs comparing IGC with CGC in patients was conducted, with an aim to evaluate the effect of IGC on mortality, neurological outcome, and other clinical outcomes in severe blunt TBI patients.
2. Methods
2.1. Retrieval strategy
The following electronic databases were retrieved until January 2018, including PubMed, Embase, and the Cochrane database, using retrieval terms of traumatic brain injury, subarachnoid hemorrhage, subdural hematoma, insulin therapy, intensive glucose control, glycemic control, conventional glucose control, and randomized controlled trial. Each step of pooled analysis was conducted by 2 investigators independently, and any disagreement was settled by mutual discussion. The current systematic review and meta-analysis of RCTs was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The current systematic review was not registered.
2.2. Selection criteria
The inclusion criteria were: comparative study (RCT), study investigating only TBI patients, and study comparing IGC with CGC. The exclusion criteria were: review article, meta-analysis, and guideline, non-RCT, study with no medical treatment control group, and study with no CGC arm, for example, IGC versus non-insulin treatment or IGC versus saline with no insulin treatment.
2.3. Data extraction
Data were extracted by 2 reviewers independently, and any disagreement was resolved by consulting with a third reviewer. The following information was extracted from the RCTs, including name of first author, country of origin, patient characteristics (such as mean age and sex), operational definitions, and outcomes. Moreover, means, standard deviations or medians, and interquartile ranges for each treatment group, together with the numbers assessed in each group, were recorded to evaluate the continuous outcomes. Besides, the primary author was reached by email to seek the clarification for the missing information.
2.4. Study outcomes
Primary outcomes of clinical importance included: 6-month mortality, and the available time frame closest to 6 months was used when it was not specifically presented, and good neurological recovery, as defined in individual studies. To be specific, a Glasgow Outcome Scale (GOS) score of 4 to 5, a modified Rankin Scale (mRS) score of 1 to 3, or an extended Glasgow Outcome Scale (eGOS) score of 5 to 8, were considered to represent good outcomes when a full range of outcomes were presented. Secondary outcomes included number of hypoglycemia episodes, length of stay (LOS) in intensive care unit (ICU), and the incidence of infections. Specifically, the major infections included wound infections, pneumonia, urinary infections, and sepsis.
2.5. Quality assessment
The risk of bias was independently evaluated by 2 reviewers using the domain-based evaluation described in the Cochrane Handbook for Systematic Reviews of Interventions. Typically, the following domains were assessed, including selection bias (random sequence generation and allocation concealment), attrition bias (incomplete outcome data), performance and detection bias (blinding of participants, personnel and outcome assessment), reporting bias (selective reporting), and other biases (other sources of bias).
2.6. Statistical analysis
Dichotomous and continuous variables were analyzed by risk ratio (RR) and mean difference (MD), respectively. The heterogeneity between studies was accessed using Cochran Q-statistic test, and tested using I2 (P < .05 stood for significant heterogeneity). In the presence of evidence for heterogeneity between studies, the random effects model was used, since it could provide a more conservative effect than that of the fixed-effects model.[21] Meanwhile, sensitivity analysis was performed in the presence of heterogeneity through eliminating one study at a time to check the resolution of heterogeneity. Publication bias was assessed using the visual funnel plot.[22] Data were analyzed by the Review Manager (RevMan version 5.3; Cochrane Collaboration, Oxford, UK).
2.7. Ethical consideration
This is a meta-analysis article, does not involve ethical review, and ethical approval is not necessary after inquiring the ethical review committee in our hospital.
3. Results
3.1. Study selection
A diagram summarizing the study selection process was shown in Fig. 1. As could be seen, a total of 3004 potential trials were identified by the first retrieval strategy, and 7 RCTs were identified after careful full-text evaluation in the final analysis [8–11,18–20]
Figure 1.
