Abstract
The aim of this study was to compare image quality of low tube voltage cerebral computed tomography angiography (CTA) reconstructed with knowledge-based iterative model reconstruction (IMR), filtered back projection (FBP), and hybrid iterative reconstruction (HIR).
A total of 101 patients with suspected cerebrovascular diseases were enrolled and randomized into 2 groups, 100 kVp tube voltage (n = 53) and reduced tube voltage (80 kVp) (n = 48). Computed tomography data were reconstructed with IMR, FBP, and HIR algorithms. The image noise, vascular attenuation, signal-to-noise ratio (SNR), and contrast-to-noise ratio (CNR) were measured and calculated. Two blinded radiologists independently evaluated image quality based on diagnostic confidence on a 3-point scale. Quantitative and qualitative assessments were compared between different groups and reconstruction subgroups.
Vascular attenuation was higher in the reduced tube voltage group than in 100-kVp tube voltage group, but showed no significant difference within each group. In both groups, the image noise, vascular SNR, and CNR were significantly improved by IMR as compared with FBP and HIR. Inter-group comparison indicated that IMR with reduced tube voltage showed better image quality with lower image noise and higher vascular SNR and CNR than FBP and HIR at 100 kVp, but slightly inferior to IMR at 100 kVp. IMR also yields the best qualitative image quality, and improves the diagnostic confidence of atherosclerosis and aneurysm. Compared with the standard 120-kVp protocol (1.86mSv), the radiation doses of 100 kVp (1.13mSv) and 80 kVp (0.56mSv) were 39% and 70% less, respectively.
The quantitative and qualitative image quality obtained by IMR was superior to that obtained by FBP and HIR for low tube voltage cerebral CTA.
Keywords: computed tomography angiography, filtered back projection, hybrid iterative reconstruction, image quality, iterative model reconstruction
1. Introduction
Computed tomography angiography (CTA) has been widely used as a noninvasive diagnostic imaging modality for the detection of cerebrovascular diseases. However, the potential risk associated with radiation exposure from CT scans has raised concerns.[1–3] Various approaches have been attempted to reduce the radiation dose, including reductions in tube voltage and current, and innovations of reconstruction techniques.
Filtered back projection (FBP) is a fast and robust reconstruction method, but the major defect is the significant increase in image noise with the reduction of the radiation dose. Therefore, several new methods based on iterative reconstruction algorithms have been proposed as an alternative to FBP for reducing image noise. These hybrid iterative reconstruction (HIR) algorithms offer better image quality and have been used as clinical routine reconstruction methods.[4–6]
Recently, knowledge-based iterative model reconstruction (IMR) has become available in clinical practice as a fully iterative algorithm. Based on a knowledge-based approach through iterative minimization of the difference between measured raw data and the estimated image via a penalty-based cost function, IMR has the potential to improve image quality with the same radiation dose or reduce radiation dose by decreasing noise.[7,8] It has been reported that IMR significantly reduced image noise and improved overall image quality in head and neck CTA using the standard tube voltage of 120 kVp.[9] A previous study showed that cerebral CTA with 100 kVp and adaptive iterative reconstruction provide sufficient image quality with a lower radiation dose.[10] However, there is no consensus whether cerebral CTA at 80 kVp can be accepted because of the known potential disadvantage of increased noise. On this basis, the purpose of our study was to evaluate the quantitative and qualitative image quality with IMR, FBP, and HIR for cerebral CTA at low tube voltage levels.
2. Material and methods
2.1. Study population
This prospective study was approved by our institutional review board. Informed consent was obtained from all patients before data colloection. All available data were contained in the article. We enrolled a total of 114 consecutive patients between March and September 2016. Inclusion criteria for this study were the following: age >18; clinically suspected cerebrovascular diseases; clinical referral for cerebral CTA. Exclusion criteria were renal dysfunction (glomerular filtration rate <40 mL/min), previous reaction to the iodinated contrast material, pregnancy, cerebral artery occlusion, intracranial clipping, and coiling that may affect computed tomography (CT) measurements. Patients were randomized to tube voltages of 100 kVp (group A) and 80 kVp (group B).
