Abstract
Touch is central to interpersonal interactions. Touch conveys specific emotions about the touch provider, but it is not clear whether this is a purely socially learned function or whether it has neurophysiological specificity. In two experiments with healthy participants (N = 76 and 61) and one neuropsychological single case study, we investigated whether a type of touch characterised by peripheral and central neurophysiological specificity, namely the C tactile (CT) system, can communicate specific emotions and mental states. We examined the specificity of emotions elicited by touch delivered at CT-optimal (3 cm/s) and CT-suboptimal (18 cm/s) velocities (Experiment 1) at different body sites which contain (forearm) vs. do not contain (palm of the hand) CT fibres (Experiment 2). Blindfolded participants were touched without any contextual cues, and were asked to identify the touch provider's emotion and intention. Overall, CT-optimal touch (slow, gentle touch on the forearm) was significantly more likely than other types of touch to convey arousal, lust or desire. Affiliative emotions such as love and related intentions such as social support were instead reliably elicited by gentle touch, irrespective of CT-optimality, suggesting that other top-down factors contribute to these aspects of tactile social communication. To explore the neural basis of this communication, we also tested this paradigm in a stroke patient with right perisylvian damage, including the posterior insular cortex, which is considered as the primary cortical target of CT afferents, but excluding temporal cortex involvement that has been linked to more affiliative aspects of CT-optimal touch. His performance suggested an impairment in ‘reading’ emotions based on CT-optimal touch. Taken together, our results suggest that the CT system can add specificity to emotional and social communication, particularly with regards to feelings of desire and arousal. On the basis of these findings, we speculate that its primary functional role may be to enhance the ‘sensual salience’ of tactile interactions.
Keywords: Affective touch, Emotion, Interpersonal interactions, Tactile communication, Insula, Interoception
Highlights
-
•
Touch can convey specific emotions and intentions.
-
•
Slow gentle touch communicates love and intimacy regardless of CT fibre activation.
-
•
The CT system plays a specific role in mediating sensual touch.
-
•
Insula activation might be necessary in the arousing function of the CT system.
1. Introduction
We are in constant interaction with a multisensory environment, where touch is an important but often neglected component. Touch is discriminative in that it can be used to acquire information regarding textures and shapes, and hence help to infer the material and identify objects. Additionally, touch possesses an affective component, in that tactile experiences can be perceived as pleasant or unpleasant. Moreover, touch has been linked with social cognition and affiliation in the sense that interpersonal touch can promote affiliative, collaborative and sexual behaviour (Löken et al., 2009). Furthermore, tactile social interactions have beneficial effects on mental and physical health (Field, 2010 for a review).
Recently, the affective and affiliative aspects of touch have been linked to the activation of specific afferent fibres (McGlone et al., 2007; Vallbo and Hagbarth, 1968). Specifically, a system of unmyelinated, mechanosensitive C-tactile (CT) nerve afferents responding preferentially to slow gentle touch (1–10 cm/s; Löken et al., 2009; range of pressure .3–2.5 mN; Vallbo et al., 1999; Cole et al., 2006) was only found on hairy skin and not glabrous skin (Olausson et al., 2002, Olausson et al., 2010, Morrison et al., 2011, McGlone et al., 2014). CT-afferent activation is linearly correlated with perceived pleasantness (Shaikh et al., 2015, Löken et al., 2009), and subjective ratings of pleasantness following affective touch lead to the activation of limbic cortical areas (Case et al., 2016, McGlone et al., 2012). In healthy subjects, soft brush stroking activates S1, S2, and insular cortex (Olausson et al., 2002), whereas in a subject lacking A-beta afferents, soft brush stroking activates the posterior insular region, but not somatosensory areas (S1 and S2; Olausson et al., 2002), corroborating the importance of the insula in CT tactile behaviours (Björndotter et al., 2009). Moreover, Gordon et al. (2013) and Voos et al. (2013) have demonstrated the involvement of key nodes of the social brain network in processing CT-targeted touch including the posterior superior temporal sulcus (pSTS) and prefrontal regions.
An especially notable aspect of touch is the fact that one cannot touch without being touched in return. Interestingly, it appears that CT-optimal touch has emotional effects on the touch giver (Gentsch et al., 2015). This finding raises the possibility that CT-optimal touch may be implicated in the communication of emotions between individuals. Indeed, it has long been proposed that touch may be an independent channel of communication with its own language (Weiss, 1979, Weiss, 1986, Vortherms, 1991). Nevertheless, only a handful of studies have examined the communicative facets of touch (Stack, 2001, Hertenstein et al., 2009, App et al., 2011) in comparison to the vast literature on facial and vocal expression of emotions.
Specifically, Hertenstein et al. (2006a) tested the power of touch to convey distinct emotions. They showed that distinct emotions were communicated through specific tactile behaviour that varied in duration and intensity (Hertenstein et al., 2006a), demonstrating diversity of physical qualities of touch (Hertenstein, 2002) used as symbols of touch language (Weiss, 1979). Hertenstein et al. (2006b) have suggested an evolutionary importance of touch and that social grooming might have led to the development of a tactile communicative system. Expanding this line of thought, Hertenstein et al. (2009) suggested that humans show the ability to communicate pro-social emotions, meaning love, gratitude and sympathy, with tactile but not facial or auditory expressions. Thus, tactile communication, especially affective touch, might be important in conveying meaning that is not communicated through any other modality because it is based on a reciprocal pleasurable experience. In support of this idea, the tactile channel of communication is preferred to communicate intimacy (e.g. love, sympathy), while survival emotions (e.g. anger, happiness) are communicated via the face and social status (e.g. embarrassment, pride) by body actions (App et al., 2011).
These findings suggest also that touch might contribute to humans’ ability to infer mental states, such as thoughts, beliefs, knowledge, desires and intentions to an actor's behaviour (Baron-Cohen, 1995) which is essential for the development and maintenance of most communicative and social interactions (Ahmed and Miller, 2011, Flavell, 2004). This ‘theory of mind’ (ToM), or the ability to ‘mentalize’ has been shown to develop in young age, when children start to show understanding that another person can hold a (false) belief that is different to their own, and predict that person's behaviour accordingly (Buttelmann et al., 2009; Rubio-Fernández and Geurts, 2013). In adults, ToM is commonly tested by the “Reading the Mind in the Eyes” task (RMET), designed by Baron-Cohen et al. (2001). When presented with picture stimuli of the ‘eyes region’ of actors expressing a particular emotion, the emotional state of another person can be inferred from as little information as the eye region. The perception of emotions through touch might contribute to this ability by providing additional information about emotions that are not readily perceived by other sensory modalities.
However, to date no study has explored the role of CT-optimal touch in the communication of emotions or other mental states in the context of social intentions. In studies by Hertenstein and colleagues, participants were able to express the different emotions with no restriction of type or location of the touch, nor any control of touch velocities. While stroking was associated with love and sympathy, there was no intention or means to assess whether the stroking was CT-optimal or not. In addition, in their studies, no differentiation between basic emotions and other mental states is made, as the authors themselves acknowledge (Hertenstein et al., 2006a, Hertenstein et al., 2006b, Hertenstein et al., 2009). More generally, in the literature on the perception of CT-optimal touch, the activation of the CT system has been linked with both ‘sensual’ or ‘erotic’ (Jönsson et al., 2015, Ebisch et al., 2014) and ‘affiliative’ feelings and perceptions (Olausson, 2010; Morrison et al., 2010). It thus remains unknown whether CT-optimal touch per se has any specificity in conveying either affiliative or sensual emotions and corresponding interpersonal intentions, or whether the reliable communication of such emotions depends on more general multisensory or contextual factors. Moreover, to our knowledge, the neural mechanisms by which touch, CT-optimal or not, may communicate emotions have never been investigated, nor has the specific functional role of the CT system been examined.
Accordingly, in a series of experiments, we set out to investigate for the first time the role of slow, CT-optimal touch in the interpersonal communication of selective emotions and intentions and further explore whether the functional role of the CT system is linked to sensory pleasure and sensual emotions, or more to social emotions of care and support. In particular, we explored whether the activation of the CT system plays a role in the understanding of emotions, depending on whether the target of the touch communication was the touch giver (‘emotion’) or the touch receiver (‘intention’). In the latter case, we thus tested whether this type of touch could communicate other mental states, such as interpersonal intentions.
In a first experiment, we investigated whether CT-optimal touch on the forearm conveyed more positive and specific emotions and intentions such as arousal and social support, respectively, than fast (non-CT-optimal) touch. A second experiment aimed to replicate and further specify the role of the CT system in emotional communication, in comparison to more top-down factors. We thus expected that gentle, slow touch to the palm of the hand, that does not contain CT-fibres, would not be reliably associated with the above emotional ‘readings’. Finally, an exploratory neuropsychological single case study aimed to examine whether the cortical areas typically associated with the processing of CT signals from the periphery, and particularly the right posterior and mid insula, would be necessary for the ability to read emotions via CT-optimal touch, even without the involvement of other cortical areas such as the superior temporal sulcus (STS) and the ventromedial prefrontal cortex that are considered key nodes of the social brain network.
2. Experiment 1 – Reading emotions and intentions from touch – the role of touch velocity
This first experiment investigated the ability to attribute emotions and intentions to the touch giver. Participants were stroked on their forearm at C-tactile optimal (CT: 3 cm/s) and fast sub-optimal (non-CT: 18 cm/s) velocities, and were asked to determine the emotion and intention of the touch giver by choosing between four word categories. We chose the categories according to three ‘basic’ emotions (Happiness, Fear, Anger) as identified in various, influential taxonomies (Ekman, 1993, Panksepp, 1998), adding Arousal as a fourth positive emotion that could be read via touch (as a possible distinction between affiliative and sexual functions of the CT system – as mentioned in the discussion of Löken et al. (2009), but also in some recent papers suggesting a possible erotic role of affective touch; Ebisch et al., 2014; Jönsson et al., 2015). We matched these four categories with four intentions (emotion towards the touch receiver): two positive and two negative, which were linked to emotions of the touch giver (i.e. Warning – Fear; Aggression – Anger; Support – Happiness; Reward – Arousal; see Table 1). The selection of categories corresponding to primary emotions was motivated by the fact that our study was aiming to disentangle two potential communicative functions of the CT system, namely the communication of emotions of the touch giver and the communication of social intentions by the touch giver towards the touch receiver. These two aspects of emotional communication correspond to many theories regarding the potential differences between primary emotions and social emotions (e.g. Panksepp, 1998), as well as the potential difference between emotional perception and mind reading (e.g. Baron-Cohen, 1995). Unfortunately, these functions have been potentially conflated in previous studies on touch communication (Hertenstein et al., 2006a, Hertenstein et al., 2006b, Hertenstein et al., 2009) and are examined explicitly here for the first time. Moreover, previous questionnaires (Guest et al., 2011; Ackerley et al., 2014) that have explored the emotional aspects of touch are based on a different theoretical tradition looking at the intra-individual, sensory or homeostatic aspects of tactile pleasure. Thus, they are based on asking people to describe what emotions touch elicits in them, i.e. the touch receiver, not the emotions or the social intentions of the touch giver as in our study, and hence they were not testing the communicative functions of touch. We hypothesised that CT-optimal touch would be read as positive and communicate positive intentions whereas non-CT-optimal touch, as less clearly linked to affiliation and bonding, should be linked to positive intentions to a lesser extent.