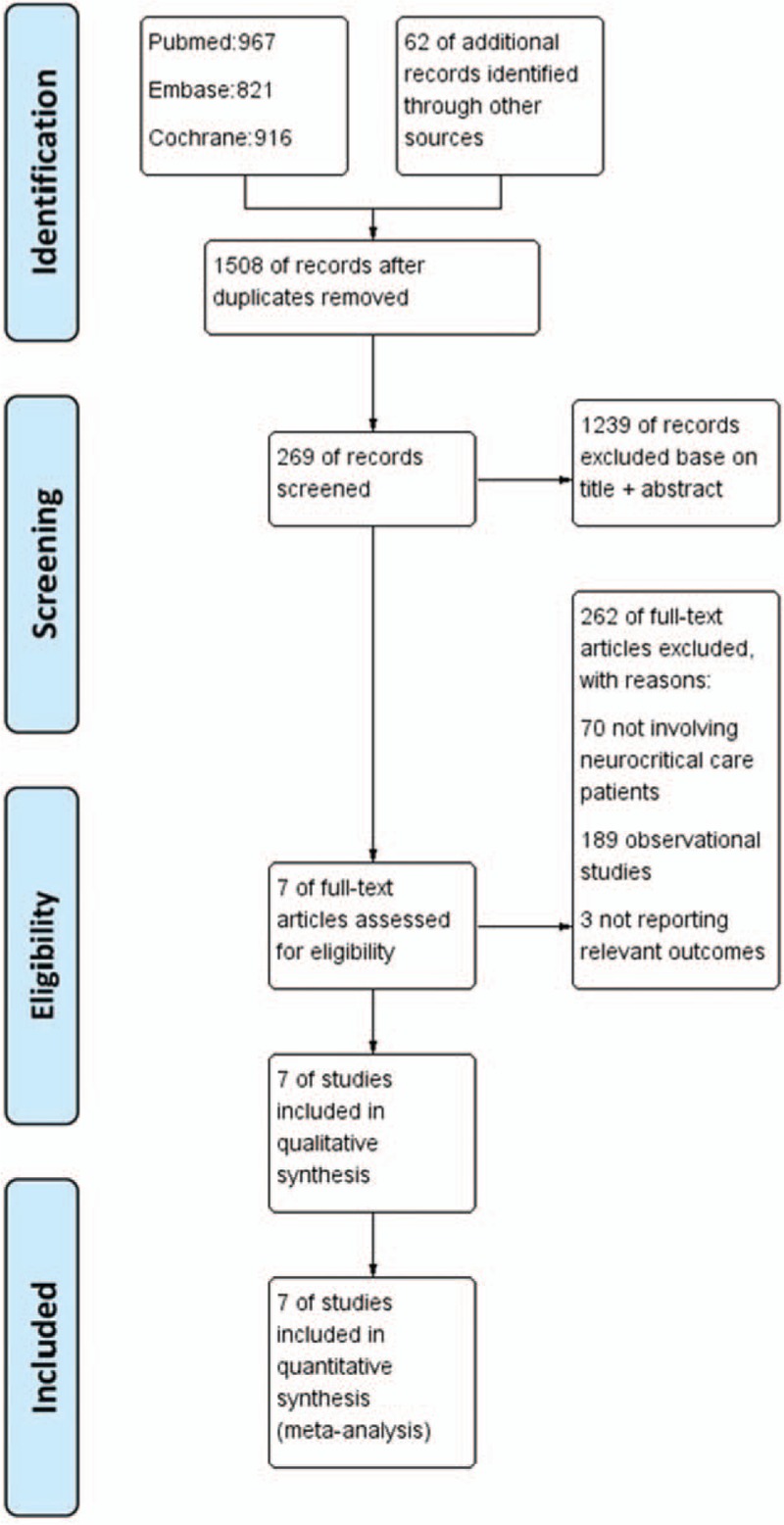
The flow diagram of study selection for meta-analysis.
3.2. Trial characteristics
Together, a total of 1013 patients were enrolled in the identified RCTs published from 2007 to 2017, including adults diagnosed with TBI. The follow-up period ranged from 6 to 24 months, and the detail information was summarized in Table 1. Five out of the 7 studies had an intensive target range of 80 to 108 (or 110) mg/dL,[8,10,11,19,20] and only 2 studies by Bilotta et al[9,18] had an intensive target range of 80 to 120 mg/dL. In comparison, the target range for CGC was more variable, among which, 2 studies had an intensive target range of <220 mg/dL,[9,18] 2 of 180–200 mg/dL,[11,20] 2 of <180 mg/dL,[8,19] and 1 of <150 mg/dL.[10] Moreover, most studies had displayed a low risk of bias; however, a few studies had an unclear risk (Fig. 2). In the meantime, the risk of bias among studies was assessed to be low.
Table 1.
Characteristics of studies included in the meta-analyses.
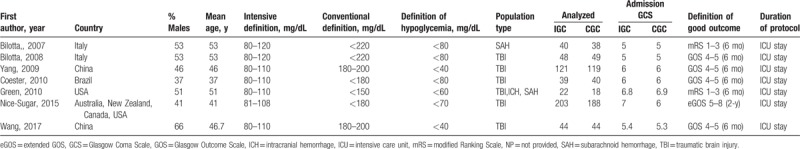
Figure 2.
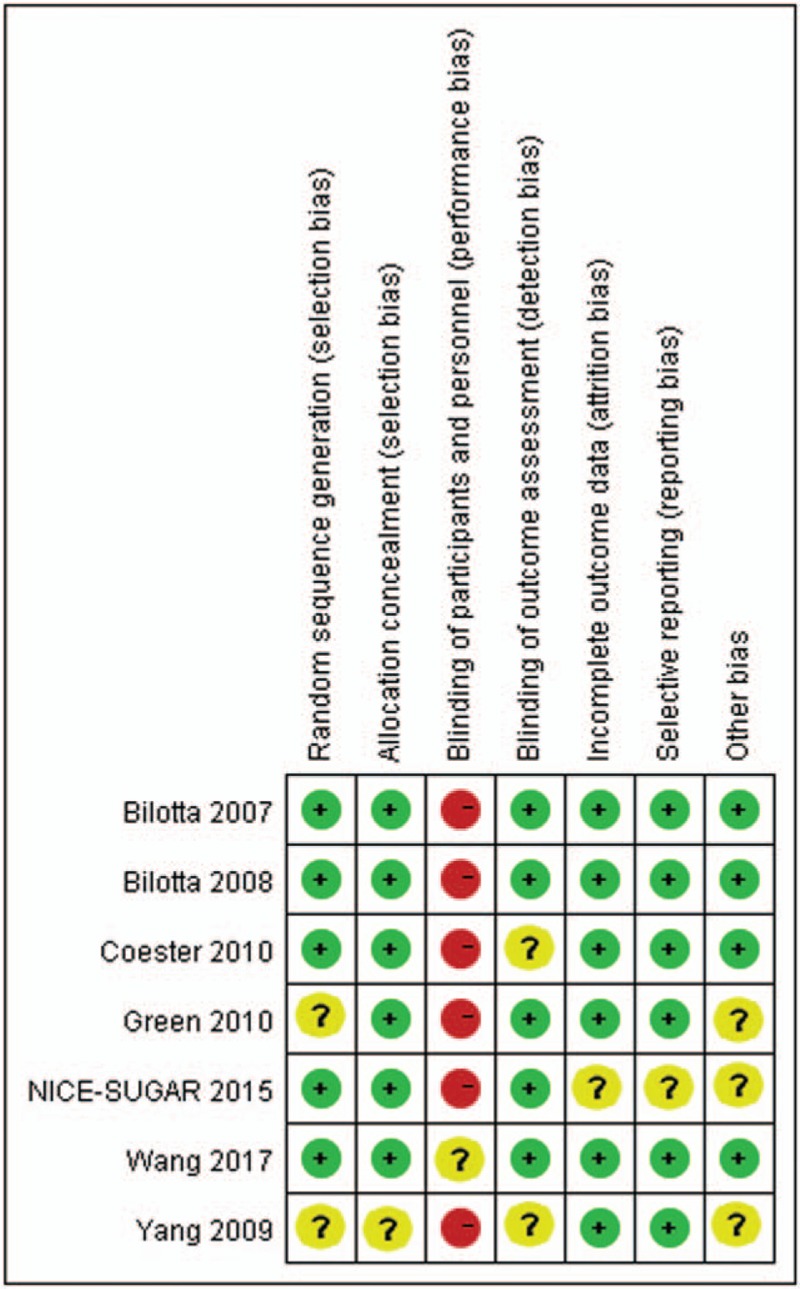
Risk of bias assessment for randomized controlled trials. “+”: low risk of bias, “–”: high risk of bias, and “?”: unclear risk of bias.
3.3. Primary outcomes
3.3.1. Mortality
A meta-analysis was performed to calculate the RR of mortality related to IGC versus CGC following TBI. Typically, 6 studies had reported mortality as the primary outcome. A total of 286 related deaths were reported, including 139 in the IGC arm and 147 in the CGC arm. Besides, the results of pooled analysis demonstrated no difference in the risk between IGC and CGC (RR, 0.92; 95% CI 0.76 to 1.10; P = .34). No significant heterogeneity was observed among the identified studies after evaluating mortality (I2 = 0, P = .98). (Fig. 3A)
