Abstract
We aim to investigate the mutation types of glucose-6-phosphate dehydrogenase (G6PD) deficiency in Chinese Han children in eastern Fujian Province.
A total of 904 Chinese Han neonates (male: 733 with positive G6PD deficiency and 28 with weakly positive deficiency; female: 73 with positive G6PD deficiency and 70 with weakly positive deficiency) received G6PD screening in our center from January 2014 to December 2016 were included in this study. Additionally, 904 age-matched normal Chinese Han individuals (male: 761; female: 143) were selected as control. Neonatal G6PD deficiency screening was performed through blood sample collection from the heels, using the commercial kits. Multicolor melting curve analysis (MMCA) method was used to determine the G6PD mutation type in the 904 cases. If it failed to detect mutations in the cases with abnormal enzyme activity, the polymerase chain reaction (PCR) and gene sequencing were used to determine the mutation sites. PCR and gene sequencing were used to determine the mutation sites in the 904 individuals with normal enzyme activity. Three most common mutation types in Chinese population were compared between Fujian and other provinces.
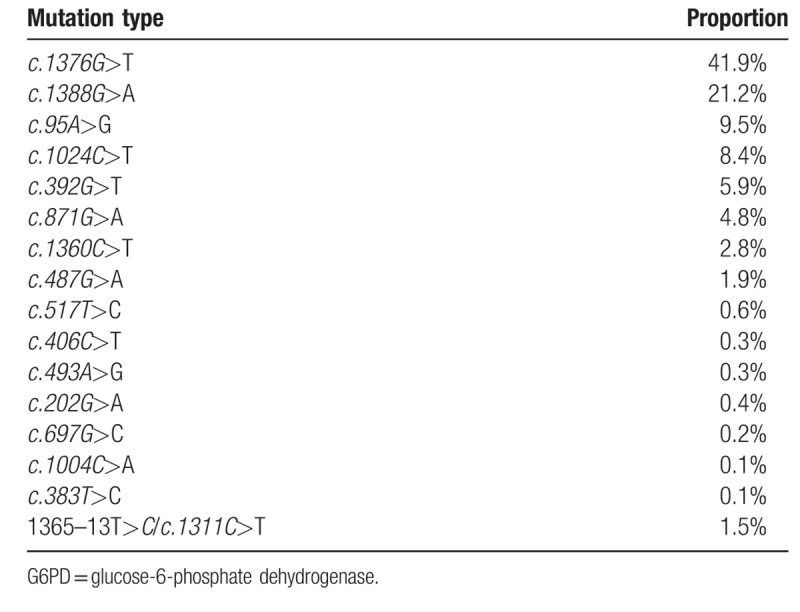
Among the 904 neonates with abnormal G6PD enzyme activity, 17 mutation types were detected including 15 single point mutations and 7 complex mutations. Three most common mutation types were c.1376G > T, c.1388G > A, and c.95A > G accounted for 72.6% of the total mutations in eastern Fujian.
The proportion of mutational types in G6PD and the degree of enzyme activity change in various mutational types were found in the neonates of Fujian Province. Our study may enrich the molecular diagnosis of G6PD deficiency meaning Fujian Province.
Keywords: gene mutation, gene sequencing, glucose-6-phosphate dehydrogenase, multicolor melting curve analysis, polymerase chain reaction
1. Introduction
Glucose-6-phosphate dehydrogenase (G6PD) deficiency, is an X-linked incomplete dominant genetic disease mainly characterized by hemolysis caused by the decreased G6PD activity or the changed enzyme properties in erythrocytes.[1] Males are hemizygous for having only one X chromosome. Females, with 2 X chromosomes, may be heterozygous or homozygous having less severe clinical manifestations. To date, more than 400 G6PD biochemical mutants have been reported worldwide, and more than 140 kinds of G6PD mutations have been identified with at least 31 mutations identified in Chinese population.[2,3] Nowadays, rare studies have been focused on G6PD mutations in Han children in China mainland. In this study, we aim to investigate the mutation type of G6PD gene deficiency in Chinese Han children in eastern Fujian Pronvince.
