Abstract
Recently, new techniques and devices in transjugular intrahepatic portosystemic shunt (TIPS) placement have emerged that can improve upon the standard procedure. Ultrasound guidance during TIPS with intracardiac echocardiography (ICE), placement of controlled expansion (CX) stents, and portal vein recanalization (PVR) via transsplenic access are three techniques with new data supporting their implementation. ICE guidance can improve the technical success of difficult cases, decrease procedure time, and decrease complications such as capsular puncture, hemobilia, and hepatic artery injury. CX stents offer the operator better control over the final portosystemic gradient, which is particularly useful in patients with a high risk of post-TIPS hepatic encephalopathy. Finally, transsplenic access provides a stable, antegrade route for PVR, which can be used to optimize transplant candidacy as well as treat the sequelae of portal hypertension in patients with portal vein thrombosis. This article will describe the benefits, technical parameters, and patient selection criteria for each of these new techniques.
Keywords: TIPS, portal hypertension, intravascular ultrasound, portal vein thrombosis, transsplenic
Objectives: Upon completion of this article, the reader will be able to explain some of the recent advancements in TIPS placement, including the roles of the new techniques and devices, the procedural impact, and the data supporting their use.
Accreditation: This activity has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit ™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Transjugular intrahepatic portosystemic shunt (TIPS) placement is a well-validated treatment for patients with refractory ascites and variceal bleeding related to portal hypertension, and more recently has been used to treat hepatic hydrothorax, hepatorenal syndrome, portal hypertensive gastropathy, hepatic venoocclusive disease, and Budd–Chiari syndrome. Rösch et al first proposed the process of accessing a hepatic vein, puncturing through the liver for retrograde portal venous access, and placing tubing to create a portosystemic shunt in 1969. 1 Large clinical trials in the 1990s validated TIPS safety and efficacy, thereby contributing to widespread acceptance. Since then, an abundance of peer-reviewed literature has helped refine the procedure, such as with the introduction of polytetrafluoroethylene (PTFE) stent grafts to preserve patency, and a better understanding of patient selection criteria.
Recently, new TIPS techniques and devices have emerged with potential to further improve the standard procedure. Intravascular sonographic guidance during TIPS placement, controlled expansion (CX) stents, and transsplenic access for portal vein recanalization (PVR) are three techniques with new data supporting their implementation. In experienced centers with high technical success rates of TIPS creation, intravascular sonography and CX stents can further improve the technical success of difficult cases while also decreasing some of the known procedural and clinical complications such as capsular puncture, hemobilia, hepatic artery injury, and post-TIPS refractory encephalopathy. Furthermore, transsplenic access provides a direct anatomic route for PVR to improve transplant eligibility in some patients. Herein, we describe the benefits, technical parameters, and patient selection criteria for each of these new techniques.
Intravascular Ultrasound with Intracardiac Echocardiography Catheters
Procedural Impact
Clinical trials using intravascular sonography were first published in the 1980s to evaluate atherosclerotic plaque burden of arterial lumens. 2 Since that time, intravascular sonography has evolved into a robust tool with two distinct transducer categories—a 360-degree intravascular ultrasound (IVUS) catheter and a 90-degree side-fire intracardiac echocardiography (ICE) catheter. Retrospective comparison studies have begun to demonstrate that the use of ICE during TIPS creation can decrease complications and procedure times.
Several steps during TIPS creation are performed with indirect imaging guidance and/or with single plane visualization. The current authors have found ICE to be beneficial during three steps: (1) hepatic vein cannulation and confirmation of which vein was catheterized, (2) portal vein access, and (3) stent deployment. Cannulation of a hepatic vein is the first step in TIPS creation and establishes the direction of portal vein puncture. Review of cross-sectional imaging prior to TIPS is critical for procedural planning and identifies patients with variant or difficult anatomy. However, even with pre-TIPS imaging review, cannulating a hepatic vein and confidently differentiating the right from middle hepatic vein can be difficult in certain patients. In a consecutive series of 200 patients with normal liver function, 61% of patients had an independent right hepatic vein and a common trunk for the middle and left hepatic veins. 3 The remaining 39% had three separate hepatic veins draining into the inferior vena cava (IVC). Notably, 39% of patients had a small right hepatic vein which was compensated for by a large right inferior hepatic vein, accessory hepatic vein, or well-developed middle hepatic vein. Such cases may require additional time or operator skill during this initial step of the procedure. Other patients with small, stenotic, and/or thrombosed hepatic veins such as in Budd–Chiari syndrome are also cases in which direct visualization with ICE is beneficial.
Portal vein puncture is one of the technically challenging steps during TIPS placement. After selection of a suitable hepatic vein, a wedged hepatic venogram is typically performed with iodinated contrast or carbon dioxide to identify the portal vein with retrograde opacification. A TIPS access needle is then used to target the portal vein branch while using preprocedure imaging and the wedged portal venogram as a guide. This step may take multiple passes through the liver and presents an opportunity to cause injury, prolong procedural time, and increase radiation exposure. Several procedural complications—including transcapsular puncture, hemoperitoneum (0.5%), hemobilia (2%), and hepatic artery puncture (1%)—are known to occur during portal vein puncture. 4 5 Such complications can be devastating, particularly in patients with advanced liver disease who already have an underlying coagulopathy and are at higher risk of mortality after TIPS. 6
In a retrospective series of 89 patients who underwent conventional TIPS or TIPS with ICE guidance, Kao et al reported that a median of 2 needle passes were required to access the portal vein when using ICE guidance compared with 6 passes in the conventional group ( p < 0.01). 7 Fewer needle passes during portal vein cannulation would theoretically result in reduced procedural complications. Indeed, a similar retrospective study by Pillai et al analyzing 109 patients with either conventional TIPS or TIPS with ICE reported capsular perforations occurring in 31% of conventional cases compared with only 9% when ICE was used during TIPS ( p = 0.003). 8 Further study will be needed to determine if a decrease in needle passes or capsular punctures translates to improved patient outcomes and survival.