Table 1.
Word categories for both other's emotion and other's intention.
| Categories and words for other's emotion | Categories and words for other's intention |
|---|---|
Denotes word categories added only for Experiment 2. Italics words were used only in Experiment 1.
2.1. Methods
2.1.1. Participants
Seventy-six healthy participants took part in Experiment 1 during a public event at the Royal Institution, London. Due to a technical error, only partial data were available for eight of these participants so they were excluded from the analyses, yielding a final sample of 68 participants (39 females, age range 19 – 71; M = 32.27 years, SD = 12.34 years; 56 right-handed). The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee of University College London.
2.1.2. Material and touch stimulation
2.1.2.1. Touch stimuli
The stimuli consisted of gentle touch manually applied using the fingertips of both index and middle fingers of the dominant hand of the experimenter. All touches were applied from a proximal to distal direction (participant centred), gradually and with low intensity on the participant's left forearm in dynamic, linear stroking movements. Stimulations were executed by four different female experimenters who were trained to apply the touch. Touch was delivered at two different stroking velocities (3 cm/s and 18 cm/s). The duration of each touch (i.e., the temporal length from first skin contact to cessation of contact after the appropriate number of strokes, depending on the condition) was held constant at 3 seconds. On the forearm, the touch was applied within a 9 cm marked area, leading to one stroke in the 3 cm/s condition and six strokes in the 18 cm/s condition. For each trial, each touch was repeated twice. Importantly, the touch giver had no particular intention or emotion in mind when applying the touch.
2.1.2.2. Word categories
Two positive and two negative word categories were selected for conveyed emotions (Happiness, Arousal, Anger, Fear) and intentions (Support, Reward, Aggression, Warning), all containing three semantically related words (e.g. three different words for the same category such as joy, delight and happiness). Valence and arousal scores were obtained from Warringer et al. data (2013), and each category was homogeneous in terms of valence, arousal and dominance scores (see Supplementary Table 1).
2.1.3. Design
Velocity and emotion reference were manipulated leading to a 2 x 2 design. There was a total of 3 trials per condition (2 emotion reference: Intention/Emotion; and 2 velocities: CT-optimal/non-CT-optimal), leading to a total of 12 trials. Each word was presented once for each condition. Intention or emotion words were presented in a mixed order and the order of touch applied was counterbalanced between participants; with one order per experimenter, leading to 4 different orders.
2.1.4. Procedure
Participants were tested individually, seating in front of the experimenter. The experimenter explained to the participants that they would be stroked at different speeds on the left forearm. Experimenters demonstrated the touch on themselves. Participants were instructed to focus on the sensation arising from the touch, and imagine what the experimenter was feeling in themselves and what she was trying to communicate. It was emphasised that communicated emotions were unrelated to the participant, however intentions were directed at the participant.
Participants were asked to sit in a comfortable position and place their left arm on the table with their palm facing down. They were asked to wear a blindfold while the touch was applied. The experimenter applied the touch, waited one second, and repeated the touch. After each trial, the participant took off the blindfold to make judgements about the received touch in the provided booklet. For each trial, one word per category was presented, leading to a choice between four words. Participants were encouraged to make use of a provided glossary if word meanings were unclear. After each touch, participants also rated how pleasant the touch was on a 10-point scale (from 1 ‘not at all’ to 10 ‘extremely pleasant’). Both word choices and ratings were entered in a booklet (paper and pencil task). Two practice trials were added at the beginning in order to familiarise the participant with the task, but were not included in the analysis.
2.1.5. Data analysis
To analyse whether the distribution of the word categories chosen by participants was not randomly distributed, separate Chi-square goodness of fit tests were first run for each of the conditions (Table 2A&B). Then, to establish which word groups showed the highest frequencies, residuals were examined, as they present the difference between the observed and expected values for a cell. Large residuals indicate a greater contribution of the cell to the magnitude of the obtained Chi-square value (Delucchi, 1993). Therefore, the higher the residual of a specific cell, the greater the likelihood that the emotion or intention related to the cell was perceived (Table 2A&B).
Table 2.
Summary results of Experiment 1. (A) Chi-square goodness-of-fit values, observed frequencies and residuals for emotion words categories. (B) Chi-square goodness-of-fit values, observed frequencies and residuals for intention words categories. (C) Wilcoxon signed-rank test results to compare difference of obtained frequencies between emotion word categories and between intention word categories.
| (A) Emotions | ||||||||||||
| Condition | Chi-square | DF | p-value | Joy | Arousal | Anger | Fear | Total N | ||||
| 3 cm/s | 175.53 | 3 | <.001* | Observed N | 30 | 127 | 2 | 40 | 199 | |||
| Forearm | Category % | 15.08 | 63.82 | 1 | 20.1 | |||||||
| Residual | −19.8 | 77.3 | −47.8 | −9.8 | ||||||||
| 18 cm/s | 104.43 | 3 | <.001* | Observed N | 71 | 8 | 25 | 100 | 204 | |||
| Forearm | Category % | 34.8 | 3.92 | 12.25 | 49.02 | |||||||
| Residual | 20 | −43 | −26 | 49 | ||||||||
| (B) Intentions | ||||||||||||
| Condition | Chi-square | DF | p-value | Reward | Support | Aggression | Warning | Total N | ||||
| 3 cm/s | 162.98 | 3 | <.001* | Observed N | 51 | 125 | 7 | 21 | 204 | |||
| Forearm | Category % | 25 | 61.27 | 3.43 | 10.29 | |||||||
| Residual | 0 | 74 | −44 | −30 | ||||||||
| 18 cm/s | 138.14 | 3 | <.001* | Observed N | 26 | 32 | 22 | 123 | 203 | |||
| Forearm | Category % | 12.8 | 15.76 | 10.84 | 60.59 | |||||||
| Residual | −24.8 | −18.8 | −28.8 | 72.3 | ||||||||
| (C) | Velocity | Compared Emotions/Intentions | Z | p | ||||||||
| Emotions | 3 cm/s | Arousal vs Fear | −4.749 | < .001* | ||||||||
| forearm | Fear vs Joy | −.956 | .339 | |||||||||
| Joy vs Anger | −4.160 | < .001* | ||||||||||
| 18 cm/s | Fear vs Joy | −1.980 | .048 | |||||||||
| forearm | Joy vs Anger | −3.642 | < .001* | |||||||||
| Anger vs Arousal | −2.824 | .005 * | ||||||||||
| Intentions | 3 cm/s | Support vs Reward | −4.284 | < .001* | ||||||||
| forearm | Reward vs Alarm | −2.830 | .005* | |||||||||
| Warning vs Aggression | −2.401 | 0.016* | ||||||||||
| 18 cm/s | Warning vs Support | −4.979 | < .001* | |||||||||
| forearm | Support vs Reward | −.601 | .548 | |||||||||
| Reward vs Aggression | −.731 | .465 | ||||||||||
DF = degree of freedom.
Denotes significant test, for (C) after Bonferroni correction for multiple comparison (alpha = 0.017).
Chi-square results were analysed post-hoc using Wilcoxon signed-rank tests to find the preferred category for each condition. To do so, the groups were compared in descending order, i.e., the group with the highest frequency was compared to the group with the next highest frequency, then this group in turn was compared to the next highest etc. Bonferroni-corrected multiple comparisons for emotions are presented in Table 2C (p-value considered as significant if p<.017).
As a manipulation check, pleasantness ratings were analysed using a paired t-test, averaging across communication conditions, to make sure that slow CT-optimal touch was perceived as more pleasant than fast non-CT-optimal touch overall.
2.2. Results and discussion – Experiment 1
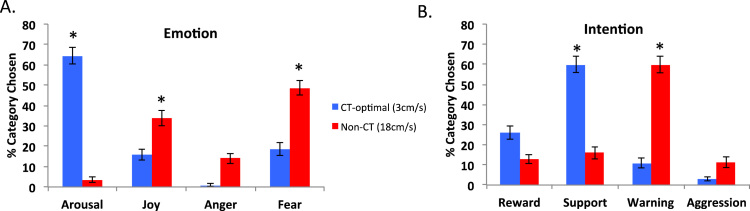
First, as shown in previous studies, and as an experimental manipulation check, pleasantness of touch was rated higher for slow CT-optimal touch than for fast touch non-CT-optimal touch (Supplementary Figure 1. A), confirming that participants were perceiving each particular touch differently (MCT = 6.95, SDCT = 1.56; MnonCT = 5.04, SDnonCT = 1.67; t(66) = 7.241, p<.001), with CT-optimal touch being more pleasant.
As expected, observed frequencies were non-randomly distributed, i.e., specific category/ies were preferred for each condition (see details in Table 2; Fig. 1). Although the touch giver had no particular intention or emotion in mind when applying the touch and hence they could not provide any cues to the touch receiver other than the touch, the results showed a preference for participants to interpret slow, C-tactile optimal dynamic touch as communicating mainly the positive emotion Arousal (63.82% of the trials), and the positive intention Support (61.27% of the trials). By contrast, fast touch was interpreted as conveying less specific emotions as communicating both Fear (49.02%) and Joy (34.80%); but specifically communicating a Warning intention (60.59%).
Fig. 1.
Average percentage of categories chosen. (A) for other's emotion, (B) for other's intention; for both CT and non-CT velocities. Error bars represent the standard error of the mean. * denotes the category significantly most chosen.
This first experiment supports Hertenstein et al. (2006a) findings that distinct emotions can be communicated through touch, but crucially it enriches the picture by showing that different intentions can also be communicated through touch. Moreover, this experiment supports the idea that slow touch can communicate positive emotions and intentions, adding to the affiliative affective touch literature.
