Supplemental Digital Content is available in the text
Keywords: dementia, dose–response relationship, meta analysis, observational study, statins
Abstract
Previous studies have indicated that statins use is associated with risk of dementia, but presented controversial results. Medline, Embase, Web of Science, and the Cochrane Database were searched update to November 2017 to identify the potential relationship between statins use and dementia. Thirty-one eligible studies involving a total of 3332,706 participants with 184,666 incident cases were included in this meta-analysis. Statins use was associated with dementia risk decrement (relevant risk [RR]: 0.85; 95% confidence interval [CI], 0.80–0.89). Subgroup analysis showed statins use was associated with Alzheimer disease (AD) (RR: 0.81; 95% CI, 0.73–0.89) and non-AD dementia (RR: 0.81; 95% CI, 0.73–0.89) risk decrement. Furthermore, statins use was associated with dementia risk decrement in female (RR: 0.89; 95% CI, 0.80–0.98) and male (RR: 0.88; 95% CI, 0.83–0.93). In addition, a dose–response showed per 1 year of duration of statins use incremental increase was associated with 20% dementia risk decrement (RR: 0.80; 95% CI, 0.73–0.87), and per 5-mg mean daily dose incremental increase in statins use was associated with 11% dementia risk decrement (RR: 0.89; 95% CI, 0.83–0.96). Statins use was associated with dementia risk decrement. The potency and the cumulative duration of statin utilized played critical roles.
1. Introduction
Statins is commonly known 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, and is the first-line drug therapy for the treatment of hyperlipidemia and the first choice for the prevention of coronary heart disease.[1] Statins have been widely used in clinically. In addition to reducing blood lipids, it's prevention and treatment of dementia gradually drawing people's attention.[2] Hypercholesterolemia may be positively correlated with dementia in middle-aged people. Statins may inhibit cholesterol metabolism by regulating cholesterol metabolism in the brain.[3]
According recent data, 93% of United States person use statins as the first choice to lower cholesterol levels, and statin use increased from 17.8% to 25.9% among the population 40 years of age or older, and it is still increasing.[4] Although, statins use has a potential to prevent dementia, there have been safety concerns regarding their effects on statins.[5] Currently, there are continued concerns, partly due to the conflicting results of the association between statins use and dementia.
Considering increasing number of patients being prescribed statins use and clinicians, pharmacists, patients, society, and governments pay more attention to the safety of these drugs; we conducted meta-analysis based on observational studies to determine whether statins use is associated with dementia risk.
2. Methods
Our meta-analysis was conducted according to the meta-analysis of observational studies in epidemiology checklist.[6] There are no ethical issues involved in our study for our data were based on published studies.
2.1. Search strategy
“Dementia” [MeSH] OR “Amentia” [MeSH] OR “Senile Paranoid Dementia” [MeSH] OR “Alzheimer's disease” [MeSH] OR “Parkinson's disease” [MeSH] OR “Huntington's disease” [MeSH] AND “Hydroxymethylglutaryl-CoA Reductase Inhibitors” [MeSH] OR “Statins” [MeSH] OR “Atorvastatin Calcium” [MeSH] OR “Lovastatin” [MeSH] OR “Meglutol” [MeSH] OR “Pravastatin” [MeSH] OR “Rosuvastatin Calcium” [MeSH] OR “Simvastatin” [MeSH] as the retrieval words, the databases such as Medline, Embase, Web of Science, and the Cochrane Database were retrieved from the time of the database established to November 2017.
2.2. Inclusion and exclusion criteria
Literature screening, inclusion, and quality evaluation were conducted independently by 2 researchers. The contents of our data extraction include the outcome was dementia. Risk estimates on the association between statins use and dementia risk. For nonrandomized controlled trials (RCTs), quality assessment was used Newcastle–Ottawa scale to score.[7]
2.3. Statistical analysis
Dose–response meta analysis using the method recommended by Greenland, Longnecker, and Orsini et al[8] by using STATA software 14.0 (STATA Corp, College Station, TX).
3. Results
3.1. Literature search results
A total of 2061 articles were screened through database retrieval and manual screening. After reading the topics and abstracts, most animal experiments and no related articles, meta-analyses, reviews, systematic reviews, randomized controls, and other forms of research were excluded and included in 51 articles. Full texts were further read and 21 articles removed. After review reference of studies, 1 article was identified. Finally, 31 studies were used for the final data synthesis.[9–39] The flowchart of literature searching was presented in Fig. 1. The characteristics of the included studies are shown in Tables 1 and 2.