Finally, ICE is useful during stent deployment to confirm stent placement relative to adjacent structures, such as large bile ducts or hepatic arterial branches. Rare cases of hepatic ischemia and infarction from stent compression of the hepatic artery have been reported, due to portal flow shunting and limited arterial reserve in patients with high Child–Pugh scores. 9 Additionally, using intravascular ultrasound, the uncovered portion of the TIPS stent is easily visible within the portal vein, which allows the operator to position it optimally at the portal vein entry site. This ensures that no more than 1 to 2 cm of the stent extends into the extrahepatic portal vein to allow for surgical cross-clamping during liver transplantation and the use of the main portal vein for the transplant anastomosis. Lastly, after deployment, the relationship and angulation between the hepatic venous end of the TIPS stent and the hepatic vein ostium can be assessed sonographically to prevent future TIPS stenosis or occlusion.
Given the variable difficulty of hepatic and portal vein cannulation, TIPS creation has the potential for prolonged procedure times and high ionizing radiation exposure to the patient and operator. Patients with variant anatomy or complicating factors such as portal vein thrombosis (PVT) may surpass thresholds for transient erythema (2 Gy) and dry desquamation or transient epilation (3–5 Gy). Analysis of fluoroscopy time (minute), dose area product (µGy*m 2 ), air kerma or cumulative dose (mGy), and total procedural time (minute) showed these technical factors to be significantly less in TIPS with ICE compared with conventional TIPS cases which used marker wires or fluoroscopy for guidance. 10 Specifically, Gipson et al showed a median air kerma of 1,442 and 1,421 mGy in TIPS using fluoroscopy or marker wires, respectively, compared with 850 mGy when ICE was used. 10
Patients undergoing TIPS placement for cirrhosis often have a myriad of sequelae related to liver disease. Concomitant renal disease, in particular, may be an indication for TIPS (e.g., hepatorenal syndrome) or a complicating factor in TIPS creation requiring judicious use of iodinated contrast. ICE guidance may benefit patients with compromised renal function by decreasing the volume of contrast used throughout the procedure. It may also reduce the risk of acute renal failure or nephrotoxicity, which is reported in up to 4% of cases. 5 The study by Kao et al showed that a median of 140 mL of contrast media was used during conventional TIPS compared with only 57 mL during TIPS with ICE. 7 Further studies would be necessary to determine whether the difference in contrast volume impacted renal function in the patients undergoing TIPS.
Technique
There are several commercial manufacturers of ICE transducer catheters. Available transducer frequencies are typically 5 to 10 MHz and produce 90-degree sector images. The transducer catheter can be introduced into 8- or 10-Fr systems at a second internal jugular access site or a common femoral vein access. A complementary ICE-compatible ultrasound machine is required to input and display the sonographic images. The current authors place a 9-Fr, 30-cm sheath into the right common femoral vein to accommodate an 8-Fr AcuNav catheter (Siemens, Washington, DC). While this technique requires a second operator, the ultrasound catheter can also be placed into a second internal jugular access and controlled by the primary operator during the procedure.
The transducer is placed at the level of the suprahepatic IVC–hepatic venous confluence for hepatic vein catheterization. Prior to portal vein cannulation, the transducer is moved slightly caudally within the retrohepatic IVC to inspect the adjacent vasculature, biliary tree, and parenchyma. The portal vein branch nearest to the cannulated hepatic vein is assessed and intervening parenchyma assessed for findings that would preclude this target such as a hepatic artery branch, large bile duct, or mass lesion ( Fig. 1a, b ). Needle access of the portal vein is performed with direct ultrasound guidance ( Fig. 1c, d ). Finally, the covered PTFE stent is deployed under direct visualization, with careful attention to the location of the uncovered portion and the hepatic venous end of the stent.
Fig. 1.