As an additional point, it should be noted that in this experiment all touch givers were female whereas touch receivers were either male or female. To examine whether participant gender might influence the results, we ran gender differences analyses and found that male and female participants chose the same categories when reading emotions, whereas for reading intentions, in particular during CT optimal touch, male participants tended to read CT optimal touch as communicating both Support and Reward, whereas female participants read CT optimal touch as Support more than other categories (for full details of the data analysis, and results, see Supplementary material). Taking these results into account, we decided to keep the gender of the experimenter constant (female) for Experiment 2, and to include only female participants to avoid any gender effects, and to add to the results of Experiment 1 by investigating the CT specificity of reading intentions and emotions via touch (also in line with Suvilehto et al., 2015; Gazzola et al., 2012). Furthermore, in this first experiment, participants had to choose between four specific words. The specific forced-choice categories might have biased participants and so we increased our categories in Experiment 2. Moreover, from this experiment we were not able to infer specificity of the CT system, as the difference found between fast and slow touch could just be a matter of velocity and not due to the activation of the CT fibres; such as due to top-down manipulation and previous experience of touch (such as slow = good; fast = bad). In light of these findings, in order to specifically address the role of the CT afferents system, a body site that lacks CT fibres must be tested, and thus we included the palm as an additional body site in Experiment 2.
3. Experiment 2 – Reading emotions and intentions from touch – the role of the CT system
Experiment 2 was designed to further explore the role of the CT system in emotion and intention communication via touch. As past research has shown an absence of CT-fibres in non-hairy (glabrous) skin (e.g., the palm of the hand; Johansson and Vallbo, 1979; Vallbo et al., 1999), the palm offers a unique opportunity to specifically investigate the role of the C-tactile afferent system in the perception of emotions and intentions by means of tactile interactions. As a comparison, we administered touch to a body site containing CT fibres, choosing to use the forearm in order to maintain consistency with Experiment 1, and because this is the body site that has been the most studied in the CT fibres literature (Lö̈ken et al., 2009; Crucianelli et al., 2013; Ackerley et al., 2014; Krahé et al., 2016). The back of the hand might also be suggested as an alternative CT-fibre site to use in this study; however, this location has its own limitations (e.g. it is not a habitual site for interpersonal hand stroking), and so we decided to keep the forearm as the body site containing CT fibres. If results stand only for the forearm, this could suggest a CT-specificity of touch communication, and not a ‘reading’ that would be top-down, velocity dependent. The C-tactile optimal and non-optimal velocities were maintained as in Experiment 1, while one further positive and one negative emotion and intention category, respectively, were added to further investigate the specificity of categories shown in Experiment 1. Moreover, to reduce variability within each word category, we chose to reduce the choice of word in each category to only two words (informed by the results of Experiment 1, we chose the two words that were the closest in terms of meaning within each category).
Given the results of Experiment 1, we expected that participants would be able to read both emotions and intentions through touch, choosing more positive emotions and intentions when touched at CT-optimal speeds. We also expected that the palm would not convey a clear emotion and intention reading pattern, as it does not contain CT fibres and the functional role of other tactile fibres such as Aβ is linked more with the processing of sensory, discriminatory aspects of touch (McGlone et al., 2012).
3.1. Methods
3.1.1. Participants
Sixty-one healthy female volunteers, age range 18–55 years (M = 21.33, SD = 6.56), from the University of Hertfordshire took part in exchange for course credit. According to the Edinburgh Handedness questionnaire (Oldfield, 1971), 58 participants were right handed, 1 left handed and 2 ambidextrous. Participants with scars, tattoos or skin conditions in the touched area were excluded, in order to avoid any misreading of the touch due to skin oversensitivity. All participants were native or fluent English speakers. The University of Hertfordshire Ethics Committee approved all experimental procedures.
3.1.2. Design
The same design as Experiment 1 was used, adding one factor: location (forearm vs palm), leading to a 2 × 2 × 2 within-subjects design, with factors velocity of touch (3 cm/s and 18 cm/s), location of touch (forearm and palm) and the emotion reference (emotion/intention).
A total of 32 trials, with 4 trials per condition, were divided into four blocks of 8 trials with two questions about emotion conveyed and two about intention for each velocity (pseudo-randomized). The starting location of the touch (forearm and palm) alternated between blocks. Starting location and velocity were counterbalanced between participants, leading to eight different possible orders. Participants were randomly assigned to one pseudo-randomized order. The outcome measures were words chosen, pleasantness ratings of the touch, measured on a 10-point scale (as in Experiment 1); but also confidence ratings (how confident participants were with their word choice on a 10-point scale). Confidence ratings on a 10-point scale were added in this second experiment, to explore whether participants’ confidence changed in function of body sites and velocities. Responses were recorded via a booklet that presented for each trial, six words (one per category), the confidence and the pleasantness scales.
3.1.3. Material and touch stimulation
3.1.3.1. Stroking / touch stimuli
Stimulation was delivered at two different stroking velocities (3 cm/s and 18 cm/s), on two different body sites (forearm and palm). Touch was delivered the same way as in Experiment 1, except that on the palm the touch was applied within a 4.5 cm area from wrist to fingers thus two strokes were applied for the 3 cm/s condition and 12 for the 18 cm/s condition.
3.1.3.2. Word categories
Words categories represented a revised version of the set used in Experiment 1, consisting of six emotions and six intentions word categories (see Table 1), three of which were positive and three negative. In contrast to Experiment 1, each word category contained two semantically related words and not three. The emotion ‘Love’ (category: love, affection) and the intention ‘Intimacy’ (category: intimacy, closeness) were added, based on previous research suggesting that they are especially communicated through touch, and adding a social component. Further, the emotion ‘Depression’ (category: depression, desperation) representing sadness, and the intention ‘Belittlement’ (category: belittlement, degradation), adding a social dominance dimension, were included to add further dimensions of emotion and intention. The ‘Depression’ category was thus added to represent sadness as another negative emotion that was missing from Experiment 1 (where we had Fear, Anger and Happy, but not Sad), and the ‘Belittlement’ category (representing a social intention tapping into social dimensions of dominance) was added as another negative intention to complement the existing ones (complementing for ‘Aggression’, i.e. adding a negative category in both the emotion and intention reading). Note that the word sadness itself was not suitable for inclusion as it was not matched with the rest of the words in the experiment. The length of these words, their frequency in everyday language, as well as their matching for valence and arousal among emotions and intentions was taken into account in all these choices (along Warringer et al. data (2013)).
3.1.4. Procedure
The procedure was the same as in Experiment 1, except that only one female experimenter delivered the touch. Each participant was tested individually in a small, quiet room. The experimenter explained that the participant would be stroked at different speeds on the left forearm and palm and then demonstrated the touch on herself. It was stressed that communicated emotions were unrelated to the participant, however intentions were directed at the participant.
Participants were asked to sit in a comfortable position and place their left arm on the table with their palm facing down or up, depending on the touch starting location. They were encouraged to make use of a provided glossary if word meanings were unclear and asked to wear a blindfold while the touch was applied. The experimenter applied the touch, waited one second and repeated the touch. After each trial the participant took off the blindfold to make judgements about the received touch in the provided booklet. Two practice trials were added at the beginning but not included in the analysis.
3.1.5. Data analysis
Data Analysis followed a similar format as in Experiment 1. To analyse whether the distribution of the word categories chosen by participants was not randomly distributed, separate Chi-square goodness of fit tests were first run for each of the velocity/location/question conditions (Tables 3A&4A). To establish which groups showed the highest frequencies the residuals were examined, as they present the difference between the observed and expected values for a cell.
Table 3.
Summary results of Experiment 2 – Reading Emotions. (A) Chi-square goodness-of-fit values, observed frequencies and residuals for emotion words categories. (B) Wilcoxon signed-rank test results to compare difference of obtained frequencies between emotion word categories.
| (A) | ||||||||||||
| Condition | Chi-square | DF | p | Love | Joy | Arousal | Anger | Fear | Depression | Total N | ||
| 3 cm/s | 178.46 | 5 | <.001 | Observed N | 85 | 24 | 79 | 3 | 25 | 23 | 239 | |
| Forearm | Column % | 35.6 | 10.0 | 33.1 | 1.3 | 10.5 | 9.6 | |||||
| Residual | 45.2 | −15.8 | 39.2 | −36.8 | −14.8 | −16.8 | ||||||
| 18 cm/s | 94.01 | 5 | <.001 | Observed N | 35 | 54 | 23 | 17 | 58 | 54 | 241 | |
| Forearm | Column % | 14.5 | 22.4 | 9.5 | 7.1 | 24.1 | 22.4 | |||||
| Residual | −5.2 | 13.8 | −17.2 | −23.2 | 17.8 | 13.8 | ||||||
| 3 cm/s | 128.44 | 5 | <.001 | Observed N | 120 | 26 | 41 | 6 | 22 | 24 | 239 | |
| Palm | Column % | 50.2 | 10.9 | 17.2 | 2.5 | 9.2 | 10 | |||||
| Residual | 80.2 | −13.8 | 1.2 | −33.8 | −17.8 | −15.8 | ||||||
| 18 cm/s | 167.65 | 5 | <.001 | Observed N | 17 | 46 | 11 | 38 | 77 | 54 | 243 | |
| Palm | Column % | 7.0 | 18.9 | 4.5 | 15.6 | 31.7 | 22.2 | |||||
| Residual | −23.5 | 5.5 | −29.5 | −2.5 | 36.5 | 13.5 | ||||||
| (B) | ||||||||||||
| Condition | Compared Emotions | Z | p | |||||||||
| 3 cm/s | Love vs Arousal | −.466 | .64 | |||||||||
| Forearm | Arousal vs Fear | −3.98 | < .001* | |||||||||
| Fear vs Joy | −.030 | .976 | ||||||||||
| Joy vs Depression | −.291 | .771 | ||||||||||
| Depression vs Anger | −3.625 | < .001* | ||||||||||
| 18 cm/s | Fear vs Joy | −.290 | .772 | |||||||||
| Forearm | Joy vs Depression | −.084 | .933 | |||||||||
| Depression vs Love | −1.531 | .126 | ||||||||||
| Love vs Arousal | −1.785 | .074 | ||||||||||
| Arousal vs Anger | −.933 | .351 | ||||||||||
| 3 cm/s | Love vs Arousal | −4.954 | < .001* | |||||||||
| Palm | Arousal vs Joy | −1.541 | .123 | |||||||||
| Joy vs Depression | −.308 | .758 | ||||||||||
| Depression vs Fear | −.264 | .792 | ||||||||||
| Fear vs Anger | −2.751 | .006* | ||||||||||
| 18 cm/s | Fear vs Depression | −2.099 | .036 | |||||||||
| Palm | Depression vs Joy | −.846 | .398 | |||||||||
| Joy vs Anger | −.593 | .553 | ||||||||||
| Anger vs Love | −2.338 | .019 | ||||||||||
| Love vs Arousal | −.947 | .343 | ||||||||||
DF = degree of freedom.