Figure 3.
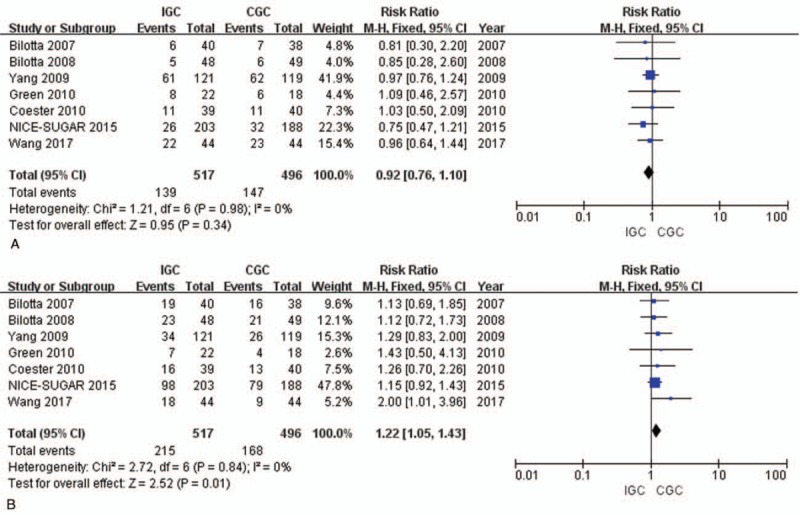
Forest plot of all included trials examining the effect of IGC versus CGC on mortality and favorable neurological outcomes in TBI patients. (A) Mortality; and (B) favorable neurological outcomes. CGC = conventional glycemic control; IGC = intensive glycemic control; M-H, Random = M-H, Fixed = Mantel-Haenszel, Fixed-effects model; TBI = traumatic brain injury.
3.3.2. Favorable neurological outcomes
Results of pooled analysis on the 7 studies reporting neurological recovery showed that, IGC therapy was superior to CGC therapy among patients (RR, 1.22; 95% CI 1.05–1.43; P = .01). At the same time, no significant heterogeneity was found (I2 = 0%, P = .84). (Fig. 3B)
3.4. Secondary outcomes
3.4.1. Infection rate
Five trials had reported that, the incidence of overall infectious complications in the IGC group and CGC group was 36.6% (107/292) and 50% (145/290), respectively.[8,9,11,18] Meanwhile, pneumonia, urinary infection, wound infection, and sepsis were the 4 most common infections, which were included in our meta-analysis. Our results suggested that IGC showed no protective effect (RR 0.73, 95% CI 0.52–1.03, P = .07). At the same time, there was significant heterogeneity among the selected studies when evaluating the clinical efficacy (I2 = 70%, P = .009) (Fig. 4A). Upon sensitivity analysis, such heterogeneity could be eliminated after removing the study by Coester et al[8] (I2 = 0%). Interestingly, the protective effect of IGC on infection was increased in magnitude after removing the RCT by Coester et al[8] (RR, 0.65; 95% CI 0.51–0.82; P = .0003) (Fig. 4B).