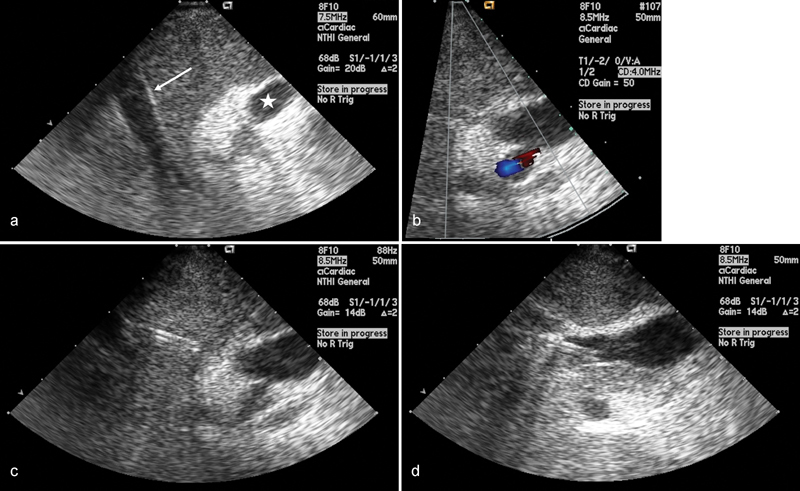
( a ) Intravascular ultrasound image with an ICE catheter positioned within the IVC shows the relationship between the middle hepatic vein (arrow) and the right portal vein (star). ( b ) Color Doppler image demonstrates the position of the hepatic artery relative to the portal vein. ( c ) Intravascular ultrasound image showing the TIPS needle traversing the liver parenchyma toward the right portal vein and ( d ) within the right portal vein.
Indications for Use
Ultrasound guidance is beneficial during several steps of TIPS creation. Although there is additional cost associated with the ultrasound catheter, and the potential risks associated with a second venous access site, TIPS creation with ICE has been shown to decrease procedural complications, procedure time, and radiation exposure. Therefore, when available, the current authors advocate routine use of ICE during TIPS. Special priority for ICE should be given to cases with variant anatomy, coagulopathy, impaired renal function necessitating limited contrast use, or other factors which might contribute to procedural complexity.
Controlled Expansion Transjugular Intrahepatic Portosystemic Shunt
Procedural Impact
Hepatic encephalopathy (HE) is the most common clinical complication of elective TIPS and occurs in up to 43% of patients. 11 12 Ammonia is produced by colonic bacterial catabolism and enterocytes from glutamine before being absorbed into the portal circulation and filtered by the liver. HE due to shunting of ammonia to the systemic circulation can be due to underlying liver disease or precipitated by or worsened after TIPS creation. Symptoms are graded from 0 to 4 based on the West Haven Grading System, where 1 corresponds to mild confusion and shortened attention span and 4 is late-stage comatose encephalopathy.
A multivariate analysis of an 82-patient cohort identified that advanced age (mean: 61.9 vs. 54.8 years), higher Child–Pugh score, and covert HE (i.e., subclinical HE, as defined by psychometric testing) were independently associated with post-TIPS HE. 12 Not surprisingly, patients with preexisting HE, including subclinical encephalopathy, were at the highest risk of worsening or refractory encephalopathy post-TIPS. Other studies have reported similar findings and also noted that MELD score greater than 18 was independently associated with HE (58 vs. 37%, p = 0.009). 11 Patients who undergo elective TIPS for an indication of refractory ascites have also shown to have a higher risk for encephalopathy compared with patients undergoing TIPS for variceal bleeding. 13
In the retrospective review by Casadaban et al, 42% of patients had encephalopathy after TIPS: 22% with de novo HE, 8% with worsening symptoms of previous encephalopathy, and 12% with stable encephalopathy. 11 Patients with worsening HE post-TIPS had a significantly higher 90-day mortality ( p = 0.001). Patients were treated for post-TIPS HE with a standard combination of lactulose with or without rifaximin and metronidazole. Encephalopathy refractory to medical therapy occurs in up to 7% of patients after TIPS. Of this series, 4% of patients with refractory encephalopathy were eventually treated with TIPS revision.
For patients with refractory HE, several methods of TIPS reduction have been described to increase the portosystemic gradient and decrease ammonia shunting to the systemic circulation. A few techniques, for example, include parallel deployment of a second stent graft or balloon, intramural reduction with sutures, and temporary occlusion with thrombosis. 14 15 These procedures decrease or obliterate the TIPS luminal diameter and divert blood through the portal circulation. As a result of TIPS reduction, two separate series reported an increase in portosystemic gradient to a final mean of 17 mm Hg, representing mean increases of 8 and 5 mm Hg, respectively. 11 16 Although TIPS reduction has been technically successful when indicated, it would be preferable to avoid the clinical ramifications of HE as well as the need to re-access and modify a functioning TIPS.
Some authors have reported success with preemptive underdilation of the PTFE-covered VIATORR (Gore, Newark, DE) stents in patients with a high risk for post-TIPS encephalopathy, such as by dilating the 10-mm stent with an 8-mm balloon or varying balloon dilation within the stent to create an hourglass shape. 17 However, a recent study obtained serial imaging on 39 patients with underdilated VIATORR or WALLSTENT (Boston Scientific) endoprostheses and found that passive shunt expansion occurred within the first 30 days and continued over 180 days due to intrinsic properties of the stent. 13 The stents that were originally dilated to approximately 65% of potential diameter were expanded to 82 to 92% at last follow-up. Authors of the study suggested that the delayed, passive shunt expansion might allow for more gradual adaptation to hemodynamic changes, but the unpredictable nature of passive expansion negates the operator's desired stent diameter for an individual patient.
