Abstract
Background:
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are the preferred first-line treatment for nonsmall-cell lung cancer (NSCLC) patients with an activating EGFR mutation. Osimertinib, compared with erlotinib or gefitinib, showed an improvement in progression-free survival (PFS) in a recent trial. The authors compared EGFR TKIs in terms of PFS in a network meta-analysis.
Methods:
The PubMed and Embase databases and meeting abstracts were screened for relevant studies between January 2009 and November 2017. A random-effect frequentist network meta-analysis model was conducted to assess PFS. P-score was used to rank treatment effects.
Results:
Eleven trials with 3145 patients and 5 TKIs (gefitinib, erlotinib, afatinib, dacomitinib, and osimertinib) were included. Heterogeneity and inconsistency existed in the network analysis. Gefitinib and erlotinib had similar effects (hazard ratio [HR] 0.94, 95% confidence interval [CI] 0.76–1.15). For all patients, the 3 TKIs with the highest probability of benefit were osimertinib, dacomitinib, and afatinib, with P-scores of 91%, 78%, and 46%, respectively. Compared with erlotinib or gefitinib, osimertinib was associated with improvement in men (HR = 0.79, 95% CI, 0.68–0.92), non-Asians (HR = 0.63, 95% CI, 0.40–0.98), smokers (HR = 0.73, 95% CI, 0.56–0.95), and those with a Del19 mutation (HR = 0.69, 95% CI, 0.54–0.90); dacomitinib and afatinib showed no improvement. Toxicity profiles mostly overlapped in all the EGFR TKIs. Toxicity-related death was rare.
Conclusions:
Osimertinib was shown to be the best agent to achieve the longest PFS in NSCLC patients with an activating EGFR mutation. However, the benefit of osimertinib might be restricted to certain subgroups.
Keywords: EGFR, meta-analysis, nonsmall-cell lung cancer, targeted therapy
1. Introduction
The incidence of an activating epidermal growth factor receptor (EGFR) mutation in patients with nonsmall-cell lung cancer (NSCLC) is 15% to 50%, depending on race, gender, and smoking status.[1] As first-generation tyrosine kinase inhibitors (TKIs), gefitinib and erlotinib have consistently shown a greater response, longer progression-free survival (PFS), and improved quality of life compared to chemotherapy in patients who have a driver mutation in the EGFR gene for first-line treatment.[2–5] These results have established either of the 2 TKIs as the standard of care (SoC).
Unlike the reversible first-generation EGFR TKIs, second-generation TKIs (afatinib and dacomitinib) irreversibly bind to ErbB receptors.[6] Two prospective trials have confirmed the superiority of afatinib over chemotherapy, and the extent of the benefit of PFS was similar to that observed in trials comparing first-generation TKIs with chemotherapy.[7,8] A subsequent head-to-head study compared afatinib with gefitinib as first-line treatments and showed a statistically significant improvement in PFS with afatinib,[9] but the difference was not clinically meaningful (median PFS of 11.0 and 10.9 months for afatinib and gefitinib, respectively). By contrast, in another head-to-head trial, the irreversible EGFR blocker dacomitinib was associated with an absolute difference of 5.5 months in PFS compared with gefitinib, though at the cost of increased toxicity.[10]
Osimertinib, a third generation, irreversible EGFR TKI that targets primary activating EGFR mutations and secondary T790M mutations, has been approved as a preferred second-line therapy in patients who developed the T790M mutation after first-line TKI treatment.[11–13] Then, osimertinib was used as a first-line treatment to maximize its effect on delaying progression. In the FLAURA study, compared with SoC, osimertinib showed remarkably improved PFS and more favorable tolerability.[14]
We performed a network meta-analysis by including relevant trials investigating EGFR TKIs to compare efficacy in terms of PFS and toxicity and focused primarily on whether osimertinib was superior to first-generation EGFR TKIs.
