Abstract
The aim of this study is to evaluate if low prealbumin levels on admission predict subsequent adverse cardiac events in patients hospitalized with acute coronary syndrome (ACS).
We designed a cohort study and enrolled 610 consecutive patients with ACS from whom venous blood for serum prealbumin measurement was drawn immediately upon hospital admission. Patients were classified in two groups according to prealbumin level: “normal” prealbumin levels (≥17 mg/dL, n=413) and “low” prealbumin (<17 mg/dL, n = 197). In-hospital adverse cardiac events were death, acute heart failure, reinfarction, and cardiogenic shock. Univariate and multivariable analyses were applied to evaluate the prediction value of low prealbumin.
The incidence of in hospital adverse cardiac events is 10.8%. The proportion of adverse cardiac events was significantly higher in low prealbumin group as compared with normal prealbumin group (20.8% versus 6.1%, P < .001). Univariate analysis indicates that low prealbumin levels can predict in hospital adverse cardiac events (odds ratio [OR]: 0.834, 95% confidence interval [CI]: 0.785–0.886, P < .001). Multivariable analysis shows that low prealbumin level was an independent predictor for in hospital adverse cardiac events (adjusted OR: 0.918, 95% CI: 0.848–0.993, P = .033). Other independent predictors were lower in average hemoglobin level and Killip class II-IV on admission.
Therefore, lower serum prealbumin levels on admission can independently predicts subsequent in hospital major adverse cardiac events in patients with ACS.
Keywords: acute coronary syndrome, adverse cardiac events, prealbumin
1. Introduction
Acute coronary syndrome (ACS) is one of the leading causes of cardiovascular disease-related death; therefore, identifying risk factors of ACS is urgent for prevention. Studies have shown a correlation between prealbumin levels and patient recovery and an increase in prealbumin levels is an early predictor of patient recovery. In high-risk patients, assessing prealbumin levels twice a week during hospitalization can remind the physician to prevent declining nutritional status, which shortens hospitalization and improves patient outcomes.[1] Prealbumin is mainly biosynthesized in the liver. Its half life is about 2 days while 20 days for albumin. It has been reported that blood albumin can be affected by multifactors, such as nourishment state and renal function.[2]
More and more studies have revealed that serum albumin levels were associated with coronary heart disease and mortality. Moreover, an increasing number of investigations have shown that prealbumin is closely associated with a variety of conditions, such as recurrence of malignant carcinoma,[3] postoperative complications,[4] hemorrhagic stroke,[5] ischemic stroke,[6] systemic sclerosis outpatients,[7] atherosclerotic vascular disease,[8] prognosis of heart failure,[9–11] and acute kidney injury.[12] In addition, one study showed that in coronary artery disease patients undergoing chronic hemodialysis have lower serum prealbumin levels.[13] Also, one other study reported that prealbumin was negatively correlated with angiographic severity in patients with ACS.[14] However, the association between prealbumin and adverse cardiac events of ACS has not been investigated in previous research.
Therefore, the aim of this research was to determine the association between low serum prealbumin level measured immediately on admission and in-hospital adverse cardiac events in patients with ACS. Furthermore, we combined this association with other variables to assess the value of prealbumin in predicting adverse outcomes.
2. Patients and methods
2.1. Patient population
The present study included patients with ACS who were admitted to the Department of Cardiology, Liaocheng People's Hospital and Clinical School of Taishan Medical University. We recruited the subjects consecutively between April 2017 and October 2017. The inclusion criteria of recruitment were patients diagnosed with ACS, which was based on clinical symptoms and some accessory examinations, and the diagnosis of ACS is based on criteria of ACS in ACCF/AHA guidelines,[15,16]patients with the onset of angina ≤24 hours, and patients agreed to participate by signing an informed consent. The exclusion criteria were patients previously diagnosed with chronic heart failure and NYHA class >II, patients had hepatic cirrhosis or malignancy, patients who had been already on regular dialysis or whose estimated glomerular filtration rate (eGFR) was <15 mL/kg/m2, patients with known valvular heart disease, acute comorbidity such as acute stroke, acute infection and sepsis, and patients with known chronic inflammatory disease and venous thromboembolism. All procedures were approved by the Ethics Committee of Liaocheng People's Hospital and Clinical School of Taishan Medical University in accordance with principles of the Helsinki Declaration.
