Abstract
Background:
A number of studies have investigated the prognostic impact of the neutrophil-to-lymphocyte ratio (NLR) in patients with melanoma but the results were controversial. Therefore, we conducted a meta-analysis to explore the prognostic value of NLR in melanoma.
Methods:
The databases of PubMed, Embase, and Web of Science were thoroughly searched. Associations between NLR and overall survival (OS) and progression-free survival (PFS) were investigated by pooling hazard ratio (HR) and 95% confidence interval (CI).
Results:
A total of 12 studies comprising 3207 patients were finally included in the meta-analysis. The results showed that a high NLR was associated with poor OS (HR = 2.23, 95% CI = 1.64–3.04, P < .001, random-effects model) and PFS (HR = 2.19, 95% CI = 1.78–2.69, P < .001, fixed-effects model). Subgroup analyses demonstrated that NLR was still associated with poor OS and PFS for patients in Western countries who were treated with ipilimumab. No significant publication bias was found in this meta-analysis.
Conclusion:
This meta-analysis demonstrated that a high NLR was predictive of poor OS and PFS in patients with melanoma.
Keywords: melanoma, meta-analysis, neutrophil-to-lymphocyte ratio, prognosis, survival
1. Introduction
Melanoma is the most aggressive form of skin cancer and the incidence of melanoma is still increasing.[1,2] It is estimated that there will be 87,110 new cases and 9730 deaths from melanoma in 2017 in the United States.[3] Several factors have been revealed to be predictive of poor survival in patients with melanoma, such as age, sex, Breslow tumor thickness, ulceration, and mitotic rate.[4–6] Most patients presenting at a localized stage are potentially curable. However, patients with advanced melanoma have a poor prognosis, with a 5-year survival rate of 10%.[7] Therefore, novel and efficient prognostic markers are of great importance to clinicians.
Inflammatory responses are considered to play important roles in tumor initiation and development.[8,9] In recent years, hematologic parameters of the systemic inflammatory response have been shown to be of prognostic value in various cancers.[10,11] These hematologic indices include C-reactive protein,[11,12] the platelet-to-lymphocyte ratio,[13,14] and the neutrophil-to-lymphocyte ratio (NLR).[15–17] The NLR, which is presented as the number of circulating neutrophils divided by lymphocyte counts, has gained much attention.[10] This is because the NLR is derived from routine blood tests and is cost free. A variety of studies also investigated the prognostic value of the NLR in melanoma but the results were inconsistent.[18–22] The conflicting data among studies may be due to different patient populations, different regions, and various treatment methods. We thus collected the available data and conducted a rigorous quantitative meta-analysis to shed light on the prognostic role of the NLR in melanoma.
2. Materials and methods
2.1. Literature search
This study was designed and performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.[23] The electronic databases of PubMed, Embase, and Web of Science were searched updated to May 2018. The following search terms were used: “neutrophil-to-lymphocyte ratio” or “neutrophil-lymphocyte ratio” or “NLR” “melanoma∗” (MeSH) or “malignant melanoma∗” (MeSH). Other resources were also manually checked for potentially eligible studies. An ethical approval was not necessary since meta-analysis was based on secondary data.
2.2. Inclusion and exclusion criteria
Inclusion criteria for eligible studies were as follows: patients with pathologically confirmed melanoma; the data of overall survival (OS) or progression-free survival (PFS) was reported in the text or sufficient data were provided to calculate the HR and 95% confidence interval (CI) using Tierney method[24]; a definite cut-off value of the NLR was provided; the patients were in any stages; and articles published as full-text in English. The exclusion criteria were as follows: meeting abstracts, reviews, case reports, or letters; duplicate studies; studies lacking necessary information; and animal studies. Two investigators (YD and SZ) independently evaluated all the candidate articles. Disagreements were resolved by discussion. OS was calculated from date of treatment initiation to the date of death from any cause of disease. Patients who were still alive were censored at the last follow-up. PFS was calculated from the date of treatment initiation until progression, as documented by imaging, according to response evaluation criteria in solid tumors or clinical examination or death.