Figure 4.
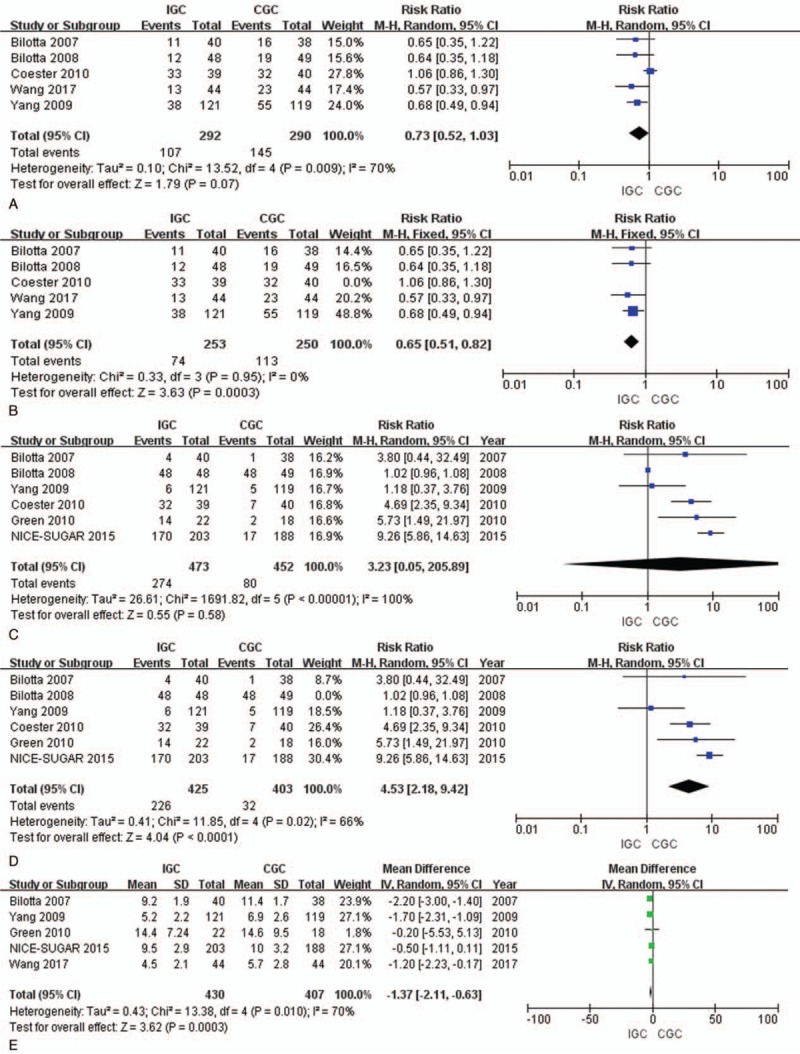
Comparison of the infection rates, hypoglycemia episodes and LOS in ICU among TBI patients. (A) Infection rates; (B) infection rates excluding the study by Coester; (C) hypoglycemia episodes; (D) hypoglycemia episodes excluding the study by Bilotta; and (E) LOS in ICU. CGC = conventional glycemic control; IGC = intensive glycemic control; IV, Random = inverse variance, random-effects model; LOS = length of stay; M-H, Fixed = Mantel-Haenszel, Fixed-effects model; M-H, Random = Mantel-Haenszel, Random-effects model; TBI, traumatic brain injury.
3.4.2. Hypoglycemia episode
The pooled RRs from 6 studies indicated that no significant difference in hypoglycemia episode between the IGC and CGC groups (RR = 3.23; 95% CI = 0.05–205.89, P = .58). However, there was a large degree of heterogeneity between studies (P < .001, I2 = 100%) (Fig. 4C). Upon sensitivity analysis, such heterogeneity could be eliminated after removing the study by Bilotta et al[9] (I2 = 66%). Similarly, the protective effect of IGC on hypoglycemia was also increased in magnitude after removing the RCT by Bilotta et al (RR, 4.53; 95% CI 2.18–9.42; P < .001) (Fig. 4D).
3.4.3. LOS in ICU
Five studies had reported the LOS in ICU after TBI.[10,11,18,19] Results of pooled analysis revealed that the LOS in ICU was shorter in IGC group than in CGC group, and the difference between these 2 groups was statistically significant (MD = –1.37; 95%CI = –2.11, –0.63; P = .0003) (Fig. 4E).
3.5. Sensitivity analysis and publication bias
Sensitivity analysis was performed by randomly excluding one trial and interchanging the fixed-effects model and random-effects model based on the pooled analysis, and the outcomes were confirmed to be stable. Publication bias was assessed using the funnel plots and Egger test. Typically, mortality was used as an exemplary indicator for publication bias assessment. The shape of funnel plot revealed no indication of obvious asymmetry. In addition, Egger test was also employed to provide statistical evidence of funnel plot symmetry, which revealed no proof of publication bias. (Fig. 5)
Figure 5.