2. Patients and methods
2.1. Patients
From January 2014 to December 2016, we screened 200,260 G6PD samples. In total, 1813 samples (male: 1489; female: 324) with decrease in G6PD activity were included. Using the random digits table, a total of 904 Chinese Han neonates (male: 733 with positive G6PD deficiency and 28 with weakly positive deficiency; female: 73 with positive G6PD deficiency and 70 with weakly positive deficiency) received G6PD screening using fluorimetric method in Fujian Province were randomly selected in this study. Their parents are Chinese Han in Fujian confirmed by telephone. Among these positive cases, 524 males and 15 females showed a G6PD concentration of 0 to 1.0 U/g Hb, while 209 males and 58 females showed a concentration of 1.0 to 2.1 U/g Hb. Among the patients with weakly positive deficiency, 28 males and 70 females showed a G6PD concentration of 2.1 to 2.6 U/g Hb. Besides, 904 age-matched normal Chinese Han individuals (male: 761; female: 143) were selected as control. The G6PD activity was > 2.6 U/g Hb. Written consent was obtained from the patients’ guardians. The study protocols were approved by the Ethical Committee of the Fujian Provincial Maternity and Children's Hospital, Affiliated Hospital of Fujian Medical University (No. 2015069).
Blood specimens were collected from the heels, and then were dripped on special filter paper (Schleicher and Schull 903, Wortmann Co., Britain). Three blood spots with diameters of 10 to 12 mm were used for multicolor melting curve analysis (MMCA) detection. Two tubes of EDTA-K2 anticoagulant vein blood (1 mL) were used for G6PD activity determination, genomic DNA extraction, polymerase chain reaction (PCR), and gene sequencing. All the G6PD deficiency cases showed no following aspects after a face-to-face conversation with their parents: eating fava bean; contacting the mothballs; and drug-induced hemolysis.
Subjects were divided into 2 groups according to the screening results, including G6PD deficiency group and normal control. They were numbered according to the birth time, and selected according to the random number table. Blood samples were collected for laboratory tests.
2.2. Determination of G6PD enzymatic activity
G6PD enzymatic activity was measured with G6P/6PG ratio method using the commercial kit (Mickey Co. Ltd, Guangzhou, China) according to the manufacturer's instructions. DNA was extracted from dried blood spots. Three blood spots with a diameter of 3.2 mm were made with a punch. DNA was extracted from the dried blood specimens obtained from 904 cases with aberrant G6PD activity based on the Lab-Aid 820 Nucleic Acid Extraction System (Zeesan Biotech, Xiamen, China).
2.3. PCR amplification
DNA samples isolated from cases with G6PD deficiency were detected using commercial kit (Access Bio, NJ) according to the manufacturer's instructions. This kit could be used to qualitatively detect the 12 common G6PD mutations in human peripheral blood genomic DNA in China: c.95A > G, c.383T > C, c.392G > T, c.487G > A, c.517T > C, c.592C > T, c.871G > A, c.1004C > A, c.1024C > T, c.1360C > T, c.1376G > T, and c.1388G > A.