Newly available VIATORR TIPS Endoprosthesis with Controlled Expansion (VIATORR CX) offers the operator the option to dilate the TIPS between 8 and 10 mm. Although results with this new device are limited, Praktiknjo et al compared VIATORR CX to regular VIATORR and bare metal stents in a series of 105 patients. 18 Their initial data showed lower rates of sepsis and ascites in the VIATORR CX recipients when compared with either the regular VIATORR or bare metal stent patients. MELD scores also significantly improved in the VIATORR CX patients compared with the other two groups (8 vs. 11 vs. 15, respectively, p = 0.019). Another early experience using VIATORR CX to reduce existing TIPS from 10 to 8 mm in two patients with refractory HE was also successful. 19
Other complications related to portosystemic shunting are much less common than HE but can result in major morbidity or mortality. Some case series have reported accelerated liver failure or hepatic insufficiency in up to 3% of patients after surgical portosystemic shunting and TIPS. 4 Preexisting comorbid factors, high MELD and Child–Pugh scores, and final shunt diameter each affected the rate of accelerated liver failure in these patients. 4 Thus, smaller shunt diameter using CX TIPS may have mitigated this complication in high-risk patients.
Technique
The process of hepatic vein cannulation, portal vein access, and stent positioning are as previously described. Dilation of the CX stent can be performed with an 8-, 9, or 10-mm balloon to achieve the target diameter. The current authors initially dilate to 8 mm in almost all elective TIPS patients regardless of the portosystemic gradient ( Fig. 2 ). Post-TIPS patients are closely monitored by a multidisciplinary team of interventional and diagnostic radiologists, hepatologists, and transplant surgeons for persistent signs and symptoms related to portal hypertension. Patients who need increased portosystemic shunting can be brought back to the interventional radiology suite for expansion of the TIPS to 9 or 10 mm. Balloon dilation of the CX TIPS in a patient requiring more shunting is a simpler procedure compared with TIPS reduction in a patient needing less shunting.
Fig. 2.
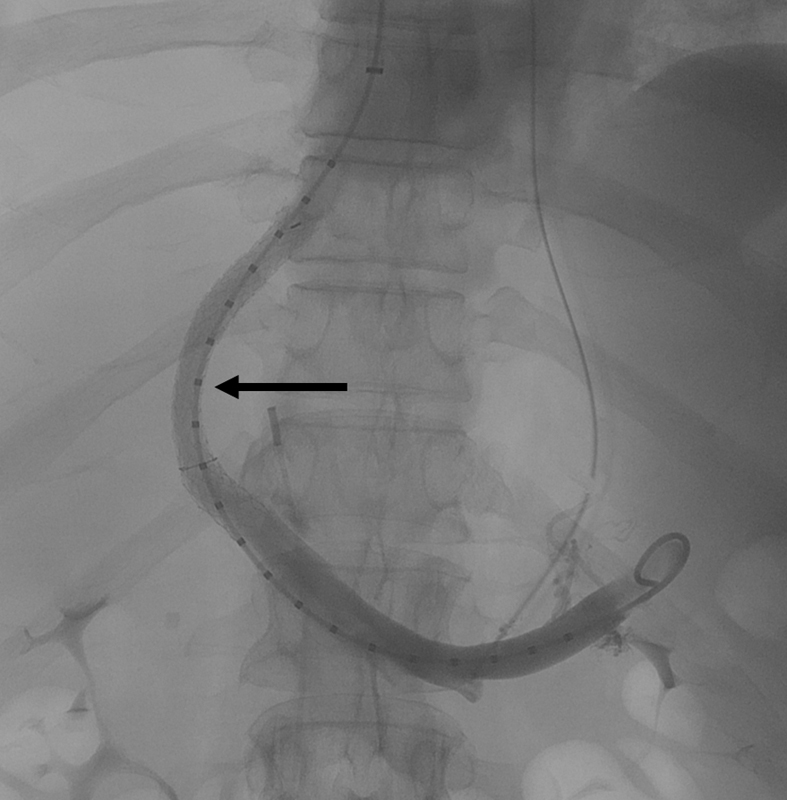
Portal venogram after TIPS creation with a controlled expansion stent dilated to 8 mm. The mid portion of the TIPS stent has a decreased diameter (arrow) compared with the 10-mm self-expanding portion of the stent within the portal vein and the trailing edge within the hepatic vein.
Indications for Use
The VIATORR CX stents have recently phased out the original VIATORR stents, giving the operator a choice of TIPS diameter for each case. Although the current authors routinely dilate to 8 mm in elective cases, there are several clinical factors which could help differentiate patients in whom a smaller diameter is likely to be beneficial. Patients with prior episodes of HE, age over 65 years, high Child–Pugh or MELD score, and comorbidities are at particularly high risk for HE and/or accelerated liver failure related to portosystemic shunting. Additionally, baseline ammonia levels, while not statistically significant in a small series, might also be predictive of patients at risk for de novo HE (median: 133 vs. 92 µg/dL). 11 Use of smaller shunt diameter should certainly be considered in elective cases for patients meeting these criteria.
