Abstract
Background:
It is thought that expression of programmed death ligand-1 (PD-L1) in esophageal cancer (EC) might compromise patient survival. However, the association between PD-L1 expression and survival of patients with EC remains controversial.
Methods:
A meta-analysis combining eligible published studies was performed to evaluate the effect of PD-L1 expression in tumor cells detected by immunohistochemistry (IHC) on overall survival (OS) and disease-free survival (DFS) in patients with EC, using pooled hazard ratio (HR) with its 95% confidence interval (CI).
Results:
The pooled HR for 19 eligible studies (18 publications, n = 3306) suggested that PD-L1 overexpression had an unfavorable impact on OS (HR = 1.42, 95% CI: 1.09–1.86). No significant effect of PD-L1 overexpression on DFS was observed, and the combined HR was 1.08 (95% CI: 0.76–1.53) for 12 eligible studies (11 publications, n = 2260).
Conclusion:
PD-L1 expression in tumor cells detected by IHC was associated with worse OS in EC. However, the prognostic value of PD-L1 expression in tumor cells on OS in EC still needs further large prospective trials to be clarified.
Keywords: biomarker, esophageal cancer, meta-analysis, prognosis, programmed death ligand-1
1. Introduction
Despite improvements in multimodality therapy, including surgery combined with chemotherapy and/or radiotherapy, the prognosis for esophageal cancer (EC) is still rather dismal.[1] It is a pressing need for developing new therapy modalities. In the past decade, great interest has been directed toward the cancer immunotherapy.
It is well known that the development and prognosis of malignant tumors are closely related to host immune functions.[2] Recent advances in cancer immunology have revealed the importance of the programmed cell death protein-1 (PD-1) signaling pathway.[3] PD-1 is a negative costimulatory receptor expressed primarily on activated T cells. The interaction of PD-1 with its specific ligands, programmed cell death ligand 1 or 2 (PD-L1 or PD-L2), plays a pivotal role in antigen-specific T-cell response, mediating PD-1-dependent immune suppression, which facilitates tumor cell to escape from immunosurveillance and promotes tumor progression.[4–6]
Up to now, PD-L1 expression has been observed in a wide variety of malignancies, and several studies suggested that PD-L1 high expression in tumor cells indicates poor prognosis in patients with numerous types of malignancies, including EC.[7–11] However, reports on the influence of PD-L1 expression in patients with EC have been equivocal.[10–13] The aim of this study therefore was to perform a meta-analysis of the influence of PD-L1 expression on overall survival (OS) and disease-free survival (DFS) in patients with EC.
2. Materials and methods
2.1. Search strategy and selection criteria
This study was approved by the Institutional Review Board of Shanghai Jiao Tong University Affiliated Sixth People's Hospital. PubMed and Web of Science were searched (last search was updated on December 31, 2017), using a search algorithm that was based on a combination of the terms: esophageal OR esophagus and cancer OR carcinoma, and programmed cell death ligand 1 OR PD-L1 OR B7-H1. All searched studies were retrieved, and their bibliographies were checked for other relevant publications. Reference lists from identified primary studies and review articles were then searched to identify additional eligible studies missed by electronic search strategies.
Two independent reviewers assessed the eligibility of studies by reviewing titles and abstracts identified by the search. Studies were included in the meta-analysis if they met the following criteria: patients included had surgery and their disease was identified as EC by postoperative pathologic check, evaluate the expression of PD-L1 in the primary tumor cells rather than in sera or metastatic tissue or in tissue adjacent to the tumor, nor in the tumor-infiltrating immune cells, the expression of PD-L1 was measured by immunohistochemistry (IHC) of protein only, association of PD-L1 expression with OS and/or DFS, articles published as a full paper in English, studies provided sufficient information to estimate hazard ratio (HR) and 95% confidence interval (CI). When multiple articles pertained to overlapping populations of patients, only the newest, largest, or most informative single article was selected.
2.2. Data extraction and quality assessment
Two investigators extracted data from eligible studies independently, discussed discrepancies, and reached consensus for all items. The data collection and assessment of methodologic quality followed the quality of reporting of meta-analysis (QUORUM) and the Cochrane Collaboration guidelines (http://www.cochrane.de). The following data were collected from each study: first author's name, year of publication, country of study, tumor cell pathologic type, number of patients analyzed, clinic stage, treatment received, follow-up time, IHC evaluation method and cut-off value for overexpression, antibody used, antibody dilution, rate of PD-L1 overexpression, and prognostic outcomes of interest (OS and DFS). Duplication of data was avoided by matching the author's name and the name of research centers.
2.3. Statistical analysis
Included studies were divided into 2 groups for analysis: those with data regarding OS and those regarding DFS. For the quantitative aggregation of survival results, the impact of PD-L1 overexpression on survival was estimated for each study by the hazard ratio (HR), with its 95% CI, respectively. When HRs and their 95% CIs were described in text or tables, we obtained these values directly. When these statistical variables were not given explicitly in an article, they were calculated from available numerical data using methods described by Parmar et al.[14] When the only available data were in the form of graphical representations, they were calculated from Kaplan–Meier survival curves; the Kaplan–Meier curves were read by 2 persons using Engauge Digitizer 4.1 version independently to reduce inaccuracy in extracted survival rates. By convention, an observed HR of >1 implied a worse survival in the group of PD-L1 overexpression. The impact of PD-L1 on survival was considered to be statistically significant if the 95% CI for the HR did not overlap 1.