Denotes significant test, for (B) after Bonferroni correction for multiple comparison (alpha = .01).
Table 4.
Summary results of Experiment 2 – Reading Intentions. (A) Chi-square goodness-of-fit values and observed frequencies and residuals for intention words categories. (B) Wilcoxon signed-rank test results to compare difference of obtained frequencies between intention word categories.
| (A) | ||||||||||||
| Condition | Chi-square | DF | p | Reward | Support | Intimacy | Aggression | Warning | Degradation | Total N | ||
| 3 cm/s | 142.72 | 5 | <.001 | Observed N | 20 | 36 | 114 | 18 | 38 | 13 | 239 | |
| Forearm | Column % | 8.4 | 15.0 | 47.7 | 7.5 | 15.9 | 5.4 | |||||
| Residual | −19.8 | −3.8 | 74.2 | −21.8 | −1.8 | −26.8 | ||||||
| 18 cm/s | 38.81 | 5 | <.001 | Observed N | 25 | 79 | 22 | 21 | 73 | 23 | 243 | |
| Forearm | Column % | 10.3 | 32.5 | 9.1 | 8.6 | 30.0 | 9.5 | |||||
| Residual | −15.5 | 38.5 | −18.5 | −19.5 | 32.5 | −17.5 | ||||||
| 3 cm/s | 209.19 | 5 | <.001 | Observed N | 22 | 71 | 90 | 13 | 34 | 14 | 244 | |
| Palm | Column % | 9.0 | 29.1 | 36.9 | 5.3 | 13.9 | 5.7 | |||||
| Residual | −18.7 | 30.3 | 49.3 | −27.7 | −6.7 | −26.7 | ||||||
| 18 cm/s | 73.42 | 5 | <.001 | Observed N | 14 | 49 | 18 | 40 | 108 | 11 | 240 | |
| Palm | Column % | 5.8 | 20.4 | 7.5 | 16.7 | 45.0 | 4.6 | |||||
| Residual | −26 | 9 | −22 | 0 | 68 | −29 | ||||||
| (B) | ||||||||||||
| Condition | Compared Intentions | Z | p | |||||||||
| 3 cm/s | Intimacy vs Warning | −4.338 | < .001** | |||||||||
| Forearm | Warning vs Support | −.111 | .911 | |||||||||
| Support vs Reward | −1.962 | .050 | ||||||||||
| Reward vs Aggression | −.251 | .802 | ||||||||||
| Aggression vs Degradation | −.994 | .320 | ||||||||||
| 18 cm/s | Support vs Warning | −.445 | .657 | |||||||||
| Forearm | Warning vs Aggression | −3.60 | < .001* | |||||||||
| Aggression vs Reward | −.300 | .764 | ||||||||||
| Reward vs Intimacy | −.140 | .888 | ||||||||||
| Intimacy vs Degradation | −.074 | .941 | ||||||||||
| 3 cm/s | Intimacy vs Support | −3.129 | .002* | |||||||||
| Palm | Support vs Warning | −4.831 | <.001* | |||||||||
| Warning vs Reward | −5.121 | <.001* | ||||||||||
| Reward vs Degradation | −4.768 | <.001* | ||||||||||
| Degradation vs Aggression | −.908 | .364 | ||||||||||
| 18 cm/s | Warning vs Support | −3.737 | < .001* | |||||||||
| Palm | Support vs Aggression | −.737 | .461 | |||||||||
| Aggression vs Intimacy | −2.385 | .017 | ||||||||||
| Intimacy vs Reward | −.507 | .612 | ||||||||||
| Reward vs Degradation | −.557 | .577 | ||||||||||
DF = degree of freedom; p = p-value.
Denotes significant test, for (B) after Bonferroni correction for multiple comparison (alpha = .01).
Chi-square results were analysed post-hoc using Wilcoxon signed- rank tests to find the preferred category for each condition. To do so the groups were compared in descending order, i.e., the group with the highest frequency was compared to the group with the next highest frequency, then this group in turn was compared to the next highest etc. Bonferroni-corrected multiple comparisons for emotions are presented in Table 3B and for intentions in Table 4B (p-value < .01).
In addition, confidence ratings were analysed with a repeated measure ANOVA. Finally, pleasantness ratings were analysed with a repeated measure ANOVA (taking the average between intention and emotion for each velocity/site, as no assumption on that level).
3.2. Results Experiment 2
Chi-square goodness-of-fit tests were conducted for each of the velocity/location conditions to confirm that frequencies for the word categories were non-randomly distributed. As intended, observed frequencies (see Tables 3A&4A) were not equal, i.e., specific category/ies were preferred for each condition.
3.2.1. Reading emotions
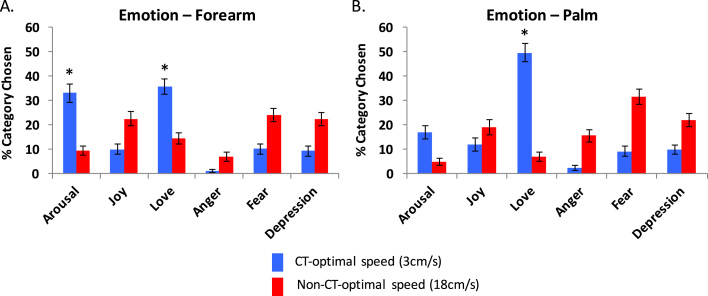
CT-optimal touch to the (non-CT-containing) palm of the hand mainly conveyed Love (50.2% of trials) whereas CT-optimal velocity touch to the forearm additionally conveyed Arousal (33.1%) and Love (35.6%) (Fig. 2; see Table 3 for Chi Square and Wilcoxon signed rank test results). At both touch locations, Anger was the emotion least likely to be communicated. This overall suggests a specificity of slow touch towards positive emotions, and in particular arousal being conveyed via CT-fibres (in the forearm especially).
Fig. 2.
Average percentage of category chosen for other's emotion, for both CT and non-CT velocities, for the forearm (A) and palm (B). Error bars represent the standard error of the mean. * denotes the category significantly most chosen.
For non-CT-optimal velocities touch, both on the forearm and palm, even though the distribution between the different categories was not equal, no category was chosen significantly more frequently than any other; showing the non-specificity of non-CT-optimal touch.
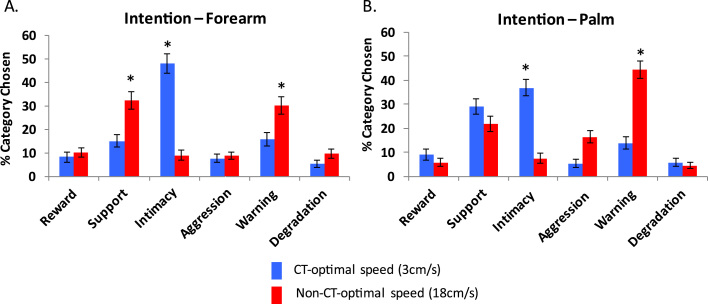
3.2.2. Reading intentions
Both residuals and Wilcoxon signed-rank test results revealed that slow CT-optimal velocity touch on the forearm and the palm communicated significantly more Intimacy intentions than any other intentions. However, in the forearm, there was a large and significant difference between the Intimacy category choice (47.7%) and the remaining categories, which did not differ from each other. For the palm, Intimacy (36.9%) was also chosen significantly more frequently than the other categories, but the next category, Support, was also chosen with greater frequency than the other next three categories (29.1%) (see Fig. 3 and Table 4). Slow touch to the palm may communicate less specific intentions, but overall mainly positive intentions; whereas slow touch on the forearm is more specifically linked to the communication of Intimacy.
Fig. 3.
Average percentage of category chosen for other's intention, for both CT and non-CT velocities, for the forearm (A) and the palm (B). Error bars represent the standard error of the mean. * denotes the category significantly the most chosen.
Fast non-CT-optimal velocity touch on the forearm communicated both Support (32.5%) and Warning (30.0%), whereas on the palm it mainly communicated Warning (45.0% - significantly different from the other categories, with no difference between the other categories). Fast touch in general seemed to communicate a warning signal, whereas on the forearm it also communicated support. In Experiment 1, participants read fast non-CT touch as communicating Warning, whereas in Experiment 2, when given more categories, they read fast non-CT touch on the forearm as a communicating both Support and Warning. It suggests that fast touch is not as reliably read as slow CT optimal touch, and can be communicating both caring intentions (such as support and warning).
3.2.3. Confidence ratings
After each touch trial, participants rated how confident they were in their word choice. Overall, participants were more confident with their answers in slow touch trials (main effect of velocity: [F(1, 60) = 4.210, p = .045, η2p = .066]), but no main effect of body site (F(1, 60) = .193, p = .662, η2p = .003) nor interaction (F(1, 60) = .251, p = .618, η2p = .004).
This supports the intentions and emotions results, as fast touch is less specific: participants are less confident and tend to answer more randomly during fast touch trials.
3.2.4. Manipulation check: Pleasantness ratings
To check whether slow stroking was generally perceived by participants as more pleasant than fast stroking, and whether this depends on body site, we examined both interactions between velocity and body site, as well as main effects, by conducting a 2-way repeated measures ANOVA. No significant main effect of body site (palm vs forearm) was found [F(1, 59) = 1.017, p = .317, η2p = .017], suggesting similar pleasantness ratings for both locations. A main effect of velocity confirmed that slow stroking was rated as more pleasant than fast stroking [F(1, 59) = 21.554, p < .001, η2p = .268]. A significant interaction effect between velocity and location was observed [F(1, 59) = 5.608, p = .021, η2p = .087] (see Supplementary Fig. 1.b). Follow-up, Bonferroni-corrected (alpha = .025) paired t-tests were carried out comparing touch on the palm and the forearm for slow and fast touch separately, and showed that there were no difference in pleasantness rating of slow touch on the palm and the forearm [t(59) = −.915, p = .364], whereas there were a trend to significance for fast touch [t(59) = 2.145, p = .036]. This suggests that slow touch was rated as pleasant on the palm and on the forearm. This is in line with previous research suggesting the role of top-down factors in pleasure from the palm (McGlone et al., 2012, but also Lloyd et al., 2013; Löken et al., 2011; Ackerley et al., 2014).
