Abstract
Postoperative epidural hematoma (POEDH) is a known complication after neurosurgical procedures. Large POEDHs are life-threatening and require emergency evacuation, and open surgery is the mainstay of treatment. Most of POEDHs are hyperdense on computed tomography (CT). We herein report a subset of POEDHs requiring evacuation, which presented with isodense features on CT. The presenting symptoms of patients were severe headache accompanied by nausea and vomiting as well as unilateral limb weakness (n = 1) and consciousness disorder (n = 4). The Glasgow coma score of the patients was 8.4 ± 3.5. All patients underwent emergency bedside burr hole evacuation through a tube, rather than open surgery. The meantime for the bedside procedures is 6.0 ± 1.5 minutes. All 5 POEDHs were proven liquid and evacuated successfully. All patients recovered quickly with good outcomes. We concluded that the isodensity of the POEDHs on CT represent their liquid nature. Bedside burr hole evacuation through a tube may be a recommendable method for this subset of POEDHs requiring evacuation. Thus, an open surgery and general anesthesia may be avoided.
Keywords: burr hole, emergency evacuation, epidural hematoma, isodensity, postoperative hematoma, suction drainage
1. Introduction
Although the incidence of postoperative epidural hematomas (POEDHs) after intracranial surgery has been reduced with the development of surgical techniques and perioperative management, they remain a serious complication of neurosurgery. Most POEDHs are detected within 3 days of surgery and are characterized by a homogeneous, biconvex lens-shaped, hyperdense collection under the skull on computed tomography (CT). Large POEDHs with a significant mass effect are life-threatening and require emergency evacuation. With the ability to both remove the clot and control the bleeding sites, open surgery is the mainstay of treatment in these cases. Park et al[1] have reported a feasible bedside thrombolytic evacuation of a smaller postcraniotomy epidural hematoma (EDH) (15–30 mL) using a closed suction drain. Larger POEDHs could also potentially be treated by thrombolytic evacuation using a closed suction drain.
Although most POEDHs are hyperdense on CT, a subset of POEDHs presents with homogeneous, isodense features on CT. Differences may exist between isodense and hyperdense POEDHs in terms of clinical characteristics and treatments. Here, we report a series of large POEDHs that presented with isodense features on CT, which were successfully treated by a thrombolytic evacuation using a closed suction drain. The aim of this report was to highlight the clinical characteristics, possible causes, effective treatment, and outcome of this rare entity.
2. Materials and methods
2.1. Patient population
Ethical approvals were obtained from the Human Research Ethics Committee of Tianjin Huanhu Hospital and the PLA General Hospital of China. A retrospective review was conducted on all isodense POEDHs that were treated by emergency evacuation between January 2012 and January 2016 at the Neurosurgery Department in Tianjin Huanhu Hospital and the PLA General Hospital of China. Five patients had developed a large POEDH that was isodense on CT. The data for these 5 patients are summarized in Table 1. The median age of the patients was 42.8 ± 15.7 years (range, 23–60 years), and the female:male ratio was 1:5. Two patients had a history of severe traumatic brain injury and subsequent decompressive craniectomy and duraplasty. One patient had a 10-year history of hypertension and maintained normal pressure by receiving sustained-release nifedipine. One patient had a 3-year history of cerebral infarction and had been receiving aspirin until 4 weeks before surgery. There were no coagulopathies in any patients.
Table 1.
Patient profiles.
