Abstract
Background
Vascular damage and primary graft dysfunction increase with prolonged preservation times of transplanted donor lungs. Hence, storage and conservation of donated lungs in protein-free, dextran-containing electrolyte solutions, like Perfadex, is limited to about 6 hours. We hypothesized that transplanted lungs are protected against neutrophil-mediated proteolytic damage by adding α1-anti-trypsin (AAT), a highly abundant human plasma proteinase inhibitor, to Perfadex.
Methods
A realistic clinically oriented murine model of lung transplantation was used to simulate the ischemia–reperfusion process. Lung grafts were stored at 4°C in Perfadex solution supplemented with AAT or an AAT mutant devoid of elastase-inhibiting activity for 18 hours. We examined wild-type and proteinase 3/neutrophil elastase (PR3/NE) double-deficient mice as graft recipients. Gas exchange function and infiltrating neutrophils of the transplanted lung, as well as protein content and neutrophil numbers in the bronchoalveolar lavage fluid, were determined.
Results
AAT as a supplement to Perfadex reduced the extent of primary graft dysfunction and early neutrophil responses after extended storage for 18 hours at 4°C and 4-hour reperfusion in the recipients. Double-knockout recipients that lack elastase-like activities in neutrophils were also protected from early reperfusion injury, but not lung grafts that were perfused with a reactive center mutant of AAT devoid of elastase-inhibiting activity.
Conclusions
PR3 and NE, the principal targets of AAT, are major triggers of post-ischemic reperfusion damage. Their effective inhibition in the graft and recipient is a promising strategy for organ usage after storage for >6 hours.
Keywords: Primary graft dysfunction, ischemia-reperfusion injury, alpha-1-antitrypsin, elastase, proteinase 3, animal models, lung transplantation
Lung grafts of donors are generally considered suitable for transplantation after static cold storage in Perfadex for a period of up to 6 hours. This short preservation time limits the availability of donor lungs and reduces the area of an organ-sharing geographic region. Prolongation of cold ischemia increases the extent of metabolic and structural changes in the transplant in the absence of cellular and blood plasma and, subsequently, the intensity of the early innate immune response in the transplanted organ after the onset of reperfusion.
Irreversible damage to transplanted lung tissue after cold ischemic storage is primarily caused by the reintroduction of blood plasma and cellular blood components at body temperature after transplantation,1 particularly by infiltrating neutrophils,2, 3 and increases with the duration of oxygen deprivation.1, 4 As activated neutrophils release large amounts of elastase-related serine proteases, some peri-operative treatment options for the recipient have been explored experimentally in animal models using protease inhibitors such as bovine aprotinin,5, 6 Lex032,7, 8 and α1-antitrypsin (AAT).9, 10, 11, 12 In those studies, intravenous or intraperitoneal administration of inhibitors has been used to further raise the already high plasma concentrations of protease inhibitors in the circulation.13 We suggested that some inhibitors applied in these studies did not display the appropriate inhibitory profile, or flooded the inhibitor-depleted graft after ex vivo perfusion and in vivo reperfusion too late to efficiently protect the transplanted organ against infiltrating activated neutrophils.
The beneficial effects of protease inhibitors, particularly AAT, have been noted in certain models of ischemia–reperfusion injury7, 9, 10, 11 at warm body temperatures. However, the experimental conditions chosen in these examples could not appropriately mimic the challenges of ex vivo organ storage in a cold cell- and protein-free electrolyte solution such as Perfadex.14 Positive results from the earlier studies cannot be extrapolated to reperfusion injury after cold preservation of organs.
To develop a new clinically appropriate lung preservation protocol and to initiate a proof-of-concept study for lung transplant patients, we used an orthotopic lung transplantation model in mice and stored the transplant in AAT containing Perfadex for an extended period of 18 hours in the cold. The goal of this pre-clinical study was to test whether vascular leakage and immediate neutrophil-mediated inflammation after reperfusion could be prevented or mitigated by adding AAT to the perfusion solution during cold ischemia and whether the anticipated improvement of graft function is mediated by the direct anti-protease activity of AAT or by other anti-inflammatory, tissue-protective, and immunomodulatory properties of AAT. Our results demonstrate that AAT acts on neutrophil-derived elastase and proteinase 3 and primarily prevents immediate reperfusion damage to transplanted lung tissue by protease inhibition.
Methods
Mice
Pathogen-free C57BL/6J mice were obtained from Charles River Labs (Wilmington, MA). Ela2–/–Prtn3–/– mice were established by Pfister et al.15 They were backcrossed for more than 10 generations with C57BL/6J mice. All animals were housed in rooms maintained at constant temperature and humidity with a 12-hour light cycle, and were allowed food and water ad libitum.
Study approval
All animal experiments were conducted under strict governmental and international guidelines and were approved by the local government for the administrative region of Upper Bavaria (Project 55.2-1-54-2532-120-2015).