Figure 1.
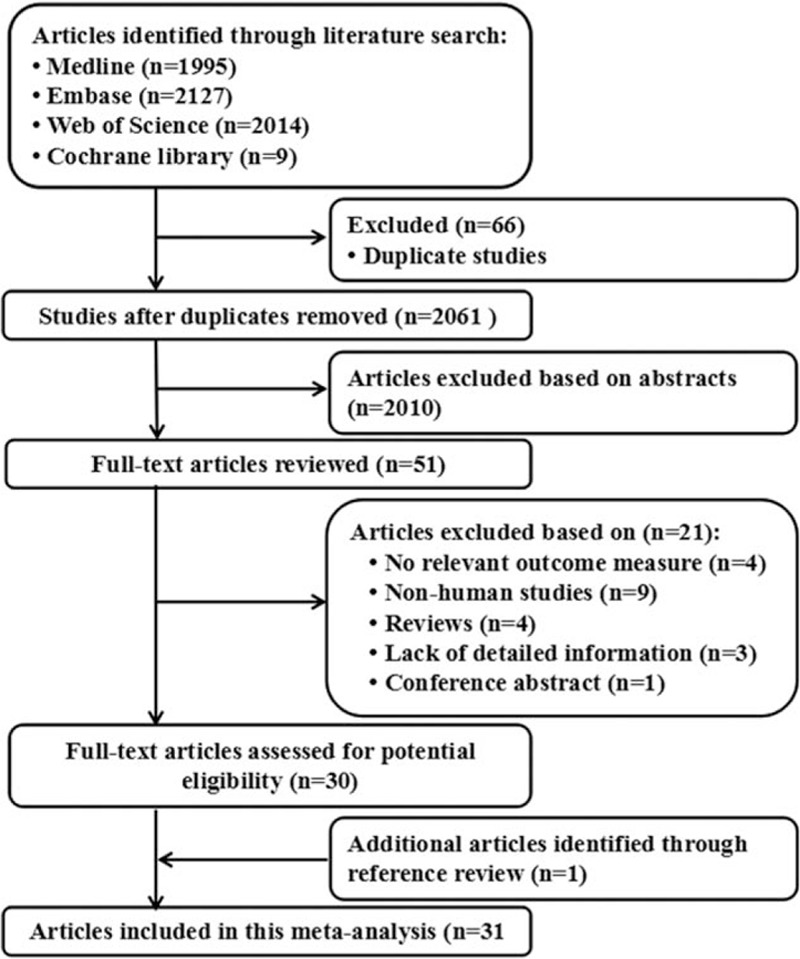
Flow diagram of the study selection process.
Table 1.
Characteristics of participants in included studies of statins use in relation to dementia.
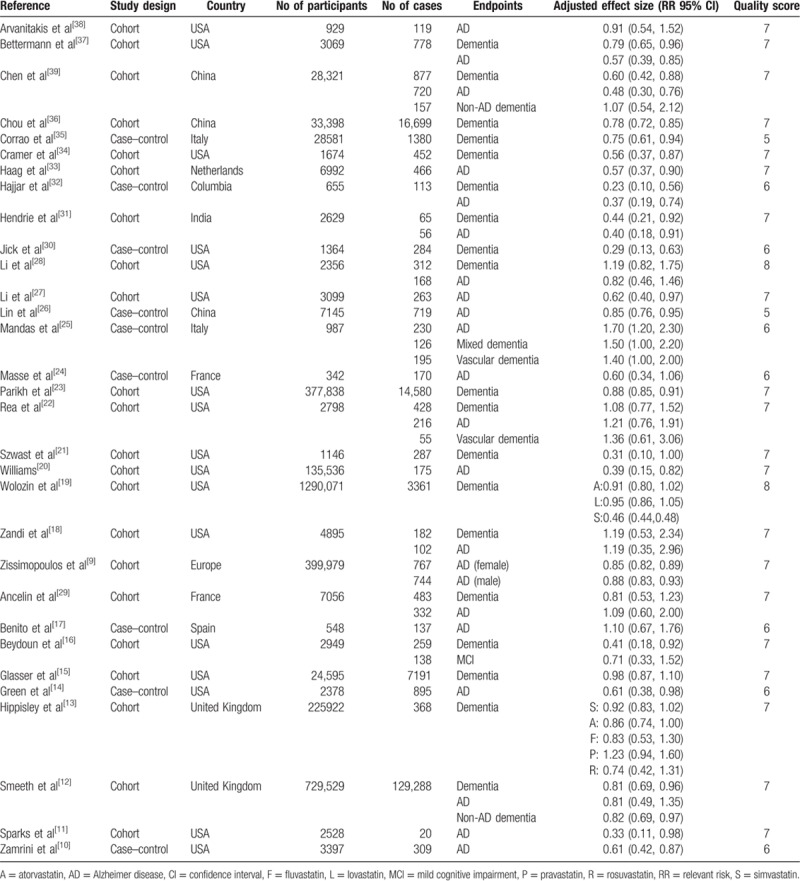
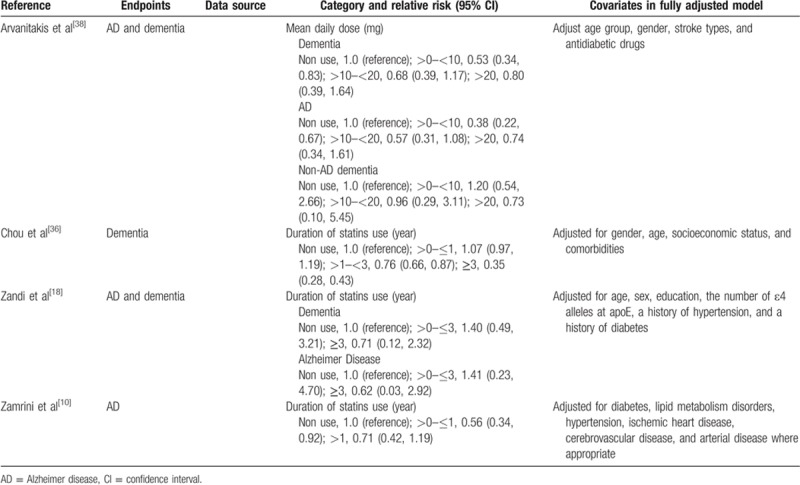
Table 2.
Outcomes and covariates of included studies of statins use in relation to dementia.
3.2. Statins use and dementia risk
Thirty-one eligible studies including 47 independent reports investigated the association between statins use and dementia risk. Compared with no statins use, statins use was associated with dementia risk decrement (relevant risk [RR]: 0.85; 95% confidence interval [CI], 0.80–0.89; P < 0.01) (Table 3). Furthermore, statins use was significantly associated with dementia risk decrement in female (RR: 0.89; 95% CI, 0.80–0.98; P < 0.01) (Table 3) and male (RR: 0.88; 95% CI, 0.83–0.93; P < 0.01) (Table 3). In addition, statins use was significantly associated with dementia risk decrement in Caucasia (RR: 0.89; 95% CI, 0.83–0.96; P < 0.01) (Table 3) and male (RR: 0.92; 95% CI, 0.84–0.97; P < 0.01) (Table 3).
Table 3.
Stratified analyses of relative risk of dementia.