2. Methods
2.1. Study search and identification
The following terms were used for the search: NSCLC, EGFR, gefitinib, erlotinib, afatinib, dacomitinib, osimertinib, rociletinib, system review, meta-analysis, randomized, and trials. Studies were prospective phase II and III randomized controlled trials that compared EGFR TKIs with standard platinum-based chemotherapy or compared different first-line EGFR TKIs in patients with newly pathologically confirmed advanced NSCLC with actionable EGFR mutations. The PubMed and Embase databases and the Cochrane Central Register of Controlled Trials were searched for published randomized controlled trials. We also searched the references of the included publications and related systemic reviews for potential publications. The search was limited to trials published between January 2009 and November 2017. Abstracts were also searched from meetings of the American Society of Clinical Oncology, the European Society for Medical Oncology, and the World Lung Cancer Conference. The study adhered to the recommendations of the PRISMA protocol.
2.2. Data extraction and quality assessment
The data extracted from the trials were as follows: name of the study, publication year, trial phase, sample size, treatments, patients’ clinical and pathological characteristics, response rates, adverse events, PFS, hazard ratio (HR), and overall survival. The subsequent subgroup analyses were performed according to sex (female vs male), ethnicity (Asian vs non-Asian), smoking status (nonsmokers vs current or former smokers), and EGFR mutation type (Del19 vs L858R). The P value, HRs, and 95% confidence intervals (CIs) were directly extracted for overall and subgroup analyses. Data extraction was conducted by 2 reviewers independently. Disagreement between reviewers was resolved by a third reviewer. The quantitative Jadad scale was used to assess study quality.[15]
2.3. Statistical analysis
We used a frequentist weighted least-squares approach described by Rücker[16,17] and implemented in the R package netmeta instead of Bayesian modeling because of easier computation and programming. Both approaches were considered to produce similar results and ranking in the network analysis.[18,19] Treatments were ranked based on a network meta-analysis. Ranking was performed by the P-score through a netrank function in the package. Higher P-scores represented a greater probability of being the best treatment.
The Qtotal statistic was the sum statistic for heterogeneity and inconsistency. The Qtotal statistic was deconstructed to assess the heterogeneity within study designs and inconsistencies between designs.
The network meta-analysis protocol stated that when significant heterogeneity was found (P < .1), a fixed-effect model was used; otherwise, a random-effect model was used. Platinum-based first-line chemotherapy was considered to have similar effects in trials. However, we acknowledged that there might be a slight difference in chemotherapy. Therefore, sensitivity analysis was performed after excluding studies that reported a median PFS longer than 6 months in the chemotherapy arm based on the recommendation from a meta-analysis comparing EGFR TKIs to chemotherapy by Lee et al.[20]
A statistical test with P < .05 was considered significant. All 95% CIs were 2-sided. Network meta-analysis was performed with R software, version i386 3.3.2.
3. Results
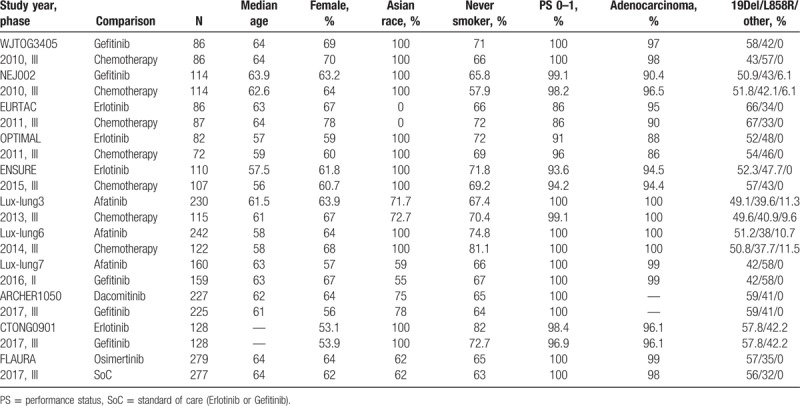
According to the flow chart in Fig. 1, after titles and abstracts were screened, 20 potential studies were evaluated further. Nine studies were excluded, namely, 2 for enrolling patients without a confirmed EGFR mutation status,[21,22] 1 for providing therapy with double targeted agents,[23] 3 for duplicate publications,[24–26] and 3 for combining TKIs with chemotherapy.[27–29] The remaining 11 trials were finally included in the network analysis.[2–5,7–10,14,30,31] Five TKIs were evaluated: first-generation gefitinib and erlotinib, second-generation afatinib and dacomitinib, and third-generation osimertinib. The study characteristics are shown in Table 1. The Chinese CTONG 0901 study allowed 91 (35.5%) patients to receive gefitinib or erlotinib as second-line treatment,[31] and these 91 patients were excluded, resulting in a total of 3145 patients in the final analysis. All patients harbored sensitive EGFR mutations, with 2 types of mutations (19Del and L858R) accounting for over 90% of the population in the analysis. The quality of included trials was high (Jadad > 3).