Orthotopic lung transplant model
Orthotopic lung transplantations were performed as described elsewhere, with minor modifications.16 C57BL/6J male mice were used as donors, and C57BL/6J and Ela2–/–Prtn3–/– male mice were used as recipients (all 8 to 12 weeks old). Briefly, donors were anesthetized with an intraperitoneal (IP) injection of ketamine/xylazine. The pulmonary artery (PA), bronchus, and pulmonary vein (PV) were carefully separated from one another with blunted forceps, before cuffing with 24G, 20G, and 22G cuffs, respectively. The left lung graft was perfused intravenously (IV) with 3 ml of Perfadex (5% dextran 40 [molecular weight 40,000), Na+ 138 mmol, K+ 6 mmol, Mg2+ 0.8 mmol, Cl– 142 mmol, SO42– 0.8 mmol, H2PO4– plus HPO42– 0.8 mmol, glucose 5 mmol). It was then perfused with Perfadex supplemented with albumin, purified recombinant wild-type AAT (AATwt), or AAT-reactive center loop mutant (AATmt) (both 1 mg/ml). To keep the solution inside the vasculature, the PA and PV were clamped with Biemer microvessel clips (B. Braun, Melsungen, Germany) and stored for 18 hours at 4°C before implantation. The recipient mouse was anesthetized with a mixture of medetomidine (1 mg/kg), midazolam (0.05 mg/kg), and fentanyl (0.02 mg/kg), then intubated and connected to a small-animal ventilator (Harvard apparatus) at a respiratory rate of 120 beats/min and a tidal volume of 300 μl. The chest was opened on the left side between ribs 3 and 4 and the native left lung retracted with a clamp. The hilar structures were carefully separated from one another with blunted forceps. The donor lung graft, prepared 18 hours earlier, was perfused IV with Perfadex to rinse away the storage solution. After arrest of the blood and air flow toward the left lung, the cuffed graft PA, bronchus, and PV were inserted into the recipient counterparts, and the connection between donor and recipient structures was secured with 9-0 sutures. The native left lung was removed and the incision in the chest was closed with a 6-0 suture, after removing all potential air bubbles from the chest. Antagonist was administered and the animal was extubated when it showed signs of spontaneous breathing. After the operation, the recipient mice were allowed to recover at 30°C and received buprenorphine (0.1 mg/kg).
Lung analyses in recipients
The mice were euthanized 4 hours after lung transplantation for assessment of primary graft dysfunction. The recipient mouse was anesthetized with a mixture of medetomidine (1 mg/kg), midazolam (0.05 mg/kg), and fentanyl (0.02 mg/kg) 4 hours after lung transplantation. After intubation (300 µl room air/120 strokes/min), a mid-line abdominal incision was performed, the diaphragm incised, and the chest opened to expose the lung and heart block. The right bronchus and PV were clamped for 5 minutes (75 µl room air, 120 strokes/min) and the oxygenation function of the transplanted left lobe was assessed by collecting blood from the left ventricle of the heart and directly measuring the partial pressure of oxygen percentage (%PO2) in a blood gas analyzer (ABL80 FLEX CO-OX Analyzer; Radiometer, Copenhagen, Denmark). Further, with the right bronchus still clamped, bronchoalveolar lavage (BAL) of the transplanted left lung was performed by instilling 3 × 200 µl cold phosphate-buffered saline (PBS) into the trachea. The BAL fluid was obtained by centrifugation (400g, 20 minutes, 4°C) and the protein concentration was measured with a bicinchoninic acid protein assay kit (Pierce, Catalog No. 23225; Thermo Fisher Scientific, Waltham, MA). Then 30,000 cells from the BAL cell pellet were used for cytospin (200g, 6 minutes, 4°C) and stained with May–Grünwald (1424; Merck, Darmstadt, Germany) and Giemsa (9204; Merck) staining solution to perform a differential cell count. The lower part of the lung was kept for protein analysis (snap freezing, storage at –80°C) and the leftover part was used for histology (inflation with and storage in 4% paraformaldehyde).
Recombinant protein expression and purification
The cDNA modification of human AATmt (A355D/I356P/P357D/M358S) was introduced by amplifying the cDNA of human AAT with the forward primer DJ3608 (5′-TCGAGGACCCTGACTCATCG-3′) and the reverse primer DJ3609 (5′-ATCGATGAGTCAGGGTCC-3′).17 The polymerase chain reaction (PCR) product was digested with Acc I and Abs I restriction enzymes (NEB) and cloned into the respective sites of the previously modified wild-type AAT pTT5 plasmid. Recombinant clones were identified by sequencing (Eurofins). The cDNA constructs of AATwt (human M1 [V213]) and AATmt were transiently expressed in human embryonic kidney 293 (HEK293) cells, as described elsewhere.18 Harvesting of supernatants and purification of recombinant AAT variants were performed as described elsewhere.17
Western blot analysis
Refer to the Supplementary Material (available online at www.jhltonline.org/) for a description of Western blot analysis.
Assay for inhibitory activity measurement
The enzymatic activity of serine proteases NE and PR3 was determined using thiobenzylester substrates and Ellman’s reagent (5,5′-dithiobis-[2-nitrobenzoic acid], or DTNB). Free thiobenzylester groups react with DTNB and form yellow 2-nitro-5-thiobenzoate ions. The rate of hydrolysis was measured at 405 nm. Inhibitory activities of 0.5 µmol/liter AATwt and AATmt were tested in an activity assay using 0.1 µmol/liter recombinant mouse and human NE and PR3. The remaining activity of the proteases was determined using thiobenzylester substrate (Boc-Ala-Pro-Nva-chloro-SBzl; 4008235.0010; Bachem) at 1 mmol/liter substrate concentration and 0.5 mmol/liter DTNB in activity assay buffer (50 mmol/liter Tris, 150 mmol/liter NaCl, 0.01% Triton X-100 [pH 8.0]).