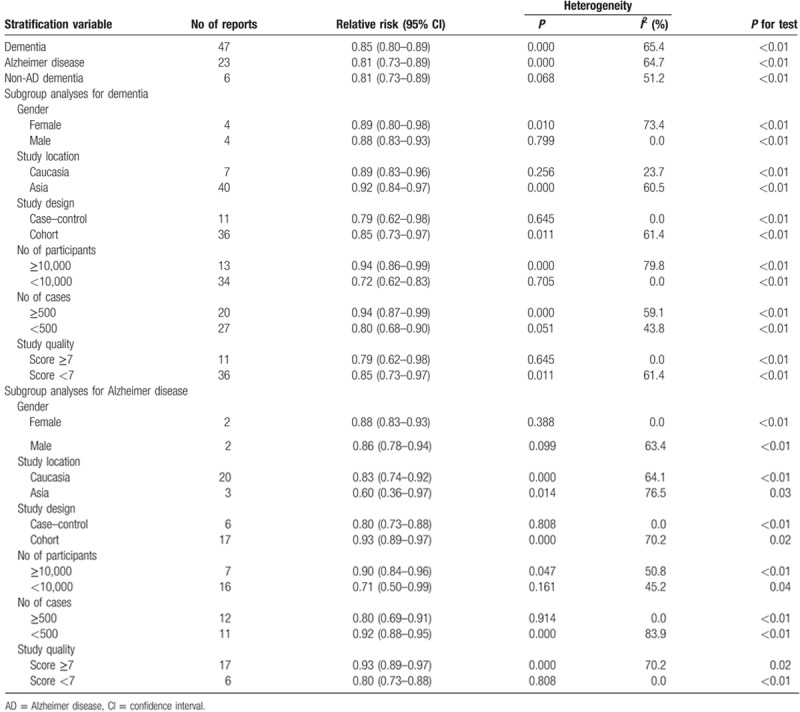
Twenty-one eligible studies including 23 independent reports investigated the association between statins use and Alzheimer disease (AD) risk. Compared with no statins use, statins use was associated with AD risk decrement (RR: 0.81; 95% CI, 0.73–0.89; P < 0.01) (Table 3). Furthermore, statins use was significantly associated with AD risk decrement in female (RR: 0.88; 95% CI, 0.83–0.93; P < 0.01) (Table 3) and male (RR: 0.86; 95% CI, 0.78–0.94; P < 0.01) (Table 3). In addition, statins use was significantly associated with AD risk decrement in Caucasia (RR: 0.83; 95% CI, 0.74–0.92; P < 0.01) (Table 3) and male (RR: 0.60; 95% CI, 0.36–0.97; P = 0.03) (Table 3).
Six eligible studies including 6 independent reports investigated the association between statins use and non-AD dementia risk. Compared with no statins use, statins use was associated with non-AD dementia risk decrement (RR: 0.81; 95% CI, 0.73–0.89; P < 0.01) (Table 3).
3.3. Dose–response between statins use and dementia risk
The test for a nonlinear dose–response relationship was significant (likelihood ratio test, P < 0.001), suggesting curvature in the relationship between statins use and dementia risk. Increasing per 1 year of duration of statins use incremental increase was associated with 20% dementia risk decrement (RR: 0.80; 95% CI, 0.73–87; P < 0.001) (Fig. 2), and per 5-mg mean daily dose incremental increase in statins was associated with 11% dementia risk decrement (RR: 0.89; 95% CI, 0.83–96; P < 0.001) (Fig. 3).
Figure 2.
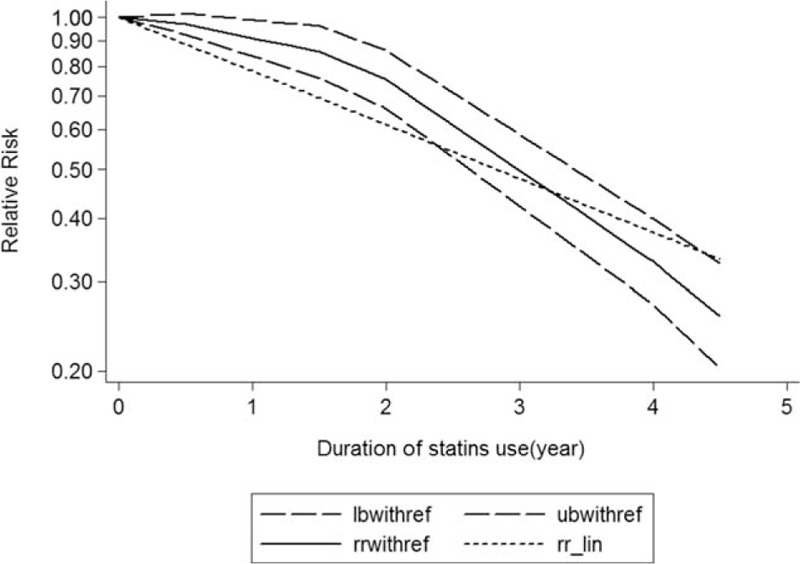
Dose–response relationship between duration of statins use in relation to risk of dementia. The solid line represents point estimates of the association of statins use and dementia risk with the use of a restricted cubic splines model, and the dashed lines indicate 95% confidence intervals.