2.2. Data collection
The blood collection was drawn immediately upon hospital admission, before any reperfusion procedure or heparin therapy. Characteristic data were collected during hospitalization. Clinical presentation including heart rate and Killip class determination was assessed on admission by the attending physician. Medical history related to cardiovascular risk factors, that is, hypertension, diabetes mellitus, previous ischemic heart disease, and smoking status were noted. Biochemical profiles including prealbumin, albumin, total cholesterol, liver, and kidney function were recorded. The “normal” prealbumin values are variable depending on the different studies: >17 mg/dL, >15 mg/dL, and >13 mg/dL.[1,17,18] For the present study, we have used 17 mg/dL as cut-off point, as this value is the most used in literature. According to this, patients are divided into two groups: those with serum prealbumin <17 mg/dL were categorized as “low” serum prealbumin group and the others with serum prealbumin ≥17 mg/dL were categorized as “normal” serum prealbumin group.
2.3. Research outcome
From admission, the treatment strategy for the patients was made by attending cardiologists. The study outcome was in-hospital adverse cardiac events. The adverse outcomes were recorded and determined as in-hospital death, acute heart failure, reinfarction and cardiogenic shock. In-hospital death was determined as all cause of death during hospitalization. Acute heart failure was based on the presence of symptom of breathlessness and clinical signs of pulmonary congestion in physical examination, or intravenous loop diuretics to treat pulmonary congestion. Reinfarction was defined as newly or on-going developed pain, a repeated elevation of troponin I or recurrent ST-segment elevation following the event. Cardiogenic shock was diagnosed based on systolic blood pressure <90 mm Hg and low perfusion signs with subsequent use of vasopressors (dopamine or/and norepinephrine). The adverse outcomes were confirmed by attending cardiologists.
2.4. Statistical analysis