Tissue preparation
Refer to the Supplementary Material (online) for a description of tissue preparation.
Hematoxylin and eosin staining
Refer to the Supplementary Material (online) for a description of hematoxylin and eosin (H&E) staining.
Immunohistochemistry
Refer to the Supplementary Material (online) for a description of the immunohistochemistry procedures.
Statistics
Results are reported as mean ± SEM. The Mann–Whitney U-test was used with GraphPad Prism version 5.00 for Windows (www.graphpad.com/). p ≤ 0.05 was considered statistically significant.
Results
Treatment of lung grafts with AAT
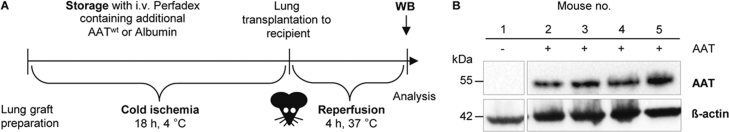
We determined the levels of AAT 4 hours after lung transplantation in transplanted mice with grafts stored in AAT (1 mg/ml in Perfadex) by Western blotting (Figure 1A and B). This procedure resulted in a sustained local deposition of AAT in the transplanted lungs in the absence of plasma proteins (Figure 1B).
Figure 1.
Storage of lung grafts in AAT containing Perfadex results in high AAT levels after 4 hours of reperfusion. (A) Experimental set-up for perfusion, cold ischemic storage, transplantation, and reperfusion. Donor lungs were perfused with AATwt or albumin in Perfadex and stored in the respective solutions at 4°C for 18 hours (cold ischemia). Thereafter, the left lung was orthotopically transplanted to the C57BL/6J recipient mice. The transplanted animals were kept alive for 4 hours (reperfusion) before Western blot analysis. (B) Western blot of total lung tissue lysates of transplanted left lungs of 4 C57BL/6J recipient mice after 4 hours of reperfusion using an AAT-specific monoclonal antibody. Immunodetection of β-actin served as a loading control (lower panel). The AAT-specific antibody did not cross-react with endogenous mouse proteins (left panel).
AAT treatment during storage improves primary graft function
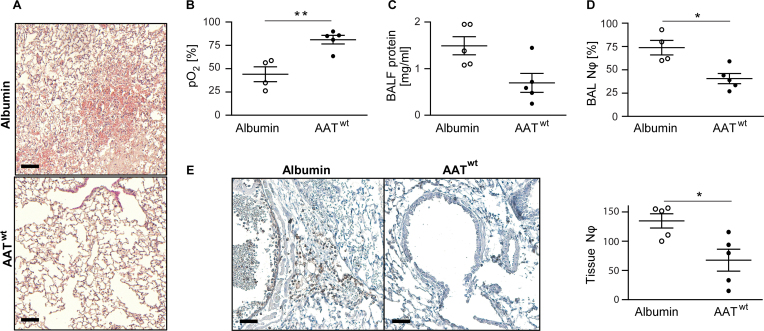
Primary graft dysfunction after prolonged cold storage of the left mouse lung in Perfadex was observed using the mouse orthotopic left lung transplantation model (Figure 1B). Cellular infiltrates in the transplanted left lung lobe and neutrophils in the BAL fluid were clearly noticed. After interrupting the blood flow from the non-transplanted right lung, the oxygen pressure in the left heart ventricle decreased, indicating graft dysfunction after prolonged ischemic storage in the cold. To prevent primary graft dysfunction after prolonged storage, we explored the potential beneficial effect of the protease inhibitor AAT by perfusing and storing the lung graft in AAT containing Perfadex at 4°C. We directly compared the AAT with albumin as 2 similar proteinaceous supplements at the same concentration in our mouse transplantation model (Figure 2). As envisaged, we found a highly significant protective effect for AAT on graft preservation at both the histopathologic (Figure 2A) and functional levels (Figure 2B and E). The addition of AAT resulted in almost 40% higher oxygenation of the blood (Figure 2B) in the transplanted left lung. The protein and neutrophil content of the BAL fluid (Figure 2C and D) and the neutrophil infiltration of the transplanted lung (Figure 2E) were much lower in AAT-treated lungs than in the albumin-treated lungs after 4 hours of reperfusion.
Figure 2.
AAT reduces primary graft dysfunction. Left lungs from C57BL/6J donor mice were stored in either Perfadex supplemented with wild-type AAT (AATwt) or albumin at 4°C for 18 hours (cold ischemia). Four hours after orthotopic transplantation, C57BL/6J recipient mice were euthanized and the outcomes of the 2 treatment groups labeled as AATwt or albumin (n = 4 or 5 per group) were compared. (A) Hematoxylin and eosin staining (H&E) staining shows the tissue morphology of a transplanted left lung (scale bars = 200 µm). (B) Partial oxygen pressure (pO2) of the oxygenated blood in the left heart ventricle was determined after clamping the right bronchus for 5 minutes. The oxygen exchange function of the transplanted lung alone was assessed during mechanically controlled ventilation. (C) Total protein concentration in the bronchoalveolar lavage fluid and (D) bronchoalveolar lavage neutrophil count in the indicated transplanted mice. (E) Immunohistochemistry (scale bars = 200 µm) and quantification of neutrophils in the transplanted left lungs from 10 randomly chosen visual microscopic fields. Data presented as mean ± SEM and compared using the Mann–Whitney U-test (**p = 0.0159, *p < 0.05).