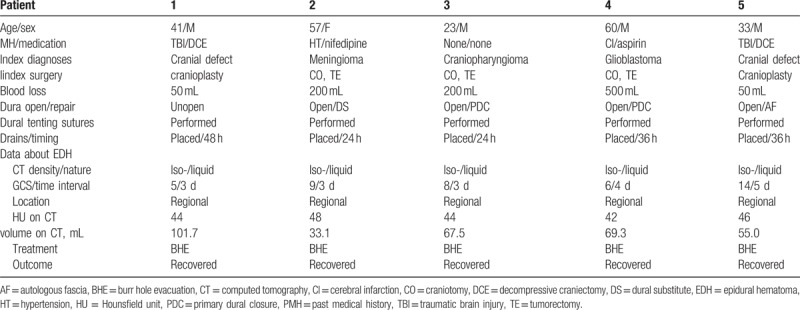
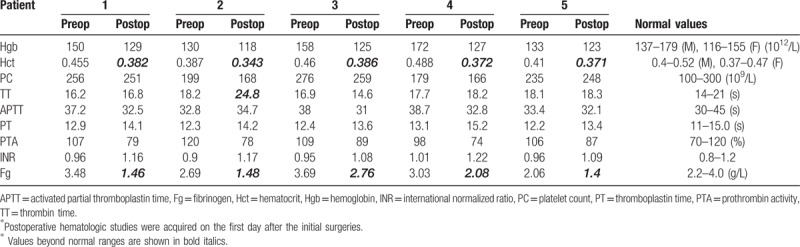
The initial diagnoses were sellar craniopharyngioma, glioblastoma in the posterior temporal lobe, meningioma (Fig. 1) at the left parietal convexity, and cranial defect (Fig. 2), resulting from a prior decompressive craniectomy (n = 2). Total tumor resections were achieved for the 3 patients with tumors via different craniotomies according to the tumor locations. For the patient with a meningioma, the dural defect was repaired with a dural substitute (Preclude Dura Substitute; W. L. Gore & Associates, Inc, Flagstaff, AZ). Primary dural closure was performed in the other 2 patients with tumors. Cranioplasties with computer-shaped titanium mesh were conducted for the 2 patients with cranial defects. During surgery, the dura-like mater was not open in 1 patient, while cerebrospinal fluid (CSF) leakage occurred via a small slit in the dura-like mater in the other patient and was repaired with autologous fascia. Multiple dural tenting sutures (circumferential and central) and complete hemostasis of the dura, bone, or scalp flap were achieved in all patients according to the surgical records. Neither open ventricles nor large CSF losses occurred in any patients. Fibrin glue was not used in any patients. Normal-pressure epidural drains were used in all 5 patients, and drains were maintained for the initial 24 to 48 hours after surgery. The postoperative hematologic study results on the first day after the initial procedure revealed some abnormalities in fibrinogen (n = 5), hematocrit (n = 5), or thrombin time (n = 1) (Table 2).