2.2. CT data acquisition
All cerebral CTA examinations were performed on a 256-slice CT scanner (Brilliance-iCT Elite FHD; Philips Healthcare, Cleveland, OH). In group A, we used a tube voltage of 100 kVp and a tube current of 220 mAs. In group B, the tube voltage was decreased to 80 kVp and the tube current remained unchanged. Data acquisition was acquired by axial scanning, with a collimation of 128 × 0.625 mm, gantry rotation time of 0.75 seconds, table feed per rotation of 80 mm, scanning range of 160 mm that encompassed the whole brain. Fifty microliters contrast agent (iopromide, 370 mg iodine/ml, Ultravist; Bayer Schering Pharma, Guangzhou, China) was injected into the antecubital vein at a flow rate of 5 mL/s, immediately followed by 30 mL of saline flash at the same injection rate. Images were reconstructed with a 0.8-mm section thickness and 0.4-mm increments using FBP, HIR (iDose4-level 4), and IMR (IMR-level 1-standard) techniques, respectively.
2.3. Radiation dose
The automatically calculated radiation dose parameters including volume CT dose index (CTDIvol) and dose-length product (DLP) were 16.9 mGy, 540.0 mGy·cm for group A, 8.3 mGy, 267.2 mGy·cm for group B, and 27.6 mGy, 884.4 mGy·cm for the standard 120 kVp protocol as a reference. Effective dose (ED) was calculated by multiplying the DLP with the international commission on radiological protection conversion factor for head CT imaging (k = 0.0021 mSv/mGy/cm).
2.4. Quantitative image analysis
Quantitative measurements were performed on Philips Intellispace Portal workstation (Philips Medical Systems Netherlands B.V.). One reader (X.W. with 4 years’ experience in neuroimaging) manually drew circular regions of interest (ROIs) as large as possible in the center of the main cerebral arteries on axial images. Care was taken to avoid atherosclerotic plaque and false registration of the vessel wall (Fig. 1). ROIs were placed in the cavernous segment of the internal cerebral artery (ICA), the M1 segment of the middle cerebral artery (MCA), the trunk of the basilar artery (BA), and the V4 segment of the vertebral artery (VA). Image noise was determined as the standard deviation of the CT attenuation of the brain parenchyma. All measurements were performed 3 times bilaterally for each patient and the averages were used for further calculation. Signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) were calculated as: SNR = vascular attenuation/image noise and CNR = vaucular contrast/image noise. Vascular contrast was calculated as the average vascular attenuation minus the average brain parenchyma attenuation.
Figure 1.
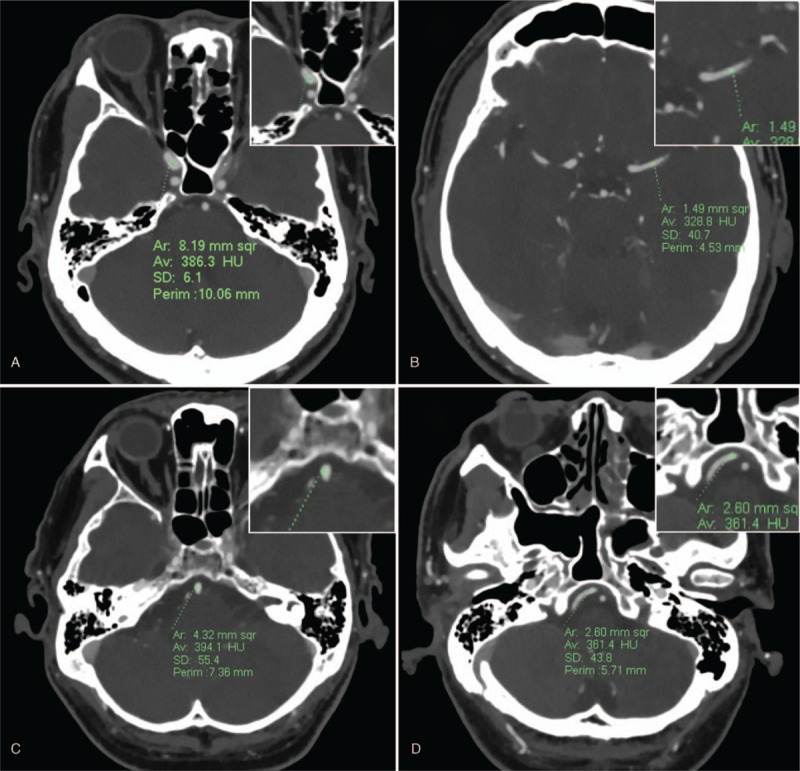
Region of interest placement in target vessels. Transverse computed tomography images show ROI placement in the right internal cerebral artery (A), left middle cerebral artery (B), basilar artery trunk (C), and right vertebral artery (D).