Transsplenic Approach
Procedural Impact
Up to 26% of patients with cirrhosis develop chronic nontumoral PVT, a complicating factor and often a relative contraindication for liver transplant. Thrombosis in liver dysfunction results from a complex imbalance of coagulation proteins, coagulation inhibitors, decreased platelets or platelet dysfunction, and abnormalities of fibrinolytic activity. 20 These acquired risk factors, combined with inherited risk factors, and local hemodynamic and anatomic factors may all contribute to thrombosis. 21 Previous studies have shown that male sex, Child–Pugh class C, low platelets, encephalopathy, ascites, and nonalcoholic steatohepatitis are each associated with increased risk of PVT. However, decreased portal venous blood flow seems to be the most important predictor of PVT in patients with cirrhosis. PVT occurred in 91.7% of patients with portal vein velocity less than 15 cm/s, compared with only 19.7% of patients with velocity greater than 15 cm/s ( p < 0.001). 21 Despite surgical management techniques for PVT during liver transplant, patients with PVT at the time of transplant have higher morbidity and 1-year mortality compared with patients with patent portal veins. 22 23 Thus, PVT is a relative contraindication for transplant at some centers. TIPS with PVR can increase a patient's candidacy for transplant and decrease posttransplant morbidity.
A few centers have reported successful TIPS placement in patients with PVT through a standard transhepatic approach and direct puncture of a portal vein branch. 24 25 Several of these cases have resulted in successful liver transplant with end-to-end portal anastomosis. However, retrograde transhepatic recanalization of the portal vein is not always feasible in cases with extensive thrombosis (including intrahepatic portal branches) or cavernous transformation.
The current authors' institution reported a series of 61 patients who underwent PVR and TIPS (PVR-TIPS) to treat their chronic PVT and improve their transplant candidacy. 26 The final 20 patients in the series had recanalization performed via a transsplenic approach. Restoration of portal flow was successful in 100% of patients in whom transsplenic access was utilized, including six with cavernous transformation. 27 28
Potential complications related to transsplenic access include hemorrhage and intraperitoneal bleeding. In the series of 61 patients, of whom 20 underwent transsplenic access, there were two cases of perisplenic hemorrhage requiring packed red blood cells transfusion without embolization or splenectomy. 28 There was no mortality attributable to transsplenic access. A theoretical risk of portal vein rupture during recanalization due to chronic scarring and fibrosis was not observed. Thus, the safety and effectiveness of a transsplenic approach for PVR has made this technique the author's preferred approach for PVR-TIPS.
Technique
In addition to usual pre-TIPS evaluation, during the workup of patients with chronic PVT who may require transsplenic recanalization, imaging is carefully reviewed to assess the degree of PVT, presence of cavernoma, and patency/anatomy of the splenic vein. If transsplenic PVR is for transplantation purposes, the transplant workup should be complete in case hepatic decompensation occurs.
The technique for transsplenic access has been previously described in detail. 26 27 Briefly, after right internal jugular vein access and selection of the right or middle hepatic vein, percutaneous puncture of the spleen is performed with a straight in-line path to the main splenic vein using a 21-G needle and ultrasound guidance ( Fig. 3 ). Transsplenic puncture of the splenic vein is visualized with ultrasound guidance, a 0.018-inch wire is advanced, and system is upsized to a 35-cm 5-Fr sheath which is advanced through the splenic parenchyma and into the splenic vein. A venogram is performed to assess the degree of PVT as well as the presence of shunts or varices ( Fig. 4 ). A glide wire is advanced through an angled catheter (e.g., KMP catheter) through the thrombosed portal vein and a snare is advanced into a portal vein branch and used as a fluoroscopic target for the TIPS needle ( Fig. 5 ). An exchange length stiff glide wire is grasped with the snare, withdrawn through the splenic sheath, providing through and through access. A TIPS stent is then deployed and both the TIPS and thrombosed portal vein are dilated. In potential transplant recipients, it is important when deploying the stent to leave an adequate length of unstented portal vein (>2 cm) for use at the time of transplant ( Fig. 5d ). At the conclusion of the procedure, the transsplenic access tract is embolized with coils or Gelfoam ( Fig. 6a, b ).
Fig. 3.
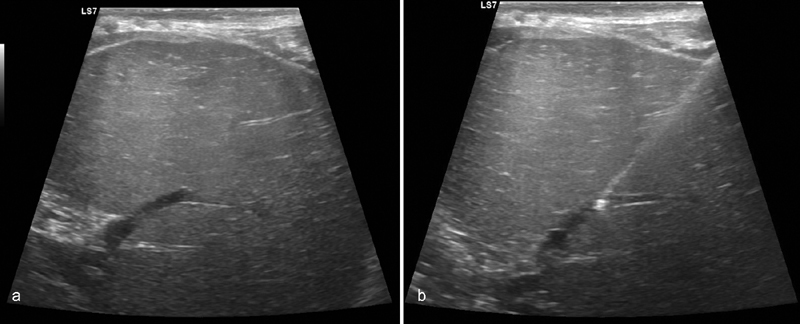
Transsplenic portal vein recanalization and TIPS placement (PVR-TIPS) performed in a patient with chronic PVT who needs a liver transplant. ( a ) Ultrasound demonstrates patency of an intraparenchymal splenic vein branch. ( b ) Ultrasound-guided needle access into the splenic vein branch is performed.
Fig. 4.