Figure 3.
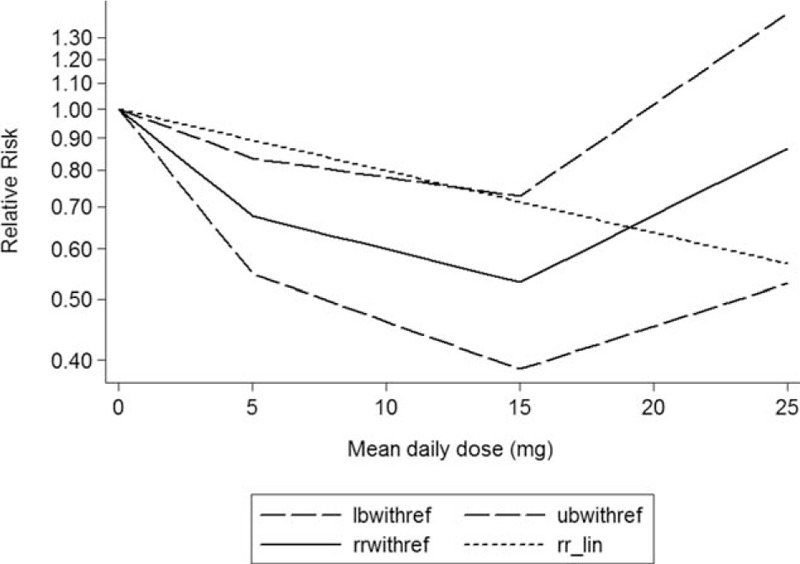
Dose–response relationship between mean daily dose of statins use in relation to risk of dementia. The solid line represents point estimates of the association of statins use and dementia risk with the use of a restricted cubic splines model, and the dashed lines indicate 95% confidence intervals.
3.4. Publication bias
Results from Egger's tests indicated no evidence of publication bias among these studies (Supplementary Table 1). A funnel plot for publication bias assessment is illustrated in Supplementary Figs. 1 to 3.
4. Discussion
Dementia is a chronic acquired progressive retardation syndrome, and slow onset of intellectual decline is the main feature in clinically, accompanied by varying degrees of personality changes. It is a group of clinical syndromes, rather than an independent disease. Dementia is mainly divided into degenerative dementia and nondegenerative dementia. Degenerative dementia includes AD, fronto-temporal dementia, Louis's dementia, Parkinson's disease, and Huntington's disease, and nondegenerative dementia mainly includes vascular dementia, traumatic brain dementia, and space occupying lesions.[40] There is no specific treatment for degenerative dementia, so as to improve cognitive and symptomatic treatment.[41] Although some of the drugs (such as cholinesterase inhibitors) can improve the ability of patients to accept new things in short term and delay the aggravation of dementia, the long-term curative effect remains to be observed. Antipsychotic drugs can be used to combat psychotic symptoms, agitation, or aggressive behavior.[42] Antidepressants can be used in patients with dementia and depression, and help to improve the dementia syndrome. But it must be noted that the anticholinergic side effects of tricyclic drugs can aggravate cognitive impairment.[43] Although benzodiazepines can control the behavior problem of the dementia, it should be specially cautious because it can cause falls and drug dependence. In general, these drugs in treatment of dementia are more or less all kinds of problems, and statins have been widely used in lipid-lowering therapy, and its pleiotropic effects have expanded its clinical value and the potential therapeutic effect of statins on dementia is remarkable.
Statins is commonly known 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, including lovastatin, pravastatin, simvastatin, mevastatin, fluvastatin, atorvastatin, cerivastatin, rosuvastatin, and is the first-line drug therapy for the treatment of hyperlipidemia and the first choice for the prevention of coronary heart disease. Recent report presents safety problems between statins use and dementia, and statins use may contribute to the potential of dementia. Currently, there are continued concerns, partly due to the conflicting results of the association between statins use and dementia.
Previous meta-analysis has investigated the association between statins use and dementia risk but presented controversial results. Wong et al[44] based on 20 observational studies found statins use was slight associated with dementia (RR: 0.82; 95% CI, 0.69–97) and AD (RR: 0.70; 95% CI, 0.60–83) risk decrement. However, Rojas-Fernandez et al[45] found statins use was not associated with dementia risk.