Figure 1.
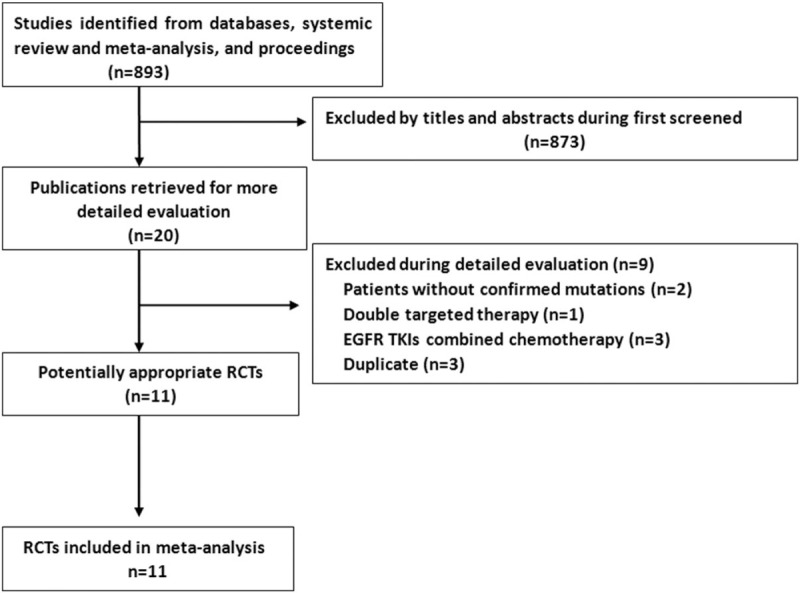
The search strategy. EGFR = epidermal growth factor receptor, RCTs = randomized controlled trials, TKI = tyrosine kinase inhibitor.
Table 1.
Baseline characteristics of included trials.
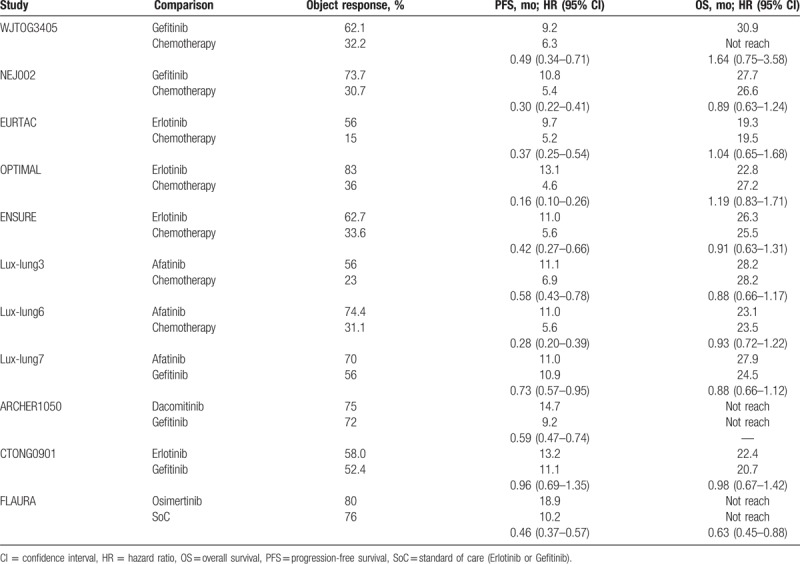
The main outcomes of included studies, including response rates, PFS, and overall survival, are presented in Table 2. Overall, TKIs had significantly higher response rates and longer PFS than chemotherapy. The second (afatinib and dacomitinib) and third (osimertinib) generations of TKIs were superior to gefitinib in PFS, though they were not accompanied by a higher response rate. No significant survival differences were demonstrated in all the comparisons among the trials.