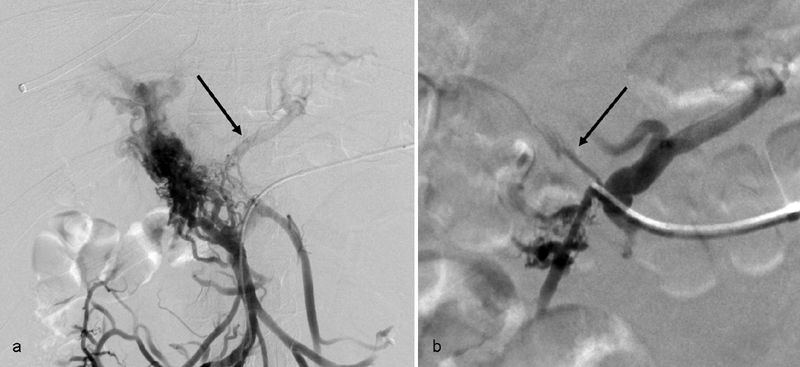
( a ) Venography during PVR-TIPS demonstrates chronic occlusion of the portal vein with extensive cavernous transformation. The SMV and IMV are patent. However, the left gastric vein (arrow) is identified which guides recanalization. ( b ) Positioning the catheter closer to the origin of the left gastric vein helps identify the thrombosed main PV (arrow).
Fig. 5.
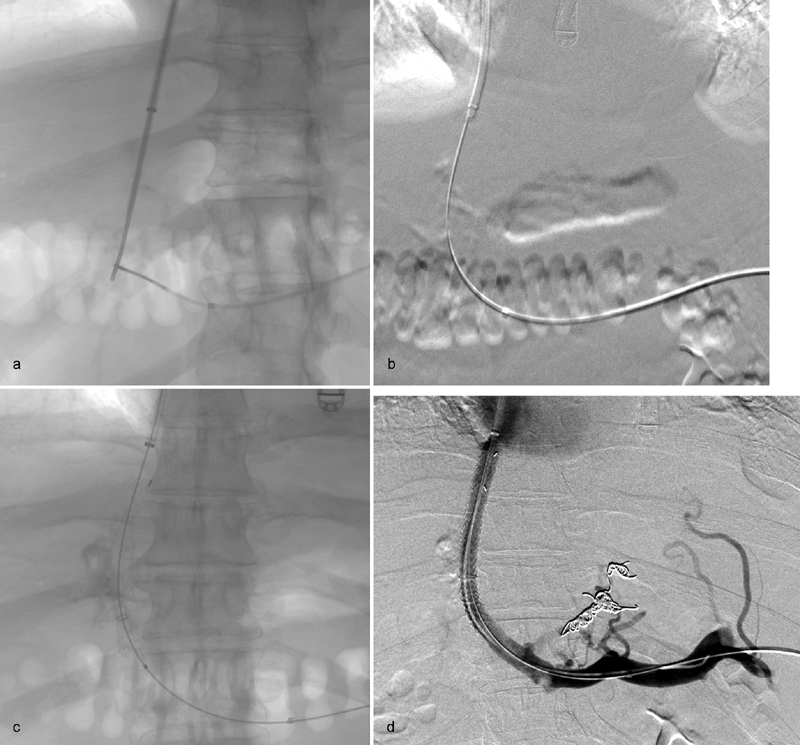
( a ) After the wire and catheter traverse the thrombosed portal vein, a snare is advanced through the catheter as a fluoroscopic target and used to grasp the TIPS needle. ( b ) An exchange length stiff glide wire is advanced through the TIPS needle, grasped with the snare, and withdrawn through the splenic sheath providing through-and-through access. ( c ) After exchange for a working wire, the TIPS is deployed. ( d ) Venography after angioplasty of the PV/TIPS and embolization of the left gastric vein varix demonstrates patency of the portal vein and TIPS.
Fig. 6.
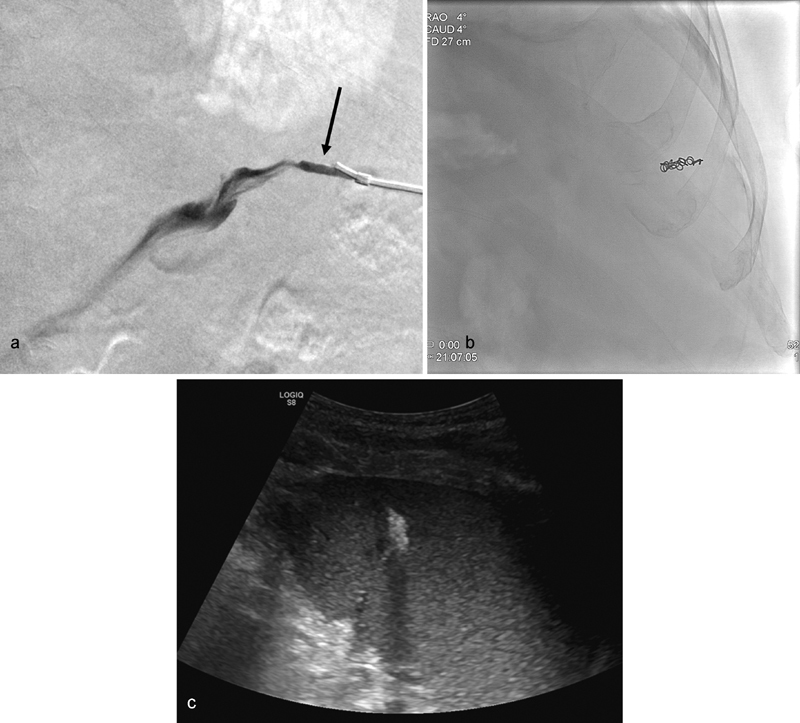
( a ) After performing transsplenic PVR-TIPS, a pullback venogram is performed until the splenic parenchymal tract is identified by seeing stagnant contrast (arrow) that does not flow into the splenic vein. ( b ) A single 4-mm coil is deployed in the parenchymal tract to achieve hemostasis. ( c ) Ultrasound guidance during and after coil deployment is helpful to assess the position of the coil in the splenic parenchyma, particularly in patient with ascites or large body habitus.