The heterogeneity was formally investigated by means of Q test and I2 statistics. If the heterogeneity was existed, we used a random-effects model in place of a fixed-effects model. Evidence of publication bias was evaluated by the funnel plot with Begg test[15] and Egger linear regression asymmetry test.[16] For all of these analyses, P-values below .05 were considered representative of statistically significant. All the data analyses were performed STATA version 12.0 (Stata Corporation, College Station, TX).
3. Results
3.1. Search results
The search results have been shown in Figure 1. The primary literature research retrieved 204 records. After screening the title of citations, 51 records were excluded because of duplicated literatures. Next, 89 citations were excluded after screening abstracts of the records due to non-English articles, meeting reports, reviews, not PD-L1 topic, and non-EC. Then we carefully read the full text of the left citations and 46 of those were excluded due to laboratory studies, insufficient OS data, irrelevant study to the current analysis, or repeat study. Finally, there were 18 published studies included in final meta-analysis.
Figure 1.
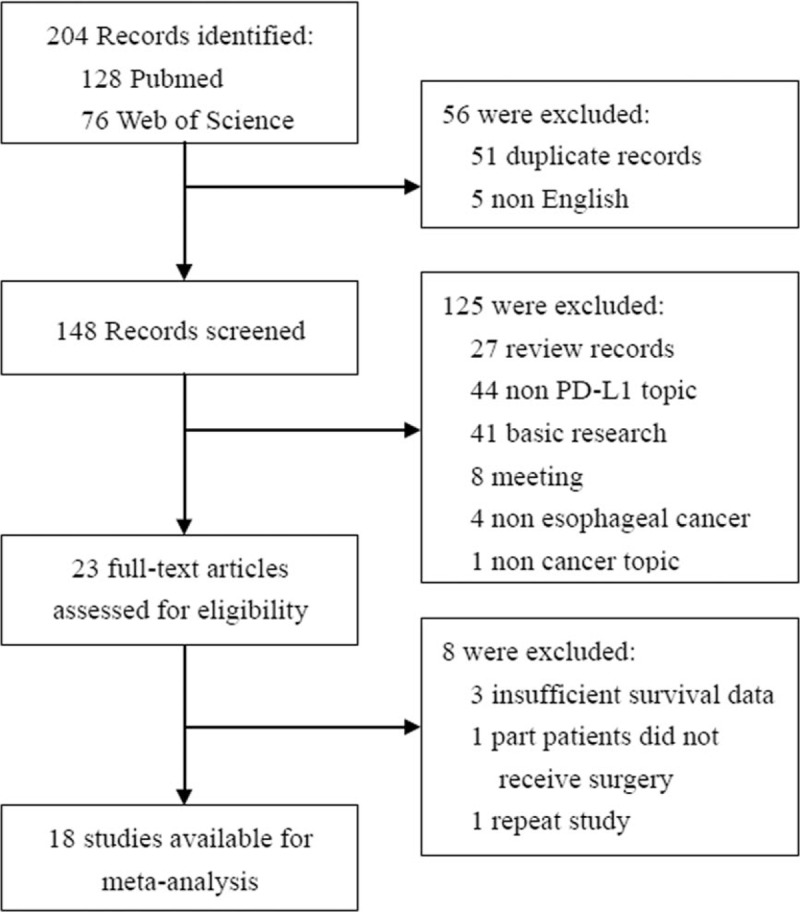
Flow chart for eligible studies.
3.2. Study characteristics
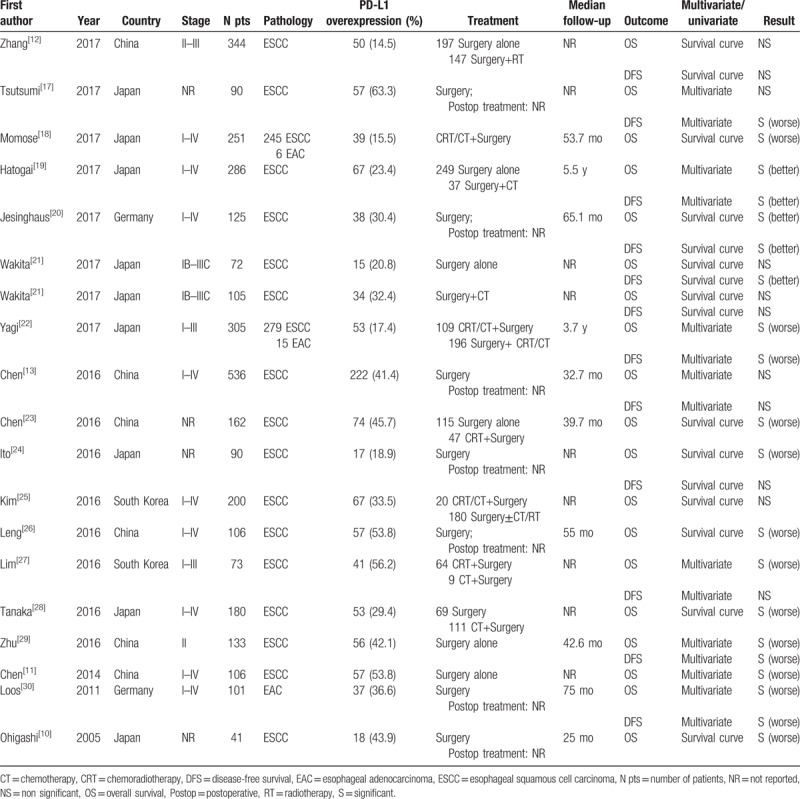
The characteristics of eligible studies are summarized in Table 1. A total of 18 studies[10–13,17–30] published from 2005 to 2017 met the criteria for this meta-analysis. All of studies were based on the data of retrospective analysis. A total of 3306 patients were subjected to the final analysis (mean: 184 per study; range: 41–536). Surgery was performed for all patients and 611 patients in 6 studies were delivered preoperative chemotherapy and/or radiotherapy. These studies were conducted in 4 countries (China, Germany, Japan, and South Korea), and 16 studies (3080 patients) were performed in Asian population, and 2 studies (226 patients) performed in non-Asian patients. Only squamous cell carcinoma was examined in 15 studies and only adenocarcinoma were analyzed in 1 study. The remaining 2 studies investigated squamous cell carcinoma and adenocarcinoma.