This meta-analysis was based on 31 studies (including nine case–control studies and 22 cohort studies) update to November 2017. Our meta-analysis supported statins use was associated with dementia risk decrements, and a dose–response showed per 1 year of duration of statins use incremental increase was associated with 20% dementia risk decrement, and per 5-mg mean daily dose incremental increase in statins use was associated with 11% dementia risk decrement. This meta-analysis included enough studies; these results should be credible.
High-cholesterol diet, elevated serum cholesterol, and high blood pressure are risk factors for coronary heart disease and AD, and hypercholesterolemia in the brain can be deposited in the hippocampus, causing amyloid precursor protein to be degraded intoamyloid precursor protein, which causes degeneration of neurons, resulting in AD. Statins may reduce the formation of β-amyloid peptide by decreasing cholesterol levels. Statins have a stable effect on the homeostasis of the nervous system cholesterol, inhibit the synthesis of cholesterol, lower the cholesterol level, and thus inhibit the beta metabolism of amyloid precursor protein.[46–49] Furthermore, the intermediate product isoprene of cholesterol biosynthesis in dementia patients is often depleted, thus affecting cell growth, mitosis, and signal transduction, while statins have a regulatory role in the above intracellular mechanisms.[50] In addition, commonly known as apoE4, an important cholesterol transporter, is an important risk factor and genetic marker of sporadic and late-onset familial AD and plays an important role in Aβ deposition and the formation of senile plaques. Astrocytes and microglia are the main sources of apoE in the brain. These cells secrete apoE, which requires the isoprenylation of key proteins, while mevalproic acid is the precursor of isoprene derivatives. Statins inhibit the synthesis of mevalonate, inhibit apoE secretion, and reduce extracellular apoE levels, thereby preventing the formation of senile plaques and improving cognitive function.[51]
This meta-analysis also has some limitations. First, we did not included RCTs due to dementia was not a prespecified endpoint in RCTs, and RCTs should be included in the further. Second, study duration was short in these studies and person included in these studies may be different from the real life. Third, the overall sample size of the study was small, which may have a certain impact on the evaluation results.
To sum up, statins use was associated with dementia risk decrement, and it is expected to become an important auxiliary means of dementia treatment. Due to the limitation of the quality and quantity of the inclusion study, more high-quality, large sample, and multicenter RCT are needed to verify the conclusions of this study in the future.
Author contributions
Data curation: Xiaoyu Zhang, Jianzhong Wen, Zhiqiang Zhang.
Formal analysis: Xiaoyu Zhang, Jianzhong Wen, Zhiqiang Zhang.
Project administration: Zhiqiang Zhang.
Software: Xiaoyu Zhang, Jianzhong Wen, Zhiqiang Zhang.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, RR = relevant risk.
Funding/support: This study received no specific external funding.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Newman T, Hulley S. Carcinogenicity of lipid-lowering drugs. JAMA 1996;275:55–60. [PubMed] [Google Scholar]
- [2].Cucchiara B, Kasner S. Use of statins in CNS disorders. J Neurol Sci 2001;187:81–9. [DOI] [PubMed] [Google Scholar]
- [3].Vaughan C. Prevention of stroke and dementia with statins: effects beyond lipid lowering. Am J Cardiol 2003;91:23B–9B. [DOI] [PubMed] [Google Scholar]
- [4].Gu Q, Paulose-Ram R, Burt V, et al. Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003–2012. NCHS Data Brief 2014;177:1–8. [PubMed] [Google Scholar]
- [5].Chong P, Seeger J, Franklin C. Clinically relevant differences between the statins: implications for therapeutic selection. Am J Med 2001;111:390–400. [DOI] [PubMed] [Google Scholar]