Indications for Use
Transsplenic access should be considered in any patient in whom traditional transhepatic portal vein access during a TIPS would be difficult, including those with thrombosed portal veins, very diminutive portal veins, or extensive cavernoma. At the current authors' institution, recanalization of a chronically thrombosed portal vein has been found to be simpler via a transsplenic route compared with transjugular or transhepatic routes. Despite this, transhepatic access may be preferable in a minority of cases, the most common being long segment chronic splenic vein thrombosis which precludes advancement of the wire and catheter to the portal confluence. Additionally, patients with splenic pathology (e.g., lymphoma with spleen involvement and autoinfarcted spleen in sickle cell disease) may not be candidates for transsplenic access due to difficulty or complications associated with splenic access in these patients. Patients with nonocclusive PVT may be treated successfully without the use of transsplenic access.
Conclusion
Major complications occurring in approximately 5% of TIPS cases may result in additional therapy, prolonged hospitalization, permanent adverse sequelae, or death. ICE has been shown to decrease needle passes during TIPS, thereby decreasing procedural complication rates in many high-risk patients undergoing TIPS. Other benefits of ICE include decreasing procedural time—resulting in less ionizing radiation to the patient and operator—and decreasing contrast use and potential nephrotoxicity. CX stents give the operator control of the stent size for patients with a high risk of post-TIPS HE. It reliably maintains a set diameter without passive expansion seen in underdilated stents. Finally, transsplenic access for PVR can improve transplant candidacy for patients with PVT. These patients subsequently undergo liver transplant with primary vasculature anastomosis and improved morbidity and mortality risks. Indications for ICE guidance, CX stents, and transsplenic access should be considered during workup evaluation of any patient for TIPS.
References
- 1.Rösch J, Hanafee W N, Snow H. Transjugular portal venography and radiologic portacaval shunt: an experimental study. Radiology. 1969;92(05):1112–1114. doi: 10.1148/92.5.1112. [DOI] [PubMed] [Google Scholar]
- 2.Tobis J M, Mallery J A, Gessert J et al. Intravascular ultrasound cross-sectional arterial imaging before and after balloon angioplasty in vitro. Circulation. 1989;80(04):873–882. doi: 10.1161/01.cir.80.4.873. [DOI] [PubMed] [Google Scholar]
- 3.Fang C H, You J H, Lau W Y et al. Anatomical variations of hepatic veins: three-dimensional computed tomography scans of 200 subjects. World J Surg. 2012;36(01):120–124. doi: 10.1007/s00268-011-1297-y. [DOI] [PubMed] [Google Scholar]
- 4.Dariushnia S R, Haskal Z J, Midia M et al. Quality improvement guidelines for transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 2016;27(01):1–7. doi: 10.1016/j.jvir.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Freedman A M, Sanyal A J, Tisnado J et al. Complications of transjugular intrahepatic portosystemic shunt: a comprehensive review. Radiographics. 1993;13(06):1185–1210. doi: 10.1148/radiographics.13.6.8290720. [DOI] [PubMed] [Google Scholar]
- 6.Copelan A, Kapoor B, Sands M. Transjugular intrahepatic portosystemic shunt: indications, contraindications, and patient work-up. Semin Intervent Radiol. 2014;31(03):235–242. doi: 10.1055/s-0034-1382790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kao S D, Morshedi M M, Narsinh K H et al. Intravascular ultrasound in the creation of transhepatic portosystemic shunts reduces needle passes, radiation dose, and procedure time: a retrospective study of a single-institution experience. J Vasc Interv Radiol. 2016;27(08):1148–1153. doi: 10.1016/j.jvir.2016.01.137. [DOI] [PubMed] [Google Scholar]
- 8.Pillai A K, Andring B, Faulconer N et al. Utility of intravascular US-guided portal vein access during transjugular intrahepatic portosystemic shunt creation: retrospective comparison with conventional technique in 109 patients. J Vasc Interv Radiol. 2016;27(08):1154–1159. doi: 10.1016/j.jvir.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Gaba R C, Khiatani V L, Knuttinen M G et al. Comprehensive review of TIPS technical complications and how to avoid them. AJR Am J Roentgenol. 2011;196(03):675–685. doi: 10.2214/AJR.10.4819. [DOI] [PubMed] [Google Scholar]
- 10.Gipson M G, Smith M T, Durham J D et al. Intravascular US-guided portal vein access: improved procedural metrics during TIPS creation. J Vasc Interv Radiol. 2016;27(08):1140–1147. doi: 10.1016/j.jvir.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Casadaban L C, Parvinian A, Minocha J et al. Clearing the confusion over hepatic encephalopathy after TIPS creation: incidence, prognostic factors, and clinical outcomes. Dig Dis Sci. 2015;60(04):1059–1066. doi: 10.1007/s10620-014-3391-0. [DOI] [PubMed] [Google Scholar]