Non-inhibitory AAT has no effect on graft preservation
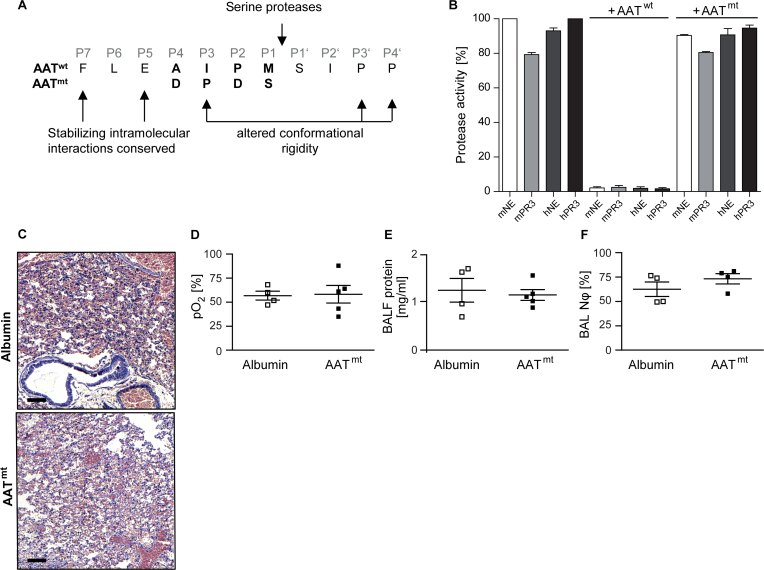
To explore the potentially pleiotropic effects of AAT in graft protection, we separated the anti-proteolytic functions of the reactive center loop from other AAT-associated modulatory functions on the remaining surface of the AAT molecule. To eliminate trypsin-, chymotrypsin- or elastase-targeting specificity, we substituted 2 residues in the reactive center loop by aspartate residues at the P4 and P2 position and the 2 residues at P3 and P1 by a proline and serine, respectively (Figure 3A). Changing the P4 Ala-355 and the P2 Pro-357 to a negatively charged Asp eliminates the hydrophobic nature of the reactive center loop and reduces potential interactions of AAT with phospholipid bilayers.19 Shifting the Pro-357 to position 356 (P3) and replacing the methionine-358 conservatively by a serine alters the canonical inhibitory conformation and β-pleated strand extension of the reactive center loop (P4 to P3′)20 and its specificity, both of which are required for inhibition of serine proteases.
Figure 3.
The non-inhibitory reactive center loop variant of AAT (AATmt) added to the preservation solution proved ineffective. (A) A non-inhibitory reactive center loop variant (mutant AAT, AATmt) was designed by replacing the residues at P4 and P2 with aspartates and P1 with a more polar residue and shifting the P2 proline to the P3 position. Although hydrophobic intramolecular interactions of the extended loop were conserved, the flexibility and conformation of the loop was altered by the proline at P3 (B) Protease activities of human (h) and murine (m) neutrophil elastase (NE) and proteinase 3 (PR3) alone or in the presence of AATwt or AATmt. Data represent 3 independent experiments (mean ± SEM). (C–F) Left donor lungs from C57BL/6J mice were stored in either AATmt or albumin-supplemented Perfadex at 4°C for 18 hours (cold ischemia). C57BL/6J recipient mice were euthanized 4 hours after orthotopic transplantation. The 2 groups are labeled as albumin and AATmt (n = 4 or 5 per group). (C) Hematoxylin and eosin staining (H&E) shows the tissue morphology of a transplanted left lung (scale bars = 200 μm). (D) Partial pressure of oxygen (pO2) measured in blood collected from the left ventricles of ventilated mice at 5 minutes after clamping of the right bronchus. (E) Total protein concentration in the bronchoalveolar lavage fluid and (F) neutrophil content of the lavage fluid in the 2 treatment groups. Data are presented as mean ± SEM.
In accordance with our predictions, murine and human neutrophil elastase (NE) and proteinase 3 (PR3) were not inhibited by AATmt (Figure 3B) produced in HEK293 cells,18 whereas the purified recombinant AATwt inhibited human and mouse NE and PR3 irreversibly. The sequence modifications of AAT introduced into the reactive center loop were intentionally designed to abolish its cleavability by various mammalian proteases, as assessed using Prosper, Cascleave 2.0, and PeptideCutter. In the lung transplantation experiments with AATmt in direct comparison to albumin, acute lung inflammation, as judged from histology sections (Figure 3C), oxygenation of blood in the transplanted left lobe (Figure 3D), and the protein content in the BAL fluid (Figure 3E and F), was indistinguishable between the 2 treatment groups.