2.5. Qualitative image analysis
All examinations were randomized and independently evaluated by 2 readers (X.W. and J.L. with 4 and 6 years’ experience) who were blinded to patient data and technical parameters. Multiplanar reconstruction, maximum intensity projection, and volume-rendered images were used to evaluate the image quality based on diagnostic confidence using a 3-point scale (1 = poor quality, non-diagnostic; 2 = moderate image quality, diagnostic; 3 = good image quality, good diagnostic confidence).[11] Discordances between the 2 readers were resolved by consensus.
2.6. Statistical analysis and sample size calculation
Statistical analyses were performed using SPSS software (SPSS, version 19, SPSS Inc, Chicago, IL). Data were tested for normality using the Shapiro-Wilk test. Continuous data were expressed as mean ± standard deviation and categorical data were expressed as counts. Student t and χ2 tests were performed to assess differences in demographic data. CT parameters, including noise, vascular attenuation, SNR, and CNR, were compared between the 2 groups and within each group among various reconstruction subgroups using analysis of variance. If a statistical difference was present, post hoc tests were performed using Turkey test. The diagnostic confidence scores were compared using the Kruskal-Wallis test and χ2 test. Inter-reader agreement of overall image quality was evaluated using Cohen κ. A κ value >0.60 was used to indicate good agreement. A P value <0.05 was considered statistically different.
In subsets of 20 patients in Group A and B, another reviewer measured the noise, vascular attenuation, and SNR/CNR on the images reconstructed by 3 methods. The reproducibility was described by coefficient of variation (CV). For each measurement, sample size calculation was based on a 2-sample unpaired t test with 90% power and 5% significance level (2-tailed) as adopted from a previous study.[12] Sample size needed to detect 5% changes in each measurement was calculated.
3. Results
3.1. Patient demographics
Of the 114 initially enrolled patients, 13 patients were ineligible because of cerebral artery occlusion (n = 9) and intracranial clipping and coiling (n = 4), and thus 101 patients (mean age 63 ± 12 years) were included for final analysis. Group A included 32 men and 21 women with a mean age of 64 ± 14 years, and Group B included 33 men and 15 women with a mean age of 62 ± 11 years. There was no significant difference in patient demographics between the 2 groups.
3.2. Quantitative image analysis
Table 1 shows the vascular attenuation, SNR, CNR, and the image noise for the 2 groups and various reconstruction subgroups. The mean vascular attenuation was higher in group B than in group A (P < .001), but there was no difference within each group (P = .56 for group A and P = .19 for group B). In group A, the image noise was decreased by 63% and 45%, and the vascular SNR and CNR were increased over 160% and 80% compared with FBP and HIR. In group B, the image noise was decreased by 58% and 38%, and the vascular SNR and CNR were increased over 120% and 50% compared with FBP and HIR. Bar charts for the image noise, mean vascular attenuation, SNR, and CNR of the 2 groups are shown in Figure 2. Intergroup comparison showed that images reconstructed with IMR in group B had better image quality with lower image noise and higher vascular SNR and CNR than images reconstructed with FBP and HIR in group A, but slightly inferior to IMR in normal-dose group (all P < .001).