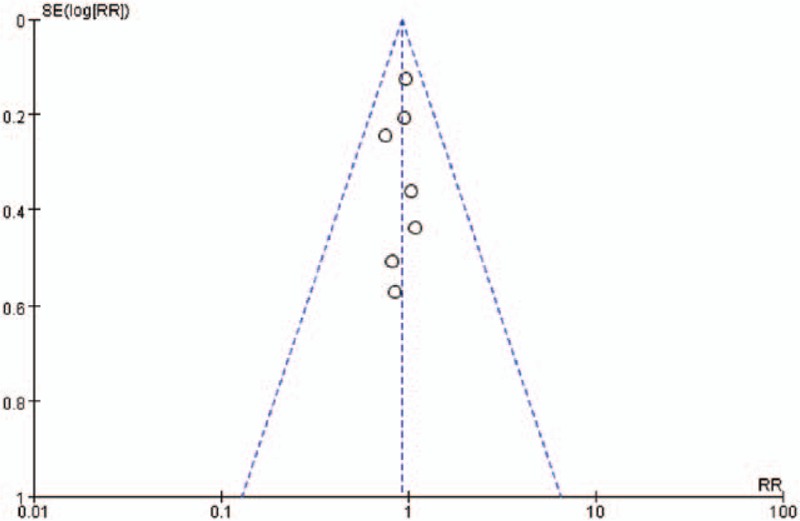
Funnel plot to detect the publication bias. No significant funnel asymmetry that could indicate publication bias was observed.
4. Discussion
The present meta-analysis demonstrates that TBI patients undergoing IGC therapy are associated with improved neurological outcome, decreased infection rate, and reduced LOS in ICU compared with those receiving CGC therapy. However, IGC does not demonstrate a mortality benefit compared with CGC. Moreover, IGC therapy will also dramatically increase the risk of hypoglycemia episode.
TBI is related to a stress response including hyperglycemia, which has been shown to worsen the neurological outcome during cerebral ischemia and hypoxia.[23–25] Studies on moderate to severe TBI patients indicate that, higher initial and postoperative glucose levels will lead to higher intracerebral lactate levels and worse outcome, especially for those with the glucose levels of greater than 160 to 200 mg/dL.[26–28] The mechanisms by which hyperglycemia exerts the harmful effect are complex. Concretely, the contributing factors may include free radical formation and oxidative injury, activation of Nmethyl-D-aspartate receptors, raised intracellular calcium, triggering of inflammatory and apoptotic pathways, and alterations in lactate metabolism with reduced tissue pH.[29] Therefore, the impact of interventions, such as more aggressive control over blood glucose with insulin control therapy, has been studied.
Notably, the target range of conventional glucose control in our meta-analysis is more variable, among which, 2 studies have the target range of <220 mg/dL,[9,18] 2 of 180 to 200 mg/dL,[11,20] 2 of <180 mg/dL,[8,19] and 1 of <150 mg/dL.[10] It is proposed in existing guidelines[30] to initiate insulin therapy when the blood glucose level exceeds 180 mg/dL to trigger a blood glucose concentration of <180 mg/dL. Moreover, the previous guidelines propose that glycemic control infusions should aim at maintaining the blood glucose levels of <200 mg/dL in neurocritically ill patients with hyperglycaemia.[31] In contrast, Jacobi et al[32] suggested that patients with the blood glucose levels of ≥150 mg/dL should be initiated the insulin therapy, so as to keep the blood glucose levels of <150 mg/dL in most adult trauma patients and to maintain the absolute blood glucose values of <180 mg/dL. Meanwhile, a protocol that could achieve a low rate of hypoglycemia (blood glucose level of ≤70 mg/dL) should be used to achieve the lower infection rates and shorter LOS in ICU in trauma patients.
In 2001, van den Berghe et al[12] had compared 783 patients receiving CGC therapy with 765 undergoing IGC therapy. Their results indicated that patients treated with IGC had dramatically lower morbidity and mortality compared with those receiving CGC therapy. Additional studies also show the benefits of IGC in reducing mortality and incidence of infections among different groups of critically ill patients.[15,33,34] Nonetheless, other studies have revealed no benefit of IGC, which fails to achieve glycemic control and potentially results in higher incidence of hypoglycemia in patients.[35–37] In our meta-analysis, no differences are observed in reducing the mortality and infection rate between IGC and CGC in TBI patients. Such observation is consistent with the results from recent large-scale and multi-center RCTs performed in critically ill patients that displayed a higher degree of heterogeneity in the neurological and diagnostic categories.[38–40] On the contrary, Zafar et al[41] had demonstrated the inconsistent results in 2011. However, they not only analyzed TBI; instead, all kinds of brain injuries were included for analysis, including tumor and stroke. Moreover, our results show that IGC is beneficial for lowering the infection rates. Nevertheless, future RCTs on infection would be needed due to the small sample size. A recent meta-analysis by Hermanides et al[42] had a similar result with us in 2018. However, they did not find a significant difference in the infection rates. The reason may be that the included trails investigated a mixed neurocritical or general ICU population. For example, Van den Berghe et al[43] included cardiac surgery, complicated lung and esophageal surgery, multiple trauma, and so on. Cinotti et al[44] included aneurysmal subarachnoid hemorrhage, intra-cerebral hemorrhage, malignant stroke, and others. Arabi et al[38,45] included mechanical ventilation, vasopressor, sepsis, and traumatic brain injury. De La Rosa Gdel et al[46] included surgery and trauma. Furthermore, 3 random control trails[9,18,20] were not included in their meta-analysis, and these trails were all about traumatic brain injury.