Statistical analysis was performed using SPSS 17.0 version (SPSS Inc, Chicago, IL). Continuous data was tested for normal distribution with Kolmogorov–Smirnov test, if applicable, with logarithmic transformation. The study population was divided based on the serum levels of serum prealbumin. Student t-test was applied to compare the normally distributed continuous data, while Mann–Whitney test was performed for not normally distributed continuous data. Comparison between categorical data was used with χ2 test and Fisher exact test. Determine whether prealbumin level was an independent predictor for adverse cardiac events use univariate and multivariable analysis (logistic regression). Statistical significance level was when P < .05.
3. Results
3.1. Patient characteristics
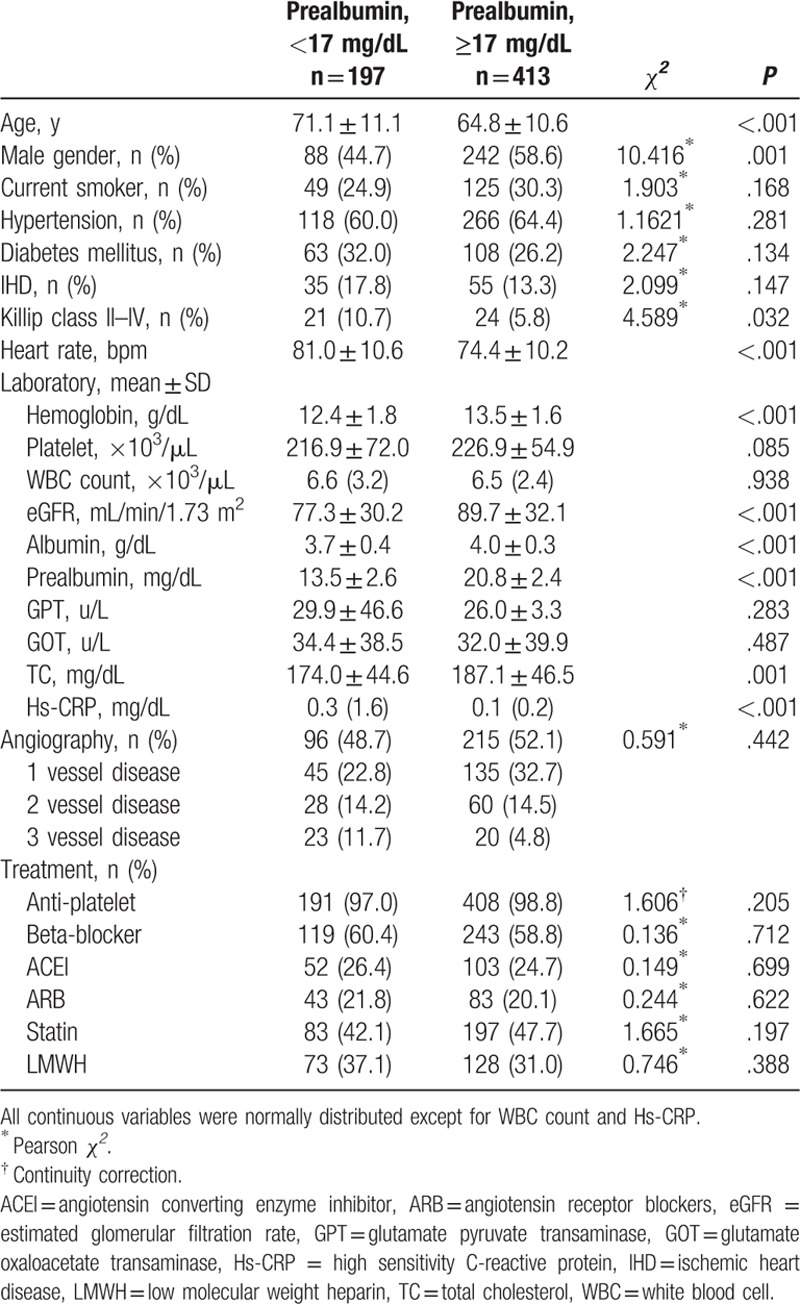
A total of 610 patients admitted due to ACS were included in this research, 54.1% of them were men and the mean age was 65.1 years old. The low prealbumin group had 197 patients, with a mean prealbumin concentration of 13.5 ± 2.6 mg/dL; the rest 413 patients were in normal prealbumin group, with a mean prealbumin concentration of 20.8 ± 2.4 mg/dL. In low prealbumin group, the patients were older, faster heart rate, lower in average hemoglobin level, albumin, eGFR, and total cholesterol, while higher in high sensitivity C-reactive protein (hs-CRP) and Killip class II to IV on admission. Coronary angiography was done in 311 subjects, the advanced coronary artery disease (3 vessel disease) proportion tended to be higher in low prealbumin group (as shown in Table 1).
Table 1.
Characteristics of patients according to serum prealbumin level.
3.2. Incidence of adverse outcomes
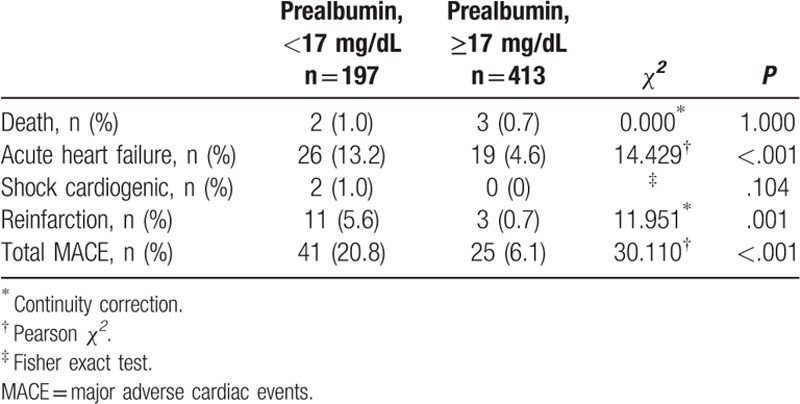
In the present study, the incidence of in hospital major adverse cardiac events is 10.8% (66 of 610 patients). The in-hospital death rate is 0.8% (5 of 610 patients). Among non-fatal outcomes, the incidence of acute heart failure is 7.4% (45 of 610 patients), cardiogenic shock is 0.3% (2 of 610 patients), and reinfarction 2.3% (14 of 610 patients). The proportion of major adverse cardiac events was significantly higher in the low prealbumin group as compared with normal prealbumin group (20.8% vs 6.1%, P < .001). Furthermore, the incidence of non-fatal outcomes was also significantly higher in the low prealbumin group (19.8% vs 5.3%, P < .001), whereas the incidence of death was not significantly different between 2 groups (1.0% vs 0.7%, P = 1.000) (as shown in Table 2).