- [6].Stroup D, Berlin J, Morton S, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) Group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [7].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [8].Orsini N, Li R, Wolk A, et al. Meta-analysis for linear and nonlinear dose–response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 2012;175:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zissimopoulos J, Barthold D, Brinton R, et al. Sex and race differences in the association between statin use and the incidence of Alzheimer disease. JAMA Neurol 2017;74:225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zamrini E, McGwin G, Roseman J. Association between statin use and Alzheimer's disease. Neuroepidemiology 2004;23:94–8. [DOI] [PubMed] [Google Scholar]
- [11].Sparks D, Kryscio R, Sabbagh M, et al. Reduced risk of incident AD with elective statin use in a clinical trial cohort. Curr Alzheimer Res 2008;5:416–21. [DOI] [PubMed] [Google Scholar]
- [12].Smeeth L, Douglas I, Hall A, et al. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br J Clin Pharmacol 2009;67:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ 2010;340:c2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Green R, McNagny S, Jayakumar P, et al. Statin use and the risk of Alzheimer's disease: the MIRAGE study. Alzheimers Dement 2006;2:96–103. [DOI] [PubMed] [Google Scholar]
- [15].Glasser S, Wadley V, Judd S, et al. The association of statin use and statin type and cognitive performance: analysis of the reasons for geographic and racial differences in stroke (REGARDS) study. Clin Cardiol 2010;33:280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Beydoun M, Beason-Held L, Kitner-Triolo M, et al. Statins and serum cholesterol's associations with incident dementia and mild cognitive impairment. J Epidemiol Community Health 2011;65:949–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Benito-León J, Louis E, Vega S, et al. Statins and cognitive functioning in the elderly: a population-based study. J Alzheimers Dis 2010;21:95–102. [DOI] [PubMed] [Google Scholar]
- [18].Zandi P, Sparks D, Khachaturian A, et al. Do statins reduce risk of incident dementia and Alzheimer disease? The Cache County Study. Arch Gen Psychiatry 2005;62:217–24. [DOI] [PubMed] [Google Scholar]
- [19].Wolozin B, Wang S, Li N, et al. Simvastatin is associated with a reduced incidence of dementia and Parkinson's disease. BMC Med 2007;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Williams P. Lower risk of Alzheimer's disease mortality with exercise, statin, and fruit intake. J Alzheimers Dis 2015;44:1121–9. [DOI] [PubMed] [Google Scholar]
- [21].Szwast S, Hendrie H, Lane K, et al. Association of statin use with cognitive decline in elderly African Americans. Neurology 2007;69:1873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rea T, Breitner J, Psaty B, et al. Statin use and the risk of incident dementia: the Cardiovascular Health Study. Arch Neurol 2005;62:1047–51. [DOI] [PubMed] [Google Scholar]
- [23].Parikh N, Morgan R, Kunik M, et al. Risk factors for dementia in patients over 65 with diabetes. Int J Geriatr Psychiatry 2011;26:749–57. [DOI] [PubMed] [Google Scholar]
- [24].Masse I, Bordet R, Deplanque D, et al. Lipid lowering agents are associated with a slower cognitive decline in Alzheimer's disease. J Neurol Neurosurg Psychiatry 2005;76:1624–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mandas A, Mereu R, Catte O, et al. Cognitive impairment and age-related vision disorders: their possible relationship and the evaluation of the use of aspirin and statins in a 65 years-and-over Sardinian population. Front Aging Neurosci 2014;6:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lin F, Chuang Y, Hsieh H, et al. Early statin use and the progression of Alzheimer disease: a total population-based case–control study. Medicine (Baltimore) 2015;94:e2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li G, Shofer J, Rhew I, et al. Age-varying association between statin use and incident Alzheimer's disease. J Am Geriatr Soc 2010;58:1311–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li G, Higdon R, Kukull W, et al. Statin therapy and risk of dementia in the elderly: a community-based prospective cohort study. Neurology 2004;63:1624–8. [DOI] [PubMed] [Google Scholar]
- [29].Ancelin M, Carrière I, Barberger-Gateau P, et al. Lipid lowering agents, cognitive decline, and dementia: the three-city study. J Alzheimers Dis 2012;30:629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jick H, Zornberg G, Jick S, et al. Statins and the risk of dementia. Lancet 2000;356:1627–31. [DOI] [PubMed] [Google Scholar]