It should be noted that, strict glycemic control with a low target range will invariably carry a risk of inadvertent hypoglycemia episode. As found in the current meta-analysis, the IGC therapy will markedly increase the incidence of hypoglycemia (RR, 4.53; 95% CI 2.18–9.42; P < .001). RCTs examining the effect of IGC have consistently reported an increased risk of both moderate and severe hypoglycemia among patients randomly assigned to IGC group; besides, the occurrence of hypoglycemia is strongly associated with dismal outcomes.[47–49] On this account, an important question occurs, which is whether hypoglycemia episode will contribute to the worsened long-term neurological outcome following TBI. Several studies have suggested that hypoglycemia is an independent mortality factor in ICU,[50] but it is not observed in the Computerized Glucose Control in Critically Ill Patients (CGAO-REA) study.[51] Most studies enrolled in our analysis reveal that, hypoglycemia episode is not associated with dismal outcomes. For instance, in the study by Bilotta et al[18] involving 97 TBI patients, IGC therapy was reported to increase the risk of hypoglycemia but would not markedly affect the 6-month mortality or neurological disability. In the study by Coester et al[8] enrolling 88 severe TBI patients, the authors reported that IGC therapy would increase the risk of hypoglycemia but would not distinctly affect the 6-month mortality or neurological disability. In the study from Yang et al[11] recruiting 240 patients, the authors reported no difference in mortality, but an increase in the proportion of patients with favorable neurological recovery at 6 months, which showed no statistical significance.
In contrast, our results suggest that IGC can promote the occurrence of favorable neurological outcomes, which are consistent with those from Kramer in 2012.[52] Such effect on neurological outcome can be explained by the central nervous system (CNS) protection of IGC. IGC therapy can protect the CNS through reducing the mean and maximal intracranial pressures in patients with isolated brain injury.[5,53,54] The beneficial effect of IGC on intracranial pressure can be attained in the presence of similar cerebral perfusion pressures achieved with notably less norepinephrine as a vasopressor. Not only normoglycemia, but also insulin itself, has been reported to improve critically ill patients, which can be attributable to its metabolic and anti-inflammatory effects.[55] Experimental data suggest that insulin can increase glucose uptake in astrocyte[56] and can play a role in cerebral cortical glucose regulation.[57] In addition, our meta-analysis also reveals that the LOS in ICU is shorter in IGC group than in CGC group.
Nonetheless, our analysis is inevitably associated with several potential limitations. Therefore, firstly, any future RCTs on IGC therapy should carefully pilot their protocol to ensure the minimized hypoglycemia. Secondly, the number of included studies is not sufficient enough to make a convincing conclusion for the secondary endpoints. Thirdly, the neurological outcomes reported in this meta-analysis are relatively crude, and it remains possible that glycemic control can have greater influence on more subtle neurocognition or indices of quality of life. Fourthly, the follow-up period of the enrolled trials is short to moderate, making it impossible to evaluate the long-term complications. Lastly, the description of allocation concealment, difficulties in the blinding of participants, and outcome assessors is lacking in these included studies, which can be attributed to the nature of intervention.
5. Conclusions
This meta-analysis suggests that IGC generally has comparable efficacy to CGC in reducing mortality following TBI. Moreover, IGC plays a protective role in improving neurological outcome, decreasing infection rate and reducing the LOS in ICU.
Author contributions
Conceptualization: Tao Xu.
Data curation: Junchen Pan, Zhichao Qiu.
Formal analysis: Junchen Pan.
Investigation: Zhichao Qiu.
Methodology: Junchen Pan, Zhichao Qiu.
Software: Jinjing Chen.
Supervision: Zhichao Qiu.
Validation: Zhichao Qiu.
Writing – original draft: Chunran Zhu.
Writing – review and editing: Zhichao Qiu.
Footnotes
Abbreviations: CGC = conventional glycemic control, CI = confidence intervals, eGOS = extended Glasgow Outcome Scale, GOS = Glasgow Outcome Scale, ICU = intensive care unit, IGC = intensive glycemic control, MD = mean difference, mRS = modified Rankin Scale, RCTs = randomized controlled trials, RR = risk ratio, TBI = traumatic brain injury.
CRZ and JJC have contributed equally to this article and listed as co-first author.
The authors declare that they have no conflicts of interest.