Table 1.
Quantitative image quality analysis.
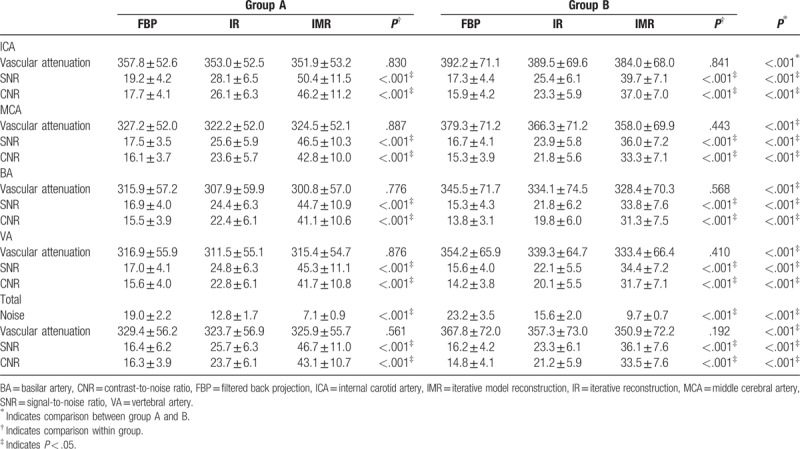
Figure 2.
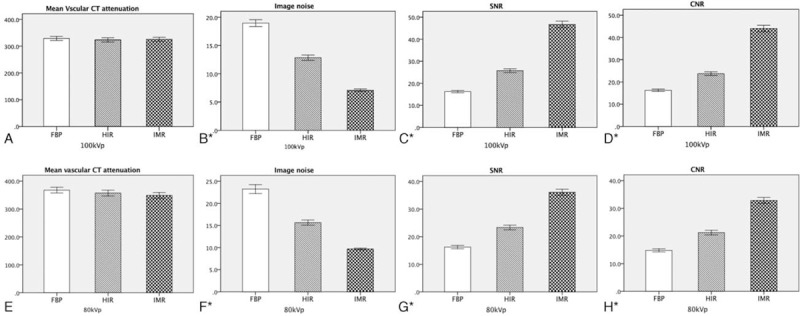
Bar charts for mean and standard deviation of the vascular CT attenuation, image noise, SNR and CNR of group A and group B. The vascular CT attenuation value shows no significant difference between FBP, hybrid IR and IMR. The image noise with IMR was much lower than FBP and hybrid IR. The vascular SNR and CNR were increased noticeably with IMR. ∗ Indicates analysis of variance test P <.05. CNR = contrast-to-noise ratio, CT = computed tomography, FBP = filtered back projection, IMR = iterative model reconstruction, IR = iterative reconstruction, SNR = signal-to-noise ratio.
3.3. Qualitative image analysis
Table 2 shows the diagnostic confidence of each group and subgroup. IMR in group A showed best overall image quality, followed by IMR in group B as second best, and then HIR in group A and B, FBP in group A, and FBP in group B (P < .001). Representative cases are shown in Figures 3 to 5. IMR improved the diagnostic confidence of atherosclerosis and aneurysm as compared with FBP and HIR. The inter-reader agreement for the diagnostic confidence was good, with a κ value of 0.75.
Table 2.
Qualitative image quality analysis.
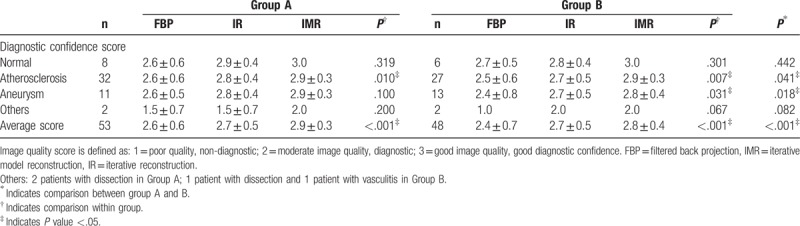
Figure 3.