Taken together with results of Experiment 1, CT-optimal touch (slow, gentle touch on the forearm) was significantly more likely than other types of touch to convey arousal, lust or desire. Affiliative emotions such as love and related intentions such as social support were instead reliably elicited by gentle touch, irrespective of CT-optimality, suggesting that other top-down factors contribute to these aspects of tactile social communication.
4. Experiment 3 – Single case study – patient with right hemisphere lesion
As aforementioned, the neural mechanism by which affective touch may communicate emotions has never been investigated. The right posterior insula, the primary somatosensory cortex and the superior temporal sulcus have all been associated with the perception of CT-optimal touch in previous neuroimaging studies (Olausson et al., 2002, Morrison et al., 2010, Gordon et al., 2013, Voos et al., 2013), however these studies cannot establish which brain areas are necessary for the processing of peripheral CT signals. To begin to address this question, we recruited a patient who had suffered brain damage to the right posterior insula and neighbouring areas in the right hemisphere, but excluding temporal cortex, or any orbitofrontal cortex areas.
Using the same paradigm as in Experiment 2, we thus aimed in a single case study to examine whether a lesion of the insula and neighbouring areas would affect the ‘reading’ of emotions (in ipsilateral areas of the body to where touch was generally perceived). We aimed to explore whether a right hemisphere lesion, and in particular a lesion of the posterior insula, would selectively disturb the perception of sensory pleasure and emotion reading, as a means to test the necessary cortical areas supporting the functional role of the CT-optimal system.
4.1. Methods
4.1.1. Patient
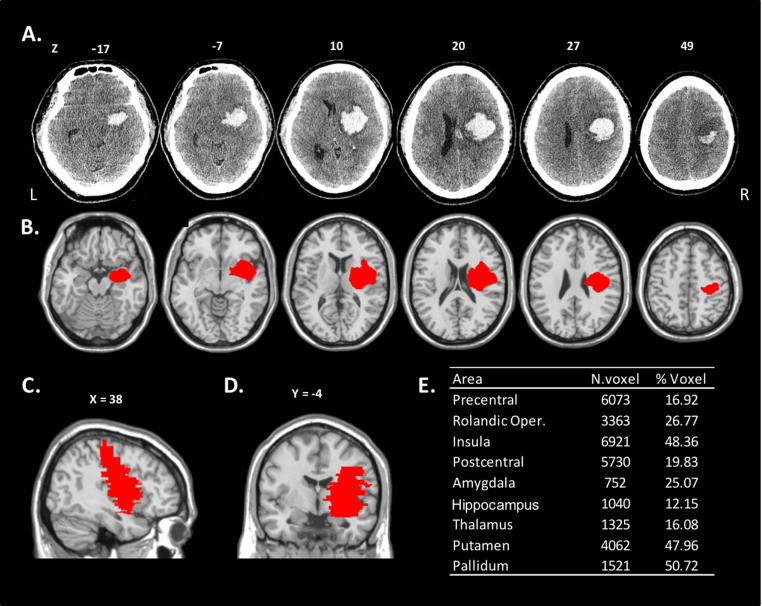
Patient NQ, a 42-year-old right-handed male, suffered a stroke with an opercular lesion involving fronto-parietal areas around the central sulcus, insula and basal ganglia (see details of regions affected in Fig. 4). He had no previous history of neurological or psychiatric illness. He gave written, informed consent to take part in the study. The local National Health System Ethics Committee approved the study, which was carried out in accordance to the Declaration of Helsinki.
Fig. 4.
NQ's lesion. A = the lesion of the patient is shown (centre of mass, x = 34, y = 10, Z = 20). B = the lesion is traced on MRI Template in the axial view (the right hemisphere is on the right); C = sagittal view; D = coronal view. The lesion (in red) mostly involves the Insula (Visible in the slices Z = −7,10, 20, X = 38, Y = −4), the Rolandic Operculum (Z = 10, 20, X = 38, Y = −4), the Precentral gyrus (Z = −17, 49, X = 38), the Postcentral gyrus (Z = 49, X = 38), the Pallidum (Y = −4), the Putamen (Z = −7,10, Y = −4), the Amygdala (Z = −17,20) the Thalamus (Z = 10, 20, Y = −4), the Hippocampus (Z = −17), and the white matter around these structures. E = The table shows the percentage and number of voxels of damaged tissue in each area.
At the time of hospitalization, the patient was alert, oriented and cooperative. His lesion was associated with (see Table 5 for patient's scores) dense left hemiparesis (assessed via the MRC scale; Guarantors of Brain, 1986), severe visuo-spatial neglect (as measured by two subsets of the Behavioural Inattention Test: the line crossing, and star cancellation; Wilson et al., 1987), but no personal neglect (as measured by the comb/razor test; McIntosh et al., 2000), impaired proprioception (assessed with eyes closed by applying small, vertical, controlled movements to three joints - middle finger, wrist and elbow - at four time intervals; correct responses were rated as 0 and incorrect ones as 1; adapted from Vocat et al. (2010)), impaired tactile sensation on the left (assessed with the revised Nottingham sensory assessment, Lincoln et al., 1998 – light touch, pressure and pinprick were not detected on the left arm), but intact on the right arm.
Table 5.
Details of patient NQ scores on different neuropsychological tests.
| Demographics and neuropsychological scores | |
|---|---|
| Age (years) | 42 |
| Days from onset | 12.00 |
| MRC Left upper limb | 0 |
| MRC left lower limb | 0 |
| Berti awareness interview (on day of testing) | 0 |
| MOCA | 16/27 |
| MOCA MEMORY | 3/5 |
| Nottingham (arm) | 0 |
| Proprioception (max 12-errors) | 8 |
| Comb/razor test left | 22 |
| Comb/razor test right | 23 |
| Comb/razor test ambiguous | 6 |
| Line crossing right | 18 |
| Line crossing left | 0 |
| Star cancelation right | 23 |
| Star cancelation left | 5 |
| HADS depression | 8 |
| HADS anxiety | 7 |
In terms of cognition, NQ scored poorly on the Montreal Cognitive Assessment and particularly on the more demanding word digit span subtest, showing post-stroke deficits, no premorbid dementia (MoCA; Nasreddine et al., 2005) and average performance on the long term verbal recall subtest (MoCA memory subscale). The Hospital Depression and Anxiety Scale (HADS; Zigmond and Snaith, 1983), was used to assess depression and anxiety. The patient showed borderline scores below the cut-off for clinical depression.
On the first day of testing, patient NQ was not aware of his motor deficit (anosognosic for hemiplegia – scored 1 on Berti Scale after a short interview following the Berti et al. (1996) method); however, when the present task was conducted he had recovered awareness (score of 0 on Berti Scale). Body ownership disturbances such as asomatognosia (the inability to recognise one's own body; Cutting, 1978) and somatoparaphrenia (body ownership delusions; Gerstmann, 1942) were assessed using the Cutting (1978) questionnaire: the patient did not show any sign of somatoparaphrenia or asomatognosia.
4.1.2. Lesion analysis methods
A CT scan was carried out two days after the lesion onset and the patient's lesion was mapped by means of the MRIcron software (Rorden and Brett, 2000) on the standard T1-weighted MRI template (ICBM152) of the Montreal Neurological Institute (MNI) coordinate system, approximately matched to the Talairach space (Talairach and Tournoux, 1988). We first oriented the template on the midsagittal and midcoronal axis to match the original scan of the patient. Then, an expert clinician (blind to the experimental purpose) manually traced the lesion using the MRIcron Software (Rorden and Brett, 2000).
The final image of the lesion was superimposed onto the Automatic Anatomical Label (AAL) template (Tzourio-Mazoyer et al., 2002) in order to ascertain the number of voxels involved in the lesion in each area and the centre of mass of the lesion.
The main (more than 10% of the voxels) areas damaged in the right hemisphere lesion were the Insula, the Precentral and Postcentral gyri, the Rolandic Operculum, and subcortical structures such as the Pallidum, the Putamen, the Amygdala, the Hippocampus and the Thalamus (see Fig. 4 for details).
4.1.3. Experimental design and procedure
A similar design to that of Experiment 2 was employed. However, we tested only the right forearm. Manipulated factors were the velocity of touch (CT-optimal: 3 cm/s and non-CT: 18 cm/s), and emotion reference (emotion or intention conveyed). A total of 16 touch stimuli were applied (4 repetitions per condition), which were divided into two blocks of 8 trials in which the number of emotion and intention questions consisted of two for each velocity.
The word set used was the same as in Experiment 2 (see Table 1). Instead of being presented horizontally, words were presented in a pseudo-random order in a vertical orientation to avoid confounds related to unilateral neglect. Touch stimuli were exactly the same as in Experiment 1.
Pleasantness and tactile acuity of each type of touch was assessed before starting the experiment. The female experimenter stroked the patient's right forearm with her fingers on a 9 cm surface for 3 s, comprising two trials at 3 cm/s, two trials at 18 cm/s, and two sham trials where no touch was delivered (just movement over the arm). Patient NQ was asked to keep his eyes closed. NQ was asked how well he was able to feel the touch and how pleasant the touch was on a vertical 10-point Likert scale from 1 (not at all felt/pleasant) to 10 (extremely well/pleasant). We used a vertical scale to avoid any bias due to neglect.
Moreover, we assessed first the patient's ability to infer the sensory emotions associated with different fabrics (in memory) as well as his ability to use the 10-point pleasantness scale correctly by asking 3 hypothetical questions – how pleasant would it be to be touched by velvet, sandpaper and soft cream, respectively.
4.2. Results
4.2.1. Pleasantness, and touch data
First of all, patient NQ was able to appropriately use the scale in terms of the pleasantness of touch, as on a scale from 1 (not at all) to 10 (extremely), he rated as 1 how pleasant it would be to be touched by sandpaper, as 6 to be touched by soft cream, and as 9 to be touched by velvet. This confirmed that patient NQ did not have a general emotion inference issue, and he could use the scale properly. Furthermore, he understood ‘touch’ related emotional judgements at higher order levels, showing a normal range of positive and negative emotions.
Moreover, patient NQ had excellent tactile acuity on his right forearm (rated as 10 -the maximum- for both slow and fast touch), and responded correctly to both sham trials (where there were no touch); while having eyes closed.
Patient NQ rated fast touch as more pleasant (M = 9.5) than slow touch (M = 8). We used the revised standardized difference test along Crawford and Garthwaite (2005, RSDT), and showed that patient NQ ratings were significantly different from healthy controls (see details in Table 6.A). This suggests that patient NQ has a deficit of the CT system, compared to healthy controls who rate slow touch as more pleasant than fast touch.