Table 1.
Main characteristics and results of the eligible studies.
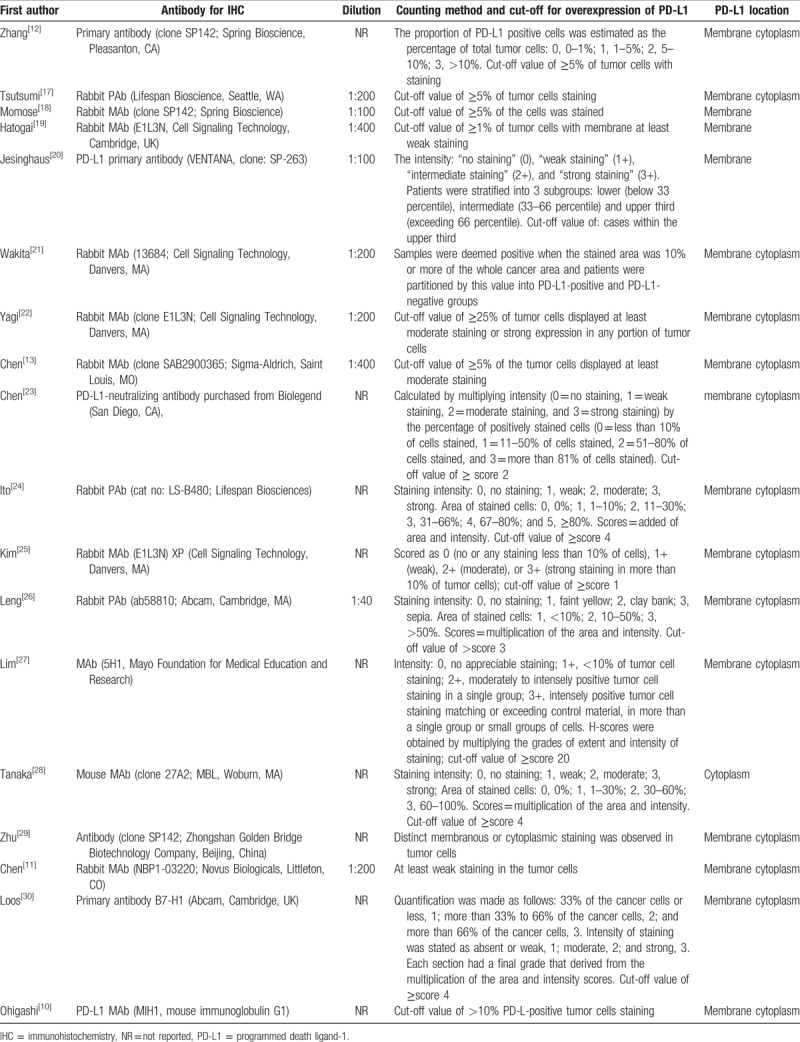
The expression of PD-L1 was measured by IHC in all publications, but the IHC techniques used varied widely among studies, with a wide range of dilutions (from 1:40 to 1:1000). The IHC technique for PD-L1 expression detection was summarized in Table 2. According to the cut-off values for PD-L1 overexpression, as defined by each study's author, 1052 patients (31.8%) in this meta-analysis had PD-L1 overexpression, with a ranged of 14.5% to 63.3%.
Table 2.
Immunohistochemical technique used in studies.
In the study of Wakita et al,[21] the impacts of PD-L1 expression on OS and DFS were analyzed in the subgroup of surgery alone and surgery plus adjuvant therapy, respectively. Hence HRs on OS and DFS could be extracted for 19 (18 publications) and 12 (11 publications) of studies, respectively. Of the 19 studies analyzing the impacts of PD-L1 overexpression on OS, 9 directly reported HRs (multivariate analysis), while the other 10 studies provided survival curves. A significant association between PD-L1 overexpression and OS was found in 13 studies, including 11 studies linking PD-L1 expression with worse OS and 2 studies linking PD-L1 expression with better OS. The remaining 6 studies yielded negative results. In the 12 studies analyzing PD-L1 overexpression on DFS, 5 directly reported HRs (multivariate analysis), while the other 7 studies provided survival curves. Four studies suggested PD-L1 overexpression indicated poor prognosis of DFS, and 3 studies resulted in a favorable DFS, and 5 studies resulted in an indeterminate role for PD-L1 overexpression on DFS.