Figure 1.
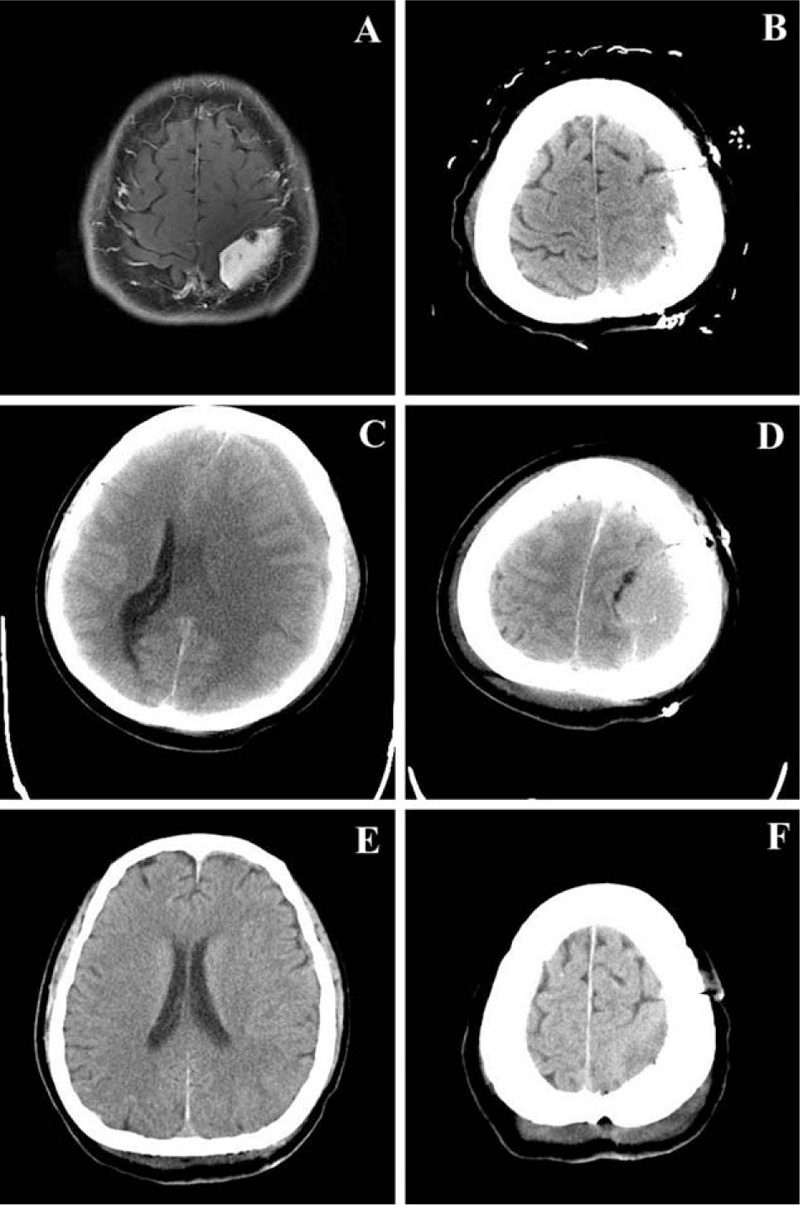
A craniotomy was conducted on a 57-year-old female patient for a meningioma at the left parietal convexity (A). Computed tomography showed a drainage tube with no hematoma at the operative site on the first postoperative day (B). Three days later, a midline shift (C) and homogeneous, isodense regional epidural hematoma (D) were detected. Emergent bedside burr hole evacuation through a tube and suction drainage were conducted. No remnant or recurrent hematoma could be seen 14 days later (E, F).
Figure 2.
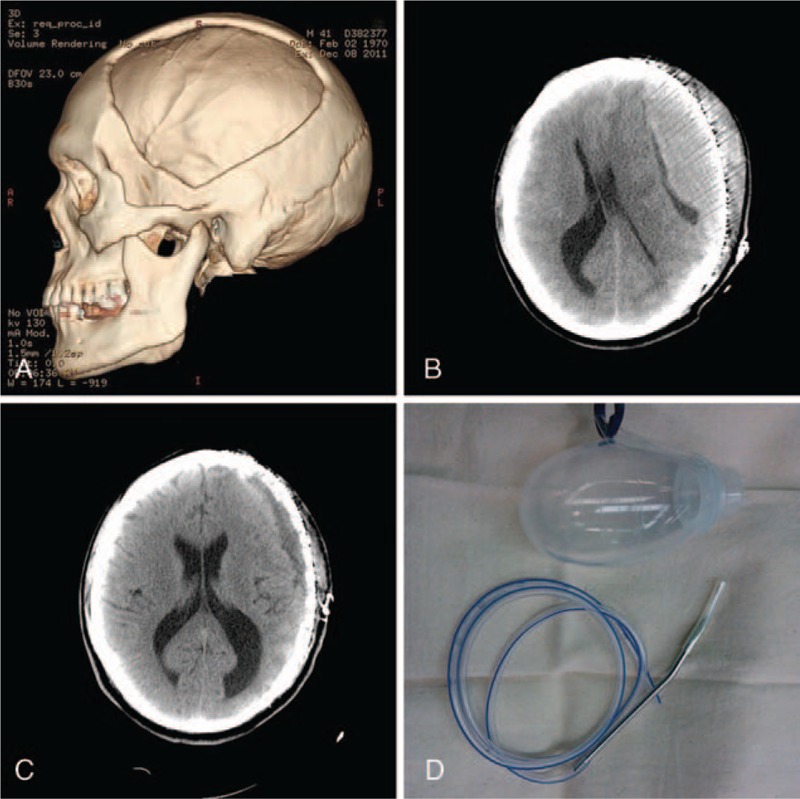
A 41-year-old male patient was admitted to the hospital with a cranial defect (A) due to a prior decompressive craniectomy for a severe traumatic brain injury. A cranioplasty with computer-shaped titanium mesh was conducted, and 3 days later, a homogeneous, isodense regional epidural hematoma (B) was detected. Emergent bedside burr hole evacuation through a tube was conducted. Suction drainage was maintained for 2 days, and recovery as evidence by computed tomography was seen 7 days later (C). (D) The tube used for bedside burr hole evacuation in all present cases and the negative pressure ball are shown.
Table 2.
Hematologic studies.