Table 6.
Summary of results comparing patient NQ scores to controls of Experiment 2 (Crawford et al., 2010). (A) Pleasantness Ratings using RSDT method; (B) Emotion and Intention Reading, using the SINGLIM_ES method.
|
Control Group |
Significance test (two-tailed) |
Estimated effect size (z-CC) |
||||||
|---|---|---|---|---|---|---|---|---|
| Condition | N | Mean | SD | NQ Score | t | p | Point | (95% CI) |
| ||||||||
| Plesantness ratings | ||||||||
| CT speed | 60 | 5.94 | 1.44 | 8 | 2.066 | .043* | −2.11 | (−2.808 to −1.454) |
| Non-CT speed | 60 | 5.43 | 1.47 | 9.5 | ||||
| ||||||||
| Emotion Reading | ||||||||
| CT – ‘Joy’ | 61 | .39 | .67 | 3 | 3.864 | .000* | 3.896 | (3.154–4.632) |
| Emotion Reading | ||||||||
| Non-CT – ‘Joy’ | 61 | .89 | .91 | 3 | 2.300 | .025* | 2.319 | (1.831–2.799) |
| Intention Reading | ||||||||
| CT – ‘Support’ | 61 | .59 | .80 | 2 | 1.748 | .085 | 1.762 | (1.356–2.161) |
| CT – ‘Warning’ | 61 | .62 | .92 | 2 | 1.488 | .142 | 1.500 | (1.130–1.864) |
| Intention Reading | ||||||||
| Non-CT – ‘Reward’ | 61 | .41 | .64 | 2 | 2.464 | .017 * | 2.484 | (1.972–2.991) |
| Non-CT – ‘Warning’ | 61 | 1.20 | 1.18 | 2 | .672 | .503 | .678 | (.397–.954) |
4.2.2. Reading emotions and intentions
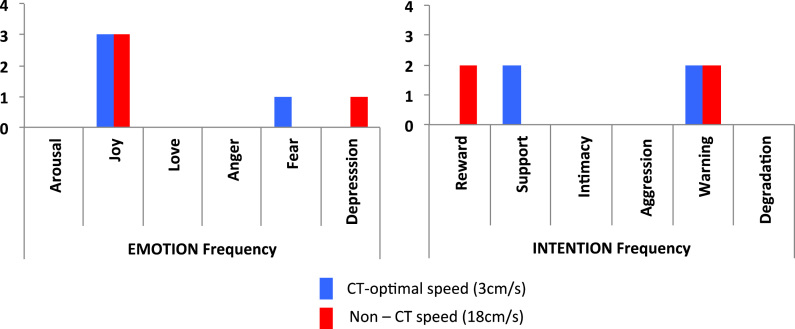
When patient NQ was asked to rate slow CT-optimal touch, he preferentially rated them as the touch giver feeling Joy (see Fig. 5). This was the same for fast non-CT touch, suggesting that NQ did not differentiate between CT and non-CT touch in terms of emotion reading. We can note that in contrast to healthy participants, NQ never chose the Arousal or Love categories. As an exploratory analysis, we compared patient NQ's scores with healthy controls using the procedure of Crawford and Garthwaite (2002, SINGLIMS_ES) for comparing a single case with a control population, separately for each condition. As patient NQ chose mostly Joy for both CT and non-CT touch conditions, we compared his answer to the average answer of controls of Experiment 2 for the category Joy, for both CT-optimal and Non-CT touch on the forearm. As shown in Table 6, the patient showed a significant deficit in reading emotions for both CT and non-CT touch.
Fig. 5.
Frequency of category chosen for other's emotion (A) and intention (B), for both CT and non-CT velocities - Number of choices for each category per condition (max of 4 trials per condition).
When NQ was asked to rate the intention of the touch giver through tactile stimulation of the right forearm, results were mixed. NQ did not have a clear preference within each condition, nor between, choosing equally Support and Warning for slow CT-optimal touch, and both Reward and Warning for fast non-CT-optimal touch. Moreover, NQ never chose Intimacy, in contrast to healthy controls. However, this lack of specificity was to a degree present in the controls. When comparing patient NQ's choice when reading intentions to that of the controls in Experiment 2, results were less clear than for emotion reading, showing a clear significant deficit only when choosing the category Reward for Non-CT touch.
Overall, from a qualitative observation, NQ seems to have a deficit in reading both the emotions and the intentions of the touch giver; however, patient NQ showed a statistically significant deficit compared to healthy controls only in emotion reading. We note as a limitation that patient NQ was male whereas control participants in Experiment 2 were all female. Referring back to the results of Experiment 1, where we found some touch receiver gender effects only for intention reading, we suggest caution in the interpretation of the intention reading results. However, we can have more confidence in the fact that patient NQ showed emotion reading deficits.
5. General discussion
In a set of three experiments, we investigated the role of slow, CT-optimal touch in interpersonal communication of selective emotions and intentions. The first experiment provided evidence of some specificity of the emotions conveyed by CT-optimal, slow velocity touch vs. fast, CT-suboptimal velocity touch on the forearm, whereas the second experiment further specified these findings, showing that the only emotional inference elicited reliably and uniquely by CT-optimal touch was the attribution of lustful, arousing emotions to the touch provider. Social emotions such as love, and related pro-social intentions such as social support were also inferred on the basis of other, non-CT based touch. Finally, in an exploratory single case study, we found that a patient with a fronto-parietal lesion including the insula but excluding the temporal cortex, could not distinguish between CT-optimal and suboptimal touch in terms of sensory pleasure and emotion reading, even though he was able to correctly infer and rate accordingly the sensory pleasantness associated with different fabrics. These findings are discussed in turn below.
5.1. Emotion reading by touch and the CT system
A first notable finding of our experiments with healthy controls is that slow gentle touch by a stranger, even in the absence of other relevant contextual or sensory cues, conveys significantly more positive rather than negative emotions. By contrast, fast touch does not show any reliable valence specificity, at least as tested here. This finding seems intuitive and is consistent with the aforementioned studies on the more general capacity of certain types of touch to communicate specific emotions reliably (Hertenstein et al., 2006a, Hertenstein et al., 2006b, Hertenstein et al., 2009). Yet to our knowledge there are no studies on how these particular parameters of tactile behaviour, i.e. low pressure on the skin, dynamic touch and slow velocity, which are typically associated with feelings of sensory pleasure in the self, are ‘translated’ into inferences about the mental states of the touch provider. Indeed, studies on facial emotion recognition have been criticized for using fixed choice tasks that might ‘create’ top-down representations of emotional categories to ambiguous stimuli (Barrett et al., 2011). The same limitations may apply to our study, with Experiment 2 aiming exactly at reducing the impact of such effect by increasing the range of available categories used. However, future studies could investigate the direct interplay between the perception of affective touch in the self and ‘emotion reading’ on its basis, as well as the contribution of different top-down factors in such readings.
A second notable finding of our study was that ‘Arousal’ (Desire/Lust) was the only emotion to be specifically ‘communicated’ by CT-optimal touch, while ‘Love’ (Affection) was also ‘communicated’ by slow touch on the palm, which is known not to contain CT-fibres. In comparison, intention reading seemed to be less CT-based, even though slow touch on the forearm communicated ‘Intimacy’ specifically, whereas for the palm, the distinction between ‘Intimacy’ and ‘Support’ was less clear. These findings will need to be corroborated in studies which compare the social context of touch (e.g. relationship type such as couples, friends or children), as previous studies on ‘spontaneous’ use of CT-optimal touch have found differences in this respect (e.g. Croy et al., 2016). Moreover, the fact that in Experiment 2 we stimulated the forearm and the palm, two different body parts, with different effectors and different distances from the torso, could have influenced the reading. Suvilehto et al. (2015) have demonstrated that the hand is in general more "official" than any other area of the body and socially more accepted. In this study, our choice to compare the palm with the forearm, and not the back of the hand, was motivated by the fact that 1) we have not found an effect of torso proximity in previous studies (Gentsch et al., 2015); 2) we wanted to maintain consistency with Experiment 1; 3) The forearm is the body site that has been the most studied in the CT fibres literature (Lö̈ken et al., 2009; Ackerley et al., 2014; Krahé et al., 2016; Crucianelli et al., 2013); and 4) testing the back of the hand has its own limitations (e.g. it is not a habitual site for interpersonal hand stroking). We therefore decided to keep the forearm as the body site containing CT fibres in our study. However future studies should examine the influence of different body sites in more detail. Finally, as we found in Experiment 1 that the gender of the touch receiver might influence the reading of intentions, future studies should investigate this further in a full factorial design, manipulating both the gender of the touch receiver and the gender of the touch giver.
Patient NQ was not able to distinguish the pleasantness of slow versus fast touch on his ipsilateral (i.e. not affected) arm, despite his intact tactile acuity and his ability to rate tactile pleasantness in the ‘imagined’ domain. This might suggest that there is a right-sided involvement in the representation of the CT system in the brain for both ipsilateral and contralateral body parts. Further target and control patients will need to be tested in order to confirm this finding. Moreover, this study suggests that deficits in perceiving the emotional effects (pleasantness) of the touch in the self may also underlie deficits in perceiving the emotions in the touch giver. However, whether a pleasantness discrimination in the self is a necessary prerequisite for emotion communication in the touch giver remains a hypothesis to be tested in future studies. Given the results of Experiments 1 and 2, one could alternatively hypothesise that the CT system gives rise to other low-level sensations (e.g. arousal) that can be used to make different inferences about the self and the other.
Provisionally however, our findings suggest that at least when the touch giver is female, the primary communicative role of the CT system may relate more with the sensual, or the erotic rather than the affiliative component of affective touch, irrespective of the gender of the touch receiver.