3.3. Impacts of PD-L1 expression on OS and DFS
The impact of PD-L1 expression in tumor cells on OS was shown in Figure 2. Overall, the pooled HR for all 19 eligible studies (18 publications, n = 3306 patients) evaluating PD-L1 overexpression on OS was 1.42 (95% CI: 1.09–1.86, Z = 2.58, P = .01), suggesting that PD-L1 overexpression detected by IHC was an indicator of poor prognosis for EC (Fig. 2A). For obvious heterogeneity was observed (Q = 94.67, I2 = 81.0%, P < .001), random effect model was used to analyze the effect size. It is necessary to analyze the value of PD-L1 subgrouped by HR provided way. In Figure 2B, the combined HR for 9 studies evaluating PD-L1 overexpression on OS by multivariate analysis was 1.52 (95% CI: 1.06–2.19, Z = 2.25, P = .024). However, the pooled HR of 10 studies provided by survival curve was 1.34 (95% CI: 0.89–2.02, Z = 1.40, P = .162). Then, stratified analysis according to countries, the pooled HRs of Asian country studies and non-Asian country studies were 1.43 (95% CI: 1.10–1.88, Z = 2.63, P = .008) and 1.40 (95% CI: 0.24–8.0, Z = 0.38, P = .706), respectively, indicating PD-L1 is an indicator of poor prognosis of OS in Asian patients but not in non-Asian patients (Fig. 2C). At last, we limited the analysis to the studies dealing mostly (>90%) with squamous cell carcinoma, the combined HR was 1.36 (95% CI: 1.04–1.78, Z = 2.26, P = .024), suggesting that PD-L1 overexpression was significantly correlated with worse OS in esophageal squamous cell carcinoma (ESCC) (Fig. 2D).
Figure 2.
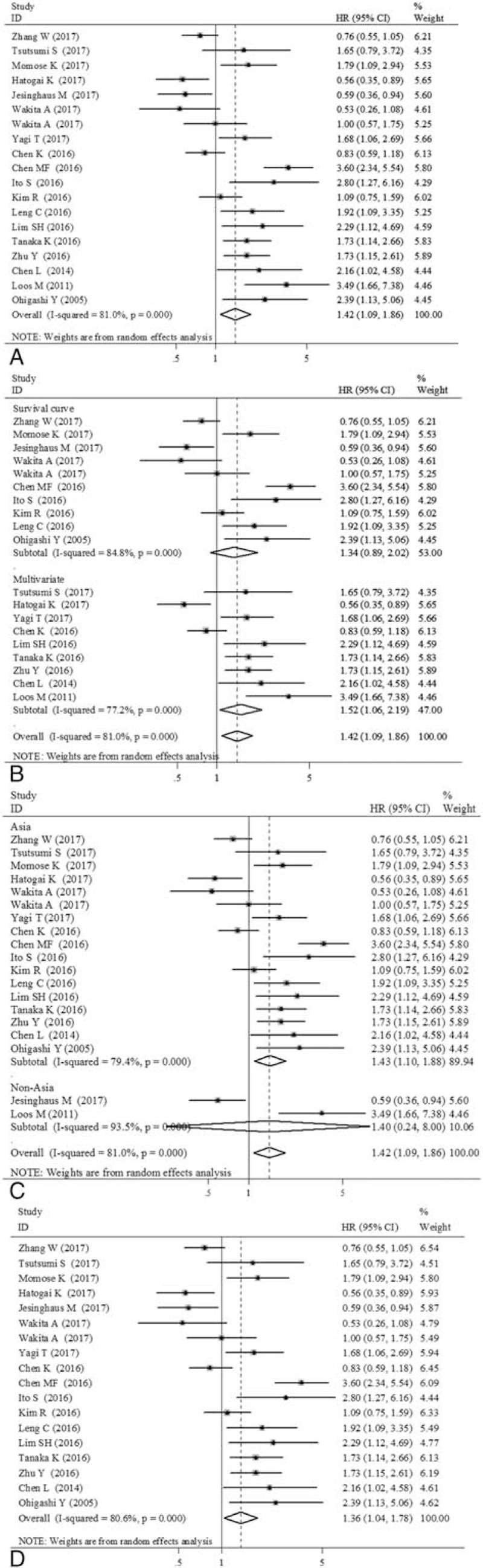
Forest plot showing the association between programmed death ligand-1 (PD-L1) expression status with overall survival (OS). (A) All 19 eligible studies (18 publications) for OS. (B) Subgrouped by hazard ratio (HR) provided way (multivariate analysis/survival curve). (C) Subgrouped by country (Asia/non-Asia). (D) 18 eligible studies (17 publications) for OS in esophageal squamous cell carcinoma (ESCC).
The impact of PD-L1 overexpression in tumor cells on DFS was shown in Figure 3. However, no statistically significant effect of PD-L1 overexpression on DFS was observed, and the combined HR for 12 eligible studies (11 publications, n = 2260 patients) involving DFS was 1.08 (95% CI: 0.76–1.53, Z = 0.41, P = .683) in patients with EC (Fig. 3A). When stratified analysis according to countries, PD-L1 overexpression had no significant impact on DFS in Asian patients (HR = 1.08, 95% CI: 0.78–1.51, Z = 0.47, P = .640) nor in non-Asian patients (HR = 1.06, 95% CI: 0.14–8.12, Z = 0.05, P = .957) (Fig. 3B). The combined HR was 0.99 (95% CI: 0.70–1.39, Z = 0.08, P = .934) in studies dealing mostly (>90%) with squamous cell carcinomas, suggesting that PD-L1 overexpression had no significant impact on DFS in ESCC (Fig. 3C).
Figure 3.