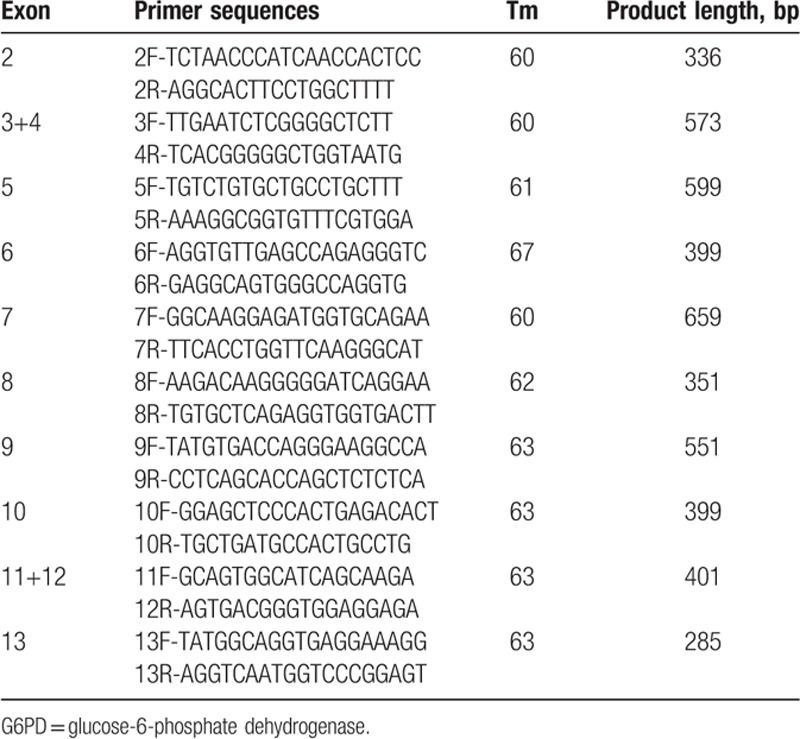
Ten pairs of PCR primers (Table 1) were designed for amplifying exon 2 to 13 (exon 1 does not encode protein) and partial introns using Primer Premier 5.0 according to the NCBI data (G6PD:NG_009015) and the previous description.[4] PCR amplification was carried out in a 25 μL volume containing 1 μL each primer, 2 μL DNA template, 8.5 μL double distilled water, and 12.5 μL TIANGEN PCR Master Mix, based on the following reaction conditions: 94°C for 5 minutes, followed by 35 cycles of 94°C for 1 minute, 60–67°C for 1 minute and 72°C for 1 minute. A final extension of 10 minutes at 72°C completed the reaction. PCR products were sent to Sangon (Shanghai, China) for DNA sequencing.
Table 1.
Primers sequence, amplification length, and temperature for G6PD.
2.4. Statistical analysis
All data were analyzed by using SPSS 17.0. As the number of homozygous and heterozygous mutations in females was small, the G6P/6PGD was compared between patients in the 6 kinds of common G6PD hemizygous mutation in Fujian. Wicoxon rank sum test was used for the comparison between patients as the above results presented non-normal distribution. Chi-square test was used for the comparison of the most common 3 G6PD mutations between eastern Fujian and other provinces in China. P < .05 was considered to be statistically significant.
3. Results
3.1. Determination of G6PD enzymatic activity
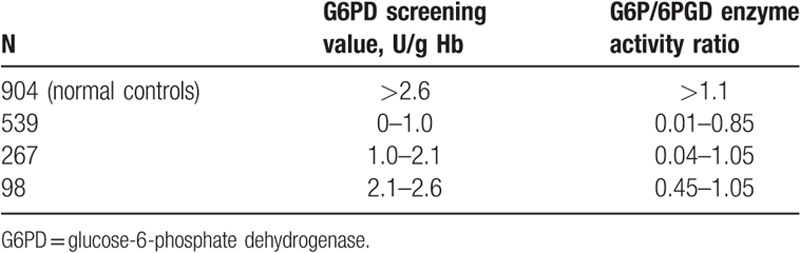
G6P/6PGD enzyme activity ratio was < 0.95 in 748 males and 104 females. A range of 0.95 to 1.05 was detected in 13 males and 39 females. According to the manufacturer's instructions, it was considered as positive in the presence of G6P/6PGD enzyme activity ratio of<0.95, and suspicious positivity in a range of 0.95 to 1.05. A value of 1.1 was detected in normal individuals. Results of G6PD enzymatic activity determination were consistent with the results of neonatal screening for G6PD activity in males. G6P/6PGD enzyme activity ratio was greater than 1.1 in the 904 normal individuals (Table 2).
Table 2.
Relation between G6PD screening value and G6PD activity value in 1808 children.