2.2. POEDH information
All patients gradually recovered in the first few days after the initial operation but then deteriorated. Three patients underwent a routine CT scan on the first postoperative day, and no intracranial hematomas were detected. The presenting symptoms that prompted a CT examination were severe headache accompanied by nausea and vomiting as well as unilateral limb weakness (n = 1) and consciousness disorder (n = 4). Brain herniation, as confirmed by unilateral mydriasis, occurred in 2 patients. The mean Glasgow coma scale (GCS) score of the patients was 8.4 ± 3.5. The mean time interval between the initial operation and development of the POEDH was 3.6 ± 0.9 days (range, 3–4 days). All 5 POEDHs presented as a homogeneous, isodense, and regional epidural collection immediately under the operative site on CT (Figs. 1 and 2). The ABC method was used to calculate the POEDH volume,[2] and Hounsfield unit (HU) values were calculated. The average HU value of the POEDHs on CT was 44.8 ± 2.3, and the average volume (the ABC method) was 65.3 ± 24.9 mL (range, 33.1–101.7 mL).
2.3. Bedside burr hole evacuation
After local disinfection and anesthesia with lidocaine, a small portion of the prior incision (1–2 cm) was opened near the burr hole, which was drilled during the initial procedure. A tube was then placed into the POEDH area via the opened incision and burr hole. For patients who underwent cranioplasty with no burr hole, the tube was positioned outside the titanium mesh. A 20-mL syringe was used to assist with evacuation if necessary. The location of the inserted tube was adjusted based on the outflow of the hematoma. After evacuation, a closed suction drainage system (Fig. 2) was placed and maintained for no more than 3 days. All patients underwent emergency bedside burr hole evacuation via a tube immediately after the POEDH was detected on CT.
Information was collected regarding demographics, past medical histories, initial diagnosis, radiologic records, details of the initial surgery, and the use and timing of drains. Underlying general factors including coagulopathies (such as coagulation factor deficiency, thrombocytopenia, and so on), medications predisposing bleeding, and results of hematologic studies (hemoglobin, hematocrit, platelet count, and coagulation results) were also collected. In terms of the isodense POEDHs, we focused on data such as presenting symptoms, time interval from the initial operation to development of the POEDH, CT features, nature of the hematoma (liquid or clotted), treatment (open surgery or other), and outcome. Statistical analysis was performed with SPSS software version 22 (SPSS Inc, Chicago, IL).
3. Results
As the tube was placed, a large volume of liquid blood (dark red) flowed through the tube and open incision in all cases. All patients immediately improved to different degrees; the unilateral mydriasis of the 2 patients returned. The mean time required for the bedside procedures was 6.0 ± 1.5 minutes. All 5 patients completely recovered soon after the emergency treatments (1–3 days). The postevacuation courses were all uneventful (Figs. 1 and 2), and no infections developed.
4. Discussion
There POEDH is a known complication after intracranial operations. Most are detected within 3 days of surgery, while some are detected during the operation.[3] Incomplete hemostasis, incomplete central stay sutures, intraoperative blood loss, and lack of drainage are thought be related to regional hematoma formation. There are also underlying systematic risk factors of hematoma, including coagulopathies, hematologic abnormalities, medications that predispose patients to bleeding, perioperative hypertension, and so on.[4–6]
All of the present cases involved regional POEDHs. Complete hemostasis, multiple dural tenting sutures, and epidural drainage were all achieved. Neither open ventricles nor large CSF losses were involved in any patients. Normal-pressure epidural drains were used in all 5 patients, and the drains were maintained for the initial 24 to 48 hours after surgery. One patient had a 3-year history of aspirin use for cerebral infarction, and another had a 10-year history of nifedipine use for hypertension. No coagulopathies were identified in any patients, while some hematologic changes were detected after the initial surgeries (Table 2). Isodensity on CT was the main feature of this subset of POEDHs, and some possible reasons for this are discussed in the following sections.