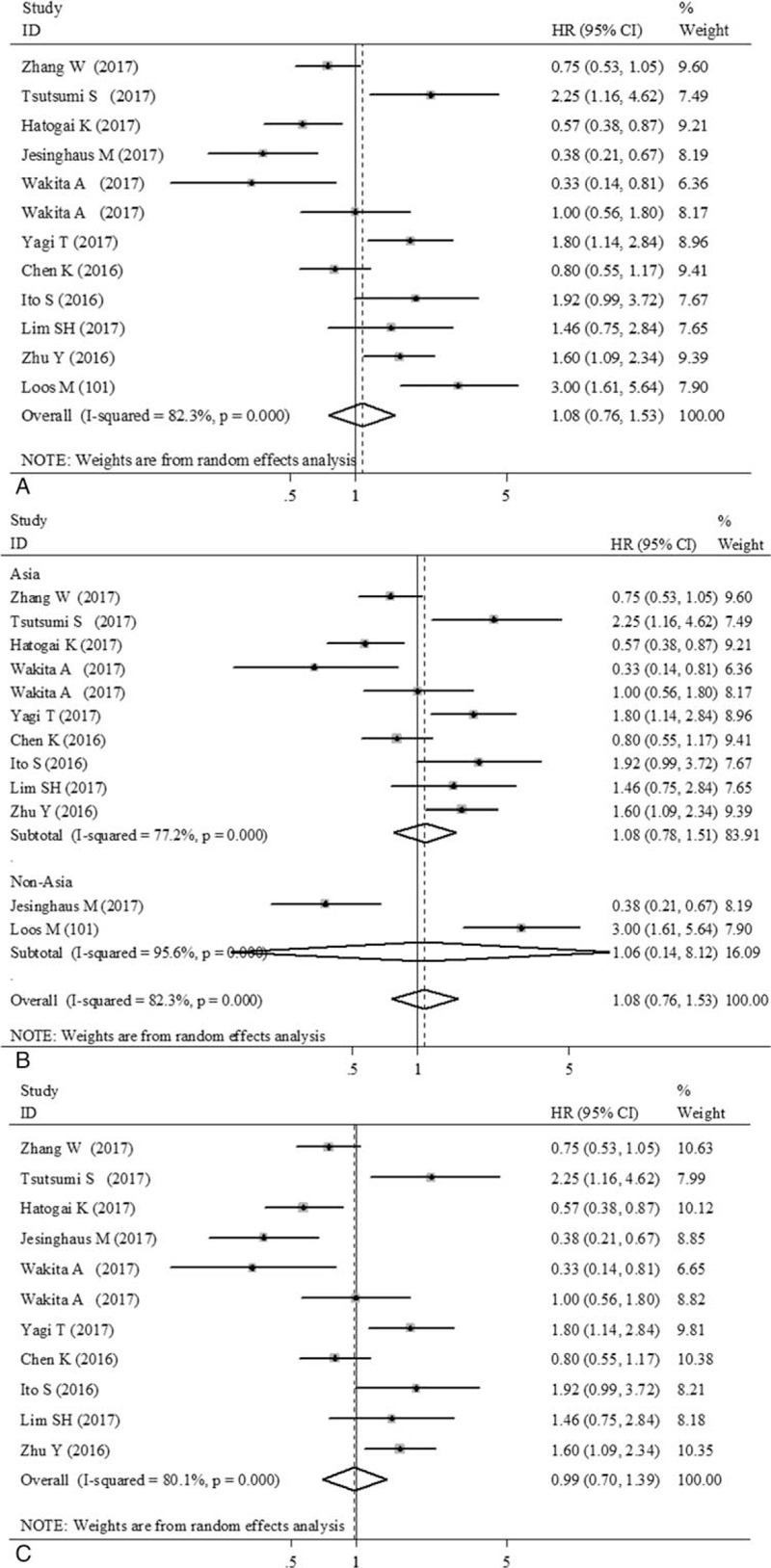
Forest plot showing the association between programmed death ligand-1 (PD-L1) expression status with disease-free survival (DFS). (A) All 12 eligible studies (11 publications) for DFS. (B) Subgrouped by country (Asia/non-Asia). (C) 11 eligible studies (10 publications) for DFS in esophageal squamous cell carcinoma (ESCC).
3.4. Publication bias
Begg funnel plot[15] and Egger test[16] were performed to evaluate the publication bias of the eligible studies (Fig. 4). About 19 and 12 studies investigating PD-L1 overexpression on OS and DFS yielded an Egger test score of P = .08 and .598 (Fig. 4A and C), respectively, indicating the absence of publication bias in the studies. About 18 and 11 studies investigating PD-L1 overexpression on OS and DFS in ESCC yielded an Egger test score of P = .144 and.856, respectively (Fig. 4B and D). Similar results were found for the subgroup analysis of PD-L1 overexpression on OS in multivariate analysis (P = .115), Asian (P = .127), respectively. These results suggested that there were no publication biases in these subgroup analyses.
Figure 4.