Table 2.
Response rates, PFS, and OS for included trails.
The whole network showed significant heterogeneity (Qtotal = 21.72, P = .001). The Qtotal statistics were further deconstructed to assess the heterogeneity within and between study designs, and the results showed significant heterogeneity within designs (Q = 18.57, P = .001) but not between designs (Q = 3.15, P = .26). After deconstructing the within-designs heterogeneity, all 3 designs of TKIs (gefitinib, erlotinib, and afatinib) versus chemotherapy were identified as the source of heterogeneity. A random-effects frequentist network model was applied to assess PFS.
First, we compared gefitinib with erlotinib in terms of PFS. Six were included. Both gefitinib and erlotinib were associated with a reduction in disease progression compared with chemotherapy. Erlotinib had a similar effect compared with gefitinib (HR 0.94, 95% CI 0.76–1.15). On this basis, treatment with erlotinib or gefitinib through all the trials was considered identical and was denoted as the SoC. This SoC arm was used as a mutual arm to connect other treatments for indirect comparison (Fig. 2).
Figure 2.
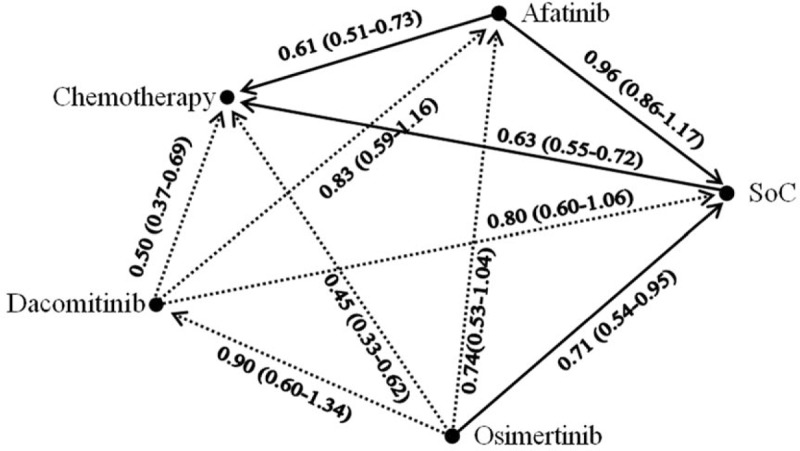
Network of comparisons. Solid lines indicate direct comparisons, and dashed lines indicate indirect comparisons. For each comparison, the arrows point to the reference arm. The hazard ratio and 95% confidence interval are presented.