PR3/NE double-deficient mice demonstrate enhanced primary graft function
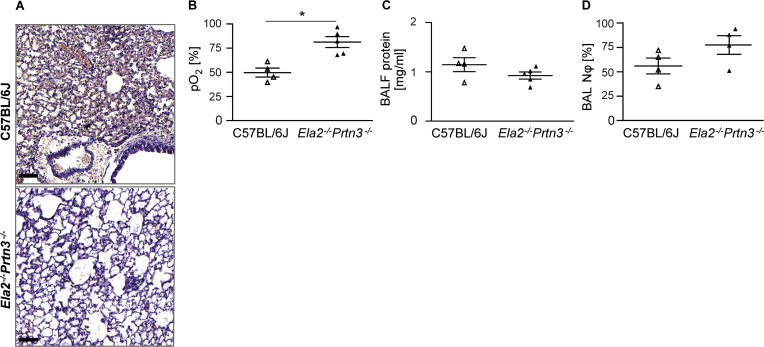
To further corroborate the detrimental role of neutrophil serine proteases in primary graft dysfunction, we examined wild-type and PR3/NE double-deficient mice as graft recipients with the same genetic background (C57BL/6J) as the donor mice. The lung tissue of the transplanted lobe in the double-knockout mice showed a healthier alveoli structure than that of wild-type mice after 4 hours of reperfusion, as assessed by H&E staining (Figure 4A). Gas-exchange function of the transplanted lobe was clearly preserved after 4 hours of exposure to blood of PR3- and NE-deficient mice, whereas the lung graft in wild-type recipient mice performed poorly (Figure 4B). The mean partial pressure of oxygen was 80% in the arterial blood of the left ventricle of knockout mice compared with 50% in wild-type recipients after clamping the arterial blood flow from the non-transplanted lung lobes. The degree of protein exudation (Figure 4C) and cellular content of neutrophils in the BAL fluid (Figure 4D) were not significantly different between the knockout and the wild-type recipient groups.
Figure 4.
Neutrophil elastase and proteinase 3 are major triggers of graft dysfunction. Left lungs were taken from wild-type C57BL/6J donor mice, perfused and stored in albumin containing Perfadex at 4°C for 18 hours (cold ischemia). Thereafter, the lungs were orthotopically transplanted either into wild-type C57BL/6J (BL/6J) mice or Ela2–/–Prtn3–/–-deficient mice with the same genetic background and euthanized 4 hours later (n = 4 or 5 per group). (A) Hematoxylin and eosin staining shows the tissue morphology of a transplanted left lung (scale bars = 200 μm). (B) Left ventricle arterial blood partial pressure of oxygen (pO2) in the transplanted lung. (C) Bronchoalveolar lavage fluid protein concentration and (D) bronchoalveolar lavage neutrophil count in the indicated transplanted mice. Data are presented as mean ± SEM and compared using the Mann–Whitney U-test (*p < 0.05).
Discussion
Cold storage of human lung grafts for >6 hours is known to increase the risk of early graft dysfunction after transplantation. Likewise, we found that the left lung in mice stored for 18 hours in a colloid-based electrolyte solution (Perfadex) displayed primary graft dysfunction after transplantation. However, when we perfused and stored the lung graft in Perfadex containing AAT (1 mg/ml), we were able to significantly reduce the immediate inflammatory response after reperfusion of the transplanted lung. Moreover, we demonstrated that the protective effect of AAT was mediated by the inhibitory function of AAT targeting the major serine proteases of neutrophils, NE and PR3.
We assume that the AAT of the preservation solution diffuses into the perivascular and interstitial space during perfusion and storage of the donor transplant and is, therefore, optimally deployed in advance to protect the lung tissue against the influx of adhering and extravasating neutrophils around the capillaries and venules. During reperfusion the anti-protease shield of AAT against elastase and other serine proteases of neutrophils appears to represent the most pivotal mechanism of lung preservation in this clinical setting. However, multiple alternative mechanisms of tissue protection and anti-inflammation by AAT unrelated to its anti-protease functions have been reported.21, 22, 23, 24, 25, 26, 27, 28
To clarify the major therapeutic mechanism of AAT in immediate graft protection, we chose to separate the anti-proteolytic functions of the reactive center loop from other potential functions on the remaining surface of the AAT molecule. The inhibitory function of AAT primarily targets NE and PR3 and, to a much lesser extent, other proteases like cathepsin G and trypsin-like enzymes.13, 29, 30, 31, 32 Serine proteases occur in the oxidizing extra- and pericellular environment or within the secretory route, including storage granules, and are active at neutral pH after regulated secretion. Thus, inhibition of proteases in the cytosol after membrane binding,19 endocytosis,33 and translocation to the cytosol34, 35 was unlikely. Inhibition of caspases,21, 36 and calpain I37 in the cytosol have been inferred to be responsible for additional anti-inflammatory properties of the wild-type AAT. Our results strongly suggest that other, non–protease-mediated functions of AAT do not contribute significantly to organ protection during the early phase of reperfusion after transplantation surgery.