Table 2.
In-hospital MACE according to serum prealbumin level.
3.3. Univariate and multivariable analyses
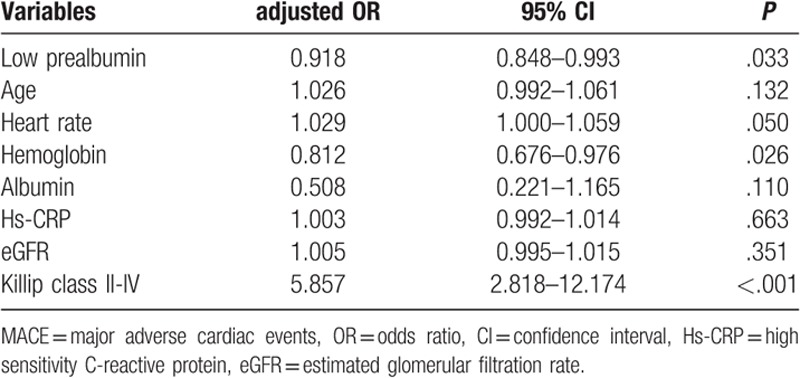
Univariate analysis showed that low prealbumin was associated with in-hospital major adverse cardiovascular events (Table 3). Other covariates that also predicted in hospital adverse cardiovascular events were older age, faster heart rate, lower hemoglobin, albumin and eGFR level, and higher hs-CRP level and Killip class II to IV on admission. We performed multivariable analysis with logistic regression test to determine whether low prealbumin level was an independent predictor for in hospital major adverse cardiovascular events adjusted by variables in univariate analysis. The result showed that prealbumin independently predicted in hospital major adverse cardiovascular events in subjects with ACS (adjusted odds ratio [OR]: 0.918, 95% confidence interval [CI]: 0.848–0.993, P = .033). Other independent predictors were lower hemoglobin level and Killip class II to IV on admission (Table 4).
Table 3.
Univariate analysis for predictors of in-hospital MACE.
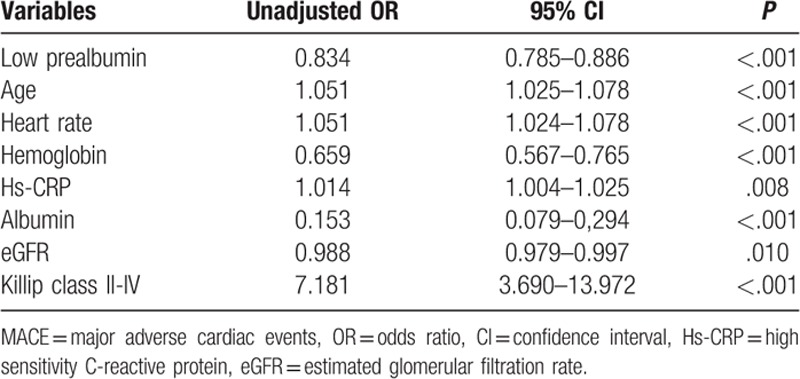
Table 4.
Multivariable analysis for predictors of in-hospital MACE.
4. Discussion
Many studies have revealed that a lower level of serum prealbumin was associated with severities and prognosis of coronary heart diseases,[9–11] higher overall mortality in critically ill patients,[17] and negatively associated with angiographic severity in patients with ACS.[14] In accordance with these studies, we have found that low level of serum prealbumin (<17 mg/dL) is associated with in-hospital adverse cardiac events. Also, other studies have suggested that lower serum prealbumin was an early stage and long-term mortality predictor for patients with acute or chronic phase of heart failure.[9–11] However, the correlations between lower prealbumin and adverse cardiac events in ACS is unclear. In this research, we have found that low levels of serum prealbumin can independent predict in-hospital adverse cardiac events in ACS patients.