4.1. CT density and EDHs
The densities on CT and their correlations with the nature of traumatic EDH have been widely reported. The densities of EDHs on CT are related to the attenuation values of the collection as a function of both the erythrocyte and hemoglobin protein concentration as well as that of the iron content of the hemoglobin molecules. The classic CT finding of an acute EDH is a homogeneous, biconvex or lenticular, hyperdense (60–90 HU), extra-axial mass. The hyperdensity represents the solid state of clotted blood and may increase during the first 3 days. A heterogeneous or mixed CT pattern of hyperdensity and hypodensity is typically observed in hyperacute EDHs. The mixed density represents a mixture of clotted and liquid blood. The hypodense appearance of the liquid content may be due to active bleeding, mixing of blood and CSF, or clotting abnormalities.[7,8] The prognosis of this type of EDH is poor compared to that of classic hyperdense EDHs, as previously reported.[9,10] Isodensity or hypodensity is often observed in subacute or chronic hematomas, which have been proven to be liquid with little or no solid clot. This type of hematoma may be attributed to the pathophysiologic process of liquefaction of the clot and is often observed in patient CT scans 4 to 21 days after injury.[7,11] Isodensity on CT can also be detected in hyperacute EDHs, typically with linear high attenuation at the inner margin of the hematoma. There are many explanations for hematoma isodensity, such as decreased hematocrit and hemoglobin, mixture of blood with CSF due to a dural tear, the presence of fresh blood, and hematologic disorders.[12–14]
All POEDHs in the present study exhibited a typical homogeneous isodensity (44.80 ± 2.28 HU) on CT and were liquid in nature. The time interval between the initial operation and detection of the POEDHs was 3.60 ± 0.89 days, which may be an insufficient period of time for complete liquefaction of a clotted EDH. Moreover, the CT on the first postoperative day revealed no EDH in 3 patients. Active bleeding was not found during the evacuation in any patients.
In terms of inducing the formation of EDHs, neurosurgical procedures differ from trauma in several aspects. First, some inevitable blood loss occurs during surgery. The blood loss, as well as the surgery itself, may lead to hematologic changes, although these may be transient. In the present patients, although observed to be within normal ranges before the initial operations, the fibrinogen (n = 5), hematocrit (n = 5), and thrombin time were abnormal postoperatively (Table 2). These changes are the potential explanation for the incomplete clot formation. Second, there is a history of dural opening in most intracranial surgeries. A watertight dural closure is not often realistically achieved, and CSF leakage may occur, which mixes with the blood and prevents it from clotting. Furthermore, a 1-way valve mechanism of the dura for movement of CSF may contribute to the enlargement of the liquid EDH in some cases. Third, the wound surface is involved in these surgeries. Complete hemostasis can be achieved to prevent large vascular bleeds; however, minimal oozing from the dura, bone, muscles, and soft tissue cannot be entirely avoided. Inflammation and effusion may contribute to the formation of the epidural collection. The osmotic pressure of the collection may also contribute to effusion of tissue fluid from the wound surface. Moreover, there may be disturbances in absorption. Two of the present patients had undergone a previous operation in the region of the initial surgery. Scar tissue may be more likely to ooze than normal tissue and may have a reduced ability to absorb blood and blood products. Lack of drainage may increase the absorptive load of the wound. Although drains were placed in all of the present cases, they may have been removed too early (within 48 hours). Above all, the mechanism of formation of isodense, liquid POEDHs remain unclear, and various factors may participate in the process.
4.2. Burr hole drainage for EDHs
Patients with small POEDHs are often asymptomatic or present with only headaches or minor neurologic deficits and can be treated conservatively. Conversely, large EDHs with a significant mass effect that are life-threatening or associated with imminent brain herniation are vital and require emergency evacuation. Open surgery is the mainstay of treatment for large POEDHs.[3,15,16] Despite the advantages of both removing the clot and controlling the bleeding sites, open surgery requires preparation time and general anesthesia. For patients with large EDHs or brain herniation, or those at risk of herniation, and particularly for patients with hematologic disturbances, the time delay for preparation for reoperation, general anesthesia, and the open surgery itself may increase the risk of a poor outcome.
Some minimally invasive techniques for evacuation of traumatic EDHs have been reported. Needle aspiration was effectively performed for the evacuation of neonatal EDHs,[17–19] which are more liquefied than their adult counterpart, and for the evacuation of an EDH in an 11-year-old boy with communicating bifrontal subgaleal hematomas.[20] Burr hole evacuation and subsequent drainage with or without urokinase has been performed for some traumatic EDHs, and the outcomes were favorable.[12,21,22] Even skull trephination was reused on “talk and deteriorate” EDH patients with a GCS of ≤8 and anisocoria at an emergency department as a beneficial adjuvant method before transferring the patient to open surgery.[23] With regard to POEDHs, Park et al[1] demonstrated that thrombolytic evacuation of a postcraniotomy EDH using a closed suction drain is feasible without complications and may be associated with better outcomes. The samples were confined to borderline cases of a symptomatic EDH with a medium (15–30 mL) volume, and the authors did not evaluate the CT density or nature (liquid or clotted) of the EDH.