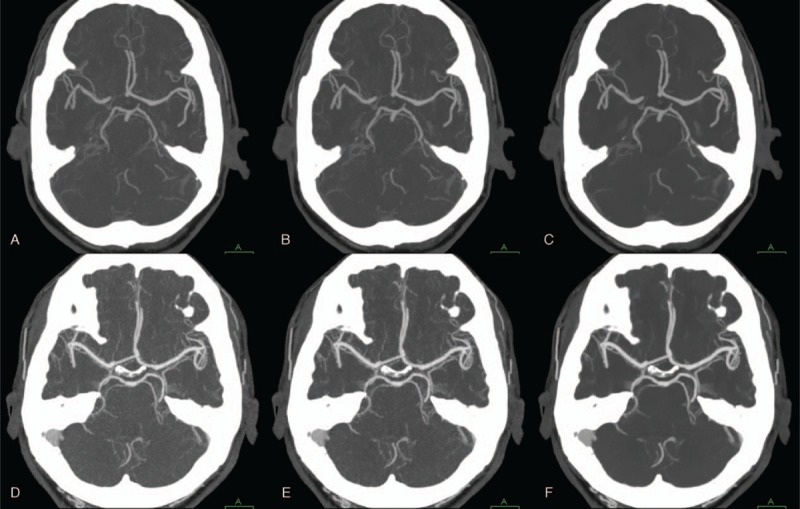
Cerebral CTA performed at 100 kVp (A–C) and 80kVp (D–F) with 3 different reconstruction algorithms (A, D: FBP; B, E: hybrid IR; C, F: IMR). Vascular attenuation at 80 kVp is higher than that at 100 kVp. Maximum intensity projection images with IMR show better visualization of the circle of Willis at both 100 kVp and 80 kVp compared with FBP and hybrid IR. CTA = computed tomography angiography, FBP = filtered back projection, IMR = iterative model reconstruction, IR = iterative reconstruction.
Figure 5.
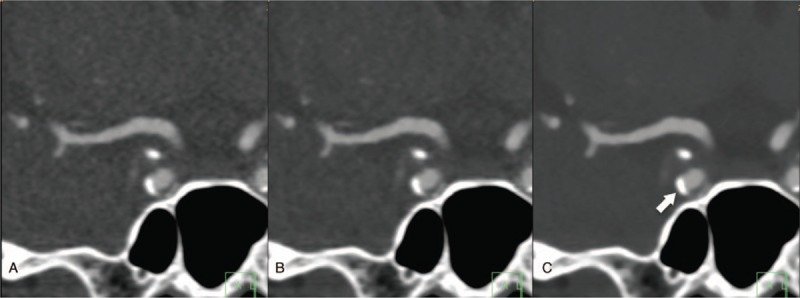
Coronal CT images of a calcified ICA plaque reconstructed with FBP (A), hybrid IR (B), and IMR (C) at 100 kVp. Images reconstructed with IMR show lower image noise compared with FBP and hybrid IR. The atherosclerotic plaque contour is clearly identified with IMR (arrow). CT = computed tomography, FBP = filtered back projection, IMR = iterative model reconstruction, IR = iterative reconstruction.
Figure 4.