References
- [1].Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc 2003;78:1471–8. [DOI] [PubMed] [Google Scholar]
- [2].Preiser JC, Devos P, Ruiz-Santana S, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med 2009;35:1738–48. [DOI] [PubMed] [Google Scholar]
- [3].Alexiou GA, Lianos G, Fotakopoulos G, et al. Admission glucose and coagulopathy occurrence in patients with traumatic brain injury. Brain Inj 2014;28:438–41. [DOI] [PubMed] [Google Scholar]
- [4].Robertson CS, Goodman JC, Narayan RK, et al. The effect of glucose administration on carbohydrate metabolism after head injury. J Neurosurg 1991;74:43–50. [DOI] [PubMed] [Google Scholar]
- [5].Jalloh I, Carpenter KL, Helmy A, et al. Glucose metabolism following human traumatic brain injury: methods of assessment and pathophysiological findings. Metab Brain Dis 2015;30:615–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rusnak M, Janciak I, Majdan M, et al. Severe traumatic brain injury in Austria VI: effects of guideline-based management. Wien Klin Wochenschr 2007;119:64–71. [DOI] [PubMed] [Google Scholar]
- [7].Bochicchio GV, Joshi M, Bochicchio KM, et al. Early hyperglycemic control is important in critically injured trauma patients. J Trauma 2007;63:1353–8. discussion 1358-9. [DOI] [PubMed] [Google Scholar]
- [8].Coester A, Neumann CR, Schmidt MI. Intensive insulin therapy in severe traumatic brain injury: a randomized trial. J Trauma 2010;68:904–11. [DOI] [PubMed] [Google Scholar]
- [9].Bilotta F, Caramia R, Cernak I, et al. Intensive insulin therapy after severe traumatic brain injury: a randomized clinical trial. Neurocrit Care 2008;9:159–66. [DOI] [PubMed] [Google Scholar]
- [10].Green DM, O’Phelan KH, Bassin SL, et al. Intensive versus conventional insulin therapy in critically ill neurologic patients. Neurocrit Care 2010;13:299–306. [DOI] [PubMed] [Google Scholar]
- [11].Yang M, Guo Q, Zhang X, et al. Intensive insulin therapy on infection rate, days in NICU, in-hospital mortality and neurological outcome in severe traumatic brain injury patients: a randomized controlled trial. Int J Nurs Stud 2009;46:753–8. [DOI] [PubMed] [Google Scholar]
- [12].van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med 2001;345:1359–67. [DOI] [PubMed] [Google Scholar]
- [13].Gandhi GY, Nuttall GA, Abel MD, et al. Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery: a randomized trial. Ann Intern Med 2007;146:233–43. [DOI] [PubMed] [Google Scholar]
- [14].Toschlog EA, Newton C, Allen N, et al. Morbidity reduction in critically ill trauma patients through use of a computerized insulin infusion protocol: a preliminary study. J Trauma 2007;62:1370–5. discussion 1375-6. [DOI] [PubMed] [Google Scholar]
- [15].Langouche L, Meersseman W, Vander Perre S, et al. Effect of insulin therapy on coagulation and fibrinolysis in medical intensive care patients. Crit Care Med 2008;36:1475–80. [DOI] [PubMed] [Google Scholar]
- [16].Vriesendorp TM, DeVries JH, van Santen S, et al. Evaluation of short-term consequences of hypoglycemia in an intensive care unit. Crit Care Med 2006;34:2714–8. [DOI] [PubMed] [Google Scholar]
- [17].Treggiari MM, Karir V, Yanez ND, et al. Intensive insulin therapy and mortality in critically ill patients. Crit Care 2008;12:R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bilotta F, Spinelli A, Giovannini F, et al. The effect of intensive insulin therapy on infection rate, vasospasm, neurologic outcome, and mortality in neurointensive care unit after intracranial aneurysm clipping in patients with acute subarachnoid hemorrhage: a randomized prospective pilot trial. J Neurosurg Anesthesiol 2007;19:156–60. [DOI] [PubMed] [Google Scholar]
- [19].Finfer S, Chittock D, Li Y, et al. Australian N-SSIft, New Zealand Intensive Care Society Clinical Trials G, the Canadian Critical Care Trials G. Intensive versus conventional glucose control in critically ill patients with traumatic brain injury: long-term follow-up of a subgroup of patients from the NICE-SUGAR study. Intensive Care Med 2015;41:1037–47. [DOI] [PubMed] [Google Scholar]
- [20].Wang Y, Li JP, Song YL, et al. Intensive insulin therapy for preventing postoperative infection in patients with traumatic brain injury: a randomized controlled trial. Medicine (Baltimore) 2017;96:e6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [22].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [23].Zygun DA, Steiner LA, Johnston AJ, et al. Hyperglycemia and brain tissue pH after traumatic brain injury. Neurosurgery 2004;55:877–81. discussion 882. [DOI] [PubMed] [Google Scholar]
- [24].Junior JR, Welling LC, Schafranski M, et al. Prognostic model for patients with traumatic brain injuries and abnormal computed tomography scans. J Clin Neurosci 2017;42:122–8. [DOI] [PubMed] [Google Scholar]
- [25].Jagannatha AT, Sriganesh K, Devi BI, et al. An equiosmolar study on early intracranial physiology and long term outcome in severe traumatic brain injury comparing mannitol and hypertonic saline. J Clin Neurosci 2016;27:68–73. [DOI] [PubMed] [Google Scholar]
- [26].Lam AM, Winn HR, Cullen BF, et al. Hyperglycemia and neurological outcome in patients with head injury. J Neurosurg 1991;75:545–51. [DOI] [PubMed] [Google Scholar]
- [27].Rovlias A, Kotsou S. The influence of hyperglycemia on neurological outcome in patients with severe head injury. Neurosurgery 2000;46:335–42. discussion 342-3. [DOI] [PubMed] [Google Scholar]