Theoretically, liquid POEDHs, regardless of the volume, could be easily evacuated via a burr hole with suction drainage. In contrast to traumatic EDHs, the initial surgery provides some advantages for this minimally invasive evacuation of isodense and liquid POEDHs. These advantages may include the presence of burr holes, incisions, and communications between the epidural and subcutaneous spaces via bone flap edges created during the initial surgery. Furthermore, rapid evacuation can be achieved with this method. Although some of the isodensities on CT may represent active bleeding, the bleeding may spontaneously cease with the shrinkage of the epidural space by evacuation of the collection. Even if persistent arterial or sinus bleeding exists and open surgery is definitely required, a prompt release of dangerously high pressure before the operation will substantially contribute to saving the patient's life and achieving a good functional outcome. Subsequent suction drainage may contribute to the evacuation of the blood and the shrinking of the epidural space. In addition, compared to open surgery, this minimally invasive evacuation may have cost advantages. In our report, all of the patients had a large (65.32 ± 24.94 mL), isodense EDH, 2 of which were life-threatening due to brain herniation. Bedside tube evacuation via a burr hole and suction drainage were conducted on all of the present patients. The outcomes of all cases were good.
However, this bedside procedure with suction drainage may have some disadvantages, including risk of infection (although this was not observed in the present cases), incomplete evacuation, prolonged hospital stays, etc.
In summary, we have reported a subset of POEDHs with homogeneous isodensity on CT with the aim of highlighting this uncommon entity. The isodensity on CT represents the liquid nature of the POEDH. The causes of these POEDHs remain unclear, and various factors may contribute to their formation. Although uncommon, large isodense POEDHs may progress to brain herniation and become life-threatening; thus, volume evacuation should be performed as soon as possible. Bedside burr hole evacuation via a tube and subsequent suction drainage could be an effective alternative treatment. Thus, open surgery and general anesthesia could be avoided. However, large samples or prospective randomized studies are required to support such a conclusion.
Author contributions
Data curation: Hecheng Ren, Lin Ma.
Formal analysis: Hecheng Ren.
Investigation: Lin Ma, Xiaodong Ma.
Methodology: Xiaodong Ma.
Project administration: Long Yin.
Resources: Xiaodong Ma.
Software: Ming Wei.
Supervision: Xiaodong Ma.
Validation: Long Yin, Xiaodong Ma.
Visualization: Long Yin.
Writing – original draft: Hecheng Ren.
Writing – review & editing: Hecheng Ren.
Footnotes
Abbreviations: CT = computed tomography, EDH = epidural hematoma, HU = Hounsfield unit, POEDH = postoperative epidural hematoma.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Park J, Kim GJ, Hwang SK. Thrombolytic evacuation of post-craniotomy epidural haematomas using closed suction drains: a pilot study. Acta Neurochir (Wien) 2008;150:359–66. [DOI] [PubMed] [Google Scholar]
- [2].Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke 1996;27:1304–5. [DOI] [PubMed] [Google Scholar]
- [3].Fukamachi A, Koizumi H, Nagaseki Y, et al. Postoperative extradural hematomas: computed tomographic survey of 1105 intracranial operations. Neurosurgery 1986;19:589–93. [DOI] [PubMed] [Google Scholar]
- [4].Seifman MA, Lewis PM, Rosenfeld JV, et al. Postoperative intracranial haemorrhage: a review. Neurosurg Rev 2011;34:393–407. [DOI] [PubMed] [Google Scholar]