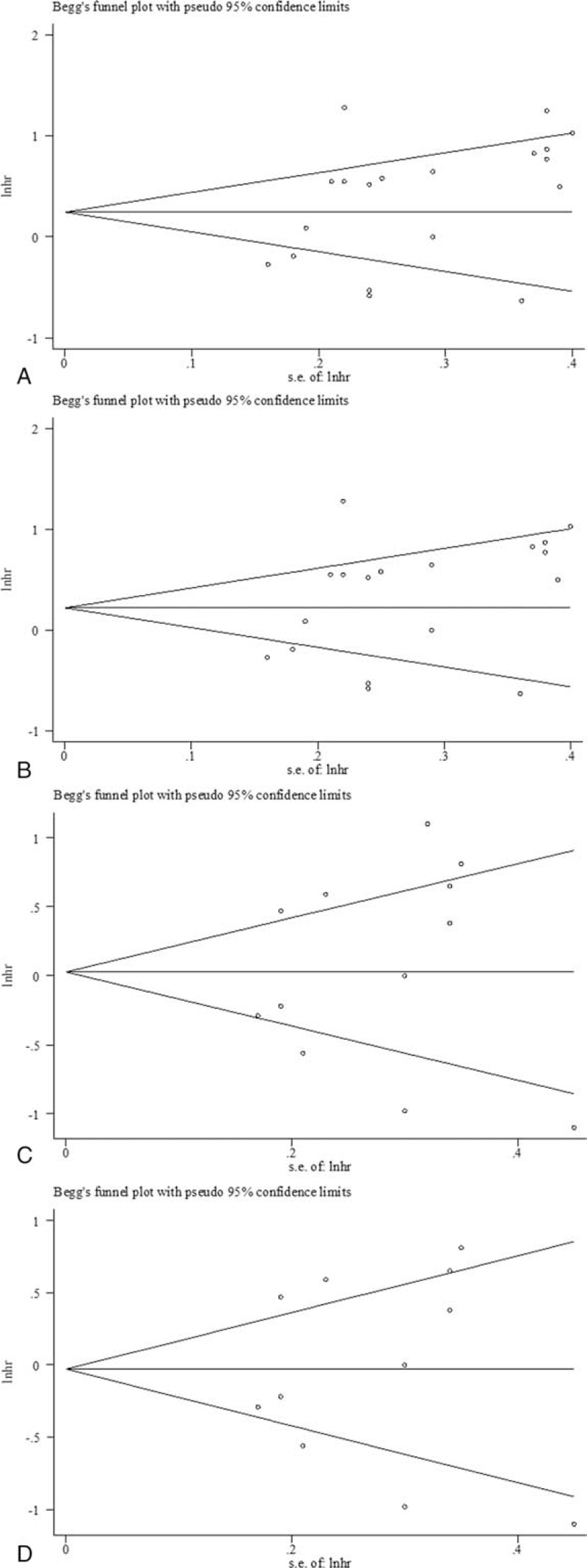
Begg funnel plot for publication bias test. Plots are arranged as follows: (A) all 19 eligible studies (18 publications) for overall survival (OS); (B) 18 studies (17 publications) for OS in esophageal squamous cell carcinoma (ESCC); (C) all 12 eligible studies (11 publications) for disease-free survival (DFS); (D) 11 studies (10 publications) for DFS in ESCC.
4. Discussion
Recently, PD-1/PD-L1 pathway has attracted much attention as an immune-based treatment of several types of malignancies. Some clinical trials using PD-L1-targeting antibodies, such as avelumab and durvalumab, were performed in gastro-ECs.[31] Understanding the mechanisms of action of anti-PD-L1 therapy requires correct evaluation of the impact of PD-L1 expression on survival of patients.
Our present analysis combining 18 published studies which included 3306 patients with EC yielded summary statistics indicating that PD-L1 overexpression has an unfavorable impact on OS, with the pooled HR of 1.42 (95% CI: 1.09–1.86), but not on DFS (HR = 1.08, 95% CI: 0.76–1.53). Conversely, 2 studies included in our analysis showed PD-L1 expression to be a factor predicting favorable OS in EC.[19,20] Similar association have also been found in different tumor types.[32,33] Several possible reasons for these discordant results have been speculated. One reason is likely that heterogeneous baseline characteristics exist in different studies. Moreover, primary antibody used, the definitions of positive staining applied and the cut-off values adopted were also different. This also explained the heterogeneity problem in our meta-analysis. Also, in certain circumstance, high PD-L1 expression might promote immune responses through interaction of PD-L1 with unknown receptors, resulting in T-cell proliferation and secretion of certain cytokines, which in turn activate strong antitumor effects.
Then, we performed analysis in subgroup of different country. The PD-L1 is a poor prognostic factor for OS in EC patients in Eastern Asian countries (China, Japan, and South Korea) (HR = 1.43, 95% CI: 1.10–1.88), but not in non-Asian patients (HR = 1.40, 95% CI: 0.24–8.0), which raises a question whether the validity of results in present meta-analysis would also be applicable to non-Asian patients. In our meta-analysis, only 2 studies included non-Asian patients,[20,30] which occupied 6.8% (n = 226), and negative result may due to the small sample. Also, variability in measurements, experimental procedures, and criteria for PD-L1 expression among countries may contribute to different results. In the study of Jesinghaus et al,[20] only membranous staining patterns were scored as positive and the intensity of PD-L1 staining was scored using a 4-tiered grading system. While in some studies from Asian countries,[10,13,17] membranous and cytoplasm staining patterns were scored as positive, and the cut-off point of high expression of PD-L1 was defined as ≥5% or 10% of the cells were stained.
In subgroup analysis of patients with ESCC, we also observed an adverse influence of PD-L1 overexpression on OS (HR = 1.36, 95% CI: 1.04–1.78). One previous meta-analysis[34] found a trend that overexpression of PD-L1 might be associated with poor survival in patients with ESCC, but the difference was not statistically significant (HR = 1.65; 95% CI 0.95–2.85; P = .07). However, that analysis only combined 7 studies and several studies showing poor survival in tumors overexpressing PD-L1 was excluded, which might have affected the results. Unfortunately, studies involving the role of PD-L1 in esophageal adenocarcinoma (EAC) are limited, so we are unable to draw similar conclusions in the subgroup of patients with EAC for the time being.