5.2. Neural networks associated with reading the mind in CT-optimal touch
CT-optimal touch has been linked with both posterior insula (a primary area for the processing of interoceptive signals from the body) and superior temporal areas that are part of the social brain (Ackerley et al., 2012, McGlone et al., 2012, Gordon et al., 2013). However, our exploratory case study (Experiment 3) suggests that the posterior and mid insula may be necessary for the processing of CT signals from the periphery and without it the possible ‘relay’ to the temporal cortex, or the modulation by the temporal cortex, is not possible. As a result, the patient could not distinguish between CT-optimal and suboptimal touch, in terms of either pleasantness or emotion reading. By contrast, he showed no difficulties or aberrant responses when he had to infer the tactile pleasantness of different fabrics, suggesting that his deficits where not the consequence of a general deficit in emotional processing. He also showed ‘borderline’ levels of self-reported, feelings of depression and anxiety, perhaps consistently with his recent stroke and hospitalization. These contrasted with his answers to the main task that instead were dominated by ‘Joy’ responses. Although CT afferents do not seem to send an excitatory signal to S1 (Olausson et al., 2008), various studies have implicated S1 in the emotional processing of touch in relation to understanding the sensations of others (Keysers et al., 2010, Gazzola et al., 2012, Bolognini et al., 2013). Although we tested patient NQ's intact, ipsilateral body side, his lesion involved the right primary somatosensory cortex (postcentral gyrus) and hence we cannot exclude that his damage could have contributed to his deficit in CT touch perception and emotion reading.
Remarkably, a lesion only in the right hemisphere was enough to create this deficit, suggesting a right hemisphere dominance for the affective functions of the CT system for both sides of the body (as the patients general tactile abilities were compromised on the left side of his body, but our CT-optimal touch assessment on the right arm also revealed a deficit). This is consistent with the known dominance of the right hemisphere for emotion perception and emotional awareness (Lane et al., 1995, Gainotti, 2012). Future, larger studies could explore this finding further, as well as specify the role of posterior versus anterior insula involvement and potential issues of inter-hemisphere disconnections following a stroke. Of course these results on the basis of one single case study will need to be expanded to involve group studies including patients with left-hemisphere lesions, before firm conclusions can be drawn. In addition, it should be noted that patient NQ also had damage further down the hierarchy, involving damaged voxels in the amygdala and the basal ganglia, which are also linked to motivation and emotion, and could contribute to patient NQ's emotion reading deficit. The role of these areas in the central processing of CT signals from the periphery remains to be established in larger studies. Moreover, even though classical literature has shown a direct connection between CT fibres (periphery) and the cortex in mammals, a recent study in mice started to question this direct link (Abraira et al., 2017). This reinforces the necessity and importance of lesion studies in humans to investigate affective touch. As a limitation of the current study, it is worth noting that tactile stimulation was applied only to the forearm of patient NQ. Future studies on patients are needed in order to test a body site without CT fibres as well (e.g. the palm of the hand), to determine better whether these deficits are specific to CT fibres or to perceiving the emotions or intentions conveyed by tactile stimuli in general. However, taken together, we believe the results of our exploratory case study warrant the hypothesis that sensory, erotic pleasure, as part of interoception, is a more basic, bottom-up function of the CT system than social affiliation or cognition.
5.3. Conclusions
There has been some ambiguity regarding the functional role of the CT system and particularly its sensual versus affiliative role (Gallace and Spence, 2010, McGlone et al., 2012; Morrison et al., 2010). Based on our findings, it seems that CT involvement can add to the specificity of the emotions that can be socially communicated via touch, but the advantage seems to concern specifically communications of sensual arousal, desire and lust, rather than other, more affiliative emotions. Moreover, our results suggest that affiliative communications, such as the provision of social support seem to entail a larger ‘top-down’ component, in the sense that it can be communicated also in body parts that do not contain CT fibres.
The findings of the present study add to the understanding of the role of touch in communication in the following ways: they provide new information about the perception of emotions through specific tactile behaviours and further show that distinct intentions can be communicated by touch alone.
Acknowledgements
We thank the healthy participants and patient NQ for his kindness and willingness to take part in the study. We are also grateful to Cristina Papadaki, Amanda Hornsby, Sonia Ponzo, and Arturo Kerbel, for help with patient recruitment and testing. We thank Sara Bertagnoli for her help regarding the lesion drawing. No conflicts of interest were reported.
This work was supported by a project grant no. (II/85 069) from the Volkswagen Foundation ‘European Platform for Life Sciences, Mind Sciences and Humanities’ and a European Research Council Starting Investigator Award (ERC-2012-STG GA313755) (to A.F.); and the Italian Ministry of Health (RF-2010-2312912) and University of Verona (to V.M).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.neuropsychologia.2017.05.024.
Appendix A. Supplementary material
Supplementary material
.
References
- Abraira V.E., Kuehn E.D., Chirila A.M., Springel M.W., Toliver A.A., Zimmerman A.L. Cell. 2017;168(1–2):295–310. doi: 10.1016/j.cell.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerley R., Hassan E., Curran A., Wessberg J., Olausson H., McGlone F. An fMRI study on cortical responses during active self-touch and passive touch from others. Front. Behav. Neurosci. 2012;6:51. doi: 10.3389/fnbeh.2012.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerley R., Saar K., McGlone F., Backlund Wasling H. Quantifying the sensory and emotional perception of touch: differences between glabrous and hairy skin. Front. Behav. Neurosci. 2014;8:34. doi: 10.3389/fnbeh.2014.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed F.S., Miller L.S. Executive function mechanisms of theory of mind. J. Autism Dev. Disord. 2011;41(5):667–678. doi: 10.1007/s10803-010-1087-7. [DOI] [PubMed] [Google Scholar]
- App B., McIntosh D., Reed C.L., Hertenstein M.J. Nonverbal channel use in communication of emotion: how may depend on why. Emotion. 2011;11(3):603–617. doi: 10.1037/a0023164. [DOI] [PubMed] [Google Scholar]
- Barrett L.F., Mesquita B., Gendron M. Context in emotion perception. Curr. Dir. Psychol. Sci. 2011;20(5):286–290. [Google Scholar]
- Baron-Cohen S. The MIT Press; Massachusetts: 1995. Mindblindness: An Essay on Autism and Theory of Mind. [Google Scholar]
- Baron-Cohen S., Wheelwright S., Skinner R., Martin J., Clubley E. The Autism-Spectrum Quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J. Autism Dev. Disord. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Berti A., Ladavas E., Della Corte D. Anosognosia for hemiplegia, neglect, dyslexia, and drawing neglect: clinical findings and theoretical considerations. Neuropsychol. Soc. 1996;2 doi: 10.1017/s135561770000151x. (426e440) [DOI] [PubMed] [Google Scholar]
- Björndotter M., Löken L., Olausson H., Vallbo A., Wessberg J. Somatotopic organization of gentle touch processing in the posterior insular cortex. J. Neurosci. 2009;29:9314–9320. doi: 10.1523/JNEUROSCI.0400-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognini N., Rossetti A., Convento S., Vallar G. Understanding others' feelings: the role of the right primary somatosensory cortex in encoding the affective valence of others' touch. J. Neurosci. 2013;33:4201–4205. doi: 10.1523/JNEUROSCI.4498-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttelmann D., Carpenter M., Tomasello M. Eighteen-month-old infants show false belief understanding in an active helping paradigm. Cognition. 2009;112:337–342. doi: 10.1016/j.cognition.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Case L.K., Laubacher C.M., Olausson H., Wang B., Spagnolo P.A., Bushnell M.C. Encoding of touch intensity but not pleasantness in human primary somatosensory cortex. J. Neurosci. 2016;36(21):5850–5860. doi: 10.1523/JNEUROSCI.1130-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J.D., Bushnell M.C., McGlone F., Elam M., Lamarre Y., Vallbo A.B., Olausson H. Unmyelinated tactile afferents underpin detection of low-force monofilaments. Muscle Nerve. 2006;34:105–107. doi: 10.1002/mus.20534. [DOI] [PubMed] [Google Scholar]
- Crawford J.R., Garthwaite P.H. Investigation of the single case in neuropsychology: confidence limits on the abnormality of test scores and test score differences. Neuropsychologia. 2002;40:1196–1208. doi: 10.1016/s0028-3932(01)00224-x. [DOI] [PubMed] [Google Scholar]
- Crawford J.R., Garthwaite P.H. Testing for suspected impairments and dissociations in single-case studies in neuropsychology: evaluation of alternatives using Monte Carlo simulations and revised tests for dissociations. Neuropsychology. 2005;19:318–331. doi: 10.1037/0894-4105.19.3.318. [DOI] [PubMed] [Google Scholar]
- Crawford J.R., Garthwaite P.H., Porter S. Point and interval estimates of effect sizes for the case‑controls design in neuropsychology: rationale, methods, implementations, and proposed reporting standards. Cogn. Neuropsychol. 2010;27:245–260. doi: 10.1080/02643294.2010.513967. [DOI] [PubMed] [Google Scholar]
- Croy I., Luong A., Triscoli C., Hofmann E., Olausson H., Sailer U. Interpersonal stroking touch is targeted to C tactile afferent activation. Behav. Brain Res. 2016;297:37–40. doi: 10.1016/j.bbr.2015.09.038. [DOI] [PubMed] [Google Scholar]
- Crucianelli L., Metcalf N.K., Fotopoulou A., Jenkinson P. Bodily pleasure matters: velocity of touch modulates body ownership during the rubber hand illusion. Front. Psychol. 2013;4:703. doi: 10.3389/fpsyg.2013.00703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting J. Study of anosognosia. J. Neurol., Neurosurg. Psychiatry. 1978;41 doi: 10.1136/jnnp.41.6.548. (548e555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delucchi K.L. On the use and misuse of chi-square. In: Keren G., Lewis C., editors. A Handbook for Data Analysis in the Behavioral Sciences. Lawrence Erlbaum; Hillsdale, NJ: 1993. pp. 294–319. [Google Scholar]