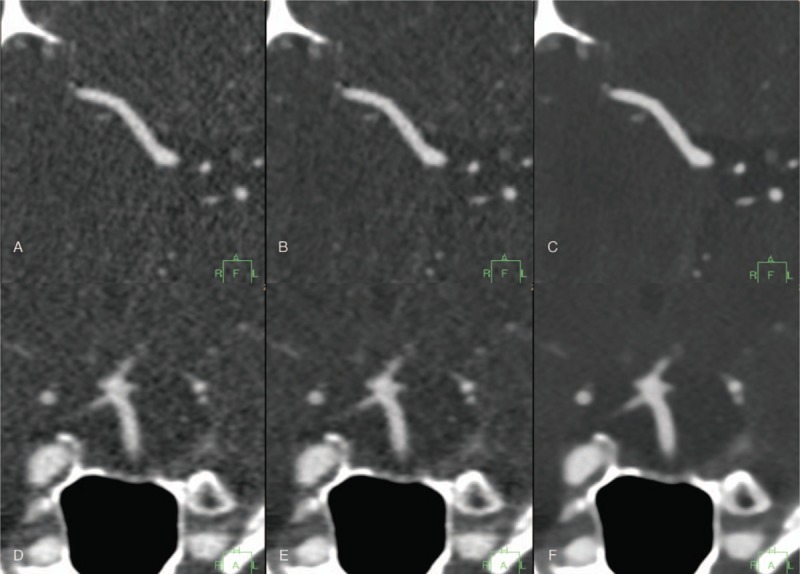
Transverse CT images of the right middle cerebral artery (A–C) and coronal CT images of the basilar artery (D–F) reconstructed with FBP (A, D); hybrid IR (B, E); and IMR (C, F) at 80 kVp. Images reconstructed with IMR offer significant noise reduction and smoother vascular structure compared with FBP and hybrid IR. CT = computed tomography, FBP = filtered back projection, IMR = iterative model reconstruction, IR = iterative reconstruction.
3.4. Radiation dose
The EDs were 1.13 mSv for group A, 0.56 mSv for group B, and 1.86 mSv for the standard 120 kVp protocol as a reference. Compared with the standard 120 kVp protocol, the EDs of groups A and B were decreased by 39% and 70%, respectively.
3.5. Sample size calculation
The average CV values of 3 reconstruction methods for measurements of vascular attenuation, image noise, SNR, and CNR were 1.0%, 3.9%, 4.7%, and 4.8% for group A, and 0.9%, 4.1%, 5.0%, and 5.1% for group B. Considering the maximal CV of 4.8% and 5.1%, sample sizes of 39 in group A and 44 in group B were required to detected 5% difference among 3 reconstruction methods. These calculated sample sizes were smaller than the size of our current study population. Considering the differences among 3 methods were >20%, the statistical power in this study was sufficient.
4. Discussion
Our study demonstrated that IMR effectively reduced image noise and improved image quality for low tube voltage cerebral CTA. IMR also yielded best overall image quality and increased the diagnostic confidence of atherosclerosis and aneurysm.
Currently, there is interest in obtaining qualified diagnostic images while reducing radiation exposure to as low as reasonably achievable. Using a decreased tube voltage is one effective and common way of reducing radiation dose. In our study, compared with the standard 120-kVp scan protocol, cerebral CTA using tube voltage of 80 kVp and 100 kVp could achieve 70% and 39% reduction in radiation dose, respectively. However, the increased image noise is a major disadvantage associated with low tube voltage CT.
The use of iterative reconstruction methods is capable of reducing image noise and improving overall image quality by incorporating statistics-model based denoising into raw and image data space.[13,14] Given this method, the latest generation of IR, knowledge-based IMR, provides an optimization process that takes into account the data statistics, image statistics, and system models. Phantom tests demonstrate that IMR enable a 1.2× to 1.7× high-contrast detectability, a 70% to 83% noise reduction and a 60% to 80% radiation reduction compared with FBP, according to a white paper.[15] Niesten et al[9] evaluated the effectiveness of IMR for cerebral CTA and indicated that IMR significantly improved the overall image quality and reduced image noise when using a conventional 120 kVp protocol. In the present study, we used low tube voltage of 100 kVp and 80 kVp for cerebral CTA. Quantitative and qualitative assessments revealed that IMR yielded superior image quality compared with FBP and HIR, and IMR would enhance the diagnostic confidence of atherosclerosis and aneurysm to some extent. In addition, the quantitative image quality obtained with 80 kVp and IMR was better than that obtained with 100 kVP and FBP/HIR. This improvement indicated that the IMR algorithm, by its global optimization, had the potential to be used with low tube voltage cerebral CTA without sacrificing image quality.