Our study show that the risk to develop in-hospital major adverse cardiac events increases 3-fold in low prealbumin patients group as compared with patients with normal prealbumin level. Among patients with low prealbumin, the occurrence of nonfatal in-hospital adverse outcomes, especially acute heart failure, is significantly higher than in patients with normal prealbumin (P < .001). In spite of a low serum prealbumin level was associated with in-hospital adverse cardiac events, the majority of the subjects survived. The occurrence of death was not significantly different between the groups. These study results suggest that a low serum prealbumin level has a deleterious impact during the acute phase of ACS, especially in the early presentation.
Prealbumin is synthesized in the liver. The prealbumin concentration level was reduced because of decreasing synthesis, extravascular distribution, higher catabolic rate, and exogenous loss.[19] Systemic inflammation and inadequate nutritional intake both affect the synthesis of prealbumin.[1,20] An inverse relationship between prealbumin and hs-CRP has been documented,[21] which has also demonstrated by our finding that patients located in the lower quartile of prealbumin had the higher level of hs-CRP. As a negative acute-phase protein, the synthesis of prealbumin is suppressed in the inflammatory settings in which cytokines, mainly tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), and IL-6, resulting in elevated generation of hs-CRP and decreased synthesis of prealbumin by the liver.[1,22] This study found that the hemoglobin level is significantly lower in the low prealbumin group (P < .001). Anemia due to inflammation is common among hospitalized patients and may also be a sign of poor nutritional intake. Investigation showed that concentration of prealbumin is low in some instances of protein energy malnutrition.[19] Therefore, malnutrition and inflammatory are responsible for the low serum prealbumin in the ACS.
The underlying mechanism between low prealbumin and adverse cardiac events in patients with ACS is considered multifactorial. First, inflammation plays a pivotal role in the pathological process of arteriosclerosis,[23] may provide a bridge between low prealbumin and poorer prognosis in ACS patients. It is established that inflammatory state can reduce the rate of prealbumin synthesis,[24] thus decreasing serum prealbumin levels. Second, it has been shown that infected patients have low serum levels of prealbumin, high levels of TNF-α,[25,26] which induced vascular cell adhesion molecule-1 expression, monocyte adhesion, and nuclear factor-kappa B activation in human aortic endothelial cells,[27] suggesting that low levels of prealbumin may induce the atherosclerosis. Third, levels of prealbumin were inversely correlated with vascular endothelial growth factor (VEGF), which plays an important role in the maintenance of endothelial integrity and increases vascular permeability to serum proteins.[28,29] Increased VEGF expression could induced atherosclerosis.[30] Fourth, prealbumin is transported by high density lipoproteins (HDL), in association with Apolipoprotein AI, and it has been suggested that the absence of prealbumin affects the properties or stability of the HDL particles and reduce cardiovascular protection of HDL.[31] The above mechanisms may jointly lead to increase the risk of atherosclerosis and adverse outcome. However, the exact mechanism still needs further verification.
In patients with severe chronic kidney disease, the combined effects of inadequate protein and inflammation result in low serum prealbumin concentration, which was an independent risk factor for mortality in hemodialysis patients.[24] In this study, we exclude those with stage 5 of chronic kidney disease and those with dialysis dependence in order to eliminate the potential confounding effect. Our result demonstrated that albumin level was significantly lower in low serum prealbumin group and it was consistent with previous studies.[32,33] It was suggested that albumin, like prealbumin, represented as a risk marker indicating underlying inflammatory or nutritional status.
Our study has several limitations. First, nutritional status at admission may influence the levels of albumin and prealbumin, body mass index or total protein are need to be assessed at admission, more data is needed. Second, as we only include patients with ACS and our conclusions to other populations, such as patients with asymptomatic coronary atherosclerosis or stable angina pectoris, is unknown. Third, this study was designed as a hospital-based study, not community-based study and this design had potentially limitation. Fourth, the prognostic predictive ability of prealbumin was provided in our study just in hospitalization, further studies are currently underway to elucidate its long-term prognostic value.