3.2. Sequencing results
PCR and gene sequencing results showed that there was no mutation in the exon and intron of G6PD in the 904 normal individuals. Results of MMCA showed that of the 904 patients with G6PD enzyme abnormality, 11 G6PD mutations including c.1376G > T, c.1388G > A, c.95A > G, c.1024C > T, c.392G > T, c.871G > A, c.1360C > T, c.487G > A, c.517T > C, c.383T > C, and c.1004C > A were detected in the 883 patients. Hemizygous mutation was identified in 742 cases, and homozygous mutation was identified in 20 cases. Single heterozygous mutation was detected in 113 cases, and 8 showed compound heterozygous mutation. No G6PD mutation was identified in the rest 21 patients with G6PD enzyme abnormality. Then whole blood DNA was extracted from the 21 cases, and 6 mutations including c.202G > A, c.697G > C, c.406C > T, c.493A > G, 1365–13T > C and c.1311C > T were detected by PCR amplification and gene sequencing. Among these patients, there were 19 hemizygous mutations and 2 homozygous mutations (Table 3). The proportion of various mutations was counted as follows: hemizygous mutation in males was defined 1, homozygous mutation in females was defined as 2, and compound heterozygous mutation was defined as 1 (Table 4).
Table 3.
G6PD mutation types and range of enzyme specific activity value.
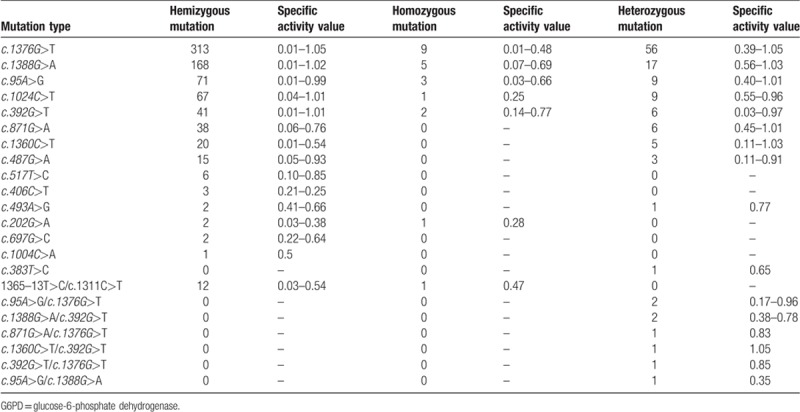
Table 4.
Proportion of various G6PD mutation types.
3.3. Results of Wilcoxon rank sum test
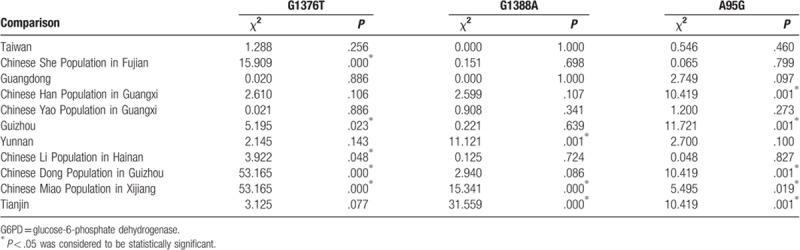
The extent of reduced enzyme activity caused by hemizygous mutation in the 6 G6PD mutations in Fujian Chinese Han children was compared. Statistical difference was noticed in the comparison between c.1376G > T and c.1388G > A. No statistical differences were observed in the other mutation types. The 3 most common mutation types in eastern Fujian were compared with the G6PD mutation in other provinces in China.[5–15] The numbers of mutation was 933 although the positive individuals were 904 (Table 5). Chi-square test and Calibration test were used for the comparison of the 3 most common G6PD mutations between Fujian and other provinces in China. Results were shown in Table 6.
Table 5.
The 3 most common G6PD mutations in China (n, %).