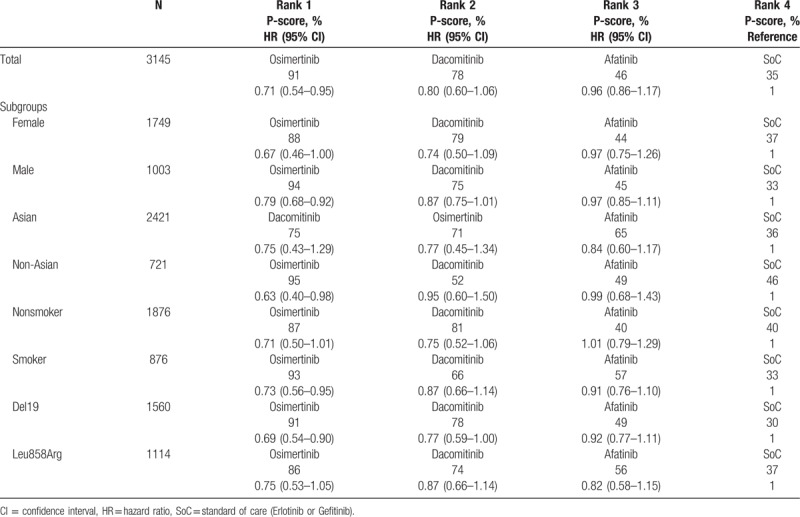
Regarding PFS, compared with SoC, the 3 TKIs with the highest probability of benefit were osimertinib, dacomitinib, and afatinib, with HRs (95% CI) of 0.71 (0.54–0.95), 0.80 (0.60–1.06), and 0.96 (0.86–1.17), respectively. The corresponding P-scores were 91%, 78%, 46%, and 35% for osimertinib, dacomitinib, afatinib, and SoC, respectively. This rank remained unchanged in females, males, non-Asians, never smokers, ever or current smokers, and those with 19Del and L858R mutations but not in the Asian subgroup. HRs and 95% CIs of direct and indirect comparisons in this network analysis are shown in Fig. 2.
Compared with SoC, osimertinib was associated with improvement in men (HR = 0.79, 95% CI, 0.68–0.92), non-Asians (HR = 0.63, 95% CI, 0.40–0.98), smokers (HR = 0.73, 95% CI, 0.56–0.95), and those with the Del19 mutation (HR = 0.69, 95% CI, 0.54–0.90). Dacomitinib and afatinib were not associated with improvement in all subgroups. The results of the rank and P-scores are presented in Table 3.
Table 3.
Rank and P-scores of subgroups.
After excluding the 2 studies that reported longer median PFS than 6 months in the chemotherapy arm, the sensitivity analysis showed consistent results with the primary results.
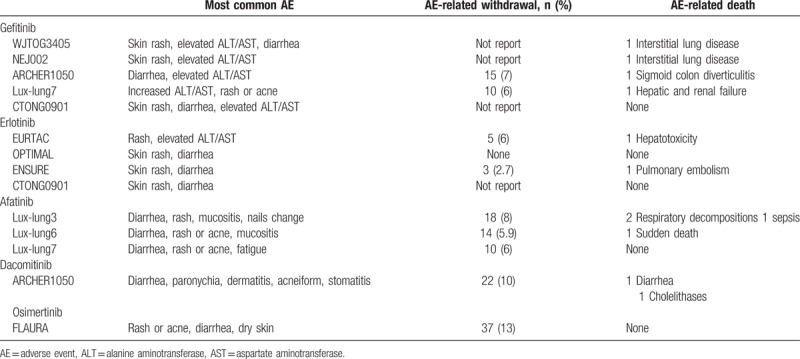
The main toxicities of different TKIs in each trial are summarized in Table 4. The first- and second-generation TKIs shared similar common adverse events, predominately rash and diarrhea, but the third-generation TKI was not associated with a significant incidence of rash. The permanent discontinuation rate due to toxicity was low for all EGFR TKIs. Toxicity-related death was rare.
Table 4.
The most common adverse events reported in each study.
4. Discussion
For patients with advanced EGFR-mutant NSCLC, 3 currently approved TKIs (gefitinib, erlotinib, and afatinib) led to dramatic tumor shrinkage and prolonged PFS compared with platinum-based chemotherapy.[20,32,33] Afatinib, dacomitinib, and osimertinib all have been evaluated against gefitinib or erlotinib in a first-line setting. Osimertinib was concluded to be the most potent TKI in terms of PFS based on results from the FLAURA study.[14] The primary results of this network meta-analysis further supported the conclusion.