In conclusion, the presence of a low serum prealbumin level (<17 mg/dL) immediately upon hospital admission in ACS independently predicts subsequent in hospital major adverse cardiac events. Measurement of serum prealbumin may aid in the risk stratification.
Author contributions
Data curation: Chun-Song Wang.
Formal analysis: Dong Ren, Tai Li.
Writing – original draft: Wei Wang.
Writing – review & editing: Heng-Chen Yao, Sheng-Jun Ma.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, ACS = acute coronary syndrome, eGFR = estimated glomerular filtration rate, HDL = high density lipoproteins, hs-CRP = high sensitivity C-reactive protein, IL-6 = interleukin-6, TNF-α = tumor necrosis factor alpha, VEGF = vascular endothelial growth factor.
This study was supported by the Natural Science Foundation of Shandong Province (ZR2016HM49 and ZR2016HB73).
The authors declare no conflicts of interest.
References
- [1].Beck FK, Rosenthal TC. Prealbumin: a marker for nutritional evaluation. Am Fam Physician 2002;65:1575–8. [PubMed] [Google Scholar]
- [2].Devoto G, Gallo F, Marchello C, et al. Prealbumin serum concentrations as a useful tool in the assessment of malnutrition in hospitalized patients. Clin Chem 2006;52:2281–5. [DOI] [PubMed] [Google Scholar]
- [3].Kawai H, Ota H. Low perioperative serum prealbumin predicts early recurrence after curative pulmonary resection for non-small-cell lung cancer. World J Surg 2012;36:2853–7. [DOI] [PubMed] [Google Scholar]
- [4].Yu PJ, Cassiere HA, Dellis SL, et al. Impact of preoperative prealbumin on outcomes after cardiac surgery. JPEN J Parenter Enteral Nutr 2015;39:870–4. [DOI] [PubMed] [Google Scholar]
- [5].Zhang SQ, Peng B, Stary CM, et al. Serum prealbumin as an effective prognostic indicator for determining clinical status and prognosis in patients with hemorrhagic stroke. Neural Regen Res 2017;12:1097–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ye S, Lin SP, Wu K, et al. Serum prealbumin is a predictive biomarker for stroke-associated infection after an ischemic stroke. Int J Neurosci 2017;127:601–5. [DOI] [PubMed] [Google Scholar]
- [7].Codullo V, Cereda E, Klersy C, et al. Serum prealbumin is an independent predictor of mortality in systemic sclerosis outpatients. Rheumatology (Oxford) 2016;55:315–9. [DOI] [PubMed] [Google Scholar]
- [8].Dalrymple LS, Johansen KL, Chertow GM, et al. Longitudinal measures of serum albumin and prealbumin concentrations in incident dialysis patients: the comprehensive dialysis study. J Ren Nutr 2013;23:91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lourenco P, Silva S, Friões F, et al. Low prealbumin is strongly associated with adverse outcome in heart failure. Heart 2014;100:1780–5. [DOI] [PubMed] [Google Scholar]
- [10].Suzuki N, Kida K, Suzuki K, et al. Assessment of transthyretin combined with mini nutritional assessment on admission provides useful prognostic information in patients with acute decompensated heart failure. Int Heart J 2015;56:226–33. [DOI] [PubMed] [Google Scholar]
- [11].Cabassi A, de Champlain J, Maggiore U, et al. Prealbumin improves death risk prediction of BNP-added Seattle Heart Failure Model: Results from a pilot study in elderly chronic heart failure patients. Int J Cardiol 2013;168:3334–9. [DOI] [PubMed] [Google Scholar]
- [12].Xie Q, Zhou Y, Xu Z, et al. The ratio of CRP to prealbumin levels predict mortality in patients with hospital-acquired acute kidney injury. BMC Nephrol 2011;12:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yeo Y, Byun SW, Lee JY, et al. Lack of association between small dense low-density lipoprotein levels and coronary artery disease in chronic hemodialysis patients. Am J Nephrol 2009;30:310–4. [DOI] [PubMed] [Google Scholar]