2.3. Data extraction and quality assessment
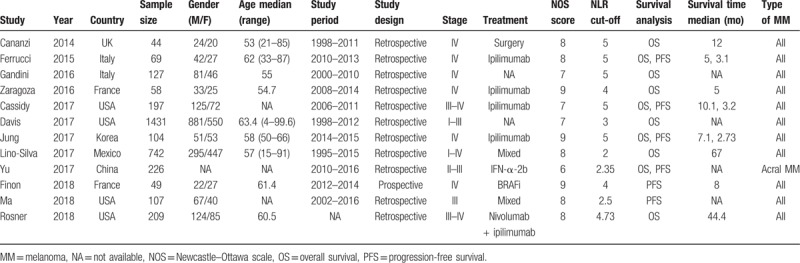
The following information was extracted from each eligible study: name of the first author, year of publication, study country, sample size, study period, sex, mean/median age, study design, tumor stage, treatment methods, cut-off value of the NLR, HR, and 95% CI for OS and/or PFS. Quality assessment for the included studies was performed according to the Newcastle–Ottawa scale (NOS).[25] The full score is 9 points and studies with ≥6 points were considered high-quality studies.
2.4. Statistical analysis
The pooled HR with its 95% CI was utilized to quantitatively assess the prognostic significance of the NLR for melanoma patients. Cochrane Q and I2 tests were used to evaluate the heterogeneity among studies. P < .10 for the Q test or I2 > 50% indicates significant heterogeneity and the random-effects model (DerSimonian–Laird method) is utilized in that situation. Otherwise, the fixed-effects model (Mantel–Haenszel method) is chosen. Subgroup analyses were conducted to examine the prognostic value of the NLR in different populations. Sensitivity analyses were performed to confirm the stability of the results. Begg funnel plot test[26] was used to evaluate the publication bias. Stata 12.0 software (Stata Corp, College Station, TX) was used for all statistical analyses. P < .05 was considered statistically significant.
3. Results
3.1. Study selection and characteristics
The initial search retrieved 260 studies. As shown in Figure 1, after duplicate records were removed, 185 records were left. After screening the titles or abstracts, 169 studies were discarded because they were animal studies, reviews, meeting abstracts, or irrelevant studies. Next, 16 full-text articles were further evaluated. Seven studies were excluded because they lacked necessary data or did not focus on melanoma. We then updated the search process and 3 eligible studies were added on May 2018. Finally, 12 studies[18–22,27–33] published between 2014 and 2018 were included in this meta-analysis. The characteristics of the included studies are shown in Table 1. The sample sizes ranged from 44 to 1431 and the total sample size was 3207. Four studies[21,28,32,33] were conducted in the United States, 2 studies[19,20] were performed in Italy, and 1 study was carried out in each of the United Kingdom,[18] France,[27] Korea,[29] Mexico,[30] France,[31] and China.[22] Five studies[18–20,28,29] selected 5 as the cut-off value of the NLR and other studies used 4,[27,31] 3,[21] 2.5,[32] 4.73,[33] 2,[30] and 2.35.[22] The NOS scores of the studies ranged from 6 to 9, with a median value of 8.
Figure 1.
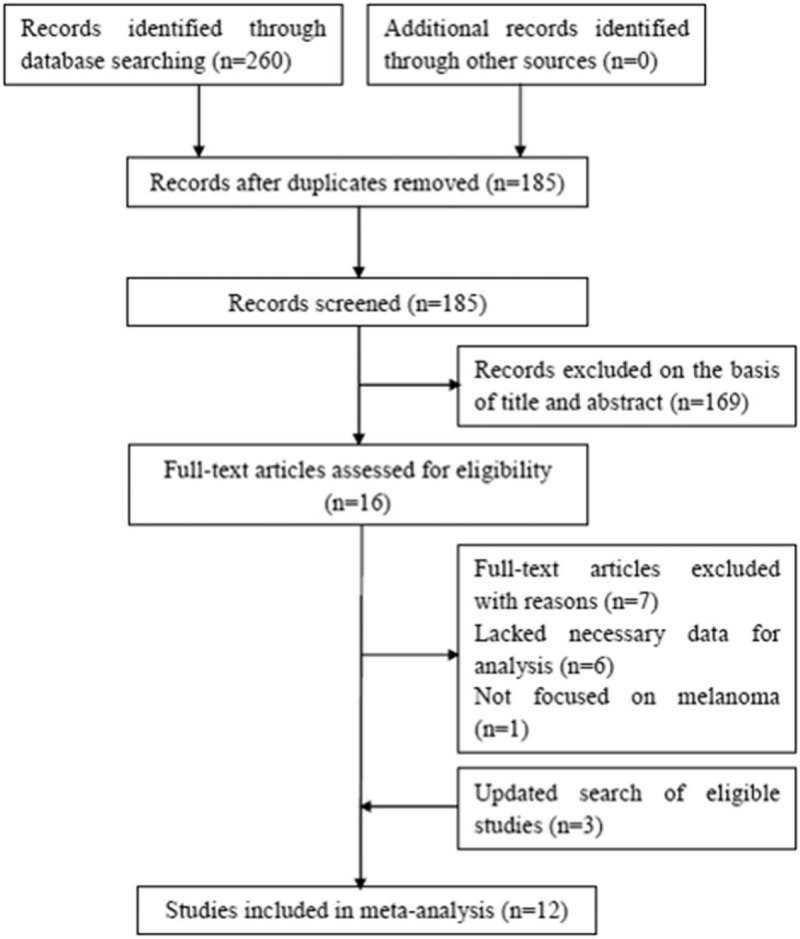
Flowchart of literature search.