- [31].Hendrie H, Hake A, Lane K, et al. Statin use, incident dementia and Alzheimer disease in elderly African Americans. Ethn Dis 2015;25:345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hajjar I, Schumpert J, Hirth V, et al. The impact of the use of statins on the prevalence of dementia and the progression of cognitive impairment. J Gerontol A Biol Sci Med Sci 2002;57:M414–418. [DOI] [PubMed] [Google Scholar]
- [33].Haag M, Hofman A, Koudstaal P, et al. Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam Study. J Neurol Neurosurg Psychiatry 2009;80:13–7. [DOI] [PubMed] [Google Scholar]
- [34].Cramer C, Haan M, Galea S, et al. Use of statins and incidence of dementia and cognitive impairment without dementia in a cohort study. Neurology 2008;71:344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Corrao G, Ibrahim B, Nicotra F, et al. Long-term use of statins reduces the risk of hospitalization for dementia. Atherosclerosis 2013;230:171–6. [DOI] [PubMed] [Google Scholar]
- [36].Chou C, Chou Y, Chou Y, et al. Statin use and incident dementia: a nationwide cohort study of Taiwan. Int J Cardiol 2014;173:305–10. [DOI] [PubMed] [Google Scholar]
- [37].Bettermann K, Arnold A, Williamson J, et al. Statins, risk of dementia, and cognitive function: secondary analysis of the ginkgo evaluation of memory study. J Stroke Cerebrovasc Dis 2012;21:436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Arvanitakis Z, Schneider J, Wilson R, et al. Statins, incident Alzheimer disease, change in cognitive function, and neuropathology. Neurology 2008;70(19 pt 2):1795–802. [DOI] [PubMed] [Google Scholar]
- [39].Chen J, Chang C, Chang T, et al. Effects of statins on incident dementia in patients with type 2 DM: a population-based retrospective cohort study in Taiwan. PLoS One 2014;9:e88434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ritchie K, Lovestone S. The dementias. Lancet 2002;360:1759–66. [DOI] [PubMed] [Google Scholar]
- [41].Scarpini E, Scheltens P, Feldman H. Treatment of Alzheimer's disease: current status and new perspectives. Lancet Neurol 2003;2:539–47. [DOI] [PubMed] [Google Scholar]
- [42].Mathis M, Muoio B, Andreason P, et al. The US Food and Drug Administration's perspective on the new antipsychotic pimavanserin. J Clin Psychiatry 2017;78:e668–73. [DOI] [PubMed] [Google Scholar]
- [43].[No authors listed]. Practice guideline for the treatment of patients with Alzheimer's disease and other dementias of late life. American Psychiatric Association. Am J Psychiatry 1997;154(5 suppl):1–39. [DOI] [PubMed] [Google Scholar]
- [44].Wong W, Lin V, Boudreau D, et al. Statins in the prevention of dementia and Alzheimer's disease: a meta-analysis of observational studies and an assessment of confounding. Pharmacoepidemiol Drug Saf 2013;22:345–58. [DOI] [PubMed] [Google Scholar]
- [45].Rojas-Fernandez C, Hudani Z, Bittner V. Statins and cognitive side effects: what cardiologists need to know. Cardiol Clin 2015;33:245–56. [DOI] [PubMed] [Google Scholar]
- [46].Petanceska S, DeRosa S, Sharma A, et al. Changes in apolipoprotein E expression in response to dietary and pharmacological modulation of cholesterol. J Mol Neurosci 2003;20:395–406. [DOI] [PubMed] [Google Scholar]
- [47].Reed B, Villeneuve S, Mack W, et al. Associations between serum cholesterol levels and cerebral amyloidosis. JAMA Neurol 2014;71:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kandiah N, Feldman H. Therapeutic potential of statins in Alzheimer's disease. J Neurol Sci 2009;283:230–4. [DOI] [PubMed] [Google Scholar]
- [49].Refolo L, Pappolla M, LaFrancois J, et al. A cholesterol-lowering drug reduces beta-amyloid pathology in a transgenic mouse model of Alzheimer's disease. Neurobiol Dis 2001;8:890–9. [DOI] [PubMed] [Google Scholar]
- [50].Gibson Wood W, Eckert G, Igbavboa U, et al. Amyloid beta-protein interactions with membranes and cholesterol: causes or casualties of Alzheimer's disease. Biochim Biophys Acta 2003;1610:281–90. [DOI] [PubMed] [Google Scholar]
- [51].Sun Y, Crisby M, Lindgren S, et al. Pravastatin inhibits pro-inflammatory effects of Alzheimer's peptide Abeta (1–42) in glioma cell culture in vitro. Pharmacol Res 2003;47:119–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