- [5].Gentleman D, Johnston RA. Postoperative extradural hematoma associated with induced hypertension. Neurosurgery 1985;17:105–6. [DOI] [PubMed] [Google Scholar]
- [6].Kim SH, Lee JH, Joo W, et al. Analysis of the risk factors for development of post-operative extradural hematoma after intracranial surgery. Br J Neurosurg 2015;29:243–8. [DOI] [PubMed] [Google Scholar]
- [7].Zimmerman RA, Bilaniuk LT. Computed tomographic staging of traumatic epidural bleeding. Radiology 1982;144:809–12. [DOI] [PubMed] [Google Scholar]
- [8].Greenberg J, Cohen WA, Cooper PR. The “hyperacute” extraaxial intracranial hematoma: computed tomographic findings and clinical significance. Neurosurgery 1985;17:48–56. [DOI] [PubMed] [Google Scholar]
- [9].Nayil K, Ramzan A, Arif S, et al. Hypodensity of extradural hematomas in children: an ominous sign. J Neurosurg Pediatr 2011;8:417–21. [DOI] [PubMed] [Google Scholar]
- [10].Pruthi N, Balasubramaniam A, Chandramouli BA, et al. Mixed-density extradural hematomas on computed tomography-prognostic significance. Surg Neurol 2009;71:202–6. [DOI] [PubMed] [Google Scholar]
- [11].Mathur PP, Dharker SR, Agarwal SK, Sharma M. Fluid chronic extradural haematoma. PMID: 7414491. https://www.ncbi.nlm.nih.gov/pubmed/?term=7414491. [PubMed] [Google Scholar]
- [12].Habibi Z, Meybodi AT, Haji MSM, et al. Burr-hole drainage for the treatment of acute epidural hematoma in coagulopathic patients: a report of eight cases. J Neurotrauma 2012;29:2103–7. [DOI] [PubMed] [Google Scholar]
- [13].Arrese I, Lobato RD, Gomez PA, et al. Hyperacute epidural haematoma isodense with the brain on computed tomography. Acta Neurochir (Wien) 2004;146:193–4. [DOI] [PubMed] [Google Scholar]
- [14].Li TC, Chen Y, Lin SM, et al. Acute posterior fossa isodense epidural hematoma: diagnostic pitfalls on computed tomography. J Trauma 2007;63:417–9. [DOI] [PubMed] [Google Scholar]
- [15].Borkar SA, Sinha S, Sharma BS. Remote site extradural haematoma. J Clin Neurosci 2009;16:1097–8. [DOI] [PubMed] [Google Scholar]
- [16].Chandra PS, Jaiswal A, Mahapatra AK. Bifrontal epidural haematomas following surgery for occipital falcine meningioma: an unusual complication of surgery in the prone position. J Clin Neurosci 2002;9:582–4. [DOI] [PubMed] [Google Scholar]
- [17].Vachharajani A, Mathur A. Ultrasound-guided needle aspiration of cranial epidural hematoma in a neonate: treating a rare complication of vacuum extraction. Am J Perinatol 2002;19:401–4. [DOI] [PubMed] [Google Scholar]
- [18].Smets KJ, Vanhauwaert D. Treatment of cranial epidural hematoma in a neonate by needle aspiration of a communicating cephalhematoma. Eur J Pediatr 2010;169:617–9. [DOI] [PubMed] [Google Scholar]
- [19].Noguchi M, Inamasu J, Kawai F, et al. Ultrasound-guided needle aspiration of epidural hematoma in a neonate after vacuum-assisted delivery. Childs Nerv Syst 2010;26:713–6. [DOI] [PubMed] [Google Scholar]
- [20].Strowitzki M, Eymann R, Schleifer J, et al. Vertex epidural hematoma with communicating bifrontal subgaleal hematomas treated by percutaneous needle aspiration. Pediatr Neurosurg 2001;35:1–4. [DOI] [PubMed] [Google Scholar]
- [21].Liu W, Ma L, Wen L, et al. Drilling skull plus injection of urokinase in the treatment of epidural haematoma: a preliminary study. Brain Inj 2008;22:199–204. [DOI] [PubMed] [Google Scholar]
- [22].Liu JT, Tyan YS, Lee YK, et al. Emergency management of epidural haematoma through burr hole evacuation and drainage. A preliminary report. Acta Neurochir (Wien) 2006;148:313–7. [DOI] [PubMed] [Google Scholar]
- [23].Smith SW, Clark M, Nelson J, et al. Emergency department skull trephination for epidural hematoma in patients who are awake but deteriorate rapidly. J Emerg Med 2010;39:377–83. [DOI] [PubMed] [Google Scholar]