Table 1.
Characteristics of the included studies for meta-analysis.
3.2. Prognostic value of NLR for OS
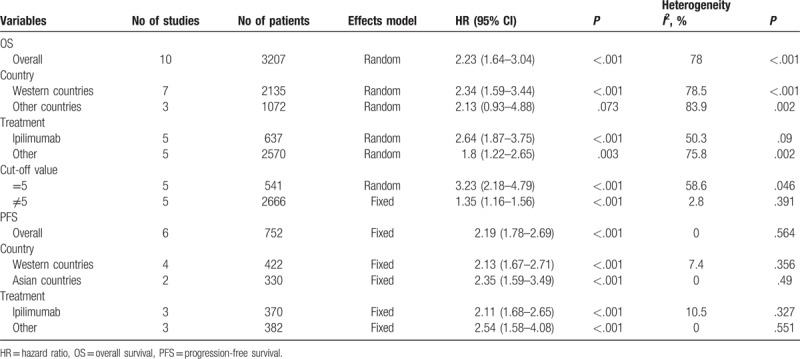
Ten studies[18–22,27–30,33] presented the hazard ratio (HR) and 95% CI for OS. As shown in Figure 2, the pooled results were HR = 2.23, 95% CI = 1.64 to 3.04, P < .001 (random-effects model), because the incidence of melanoma is quite different in Caucasian countries and non-Caucasian countries. Among non-Caucasian populations, incidence rates are quite variable and relatively low. Moreover, in this meta-analysis, most eligible studies were from Caucasian countries; therefore, we conducted subgroup analysis between Western countries and other countries.[34] The subgroup analyses showed that NLR was still associated with poor OS for patients in Western countries (HR = 2.34, 95% CI = 1.59–3.44, P < .001, random-effects model; Fig. 2, Table 2). Furthermore, the prognostic role of NLR for OS remained consistent irrespective of the treatment method (ipilimumab vs other methods) or cut-off value (NLR = 5 vs ≠5) (Table 2).
Figure 2.
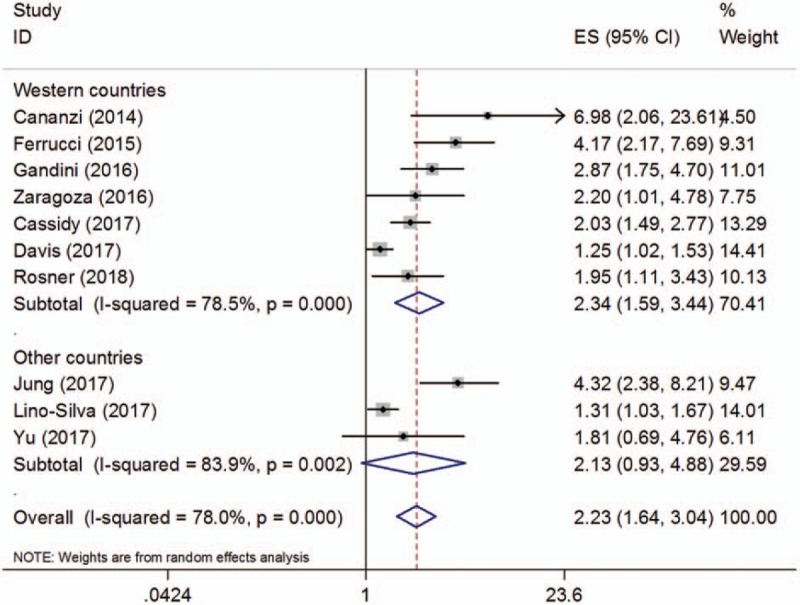
Pooled hazard ratio value for overall survival, subgroup analysis of study location.