Patient characteristics influenced outcomes in patients receiving TKIs.[20] We performed subgroup analysis by using treatment with gefitinib or erlotinib (SoC) as a comparison. Women, Asians, and nonsmokers tended to derive greater benefit from TKIs than men, non-Asians, and current or former smokers.[20] According to our subgroup analysis, substituting osimertinib for SoC did not show more favorable HRs in subpopulations of women, Asians, and nonsmokers, which suggested that these patients might be inherently sensitive to all EGFR TKIs. However, for men, non-Asians, or current or former smokers who seemed to derive less benefit, switching from SoC to osimertinib led to a significant improvement in PFS, which implied a treatment shift. Neither dacomitinib nor afatinib demonstrated superiority over SoC in all subgroups.
Specific EGFR mutations might potentially separate patients into different biological entities. Del19 and L858R mutations in EGFR have different predictive and prognostic impacts.[34,35] In a combined analysis of the Lux-lung 3 and 6 trials investigating afatinib versus chemotherapy, overall survival was improved in patients with the Del19 mutation but not in patients with the L858R mutation.[26] However, utilizing the del19 mutation to guide treatment decisions has not yet gained solid supporting evidence.[36] In the present subgroup analysis, osimertinib led to a favorable HR in patients with the del19 mutation but showed no difference in patients with the L858R mutation. A retrospective study showed that the prevalence of the secondary T790M mutation was significantly higher in del19-positive disease than in the L858R-positive disease.[37] This was probably the underlying reason for the results in this subgroup analysis.
EGFR TKIs had a favorable toxicity profile. EGFR TKIs were better tolerated than chemotherapy. Treatment-related death was not common. Dose reductions were more common for erlotinib, afatinib, and dacomitinib. In addition to its advantage in disease control, treatment with osimertinib was better tolerated than treatment with SoC.[14]
Based on the findings of this network meta-analysis, osimertinib seems to be a better option than the other EGFR TKIs. There were more merits for osimertinib. First, the activity of osimertinib in the central nervous system should lend weight to its uptake in a first-line setting. In the FLAURA study, osimertinib produced longer PFS than SoC in patients with CNS metastasis (15.2 months with osimertinib vs 9.6 months with SoC, HR = 0.47, P = .0009).[14] Responses in the cranium were not different between 2 treatments, but the median duration of response favored osimertinib (13.8 months vs 8.3 months).[14] It should be noted that the ARCHER1050 study excluded patients with central nervous system metastasis.[10] Second, only up to 60% of patients who would develop T790M mutations after first-line gefitinib and erlotinib were appropriate candidates for osimertinib treatment.[13] The remaining 40% of patients did not receive treatment with osimertinib. Only when osimertinib was introduced in front-line therapy did all patients have the chance of receiving osimertinib. In addition, confirming the T790M mutation before second-line osimertinib spared patients from re-biopsy.
However, several questions remain to be answered. First, the unquestionable effect of first-line osimertinib on overall survival outcome is still unclear from the AURA 3 study[11] and the FLAURA study.[14] There was a trend toward improvement in overall survival for osimertinib in the FLAURA study, but it was not statistically significant.[14] Second, patients who developed the T790M mutation during first-line SoC clearly benefited from second-line osimertinib. The total PFS of first-line SoC (9–13 months) plus second-line osimertinib (∼10 months) in this population was numerically equivalent to the value in the FLAURA study. Such patients did not necessarily require first-line osimertinib; however, no markers are currently available for identification.