Some studies also investigated the prognostic value of PD-L1 expression detected by IHC in tumor-infiltrating immune cells of EC. According to the search strategy in our study, we found 3 studies investigated the prognostic value of PD-L1 expression detected by IHC in tumor-infiltrating immune cells of EC.[12,18,20] In the study of Zhang et al,[12] tissue specimens from 344 patients with ESCC were obtained for IHC analysis of PD-L1 expression on tumor-infiltrating immune cells. The results demonstrated that PD-L1 expression on tumor-infiltrating immune cells is an independent prognostic factor in patients with ESCC and patients with positive immune cell PD-L1 expression had improved survival. However, the results from the study of Momose et al[18] demonstrated that high expression of PD-L1 on the immune cells was associated with unfavorable prognosis in patients with EC. Jesinghaus et al[20] also investigated the influence of immune cell PD-L1 expression on survival in ESCC. In this study, a prognostic value for PD-L1 expression on immune cells was not found for OS or DFS. The literature about the effect of immune cells PD-L1 expression on survival in EC is limited, so we did not perform a quantitative aggregation of survival results to analyzing the association between the immune cells PD-L1 expression and survival in EC.
Association of PD-L1 expression with unfavorable OS provides a rationale for antitumor use in the treatment of EC, especially in ESCC, but the association of PD-L1 expression with traditional prognostic factors such as clinical TNM stage or differentiation is still needed to investigate. Moreover, the prognostic role of PD-L1 in EC should be examined in the context with other molecular biomarkers. Some studies enrolled in this meta-analysis had already addressed the association of PD-L1 with other biomarkers, such as MLH1,[18] HLA Class I,[24] and FOXP3+.[29]
As we performed a meta-analysis, we had to deal with heterogeneity problems. Heterogeneity is a potential problem that may affects the interpretation of the results of all meta-analyses. In the present meta-analysis, obvious heterogeneity was observed between studies. Although only studies performing IHC staining were enrolled in our meta-analysis, some variations in methodologic factors may contribute to heterogeneity between studies, such as different primary antibodies and wide range of dilutions (from 1:40 to 1:1000) were used for immunodetection of PD-L1 across the studies. Some studies even did not clarify the antibody used in detail. Also, different quantitative evaluation systems for the IHC findings and cut-off value from arbitrary choices by investigators conduced to a wide range of protein overexpression. As there is no standard cut-off point for the expression of PD-L1 in the primary tumor cells in EC at present, the cut-off values are different among included studies, which may have interference in judging the prognostic value of PD-L1 expression. In this study, the rate of PD-L1 overexpression was ranged from 14.5% to 63.3%, which may also have contributed to heterogeneity.
Although we did not detect significant publication bias in this meta-analysis, some kind of potential bias still exists between studies and cannot be completely eliminated. We have restricted our analysis to published studies written in English, and a majority of studies that met eligibility criteria were excluded based on language criteria, which may have led to an overestimation of effect sizes. Another potential source of bias is related to the method for extrapolating HR. Some HRs were extrapolated from survival curves, which unavoidably developed a decrease of reliability. Therefore, the results must be interpreted with caution.
In conclusion, the results of our meta-analysis revealed that PD-L1 overexpression was significantly associated with poor OS in EC. Increased expression of PD-L1 might be a predicative factor of poor prognosis and provide a rationale for inhibiting PD-L1 in EC. In the future, higher quality studies and superior patient selection are expected.
Author contributions
Conceptualization: Weiwei Yu and Yanmei Guo.
Data curation: Weiwei Yu and Yanmei Guo.
Formal analysis: Weiwei Yu.
Funding acquisition: Weiwei Yu and Yanmei Guo.
Investigation: Weiwei Yu.
Methodology: Yanmei Guo.
Supervision: Weiwei Yu.
Writing – original draft: Weiwei Yu and Yanmei Guo.
Writing – review and editing: Weiwei Yu.
Conceptualization: Weiwei Yu.
Data curation: Weiwei Yu, Yanmei Guo.
Formal analysis: Weiwei Yu.
Funding acquisition: Weiwei Yu, Yanmei Guo.
Investigation: Yanmei Guo.
Methodology: Yanmei Guo.
Project administration: Yanmei Guo.
Resources: Weiwei Yu, Yanmei Guo.
Software: Weiwei Yu.
Supervision: Weiwei Yu.
Validation: Weiwei Yu, Yanmei Guo.
Visualization: Yanmei Guo.
Writing – original draft: Yanmei Guo.
Footnotes
Abbreviations: CI = confidence interval, DFS = disease-free survival, EC = esophageal cancer, ESCC = esophageal squamous cell carcinoma, HR = hazard ratio, IHC = immunohistochemistry, PD-1 = programmed cell death protein-1, PD-L1 = programmed death ligand-1, OS = overall survival.
WY and YG contributed equally to this work.
The study was supported by the Shanghai Committee of Science and Technology, Natural Science Foundation of Shanghai (no: 15ZR1432100), Scientific Research Found Projects of Shanghai Health Bureau (no: 20124246), and National Nature Science Foundation of China (no: 81201883).
The authors have no conflicts of interest to disclose.