Table 2.
Main results of the meta-analysis.
3.3. Prognostic value of NLR for PFS
Six studies[19,22,28,29,31,32] with a total of 752 patients investigated the correlation between the NLR and PFS. The combined results are shown in Figure 3 and Table 2. The pooled HR and 95% CI were HR = 2.19, 95% CI = 1.78 to 2.69, P < .001 (fixed-effects model). The subgroup analyses demonstrated that an elevated NLR indicated a poor PFS in both Western countries (HR = 2.13, 95% CI = 1.67–2.71, P < .001) and Asian countries (HR = 2.35, 95% CI = 1.59–3.49, P < .001). In addition, a high NLR was associated with shorted PFS for patients treated with ipilimumab (HR = 2.11, 95% CI = 1.68–2.65, P < .001) and other treatment methods (HR = 2.54, 95% CI = 1.58–4.08, P < .001). Non-significant heterogeneity was detected for all analyses of the NLR and PFS.
Figure 3.
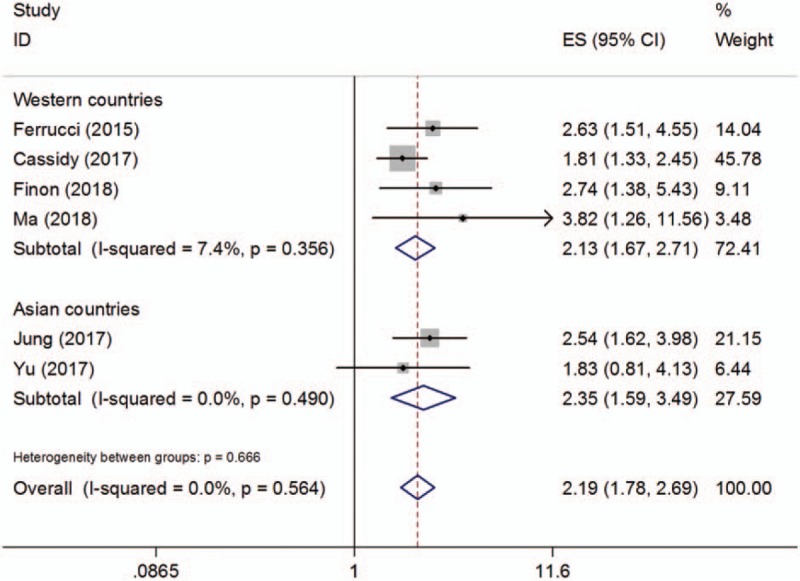
Pooled hazard ratio value for progression-free survival, subgroup analysis of study location.
3.4. Sensitivity analysis
Sensitivity analysis was conducted by excluding each included study by turn and then calculating the pooled results. As shown in Figure 4, the pooled results of both OS and PFS did not significantly change in sensitivity analysis, indicating the robustness of the results of this meta-analysis.
Figure 4.
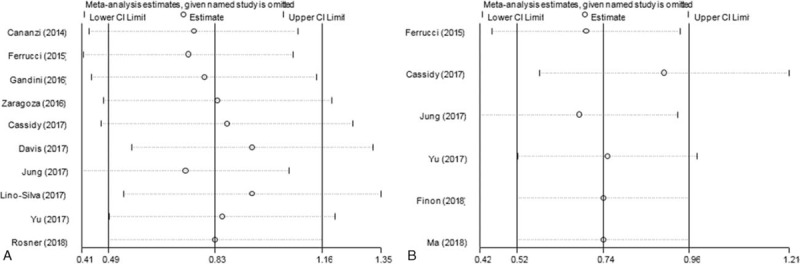
Sensitivity analyses for (A) overall survival and (B) progression-free survival.