- Ebisch S.J., Ferri F., Gallese V. Touching moments: desire modulates the neural anticipation of active romantic caress. Front. Behav. Neurosci. 2014;8:60. doi: 10.3389/fnbeh.2014.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P. Facial expression and emotion. Am. Psychol. 1993;48:384–392. doi: 10.1037//0003-066x.48.4.384. [DOI] [PubMed] [Google Scholar]
- Flavell J.H. Theory-of-mind development: retrospect and prospect. Merill-Palmer Q. 2004;50(3):274–290. [Google Scholar]
- Field T. Touch for socioemotional and physical well-being: a review. Dev. Rev. 2010;30(4):367–383. [Google Scholar]
- Gainotti G. Unconscious processing of emotions and the right hemisphere. Neuropsychologia. 2012;50:205–218. doi: 10.1016/j.neuropsychologia.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Gallace A., Spence C. The science of interpersonal touch: an overview. Neurosci. Biobehav. Rev. 2010;34(2):246–259. doi: 10.1016/j.neubiorev.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Gazzola V., Spezio M.L., Etzel J.A., Castelli F., Adolphs R., Keysers C. Primary somatosensory cortex discriminates affective significance in social touch. Proc. Natl. Acad. Sci. USA. 2012;109:E1657–E1666. doi: 10.1073/pnas.1113211109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch A., Panagiotopoulou E., Fotopoulou A. Actice interpersonal touch gives rise to the social softness illusion. Curr. Biol. 2015;25(18):2392–2397. doi: 10.1016/j.cub.2015.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I., Voos A.C., Bennett R.H., Bolling D.Z., Pelphrey K.A., Kaiser M.D. Brain mechanisms for processing affective touch. Hum. Brain Mapp. 2013;34:914–922. doi: 10.1002/hbm.21480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarantors of Brain . W.B. Saunders; London: 1986. Aids to the Examination of the Peripheral Nervous System. [Google Scholar]
- Gerstmann J. Problem of imperceptions of disease of impaired body territories with organic lesions. Arch. Neurol. Psychiatry. 1942;48 (890e913) [Google Scholar]
- Guest S., Dessirier J.M., Mehrabyan A., McGlone F., Essick G., Gescheider G., Fontana A., Xiong R., Ackerley R., Blot K. The development and validation of sensory and emotional scales of touch perception. Atten., Percept. Psychophys. 2011;73:531–550. doi: 10.3758/s13414-010-0037-y. [DOI] [PubMed] [Google Scholar]
- Hertenstein M.J. Touch: its communicative functions in infancy. Hum. Dev. 2002;45:70–94. [Google Scholar]
- Hertenstein M.J., Holmes R., McCullough M., Keltner D. The communication of emotion via touch. Emotion. 2009;9(4):566–573. doi: 10.1037/a0016108. [DOI] [PubMed] [Google Scholar]
- Hertenstein M.J., Keltner D., App B., Bulleit B.A., Jaskolka A.R. Touch communicates distinct emotions. Emotion. 2006;6(3):528–533. doi: 10.1037/1528-3542.6.3.528. [DOI] [PubMed] [Google Scholar]
- Hertenstein M.J., Verkamp J.M., Kerestes A.M., Holmes R.M. The communicative functions of touch in humans, nonhuman primates, and rats: a review and synthesis of empirical research. Genet., Soc., Gen. Psychol. Monogr. 2006;132(1):5–94. doi: 10.3200/mono.132.1.5-94. [DOI] [PubMed] [Google Scholar]
- Johansson R.S., Vallbo A.B. Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J. Physiol. 1979;286:283–300. doi: 10.1113/jphysiol.1979.sp012619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson E.H., Backlund Wasling H., Wagnbeck V., Dimitriadis M., Georgiadis J.R., Olausson H., Croy I. Unmyelinated tactile cutaneous nerves signal erotic sensations. J. Sex. Med. 2015;12(6):1338–1345. doi: 10.1111/jsm.12905. [DOI] [PubMed] [Google Scholar]
- Keysers C., Kaas J.H., Gazzola V. Somatosensation in social perception. Nat. Rev. Neurosci. 2010;11:417–428. doi: 10.1038/nrn2833. [DOI] [PubMed] [Google Scholar]
- Krahé C., Drabek M., Paloyelis Y., Fotopoulou A. Affective touch and attachment style modulate pain: a laser-evoked potentials study. Philos. Trans. R. Soc. B. 2016;371(1708):20160009. doi: 10.1098/rstb.2016.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane R.D., Kivley L.S., Du Bois M.A., Shamasundara P., Schwartz G.E. Levels of emotion- al awareness and the degree of right hemispheric dominance in the perception of facial emotion. Neuropsychologia. 1995;33(5):525–538. doi: 10.1016/0028-3932(94)00131-8. [DOI] [PubMed] [Google Scholar]
- Lincoln N.B., Jackson J.M., Adams S.A. Reliability and revision of the nottingham sensory assessment for stroke patients. Physiotherapy. 1998;84(8):358–365. [Google Scholar]
- Löken L.S., Evert M., Wessberg J. Pleasantness of touch in human glabrous and hairy skin: order effects on affective ratings. Brain Res. 2011;1417:9–15. doi: 10.1016/j.brainres.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Löken L.S., Wessberg J., Morrison I., McGlone F., Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nat. Neurosci. 2009;12:547–548. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- Lloyd D.M., Gillis V., Lewis E., Farrell M.J., Morrison I. Pleasant touch moderates the subjective but not objective aspects of body perception. Frontiers Behav. Neurosci. 2013;7:207. doi: 10.3389/fnbeh.2013.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlone F., Olausson H., Boyle J.A., Jones-Gotman M., Dancer C., Guest S., Essick G. Touching and feeling: differences in pleasant touch processing between glabrous and hairy skin in humans. Eur. J. Neurosci. 2012;35:1782–1788. doi: 10.1111/j.1460-9568.2012.08092.x. [DOI] [PubMed] [Google Scholar]
- McGlone F., Vallbo A.B., Olausson H., Löken L., Wessberg J. Discriminative touch and emotional touch. Can. J. Exp. Psychol. 2007;61(3):173–183. doi: 10.1037/cjep2007019. [DOI] [PubMed] [Google Scholar]
- McGlone F., Wessberg J., Olausson H. Discriminative and affective touch: sensing and feeling. Neuron. 2014;82:737–755. doi: 10.1016/j.neuron.2014.05.001. [DOI] [PubMed] [Google Scholar]
- McIntosh R.D., Brodie E.E., Beschin N., Robertson I.H. Improving the clinical diagnosis of personal neglect. Cortex. 2000;36 doi: 10.1016/s0010-9452(08)70530-6. (289e292) [DOI] [PubMed] [Google Scholar]
- Morrison I., Löken L.S., Olausson H. The skin as a social organ. Exp. Brain Res. 2010;204:305–314. doi: 10.1007/s00221-009-2007-y. [DOI] [PubMed] [Google Scholar]
- Morrison I., Löken L.S., Minde J., Wessberg J., Perini I., Nennesmo I., Olausson H. Reduced C-afferent fibre density affects perceived pleasantness and empathy for touch. Brain. 2011;134(2):1116–1126. doi: 10.1093/brain/awr011. [DOI] [PubMed] [Google Scholar]
- Nasreddine Z.S., Phillips N.A., Be dirian V., Charbonneau S., Whitehead V., Collin I. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53 doi: 10.1111/j.1532-5415.2005.53221.x. (695e699) [DOI] [PubMed] [Google Scholar]
- Olausson H.W., Cole J., Vallbo A. Unmyelinated tactile afferents have opposite effects on insular and somatosensory cortical processing. Neurosci. Lett. 2008;436:128–132. doi: 10.1016/j.neulet.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Olausson H., Lamarre Y., Backlund H., Morin C., Wallin B.G., Starck G. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat. Neurosci. 2002;5:900–904. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- Olausson H., Wessberg J., Morrison I., McGlone F., Vallbo A. The neurophysiology of unmyelinated tactile afferents. Neurosci. Biobehav. Rev. 2010;34:185–191. doi: 10.1016/j.neubiorev.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Oxford University Press; New York: 1998. Affective Neuroscience: The Foundations of Human and Animal Emotions. [Google Scholar]
- Rorden C., Brett M. Stereotaxic display of brain lesions. Behav. Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Rubio-Fernández P., Geurts B. How to pass the false belief task before your fourth birthday. Psychol. Sci. 2013;24(1):27–33. doi: 10.1177/0956797612447819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh S., Nagi S.S., McGlone F., Mahns D.A., Bensmaia S.J. Psychophysical investigations into the role of low-threshold C fibres in non-painful affective processing and pain modulation. PLOS ONE. 2015;10(9):e0138299. doi: 10.1371/journal.pone.0138299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack D.M. The salience of touch and physical contact during infancy: unravelling some of the mysteries of the somesthetic sense. In: Bremner G., Fogel A., editors. Blackwell Handbook of Infant Development. Blackwell; Malden, MA: 2001. pp. 351–378. [Google Scholar]
- Suvilehto J.T., Glerean E., Dunbar R.I., Hari R., Nummenmaa L. Topography of social touching depends on emotional bonds between humans. Proc. Natl. Acad. Sci. 2015;112(45):13811–13816. doi: 10.1073/pnas.1519231112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. G. Thieme; Stuttgart: 1988. Co-Planar Stereotaxic Atlas of the Human Brain 3-Dimensional Proportional System: An Approach to Cerebral Imaging. [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1) doi: 10.1006/nimg.2001.0978. (273e289) [DOI] [PubMed] [Google Scholar]
- Vallbo A.B., Olausson H., Wessberg J. Unmyelinated afferents constitute a second system coding tactile stimuli of the human hairy skin. J. Neurophysiol. 1999;81:2753–2763. doi: 10.1152/jn.1999.81.6.2753. [DOI] [PubMed] [Google Scholar]
- Vallbo Å.B., Hagbarth K.-E. Activity from skin mechanoreceptors recorded percutaneously in awake human subjects. Exp. Neurol. 1968;21:270–289. doi: 10.1016/0014-4886(68)90041-1. [DOI] [PubMed] [Google Scholar]
- Vocat R., Staub F., Stroppini T., Vuilleumier P. Anosognosia for hemiplegia: a clinical-anatomical prospective study. Brain. 2010;133 doi: 10.1093/brain/awq297. (3578e3597) [DOI] [PubMed] [Google Scholar]
- Voos A.C., Pelphrey K.A., Kaiser M.D. Autistic traits are associated with diminished neural response to affective touch. Soc. Cogn. Affect. Neurosci. 2013;8:378–386. doi: 10.1093/scan/nss009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vortherms R.C. Clinically improving communication through touch. J. Gerontol. Nurs. 1991;17(5):6–10. doi: 10.3928/0098-9134-19910501-04. [DOI] [PubMed] [Google Scholar]
- Warringer A.B., Kuperman V., Brysbaert M. Norms of valence, arousal, and dominance for 13,915 English lemmas. Behav. Res. Methods. 2013;45:1191–1207. doi: 10.3758/s13428-012-0314-x. [DOI] [PubMed] [Google Scholar]
- Weiss S.J. The language of touch. Nurs. Res. 1979;28(2):76–79. [PubMed] [Google Scholar]
- Weiss S.J. Psychophysiologic effects of caregiver touch on incidence of cardiac dysrhythmia. Heart Lung. 1986;15:495–504. [PubMed] [Google Scholar]
- Wilson, B.A., Cockburn, J., Halligan, P.W., 1987. Behavioural inattention test (BIT). Bury St Edmunds: Thames Valley Test Company.
- Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale (HADS) Acta Psychiatr. Scand. 1983;67 doi: 10.1111/j.1600-0447.1983.tb09716.x. (361e370) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material