Lowering tube voltage resulted in an increase in vascular contrast enhancement because of the characteristic absorption spectrum of iodine with markedly higher attenuation at lower photon energies, where the x-ray energy approaches the K-edge of iodine.[16,17] In our study, reducing the tube voltage to 80 kVp increased the vascular attenuation by around 10% compared with 100 kVp using the same contrast media injection protocol. As cerebral CTA with 80 kVp and IMR showed lower image noise and higher vascular attenuation, it might be practicable to decrease the contrast agent volume while maintaining a reasonable SNR and CNR, and thus reduce the possible adverse effects of the contrast materials. A previous study has examined this potential benefit of low tube voltage (100- and 80-kVp) and low contrast material volume (30 mL, 300 mg I/mL) for cerebral CTA with evidence of maintained diagnostic accuracy for aneurysm detection.[18] We posited that cerebral CTA with IMR algorithm enables further radiation dose and contrast media reduction.
Although data processing of IMR algorithm is far more complicated than FBP/HIR and requires more advanced computational capacity, the reconstruction time could be shortened to approximately 5 minutes per series by optimizing both the algorithms and reconstruction hardware, which makes IMR an acceptable reconstruction method in routine clinical practice. A previous published study mentioned the disadvantages of the full-IR technique of pixelated blotchy appearance and lack of familiarity for radiologists.[19] We found that the unfamiliar texture with IMR did exist but did not negatively affect our study.
There are several limitations in this study. First, the image quality at different tube voltage was not compared intraindividually, because it was unethical and would increase the patient's radiation exposure. However, patients were consecutively enrolled and randomly assigned in our study, and there was no significant difference in demographic data between the 2 groups. Second, as previous studies proved the feasibility of cerebral CTA at 100 kVp and this protocol was used routinely in our center, we did not set up a standard control at 120 kVp. Third, the proportion of cerebrovascular diseases in our study was unbalanced (atherosclerosis and aneurysm over 80%), thereby influencing the accuracy of diagnostic confidence analysis.
In conclusion, our study demonstrated that the IMR significantly reduced image noise and improved image quality as compared with FBP and HIR for low tube voltage cerebral CTA. It is feasible to use an 80 kVp and IMR protocol to provide satisfactory image quality and reduce radiation dose further.
Author contributions
Conceptualization: Tao Jiang, Jianping Lu.
Data curation: Xinrui Wang, Jing Li.
Formal analysis: Xinrui Wang, Chengcheng Zhu, Jing Li.
Methodology: Chengcheng Zhu, Andrew J. Degnan, Tao Jiang, Jianping Lu.
Supervision: Tao Jiang, Jianping Lu.
Writing – original draft: Xinrui Wang.
Writing – review & editing: Chengcheng Zhu, Andrew J. Degnan, Tao Jiang, Jianping Lu.
Footnotes
Abbreviations: BA = basilar artery, CNR = contrast-to-noise ratio, CTA = computed tomography angiography, CTDIvol = volume CT dose index, DLP = dose-length product, ED = effective radiation dose, FBP = filtered back projection, HIR = hybrid iterative reconstruction, ICA = internal cerebral artery, IMR = iterative model reconstruction, MCA = middle cerebral artery, SNR = signal-to-noise ratio, VA = vertebral artery.
This work was supported by the National Natural Science Foundation of China (No.31470910, No.81402680, No. 81371551). The sponsoring organization had no involvement in the study design, data collection, data analysis, data interpretation, writing of the article or decision to submit for publication.
The authors report no conflicts of interest.