3.5. Publication bias
Publication bias was examined using Begg funnel plot. The P-values for Begg test were P = .21 for OS and P = .707 for PFS (Fig. 5). The results suggested that there was no statistically significant publication bias in this study.
Figure 5.
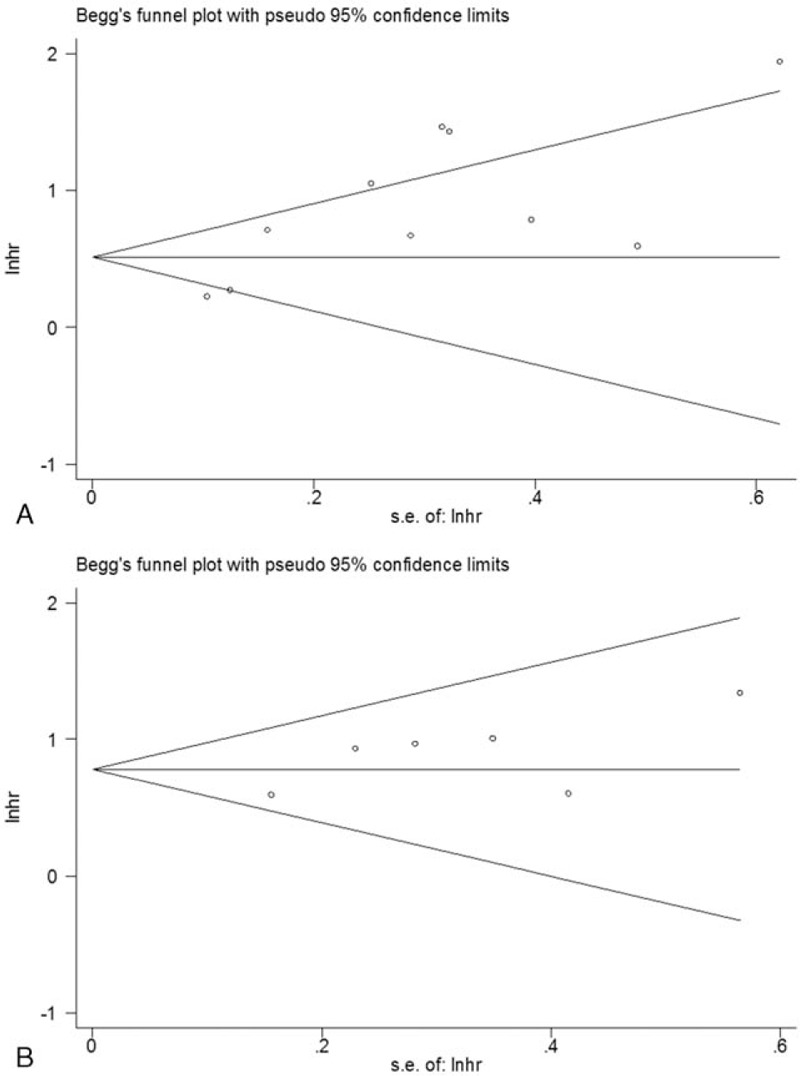
Begg funnel plots for (A) overall survival and (B) progression-free survival.
4. Discussion
In this meta-analysis, we found that an elevated NLR was predictive of poor OS and PFS in patients with melanoma. Moreover, a high NLR was associated with poor OS and PFS in patients from Western countries and for patients treated with ipilimumab. NLR = 5 is the most commonly used cut-off value for melanoma. The results of sensitivity analysis and the publication bias test confirmed the reliability of this meta-analysis. This study demonstrated that the NLR could be an efficient prognostic marker for melanoma.