In agreement with the findings using the non-inhibitory AAT variant, we found that PR3/NE double-deficient mice exhibited improved primary graft functions compared with wild-type mice. The murine homologs of human PR3 and NE are exclusively expressed in the myelomonocyte lineage and constitutively stored in primary granules of neutrophils.31 Deficiency of these 2 proteases in the germ-line does not have a biological or developmental impact on peripheral tissues and organs and does not cause any phenotype under normal housing and living conditions.15 Hence, these mice were optimally suited to clarify the role of neutrophil serine proteases after initiating reperfusion of the lung graft (Figure 4). Taken together, these findings in knockout mice are consistent with recent observations in macaques after prolonged inhibition of cathepsin C, which resulted in the depletion of serine proteases in neutrophils.38 Under these experimental pharmacologic conditions, neutrophil migration and recruitment to the lungs and bronchi was not altered in response to tracheal lipopolysaccharide instillation.
The preservation procedure assessed in this pre-clinical study corresponds very closely to the graft-handling procedure in the routine thoracosurgical setting. AAT at a final concentration of 1 mg/ml added to Perfadex solution is a safe and easily acceptable modification of the most widely used preservation solution, as this natural human plasma protein has already been approved for long-term substitution therapy in emphysema patients with constitutively low levels of AAT in their blood. In the absence of other plasma proteins, flushing and perfusion with 1 mg/ml (15 µmol/liter) AAT in Perfadex solution suffices to deliver this inhibitor to endothelial cells and the interstitial lung space after lung removal from the brain-dead donor. In the absence of competing proteins, endocytosis and transcytosis of AAT from the Perfadex solution by the lung endothelium33, 39 is presumably more efficient than its uptake from whole blood even then, when additional AAT is infused intravenously prior to the operation.
Ex vivo perfusion of the lung graft with AAT clearly has a great advantage over its systemic delivery in the donor and recipient, not only because this protocol complies with current ethical standards. Moreover, with this procedure, we achieved maximum tissue levels of AAT in advance, before the first neutrophils, together with soluble plasma components from the recipient reach the transplanted lungs after connecting the lungs to the patient’s circulation. Treatment of the graft recipient with AAT before and after surgery is clearly a complementary therapeutic option, as shown in a previous study.12 Patients with severe chronic obstructive pulmonary disease (COPD) show increased elastase activity markers in the circulation40 and may therefore benefit in particular. Safety and tolerability of high-dose AAT infusions in end-stage lung disease patients, however, is a serious concern and remains to be clarified. Our experiments with an AAT-reactive center loop mutant and with NE- and PR3-deficient mice verified our suggestion that the protease inhibiting effects of AAT on neutrophils in the early phase of blood reperfusion were essential and sufficient to protect the graft from immediate inflammatory damage after extended storage times. Other selective inhibitors of neutrophil elastases may be equally effective, but target specificity and very low toxicity of the inhibitors are crucial for drug safety.
The lung transplantation procedure performed here with isogenic donors and recipients has several limitations. In comparison to human allogenic lung grafts, the transplanted lungs are taken from a young, healthy isogenic donor without pre-existing illness, and no immunosuppressive therapy is used. The microsurgical procedure is time-consuming and challenging, even for highly skilled individuals. The inevitable blood loss in the recipient mice could be compensated by blood transfusions. Hence, the hemodynamic and circulatory status of the transplanted mice during the post-surgical phase is sub-optimal. The blood pressure in the left pulmonary artery could not be continuously monitored and measured after transplanation within the 4-hour period, as this is technically not feasible in transplanted mice. Although unlikely, initial blood flow to the AAT pre-treated lung grafts could have been lower than in untreated donor lungs leading to reduced edema formation. Despite these challenging experimental conditions, which affected all experimental groups, we believe that the effects of AAT are primarily restricted to the transplanted graft and do not change the cardiac output and systemic blood supply to the transplanted lung.
Local deposition of AAT in the graft cannot stop or significantly delay the ischemia-induced metabolic and structural changes of the graft. Cellular instability and breakdown is expected to increase even with AAT storage beyond the 6-hour period. Residential neutrophils, however, retained in the graft, are also affected by this process and presumably release a limited amount of preformed pro-inflammatory mediators, including serine and matrix metalloproteases. In contrast to matrix metalloproteases, neutrophil serine proteases are fully processed and stored in acidic primary granules, but are immediately active in a neutral (pH 7) environment in the absence of AAT. Because the PR3/NE-deficient recipient mice also showed improved graft function, we conclude that infiltrating neutrophils and macrophages of the recipient executed the majority of tissue damage during reperfusion at body temperature, and that this immediate attack by leukocytes could be counterbalanced by a local protection shield against proteases.
It remains to be clarified how long the local effects of AAT are maintained in the transplanted lung and can favorably affect a later immune response. Negative side effects of AAT in lung transplantation are conceivable in view of a delayed neutrophil response, which may favor bacterial lung infections during or directly after surgery. In a previous clinical attempt to reduce primary lung dysfunction with aprotinin, a bovine inhibitor, the study had to be stopped prematurely as renal toxicity of aprotinin was discerned.41 The organ conservation procedure, which we propose here on the basis of an appropriately designed small-animal study with human AAT, appears to be relatively safe, ethically acceptable, clinically translatable, and well-supported by long-standing clinical research on AAT. In addition, the procedure may be applicable to organ transplantation surgery in general.
Disclosure statement
The authors have no conflicts of interest to disclose. This study was supported by grants from the European Union’s Horizon 2020 Research and Innovation Program (668036, RELENT) and the CPC Research School “Lung Biology and Disease”. Responsibility for the information and views set out in this article lies entirely with the authors.