References
- [1].Nakajima M, Kato H. Treatment options for esophageal squamous cell carcinoma. Expert Opin Pharmacother 2013;14:1345–54. [DOI] [PubMed] [Google Scholar]
- [2].Markman JL, Shiao SL. Impact of the immune system and immunotherapy in colorectal cancer. J Gastrointest Oncol 2015;6:208–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 2012;24:207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Driessens G, Kline J, Gajewski TF. Costimulatory and coinhibitory receptors in anti-tumor immunity. Immunol Rev 2009;229:126–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zang X, Allison JP. The B7 family and cancer therapy: costimulation and coinhibition. Clin Cancer Res 2007;13:5271–9. [DOI] [PubMed] [Google Scholar]
- [6].Mittal D, Gubin MM, Schreiber RD, et al. New insights into cancer immunoediting and its three component phases-elimination, equilibrium and escape. Curr Opin Immunol 2014;27:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shi SJ, Wang LJ, Wang GD, et al. B7-H1 expression is associated with poor prognosis in colorectal carcinoma and regulates the proliferation and invasion of HCT116 colorectal cancer cells. PLoS One 2013;8:e76012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen J, Li G, Meng H, et al. Upregulation of B7-H1 expression is associated with macrophage infiltration in hepatocellular carcinomas. Cancer Immunol Immunother 2012;61:101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang A, Wang HY, Liu Y, et al. The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol 2015;41:450–6. [DOI] [PubMed] [Google Scholar]
- [10].Ohigashi Y, Sho M, Yamada Y, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 2005;11:2947–53. [DOI] [PubMed] [Google Scholar]
- [11].Chen L, Deng H, Lu M, et al. B7-H1 expression associates with tumor invasion and predicts patient's survival in human esophageal cancer. Int J Clin Exp Pathol 2014;7:6015–23. [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang W, Pang Q, Zhang X, et al. Programmed death-ligand 1 is prognostic factor in esophageal squamous cell carcinoma and is associated with epidermal growth factor receptor. Cancer Sci 2017;108:590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chen K, Cheng G, Zhang F, et al. Prognostic significance of programmed death-1 and programmed death-ligand 1 expression in patients with esophageal squamous cell carcinoma. Oncotarget 2016;7:30772–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Parmar MKB, Torri V, Stewart L. Extracting summary to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815–34. [DOI] [PubMed] [Google Scholar]
- [15].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [16].Egger M, Davey Smith G, Schneider M. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tsutsumi S, Saeki H, Nakashima Y, et al. Programmed death-ligand 1 expression at tumor invasive front is associated with epithelial-mesenchymal transition and poor prognosis in esophageal squamous cell carcinoma. Cancer Sci 2017;108:1119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Momose K, Yamasaki M, Tanaka K, et al. MLH1 expression predicts the response to preoperative therapy and is associated with PD-L1 expression in esophageal cancer. Oncol Lett 2017;14:958–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hatogai K, Kitano S, Fujii S, et al. Comprehensive immunohistochemical analysis of tumor microenvironment immune status in esophageal squamous cell carcinoma. Oncotarget 2016;7:47252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jesinghaus M, Steiger K, Slotta-Huspenina J, et al. Increased intraepithelial CD3+ T-lymphocytes and high PD-L1 expression on tumor cells are associated with a favorable prognosis in esophageal squamous cell carcinoma and allow prognostic immunogenic subgrouping. Oncotarget 2017;8:46756–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wakita A, Motoyama S, Nanjo H, et al. PD-L1 expression is a prognostic factor in patients with thoracic esophageal cancer treated without adjuvant chemotherapy. Anticancer Res 2017;37:1433–41. [DOI] [PubMed] [Google Scholar]
- [22].Yagi T, Baba Y, Ishimoto T, et al. PD-L1 expression, tumor-infiltrating lymphocytes, and clinical outcome in patients with surgically resected esophageal cancer. Ann Surg 2017;doi: 10.1097/SLA.0000000000002616. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [23].Chen MF, Chen PT, Chen WC, et al. The role of PD-L1 in the radiation response and prognosis for esophageal squamous cell carcinoma related to IL-6 and T-cell immunosuppression. Oncotarget 2016;7:7913–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ito S, Okano S, Morita M, et al. Expression of PD-L1 and HLA Class I in esophageal squamous cell carcinoma: prognostic factors for patient outcome. Ann Surg Oncol 2016;23:508–15. [DOI] [PubMed] [Google Scholar]
- [25].Kim R, Keam B, Kwon D, et al. Programmed death ligand-1 expression and its prognostic role in esophageal squamous cell carcinoma. World J Gastroenterol 2016;22:8389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Leng C, Li Y, Qin J, et al. Relationship between expression of PD-L1 and PD-L2 on esophageal squamous cell carcinoma and the antitumor effects of CD8(+) T cells. Oncol Rep 2016;35:699–708. [DOI] [PubMed] [Google Scholar]
- [27].Lim SH, Hong M, Ahn S, et al. Changes in tumor expression of programmed death-ligand 1 after neoadjuvant concurrent chemoradiotherapy in patients with squamous oesophageal cancer. Eur J Cancer 2016;52:1–9. [DOI] [PubMed] [Google Scholar]
- [28].Tanaka K, Miyata H, Sugimura K, et al. Negative influence of programmed death-1-ligands on the survival of esophageal cancer patients treated with chemotherapy. Cancer Sci 2016;107:726–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhu Y, Li M, Mu D, et al. CD8+/FOXP3+ ratio and PD-L1 expression associated with survival in pT3N0M0 stage esophageal squamous cell cancer. Oncotarget 2016;7:71455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Loos M, Langer R, Schuster T, et al. Clinical significance of the costimulatory molecule B7-H1 in Barrett carcinoma. Ann Thorac Surg 2011;91:1025–31. [DOI] [PubMed] [Google Scholar]
- [31].Ammannagari N, Atasoy A. Current status of immunotherapy and immune biomarkers in gastro-esophageal cancers. J Gastrointest Oncol 2018;9:196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Velcheti V, Schalper KA, Carvajal DE, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest 2014;94:107–16. doi: 10.1038/labinvest.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Karim R, Jordanova ES, Piersma SJ, et al. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res 2009;15:6341–7. [DOI] [PubMed] [Google Scholar]
- [34].Qu HX, Zhao LP, Zhan SH, et al. Clinicopathological and prognostic significance of programmed cell death ligand 1 (PD-L1) expression in patients with esophageal squamous cell carcinoma: a meta-analysis. J Thorac Dis 2016;8:3197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]