Accumulating evidence has demonstrated that inflammation was involved in the process of tumor progression.[8,9] Recent studies have revealed that the NLR could reflect the balance between tumor-promoting inflammation and anti-tumor activity.[10] Neutrophils and lymphocytes are important cell types that reflect systemic immune responses. Neutrophils play important roles in tumor progression.[35–37] They are considered the primary source of vascular endothelial growth factor, which promotes tumor angiogenesis.[38] Furthermore, tumor-associated neutrophils can contribute to tumor metastasis by enhancing the seeding of tumor cells.[37] In contrast, lymphocytes are immune cells and exhibit antitumor activity.[39] Lymphocytes could induce cytotoxic cell death and suppress tumor cell proliferation and progression.[40] Previous studies have shown that lymphocytes are barriers to tumor migration.[15] Therefore, the NLR, which combines the neutrophil count and lymphocyte count, is biologically reasonable and is predictive of poor survival outcomes for a variety of cancers.[41–45]
The present meta-analysis showed that a high NLR was an unfavorable factor for both OS and PFS in melanoma. A number of previous meta-analyses also explored the prognostic value of NLR in various solid tumors.[41,43,44,46,47] For example, Gu et al indicated that an elevated pretreatment NLR might be a predictive factor for a poor prognosis in non-small-cell lung cancer patients.[48] Wei et al demonstrated that breast cancer patients with a higher NLR had poorer prognoses.[46] Wang et al also showed that a high NLR has a strong association with worse OS and PFS in patients with diffuse large B-cell lymphoma.[45] A comprehensive meta-analysis of 100 studies showed that a high NLR is associated with an adverse OS in many solid tumors.[10] However, in that study, melanoma was not included and the prognostic value of the NLR in melanoma was not investigated in this meta-analysis. To the best of our knowledge, this study is the first meta-analysis investigating the pooled results of the prognostic role of the NLR for patients with melanoma. We noticed a recent study[49] similar to our work. We carefully read the paper and found that our study was different with Sacdalan's study in the following aspects. First, Sacdalan's study is a review and meta-analysis; however, our manuscript is a meta-analysis in accordance with PRISMA guideline. Second, Sacdalan's study only included patients receiving immune checkpoint inhibitors, while our study did not limit the treatment methods. Third, our manuscript only included full-text studies, while Sacdalan's study included several meeting abstracts. Taken together, compared with Sacdalan's study, our study is conducted with strict guideline with recruitment of more comprehensive patient population. In addition, of all eligible studies, only Yu's work recruited acral melanoma patients, while the other studies recruited all types of melanoma. Therefore, the results of this study are applicable to all types of melanoma. We also noticed that the patients were on early and advanced stages. Because most of the eligible studies included patients in advanced stages, the current meta-analysis may be more suggestive to advanced patients.
Several limitations still need to be noted in our study. First, significant heterogeneity was detected in the analysis between the NLR and OS. Although we adopted a random-effects model for analysis, heterogeneity is a universal problem in meta-analysis. Second, most eligible studies are conducted in Western countries. Therefore, the results may be more applicable for Caucasian patients and more studies on other ethnic backgrounds are still required. Third, the cut-off values of the NLR were inconsistent in the included studies, which might cause selection bias. Fourth, only 12 studies were included. The sample is small and the subgroup analysis of PFS sometimes only contained 3 studies. Further studies on the NLR and melanoma are still required.
In summary, this meta-analysis demonstrated that a high NLR was predictive of poor OS and PFS in patients with melanoma. For patients in Western countries and those who are treated with ipilimumab, the NLR has consistent prognostic significance. Because of the above-mentioned limitations, future studies should recruit patients with diverse ethnicities and use a uniform cut-off value to validate the results of our meta-analysis.
Author contributions
Conceptualization: Yingguo Ding.
Data curation: Yingguo Ding.
Formal analysis: Yingguo Ding, Shan Zhang, Jianjun Qiao.
Funding acquisition: Yingguo Ding, Shan Zhang.
Investigation: Yingguo Ding, Shan Zhang.
Methodology: Yingguo Ding, Jianjun Qiao.
Project administration: Yingguo Ding.
Resources: Yingguo Ding, Shan Zhang, Jianjun Qiao.
Software: Yingguo Ding, Jianjun Qiao.
Supervision: Yingguo Ding, Jianjun Qiao.
Validation: Yingguo Ding.
Visualization: Yingguo Ding.
Writing – original draft: Yingguo Ding, Shan Zhang.
Writing – review & editing: Yingguo Ding, Jianjun Qiao.