- 12.Nardelli S, Gioia S, Pasquale C et al. Cognitive impairment predicts the occurrence of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Am J Gastroenterol. 2016;111(04):523–528. doi: 10.1038/ajg.2016.29. [DOI] [PubMed] [Google Scholar]
- 13.Pieper C C, Sprinkart A M, Nadal J et al. Postinterventional passive expansion of partially dilated transjugular intrahepatic portosystemic shunt stents. J Vasc Interv Radiol. 2015;26(03):388–394. doi: 10.1016/j.jvir.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 14.Madoff D C, Wallace M J, Ahrar K, Saxon R R.TIPS-related hepatic encephalopathy: management options with novel endovascular techniques Radiographics 2004240121–36., discussion 36–37 [DOI] [PubMed] [Google Scholar]
- 15.Pereira K, Carrion A F, Salsamendi J, Doshi M, Baker R, Kably I. Endovascular management of refractory hepatic encephalopathy complication of transjugular intrahepatic portosystemic shunt (TIPS): comprehensive review and clinical practice algorithm. Cardiovasc Intervent Radiol. 2016;39(02):170–182. doi: 10.1007/s00270-015-1197-x. [DOI] [PubMed] [Google Scholar]
- 16.Sze D Y, Hwang G L, Kao J S et al. Bidirectionally adjustable TIPS reduction by parallel stent and stent-graft deployment. J Vasc Interv Radiol. 2008;19(11):1653–1658. doi: 10.1016/j.jvir.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Quaretti P, Michieletti E, Rossi S. Successful treatment of TIPS-induced hepatic failure with an hourglass stent-graft: a simple new technique for reducing shunt flow. J Vasc Interv Radiol. 2001;12(07):887–890. doi: 10.1016/s1051-0443(07)61516-4. [DOI] [PubMed] [Google Scholar]
- 18.Praktiknjo M, Lehmann J, Fischer S, Strassburg C P, Meyer C, Trebicka J. Novel diameter controlled expansion TIPS (Viatorr CX) graft reduces readmission compared to regular covered TIPS graft and bare metal graft. J Hepatol. 2017;66:S33–S62. [Google Scholar]
- 19.Srinivasa R N, Srinivasa R N, Chick J FB, Hage A, Saad W A. Transjugular intrahepatic portosystemic shunt reduction using the GORE VIATORR controlled expansion endoprosthesis: hemodynamics of reducing an established 10-mm TIPS to 8-mm in diameter. Cardiovasc Intervent Radiol. 2018;41(03):518–521. doi: 10.1007/s00270-017-1807-x. [DOI] [PubMed] [Google Scholar]
- 20.Kelly D A, Summerfield J A. Hemostasis in liver disease. Semin Liver Dis. 1987;7(03):182–191. doi: 10.1055/s-2008-1040575. [DOI] [PubMed] [Google Scholar]
- 21.Zocco M A, Di Stasio E, De Cristofaro R et al. Thrombotic risk factors in patients with liver cirrhosis: correlation with MELD scoring system and portal vein thrombosis development. J Hepatol. 2009;51(04):682–689. doi: 10.1016/j.jhep.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Salem R, Vouche M, Baker T et al. Pretransplant portal vein recanalization-transjugular intrahepatic portosystemic shunt in patients with complete obliterative portal vein thrombosis. Transplantation. 2015;99(11):2347–2355. doi: 10.1097/TP.0000000000000729. [DOI] [PubMed] [Google Scholar]
- 23.Hibi T, Nishida S, Levi D M et al. When and why portal vein thrombosis matters in liver transplantation: a critical audit of 174 cases. Ann Surg. 2014;259(04):760–766. doi: 10.1097/SLA.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 24.Bauer J, Johnson S, Durham J et al. The role of TIPS for portal vein patency in liver transplant patients with portal vein thrombosis. Liver Transpl. 2006;12(10):1544–1551. doi: 10.1002/lt.20869. [DOI] [PubMed] [Google Scholar]
- 25.Liatsos C, Vlachogiannakos J, Patch D et al. Successful recanalization of portal vein thrombosis before liver transplantation using transjugular intrahepatic portosystemic shunt. Liver Transpl. 2001;7(05):453–460. doi: 10.1053/jlts.2001.23914. [DOI] [PubMed] [Google Scholar]
- 26.Thornburg B, Desai K, Hickey R et al. Pretransplantation portal vein recanalization and transjugular intrahepatic portosystemic shunt creation for chronic portal vein thrombosis: final analysis of a 61-patient cohort. J Vasc Interv Radiol. 2017;28(12):1714–172100. doi: 10.1016/j.jvir.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Habib A, Desai K, Hickey R et al. Portal vein recanalization-transjugular intrahepatic portosystemic shunt using the transsplenic approach to achieve transplant candidacy in patients with chronic portal vein thrombosis. J Vasc Interv Radiol. 2015;26(04):499–506. doi: 10.1016/j.jvir.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Thornburg B, Desai K, Hickey R et al. Portal vein recanalization and transjugular intrahepatic portosystemic shunt creation for chronic portal vein thrombosis: technical considerations. Tech Vasc Interv Radiol. 2016;19(01):52–60. doi: 10.1053/j.tvir.2016.01.006. [DOI] [PubMed] [Google Scholar]