- [28].Liu-DeRyke X, Collingridge DS, Orme J, et al. Clinical impact of early hyperglycemia during acute phase of traumatic brain injury. Neurocrit Care 2009;11:151–7. [DOI] [PubMed] [Google Scholar]
- [29].Godoy DA, Di Napoli M, Rabinstein AA. Treating hyperglycemia in neurocritical patients: benefits and perils. Neurocrit Care 2010;13:425–38. [DOI] [PubMed] [Google Scholar]
- [30].Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017;43:304–77. [DOI] [PubMed] [Google Scholar]
- [31].Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 2009;32:1119–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jacobi J, Bircher N, Krinsley J, et al. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med 2012;40:3251–76. [DOI] [PubMed] [Google Scholar]
- [33].Pittas AG, Siegel RD, Lau J. Insulin therapy for critically ill hospitalized patients: a meta-analysis of randomized controlled trials. Arch Intern Med 2004;164:2005–11. [DOI] [PubMed] [Google Scholar]
- [34].Reed CC, Stewart RM, Sherman M, et al. Intensive insulin protocol improves glucose control and is associated with a reduction in intensive care unit mortality. J Am Coll Surg 2007;204:1048–54. discussion 54-5. [DOI] [PubMed] [Google Scholar]
- [35].Oksanen T, Skrifvars MB, Varpula T, et al. Strict versus moderate glucose control after resuscitation from ventricular fibrillation. Intensive Care Med 2007;33:2093–100. [DOI] [PubMed] [Google Scholar]
- [36].Shin S, Britt RC, Reed SF, et al. Early glucose normalization does not improve outcome in the critically ill trauma population. Am Surg 2007;73:769–72. discussion 72. [PubMed] [Google Scholar]
- [37].Collier B, Diaz J, Jr, Forbes R, et al. The impact of a normoglycemic management protocol on clinical outcomes in the trauma intensive care unit. JPEN J Parenter Enteral Nutr 2005;29:353–8. discussion 359. [DOI] [PubMed] [Google Scholar]
- [38].Arabi YM, Dabbagh OC, Tamim HM, et al. Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients. Crit Care Med 2008;36:3190–7. [DOI] [PubMed] [Google Scholar]
- [39].Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008;358:125–39. [DOI] [PubMed] [Google Scholar]
- [40].Kansagara D, Fu R, Freeman M, et al. Intensive insulin therapy in hospitalized patients: a systematic review. Ann Intern Med 2011;154:268–82. [DOI] [PubMed] [Google Scholar]
- [41].Zafar SN, Iqbal A, Farez MF, et al. Intensive insulin therapy in brain injury: a meta-analysis. J Neurotrauma 2011;28:1307–17. [DOI] [PubMed] [Google Scholar]
- [42].Hermanides J, Plummer MP, Finnis M, et al. Glycaemic control targets after traumatic brain injury: a systematic review and meta-analysis. Crit Care 2018;22:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Van den Berghe G, Schoonheydt K, Becx P, et al. Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology 2005;64:1348–53. [DOI] [PubMed] [Google Scholar]
- [44].Cinotti R, Ichai C, Orban JC, et al. Effects of tight computerized glucose control on neurological outcome in severely brain injured patients: a multicenter sub-group analysis of the randomized-controlled open-label CGAO-REA study. Crit Care 2014;18:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Arabi YM, Tamim HM, Dhar GS, et al. Permissive underfeeding and intensive insulin therapy in critically ill patients: a randomized controlled trial. Am J Clin Nutr 2011;93:569–77. [DOI] [PubMed] [Google Scholar]
- [46].De La Rosa Gdel C, Donado JH, Restrepo AH, et al. Strict glycaemic control in patients hospitalised in a mixed medical and surgical intensive care unit: a randomised clinical trial. Crit Care 2008;12:R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Egi M, Bellomo R, Stachowski E, et al. Hypoglycemia and outcome in critically ill patients. Mayo Clin Proc 2010;85:217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hermanides J, Bosman RJ, Vriesendorp TM, et al. Hypoglycemia is associated with intensive care unit mortality. Crit Care Med 2010;38:1430–4. [DOI] [PubMed] [Google Scholar]
- [49].Investigators N-SS, Finfer S, Liu B, et al. Hypoglycemia and risk of death in critically ill patients. N Engl J Med 2012;367:1108–18. [DOI] [PubMed] [Google Scholar]
- [50].Investigators N-SS, Finfer S, Chittock DR, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009;360:1283–97. [DOI] [PubMed] [Google Scholar]
- [51].Kalfon P, Giraudeau B, Ichai C, et al. Tight computerized versus conventional glucose control in the ICU: a randomized controlled trial. Intens Care Med 2014;40:171–81. [DOI] [PubMed] [Google Scholar]
- [52].Kramer AH, Roberts DJ, Zygun DA. Optimal glycemic control in neurocritical care patients: a systematic review and meta-analysis. Crit Care 2012;16:R203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med 2006;354:449–61. [DOI] [PubMed] [Google Scholar]
- [54].Kawabori M, Yenari MA. The role of the microglia in acute CNS injury. Metab Brain Dis 2015;30:381–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Chaudhuri A, Janicke D, Wilson MF, et al. Anti-inflammatory and profibrinolytic effect of insulin in acute ST-segment-elevation myocardial infarction. Circulation 2004;109:849–54. [DOI] [PubMed] [Google Scholar]
- [56].Kum W, Zhu SQ, Ho SK, et al. Effect of insulin on glucose and glycogen metabolism and leucine incorporation into protein in cultured mouse astrocytes. Glia 1992;6:264–8. [DOI] [PubMed] [Google Scholar]
- [57].Bingham EM, Hopkins D, Smith D, et al. The role of insulin in human brain glucose metabolism: an 18fluoro-deoxyglucose positron emission tomography study. Diabetes 2002;51:3384–90. [DOI] [PubMed] [Google Scholar]