Footnotes
Abbreviations: CI = confidence interval, HR = hazard ratio, NLR = neutrophil-to-lymphocyte ratio, NOS = Newcastle–Ottawa scale, OS= overall survival, PFS = progression-free survival, PLR = platelet-to-lymphocyte ratio, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Davey RJ, van der Westhuizen A, Bowden NA. Metastatic melanoma treatment: combining old and new therapies. Crit Rev Oncol Hematol 2016;98:242–53. [DOI] [PubMed] [Google Scholar]
- [2].Eggermont AMM, Spatz A, Robert C. Cutaneous melanoma. Lancet 2014;383:816–27. [DOI] [PubMed] [Google Scholar]
- [3].Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [4].Balch CM, Gershenwald JE, Soong SJ, et al. Multivariate analysis of prognostic factors among 2,313 patients with stage III melanoma: comparison of nodal micrometastases versus macrometastases. J Clin Oncol 2010;28:2452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Thompson JF, Soong SJ, Balch CM, et al. Prognostic significance of mitotic rate in localized primary cutaneous melanoma: an analysis of patients in the multi-institutional American Joint Committee on Cancer melanoma staging database. J Clin Oncol 2011;29:2199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Balch CM, Soong SJ, Gershenwald JE, et al. Age as a prognostic factor in patients with localized melanoma and regional metastases. Ann Surg Oncol 2013;20:3961–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Garbe C, Eigentler TK, Keilholz U, et al. Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist 2011;16:5–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436–44. [DOI] [PubMed] [Google Scholar]
- [10].Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. [DOI] [PubMed] [Google Scholar]
- [11].Shrotriya S, Walsh D, Bennani-Baiti N, et al. C-reactive protein is an important biomarker for prognosis tumor recurrence and treatment response in adult solid tumors: a systematic review. PLoS One 2015;10:e0143080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Leuzzi G, Galeone C, Gisabella M, et al. Baseline C-reactive protein level predicts survival of early-stage lung cancer: evidence from a systematic review and meta-analysis. Tumori 2016;102:441–9. [DOI] [PubMed] [Google Scholar]
- [13].Gu XB, Gao XS, Cui M, et al. Clinicopathological and prognostic significance of platelet to lymphocyte ratio in patients with gastric cancer. Oncotarget 2016;7:49878–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gu XB, Sun SQ, Gao XS, et al. Prognostic value of platelet to lymphocyte ratio in non-small cell lung cancer: evidence from 3,430 patients. Sci Rep 2016;6:23893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Marchioni M, Cindolo L, Autorino R, et al. High neutrophil-to-lymphocyte ratio as prognostic factor in patients affected by upper tract urothelial cancer: a systematic review and meta-analysis. Clin Genitourin Cancer 2017;15: 343-349.e1. [DOI] [PubMed] [Google Scholar]
- [16].Yin J, Qin Y, Luo YK, et al. Prognostic value of neutrophil-to-lymphocyte ratio for nasopharyngeal carcinoma: a meta-analysis. Medicine 2017;96:e7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Huang Y, Sun Y, Peng P, et al. Prognostic and clinicopathologic significance of neutrophil-to-lymphocyte ratio in esophageal squamous cell carcinoma: evidence from a meta-analysis. Onco Targets Ther 2017;10:1165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cananzi FC, Dalgleish A, Mudan S. Surgical management of intraabdominal metastases from melanoma: role of the neutrophil to lymphocyte ratio as a potential prognostic factor. World J Surg 2014;38:1542–50. [DOI] [PubMed] [Google Scholar]
- [19].Ferrucci PF, Gandini S, Battaglia A, et al. Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. Br J Cancer 2015;112:1904–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gandini S, Ferrucci PF, Botteri E, et al. Prognostic significance of hematological profiles in melanoma patients. Int J Cancer 2016;139:1618–25. [DOI] [PubMed] [Google Scholar]
- [21].Davis JL, Langan RC, Panageas KS, et al. Elevated blood neutrophil-to-lymphocyte ratio: a readily available biomarker associated with death due to disease in high risk nonmetastatic melanoma. Ann Surg Oncol 2017;24:1989–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yu J, Wu X, Yu H, et al. Systemic immune-inflammation index and circulating T-cell immune index predict outcomes in high-risk acral melanoma patients treated with high-dose interferon. Transl Oncol 2017;10:719–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- [24].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [26].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [27].Zaragoza J, Caille A, Beneton N, et al. High neutrophil to lymphocyte ratio measured before starting ipilimumab treatment is associated with reduced overall survival in patients with melanoma. Br J Dermatol 2016;174:146–51. [DOI] [PubMed] [Google Scholar]