Third, the mechanisms of resistance to first-line osimertinib were unknown. From the AURA1 study, potential resistance mechanisms included the amplification of MET and KRAS; mutations in MEK1, KRAS, PIK3CA, and EGFRC797S; and HER2 exon 20 insertion.[38] There was no evidence of an acquired EGFR T790M mutation.[38] For now, most of these mutations had no targeted agents; thus, clinicians would face the uncertainty of subsequent therapy after exhausting osimertinib in first-line treatment.
Finally, the Japanese study showed that the addition of bevacizumab to first-line erlotinib improved PFS from 9.7 to 16 months in EGFR mutation-positive patients without brain metastasis.[23] Relevant phase III trials are being investigated. This finding suggested that erlotinib with bevacizumab might provide a similar extent of PFS benefit as that of osimertinib in this population and push osimertinib to second-line treatment for up to 60% of patients. Such treatment sequencing might maximize the effects of currently available agents. However, the improvement was at the expense of increased toxicity.[23]
Several limitations existed in this network meta-analysis. First, heterogeneity was evident in the whole and subgroup analyses. Races, chemotherapy regimens, EGFR mutation types, and between-trial designs were intrinsic sources of heterogeneity. Heterogeneity was difficult to resolve even using the individual patient data. We used the random-effect model for the analysis and performed a sensitivity analysis. Second, the number of trials was relatively low, and data were not based on individual data. Lastly, overall survival was not assessed in our study. However, most of the included trials showed no difference in comparisons, and some overall survival results were immature.
In summary, our study supported osimertinib as a first-line treatment for NSCLC patients with an activating EGFR mutation. The analysis suggested that only some subgroups (men, non-Asians, and smokers) would really benefit from osimertinib compared with gefitinib or erlotinib.
Author contributions
Conceptualization: Jia-Zhou Lin, Shu-Han Yu, Xu-Yuan Li.
Data curation: Sheng-Xi Wu.
Formal analysis: Song-Kun Ma.
Methodology: Song-Kun Ma.
Software: Sheng-Xi Wu.
Writing – original draft: Song-Kun Ma, Sheng-Xi Wu, Shu-Han Yu.
Writing – review and editing: Jia-Zhou Lin, Xu-Yuan Li.
Footnotes
Abbreviations: CI = confidence interval, EGFR = epidermal growth factor receptor, HR = hazard ratio, NSCLC = nonsmall-cell lung cancer, PFS = progression-free survival, SoC = standard of care, TKIs = tyrosine kinase inhibitors.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Reck M, Rabe KF. Precision diagnosis and treatment for advanced non-small-cell lung cancer. N Engl J Med 2017;377:849–61. [DOI] [PubMed] [Google Scholar]
- [2].Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380–8. [DOI] [PubMed] [Google Scholar]
- [3].Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121–8. [DOI] [PubMed] [Google Scholar]
- [4].Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239–46. [DOI] [PubMed] [Google Scholar]
- [5].Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735–42. [DOI] [PubMed] [Google Scholar]
- [6].Li D, Ambrogio L, Shimamura T, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 2008;27:4702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327–34. [DOI] [PubMed] [Google Scholar]
- [8].Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213–22. [DOI] [PubMed] [Google Scholar]
- [9].Park K, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016;17:577–89. [DOI] [PubMed] [Google Scholar]
- [10].Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:1454–66. [DOI] [PubMed] [Google Scholar]
- [11].Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 2017;376:629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2016;17:1643–52. [DOI] [PubMed] [Google Scholar]
- [13].Janne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689–99. [DOI] [PubMed] [Google Scholar]
- [14].Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018;378:113–25. [DOI] [PubMed] [Google Scholar]
- [15].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [16].Rucker G. Network meta-analysis, electrical networks and graph theory. Res Synth Methods 2012;3:312–24. [DOI] [PubMed] [Google Scholar]