Footnotes
Supplementary data associated with this article can be found in the online version at www.jhltonline.org/.
Appendix A. Supplementary material
Supplementary material
References
- 1.Chen F., Date H. Update on ischemia-reperfusion injury in lung transplantation. Curr Opin Organ Transplant. 2015;20:515–520. doi: 10.1097/MOT.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 2.Meyer K.C., Nunley D.R., Dauber J.H. Neutrophils, unopposed neutrophil elastase, and alpha1-antiprotease defenses following human lung transplantation. Am J Respir Crit Care Med. 2001;164:97–102. doi: 10.1164/ajrccm.164.1.2006096. [DOI] [PubMed] [Google Scholar]
- 3.Schofield Z.V., Woodruff T.M., Halai R. Neutrophils—a key component of ischemia-reperfusion injury. Shock. 2013;40:463–470. doi: 10.1097/SHK.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 4.Laubach V.E., Sharma A.K. Mechanisms of lung ischemia-reperfusion injury. Curr Opin Organ Transplant. 2016;21:246–252. doi: 10.1097/MOT.0000000000000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan T.A., Bianchi C., Araujo E. Aprotinin preserves cellular junctions and reduces myocardial edema after regional ischemia and cardioplegic arrest. Circulation. 2005;112:I196–I201. doi: 10.1161/CIRCULATIONAHA.104.526053. [DOI] [PubMed] [Google Scholar]
- 6.Bittner H.B., Richter M., Kuntze T. Aprotinin decreases reperfusion injury and allograft dysfunction in clinical lung transplantation. Eur J Cardiothorac Surg. 2006;29:210–215. doi: 10.1016/j.ejcts.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 7.von Dobschuetz E., Hoffmann T., Messmer K. Inhibition of neutrophil proteinases by recombinant serpin Lex032 reduces capillary no-reflow in ischemia/reperfusion-induced acute pancreatitis. J Pharmacol Exp Ther. 1999;290:782–788. [PubMed] [Google Scholar]
- 8.Sands H., Tuma R.F. LEX 032: a novel recombinant human protein for the treatment of ischaemic reperfusion injury. Expert Opin Invest Drugs. 1999;8:1907–1916. doi: 10.1517/13543784.8.11.1907. [DOI] [PubMed] [Google Scholar]
- 9.Yang R., Liu Q., Collins M.H. Alpha-1-proteinase inhibitor prolongs small intestinal graft preservation and survival. J Pediatr Surg. 1996;31:1052–1055. doi: 10.1016/s0022-3468(96)90085-8. [DOI] [PubMed] [Google Scholar]
- 10.Daemen M.A.R.C., Heemskerk V.H., van't Veer C. Functional protection by acute phase proteins α1-acid glycoprotein and α1-antitrypsin against ischemia/reperfusion injury by preventing apoptosis and inflammation. Circulation. 2000;102:1420–1426. doi: 10.1161/01.cir.102.12.1420. [DOI] [PubMed] [Google Scholar]
- 11.Gao W., Zhao J., Kim H. α1-antitrypsin inhibits ischemia reperfusion-induced lung injury by reducing inflammatory response and cell death. J Heart Lung Transplant. 2014;33:309–315. doi: 10.1016/j.healun.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 12.Iskender I., Sakamoto J., Nakajima D. Human α1-antitrypsin improves early post-transplant lung function: pre-clinical studies in a pig lung transplant model. J Heart Lung Transplant. 2016;35:913–921. doi: 10.1016/j.healun.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Travis J., Salvesen G.S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- 14.de Perrot M., Liu M., Waddell T.K. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167:490–511. doi: 10.1164/rccm.200207-670SO. [DOI] [PubMed] [Google Scholar]
- 15.Pfister H., Ollert M., Fröhlich L.F. Antineutrophil cytoplasmic autoantibodies against the murine homolog of proteinase 3 (Wegener autoantigen) are pathogenic in vivo. Blood. 2004;104:1411–1418. doi: 10.1182/blood-2004-01-0267. [DOI] [PubMed] [Google Scholar]
- 16.Krupnick A.S., Lin X., Li W. Orthotopic mouse lung transplantation as experimental methodology to study transplant and tumor biology. Nat Protoc. 2009;4:86–93. doi: 10.1038/nprot.2008.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perera N.C., Wiesmüller K.H., Larsen M.T. NSP4 is stored in azurophil granules and released by activated neutrophils as active endoprotease with restricted specificity. J Immunol. 2013;191:2700–2707. doi: 10.4049/jimmunol.1301293. [DOI] [PubMed] [Google Scholar]
- 18.Malik R., Dau T., Gonik M. Common coding variant in SERPINA1 increases the risk for large artery stroke. Proc Natl Acad Sci USA. 2017;114:3613–3618. doi: 10.1073/pnas.1616301114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon S.M., McKenzie B., Kemeh G. Rosuvastatin alters the proteome of high density lipoproteins: generation of alpha-1-antitrypsin enriched particles with anti-inflammatory properties. Mol Cell Proteomics. 2015;14:3247–3257. doi: 10.1074/mcp.M115.054031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elliott P.R., Lomas D.A., Carrell R.W. Inhibitory conformation of the reactive loop of α1-antitrypsin. Nat Struct Biol. 1996;3:676–681. doi: 10.1038/nsb0896-676. [DOI] [PubMed] [Google Scholar]