References
- [1].Brenner DJ, Hall EJ. Computed tomography: an increasing source of radiation exposure. N Engl J Med 2007;357:2277–84. [DOI] [PubMed] [Google Scholar]
- [2].Sodickson A, Baeyens PF, Andriole KP, et al. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology 2009;251:175–84. [DOI] [PubMed] [Google Scholar]
- [3].Goodman DM. Initiatives focus on limiting radiation exposure to patients during CT scans. JAMA 2013;309:647–8. [DOI] [PubMed] [Google Scholar]
- [4].Hou Y, Liu X, Xv S, et al. Comparisons of image quality and radiation dose between iterative reconstruction and filtered back projection reconstruction algorithms in 256-MDCT coronary angiography. AJR Am J Roentgenol 2012;199:588–94. [DOI] [PubMed] [Google Scholar]
- [5].Leipsic J, Labounty TM, Heilbron B, et al. Adaptive statistical iterative reconstruction: assessment of image noise and image quality in coronary CT angiography. AJR Am J Roentgenol 2010;195:649–54. [DOI] [PubMed] [Google Scholar]
- [6].Löve A, Siemund R, Höglund P, et al. Hybrid iterative reconstruction algorithm improves image quality in craniocervical CT angiography. AJR Am J Roentgenol 2013;201:W861–6. [DOI] [PubMed] [Google Scholar]
- [7].Oda S, Utsunomiya D, Funama Y, et al. A knowledge-based iterative model reconstruction algorithm: can super-low-dose cardiac CT be applicable in clinical settings? Acad Radiol 2014;21:104–10. [DOI] [PubMed] [Google Scholar]
- [8].Yuki H, Utsunomiya D, Funama Y, et al. Value of knowledge-based iterative model reconstruction in low-kV 256-slice coronary CT angiography. J Cardiovasc Comput Tomogr 2014;8:115–23. [DOI] [PubMed] [Google Scholar]
- [9].Niesten JM, van der Schaaf IC, Vos PC, et al. Improving head and neck CTA with hybrid and model-based iterative reconstruction techniques. Clin Radiol 2016;70:1252–9. [DOI] [PubMed] [Google Scholar]
- [10].Yu S, Zhang L, Zheng J, et al. A comparison of adaptive iterative dose reduction 3D and filtered back projection in craniocervical CT angiography. Clin Radiol 2017;72:e1–96. [DOI] [PubMed] [Google Scholar]
- [11].Boudabbous S, Arditi D, Paulin E, et al. Model-Based Iterative Reconstruction (MBIR) for the Reduction of Metal Artifacts on CT. AJR Am J Roentgenol 2015;205:380–5. [DOI] [PubMed] [Google Scholar]
- [12].Zhang X, Zhu C, Peng W, et al. Scan-rescan reproducibility of high resolution magnetic resonance imaging of atherosclerotic plaque in the middle cerebral artery. Plos One 2015;10:e0134913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gervaise A, Osemont B, Lecocq S, et al. CT image quality improvement using adaptive iterative dose reduction with wide-volume acquisition on 320-detector CT. Eur Radiol 2012;22:295–301. [DOI] [PubMed] [Google Scholar]
- [14].Komlosi P, Zhang Y, Leivasalinas C, et al. Adaptive statistical iterative reconstruction reduces patient radiation dose in neuroradiology CT studies. Neuroradiology 2014;56:187–93. [DOI] [PubMed] [Google Scholar]
- [15].Mehta D, Thompson R, Morton T, et al. Iterative model reconstruction: Simultaneously lowered computed tomography radiation dose and improved image quality. Med Phys Int J 2013;2:147–55. [Google Scholar]
- [16].Kondratyev E, Karmazanovsky G. Low radiation dose 256-MDCT angiography of the carotid arteries: effect of hybrid iterative reconstruction technique on noise, artifacts, and image quality. Eur J Radiol 2013;82:2233–9. [DOI] [PubMed] [Google Scholar]
- [17].Huda W, Lieberman KA, Chang J, et al. Patient size and x-ray technique factors in head computed tomography examinations. II. Image quality. Med Phys 2004;31:595–601. [DOI] [PubMed] [Google Scholar]
- [18].Luo S, Zhang LJ, Meinel FG, et al. Low tube voltage and low contrast material volume cerebral CT angiography. Eur Radiol 2014;24:1677–85. [DOI] [PubMed] [Google Scholar]
- [19].Deák Z, Grimm JM, Treitl M, et al. Filtered back projection, adaptive statistical iterative reconstruction, and a model-based iterative reconstruction in abdominal CT: an experimental clinical study. Radiology 2013;266:197–206. [DOI] [PubMed] [Google Scholar]