- [28].Cassidy MR, Wolchok RE, Zheng J, et al. Neutrophil to lymphocyte ratio is associated with outcome during ipilimumab treatment. EBioMedicine 2017;18:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jung M, Lee J, Kim TM, et al. Ipilimumab real-world efficacy and safety in Korean melanoma patients from the Korean named-patient program cohort. Cancer Res Treat 2017;49:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lino-Silva LS, Salcedo-Hernandez RA, Garcia-Perez L, et al. Basal neutrophil-to-lymphocyte ratio is associated with overall survival in melanoma. Melanoma Res 2017;27:140–4. [DOI] [PubMed] [Google Scholar]
- [31].Finon A, Zaragoza J, Maillard H, et al. A high neutrophil to lymphocyte ratio prior to BRAF inhibitor treatment is a predictor of poor progression-free survival in patients with metastatic melanoma. Eur J Dermatol 2018;28:38–43. [DOI] [PubMed] [Google Scholar]
- [32].Ma J, Kuzman J, Ray A, et al. Neutrophil-to-lymphocyte ratio (NLR) as a predictor for recurrence in patients with stage III melanoma. Sci Rep 2018;8:4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rosner S, Kwong E, Shoushtari AN, et al. Peripheral blood clinical laboratory variables associated with outcomes following combination nivolumab and ipilimumab immunotherapy in melanoma. Cancer Med 2018;7:690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Berwick M. Bosserhoff A. Melanoma epidemiology. Melanoma Development: Molecular Biology, Genetics and Clinical Application. Vienna: Springer; 2011. 35–55. [Google Scholar]
- [35].Liang W, Ferrara N. The complex role of neutrophils in tumor angiogenesis and metastasis. Cancer Immunol Res 2016;4:83–91. [DOI] [PubMed] [Google Scholar]
- [36].Powell DR, Huttenlocher A. Neutrophils in the tumor microenvironment. Trends Immunol 2016;37:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Treffers LW, Hiemstra IH, Kuijpers TW, et al. Neutrophils in cancer. Immunol Rev 2016;273:312–28. [DOI] [PubMed] [Google Scholar]
- [38].Jablonska J, Leschner S, Westphal K, et al. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest 2010;120:1151–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ropponen KM, Eskelinen MJ, Lipponen PK, et al. Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol 1997;182:318–24. [DOI] [PubMed] [Google Scholar]
- [40].Yang Z, Gu JH, Guo CS, et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival of epithelial ovarian cancer: a systematic review and meta-analysis of observational studies. Oncotarget 2017;8:46414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bowen RC, Little NAB, Harmer JR, et al. Neutrophil-to-lymphocyte ratio as prognostic indicator in gastrointestinal cancers: a systematic review and meta-analysis. Oncotarget 2017;8:32171–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Takenaka Y, Kitamura T, Oya R, et al. Prognostic role of neutrophil-lymphocyte ratio in nasopharyngeal carcinoma: a meta-analysis. PLoS One 2017;12:e0181478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tang H, Lu W, Li B, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in biliary tract cancers: a systematic review and meta-analysis. Oncotarget 2017;8:36857–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tang X, Du P, Yang Y. The clinical use of neutrophil-to-lymphocyte ratio in bladder cancer patients: a systematic review and meta-analysis. Int J Clin Oncol 2017;22:817–25. [DOI] [PubMed] [Google Scholar]
- [45].Wang J, Zhou X, Liu Y, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in diffuse large B-cell lymphoma: a meta-analysis. PLoS One 2017;12:e0176008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wei B, Yao M, Xing C, et al. The neutrophil lymphocyte ratio is associated with breast cancer prognosis: an updated systematic review and meta-analysis. Onco Targets Ther 2016;9:5567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Li X, Ma X, Tang L, et al. Prognostic value of neutrophil-to-lymphocyte ratio in urothelial carcinoma of the upper urinary tract and bladder: a systematic review and meta-analysis. Oncotarget 2016;8:62681–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gu XB, Tian T, Tian XJ, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in non-small cell lung cancer: a meta-analysis. Sci Rep 2015;5:12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sacdalan DB, Lucero JA, Sacdalan DL. Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: a review and meta-analysis. Onco Targets Ther 2018;11:955–65. [DOI] [PMC free article] [PubMed] [Google Scholar]