- 21.Toldo S., Seropian I.M., Mezzaroma E. Alpha-1 antitrypsin inhibits caspase-1 and protects from acute myocardial ischemia-reperfusion injury. J Mol Cell Cardiol. 2011;51:244–251. doi: 10.1016/j.yjmcc.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Brantly M. α1-Antitrypsin: not just an antiprotease: extending the half-life of a natural anti-inflammatory molecule by conjugation with polyethylene glycol. Am J Respir Cell Mol Biol. 2002;27:652–654. doi: 10.1165/rcmb.F250. [DOI] [PubMed] [Google Scholar]
- 23.Reddy P. α-1 antitrypsin DAMPens GVHD. Blood. 2012;120:2780–2781. doi: 10.1182/blood-2012-08-442764. [DOI] [PubMed] [Google Scholar]
- 24.Lewis E.C. Expanding the clinical indications for α1-antitrypsin therapy. Mol Med. 2012;18:957–970. doi: 10.2119/molmed.2011.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jenne D.E. Off-target rewards of augmentation therapy with alpha1-antitrypsin. Am J Respir Crit Care Med. 2014;190:1203–1204. doi: 10.1164/rccm.201410-1809ED. [DOI] [PubMed] [Google Scholar]
- 26.Stockley R.A. α1-antitrypsin: a polyfunctional protein? Lancet Respir Med. 2015;3:341–343. doi: 10.1016/S2213-2600(15)00094-6. [DOI] [PubMed] [Google Scholar]
- 27.Baraldo S., Balestro E., Bazzan E. Alpha-1 antitrypsin deficiency today: new insights in the immunological pathways. Respiration. 2016;91:380–385. doi: 10.1159/000445692. [DOI] [PubMed] [Google Scholar]
- 28.Guttman O., Yossef R., Freixo-Lima G. α1-Antitrypsin modifies general NK cell interactions with dendritic cells and specific interactions with islet β-cells in favor of protection from autoimmune diabetes. Immunology. 2014;144:530–539. doi: 10.1111/imm.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reid P.T., Sallenave J.M. Neutrophil-derived elastases and their inhibitors: potential role in the pathogenesis of lung disease. Curr Opin Invest Drugs. 2001;2:59–67. [PubMed] [Google Scholar]
- 30.Pham C.T. Neutrophil serine proteases fine-tune the inflammatory response. Int J Biochem Cell Biol. 2008;40:1317–1333. doi: 10.1016/j.biocel.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korkmaz B., Horwitz M.S., Jenne D.E. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol Rev. 2010;62:726–759. doi: 10.1124/pr.110.002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kessenbrock K., Dau T., Jenne D.E. Tailor-made inflammation: how neutrophil serine proteases modulate the inflammatory response. J Mol Med (Berl) 2011;89:23–28. doi: 10.1007/s00109-010-0677-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lockett A.D., Petrusca D.N., Justice M.J. Scavenger receptor class B, type I-mediated uptake of A1AT by pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2015;309:L425–L434. doi: 10.1152/ajplung.00376.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y., He Y., Abraham B. Cytosolic, autocrine alpha-1 proteinase inhibitor (A1PI) inhibits caspase-1 and blocks IL-1β dependent cytokine release in monocytes. PLoS One. 2012;7:e51078. doi: 10.1371/journal.pone.0051078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serban K.A., Petrache I. Alpha-1 antitrypsin and lung cell apoptosis. Ann Am Thorac Soc. 2016;13(suppl 2):S146–S149. doi: 10.1513/AnnalsATS.201505-312KV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrache I., Fijalkowska I., Medler T.R. α-1 Antitrypsin inhibits caspase-3 activity, preventing lung endothelial cell apoptosis. Am J Pathol. 2006;169:1155–1166. doi: 10.2353/ajpath.2006.060058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Omari M., Korenbaum E., Ballmaier M. Acute-phase protein α1-antitrypsin inhibits neutrophil calpain I and induces random migration. Mol Med. 2011;17:865–874. doi: 10.2119/molmed.2011.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guarino C., Hamon Y., Croix C. Prolonged pharmacological inhibition of cathepsin C results in elimination of neutrophil serine proteases. Biochem Pharmacol. 2017;131:52–67. doi: 10.1016/j.bcp.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Sohrab S., Petrusca D.N., Lockett A.D. Mechanism of α-1 antitrypsin endocytosis by lung endothelium. FASEB J. 2009;23:3149–3158. doi: 10.1096/fj.09-129304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carter R.I., Ungurs M.J., Mumford R.A. Aα-Val360: a marker of neutrophil elastase and COPD disease activity. Eur Respir J. 2013;41:31–38. doi: 10.1183/09031936.00197411. [DOI] [PubMed] [Google Scholar]
- 41.Herrington C.S., Prekker M.E., Arrington A.K. A randomized, placebo-controlled trial of aprotinin to reduce primary graft dysfunction following lung transplantation. Clin Transplant. 2011;25:90–96. doi: 10.1111/j.1399-0012.2010.01319.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material