- [14].Zhang C, Liu P, Xia K, et al. Association of serum prealbumin with angiographic severity in patients with acute coronary syndrome. Med Sci Monit 2017;23:4041–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wright RS, Anderson JL, Adams CD, et al. 2011 ACCF/AHA focused update of the guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction (Updating the 2007 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2011;123:2022–260. [DOI] [PubMed] [Google Scholar]
- [16].O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127:529–55. [DOI] [PubMed] [Google Scholar]
- [17].Devakonda A, George L, Raoof S, et al. Transthyretin as a marker to predict outcome in critically ill patients. Clin Biochem 2008;41:1126–30. [DOI] [PubMed] [Google Scholar]
- [18].Bonilla-Palomas JL, Gamez-Lopez AL, Anguita-Sanchez MP, et al. Impact of malnutrition on long-term mortality in hospitalized patients with heart failure. Rev Esp Cardiol 2011;64:752–8. [DOI] [PubMed] [Google Scholar]
- [19].Myron Johnson A, Merlini G, Sheldon J, et al. Clinical indications for plasma protein assays: transthyretin (prealbumin) in inflammation and malnutrition. Clin Chem Lab Med 2007;45:419–26. [DOI] [PubMed] [Google Scholar]
- [20].Caccialanza R, Palladini G, Klersy C, et al. Serum prealbumin: an independent marker of short-term energy intake in the presence of multiple-organ disease involvement. Nutrition 2013;29:580–2. [DOI] [PubMed] [Google Scholar]
- [21].Pinilla JC, Hayes P, Laverty W, et al. The C-reactive protein to prealbumin ratio correlates with the severity of multiple organ dysfunction. Surgery 1998;124:799–805. discussion 805-796. [DOI] [PubMed] [Google Scholar]
- [22].Kalantar-Zadeh K, Anker SD, Horwich TB, et al. Nutritional and anti-inflammatory interventions in chronic heart failure. Am J Cardiol 2008;101:89E–103E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Karadeniz M, Duran M, Akyel A, et al. High sensitive CRP level is associated with intermediate and high syntax score in patients with acute coronary syndrome. Int Heart J 2015;56:377–80. [DOI] [PubMed] [Google Scholar]
- [24].Collins N. The difference between albumin and prealbumin. Adv Skin Wound Care 2001;14:235–6. [DOI] [PubMed] [Google Scholar]
- [25].Saarinen UM, Koskelo EK, Teppo AM, et al. Tumor necrosis factor in children with malignancies. Cancer Res 1990;50:592–5. [PubMed] [Google Scholar]
- [26].Fine RM. Evidence for the tumor necrosis factor/cachectin production in cancer. Int J Dermatol 1988;27:379–80. [DOI] [PubMed] [Google Scholar]
- [27].Zhang WJ, Frei B. Albumin selectively inhibits TNF alpha-induced expression of vascular cell adhesion molecule-1 in human aortic endothelial cells. Cardiovasc Res 2002;55:820–9. [DOI] [PubMed] [Google Scholar]
- [28].Eremina V, Sood M, Haigh J, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 2003;111:707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med 2000;6:389–95. [DOI] [PubMed] [Google Scholar]
- [30].Watanabe T, Shibata M, Nishiyama H, et al. Elevated serum levels of vascular endothelial growth factor is effective as a marker for malnutrition and inflammation in patients with ovarian cancer. Biomed Rep 2013;1:197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cubedo J, Padro T, Alonso R, et al. Differential proteomic distribution of TTR (pre-albumin) forms in serum and HDL of patients with high cardiovascular risk. Atherosclerosis 2012;222:263–9. [DOI] [PubMed] [Google Scholar]
- [32].Hartopo AB, Gharini PP, Setianto BY. Low serum albumin levels and in-hospital adverse outcomes in acute coronary syndrome. Int Heart J 2010;51:221–6. [DOI] [PubMed] [Google Scholar]
- [33].Horwich TB, Kalantar-Zadeh K, MacLellan RW, et al. Albumin levels predict survival in patients with systolic heart failure. Am Heart J 2008;155:883–9. [DOI] [PubMed] [Google Scholar]