- [17].Rucker G, Schwarzer G. Reduce dimension or reduce weights? Comparing two approaches to multi-arm studies in network meta-analysis. Stat Med 2014;33:4353–69. [DOI] [PubMed] [Google Scholar]
- [18].Neupane B, Richer D, Bonner AJ, et al. Network meta-analysis using R: a review of currently available automated packages. PLoS ONE 2014;9:e115065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ribassin-Majed L, Marguet S, Lee AWM, et al. What is the best treatment of locally advanced nasopharyngeal carcinoma? An individual patient data network meta-analysis. J Clin Oncol 2017;35:498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lee CK, Wu YL, Ding PN, et al. Impact of specific epidermal growth factor receptor (EGFR) mutations and clinical characteristics on outcomes after treatment with EGFR tyrosine kinase inhibitors versus chemotherapy in EGFR-mutant lung cancer: a meta-analysis. J Clin Oncol 2015;33:1958–65. [DOI] [PubMed] [Google Scholar]
- [21].Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947–57. [DOI] [PubMed] [Google Scholar]
- [22].Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 2012;30:1122–8. [DOI] [PubMed] [Google Scholar]
- [23].Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol 2014;15:1236–44. [DOI] [PubMed] [Google Scholar]
- [24].Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 2013;24:54–9. [DOI] [PubMed] [Google Scholar]
- [25].Zhou C, Wu YL, Chen G, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol 2015;26:1877–83. [DOI] [PubMed] [Google Scholar]
- [26].Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141–51. [DOI] [PubMed] [Google Scholar]
- [27].Wu YL, Lee JS, Thongprasert S, et al. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trial. Lancet Oncol 2013;14:777–86. [DOI] [PubMed] [Google Scholar]
- [28].Cheng Y, Murakami H, Yang PC, et al. Randomized phase II trial of gefitinib with and without pemetrexed as first-line therapy in patients with advanced nonsquamous non-small-cell lung cancer with activating epidermal growth factor receptor mutations. J Clin Oncol 2016;34:3258–66. [DOI] [PubMed] [Google Scholar]
- [29].Mok TS, Wu YL, Yu CJ, et al. Randomized, placebo-controlled, phase II study of sequential erlotinib and chemotherapy as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol 2009;27:5080–7. [DOI] [PubMed] [Google Scholar]
- [30].Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 2015;26:1883–9. [DOI] [PubMed] [Google Scholar]
- [31].Yang JJ, Zhou Q, Yan HH, et al. A phase III randomised controlled trial of erlotinib vs gefitinib in advanced non-small cell lung cancer with EGFR mutations. Br J Cancer 2017;116:568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lee CK, Brown C, Gralla RJ, et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst 2013;105:595–605. [DOI] [PubMed] [Google Scholar]
- [33].Tan DS, Yom SS, Tsao MS, et al. The International Association for the Study of Lung Cancer Consensus Statement on optimizing management of EGFR mutation-positive non-small cell lung cancer: status in 2016. J Thorac Oncol 2016;11:946–63. [DOI] [PubMed] [Google Scholar]
- [34].Davies RS, Nelmes DJ, Butler R, et al. Non-small cell lung cancer in South Wales: are exon 19 deletions and L858R different? Anticancer Res 2016;36:4267–71. [PubMed] [Google Scholar]
- [35].Koyama N, Watanabe Y, Iwai Y, et al. Distinct benefit of overall survival between patients with non-small-cell lung cancer harboring EGFR exon 19 deletion and exon 21 L858R substitution. Chemotherapy 2017;62:151–8. [DOI] [PubMed] [Google Scholar]
- [36].Paz-Ares L, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol 2017;28:270–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ke EE, Zhou Q, Zhang QY, et al. A higher proportion of the EGFR T790M mutation may contribute to the better survival of patients with exon 19 deletions compared with those with L858R. J Thorac Oncol 2017;12:1368–75. [DOI] [PubMed] [Google Scholar]
- [38].Ramalingam SS, Yang JC, Lee CK, et al. Osimertinib as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer. J Clin Oncol 2018;36:841–9. [DOI] [PubMed] [Google Scholar]