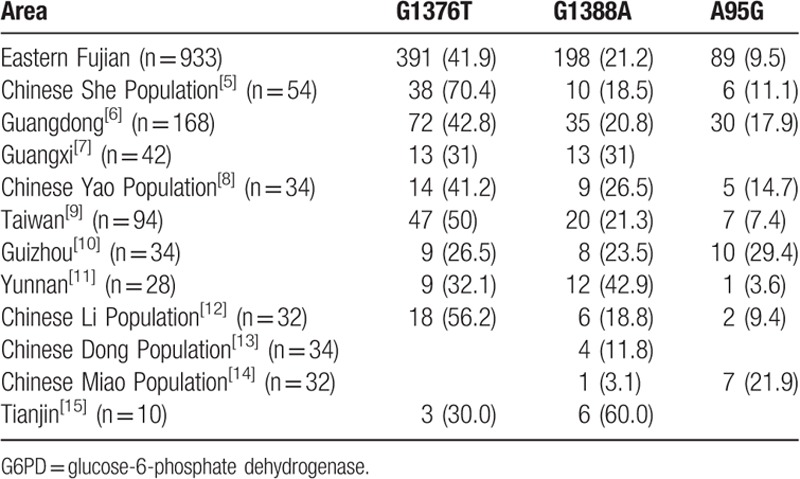
Table 6.
Comparison of the 3 most common G6PD mutations between Fujian and other provinces in China.
3.4. Sequencing map
The mutation sites of G6PD were listed in Figures 1 and 2. The c.DNA202G > A was noticed in exon 4, and c.DNA406C > T was notice in exon 5 (Fig. 1A and B). Additionally, c.DNA697G > C was noticed in exon 7 and c.DNA1311C > T was observed in exon 11 (Fig. 1C and D). Furthermore, 1365-13T > C site was identified in intron 11 (Fig. 2A) and 493A > G was noticed in intron 6 (Fig. 2B).
Figure 1.
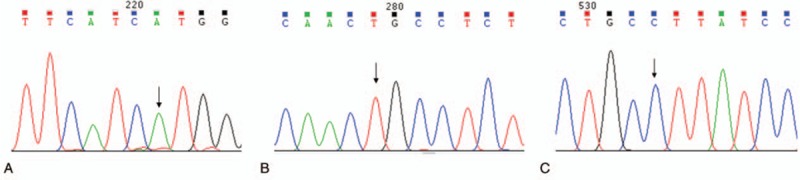
Mutation analysis of exon 4, 5, and 7. (A) The hemizygous and homozygous mutations in the c.DNA202G > A site in exon 4. (B) The hemizygous and homozygous mutation in the c.DNA406C > T site in exon 5. (C) The hemizygous and homozygous mutation in the c.DNA697G > C site in exon 7.
Figure 2.
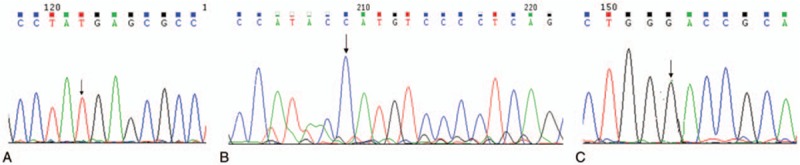
Mutation of extron 6, 11, and intron 11. (A) The hemizygous and homozygous samesense mutation in the c.DNA1311C > T site in extron11. (B) The hemizygous and homozygous mutation in the 1365-13T > C site in intron (C) The hemizygous/homozygous mutation in the c.DNA493A > G site in extron 6.
4. Discussion
From January 2014 to December 2016, a total of 200,260 G6PD samples were screened. Among these samples, 1813 (0.91%) samples (male: 1,489; female: 324) with decreased activity of G6PD were identified. G6PD deficiency, the most common enzyme defect, has a high prevalence in China especially in boys. The prevalence of G6PD deficiency in northern China was comparatively lower than that of southern China. The south regions of the Yangtze River Valley including Guangdong, Guangxi, Guizhou, Hainan, Yunnan, and Taiwan had a high risk of G6PD deficiency.[6–11] Incidence of G6PD deficiency in the north of Chongqing and Wuhan showed gradual decline and was rarely reported in Shandong Province, which was similar with that of the studies in China mainland [16] and abroad.[17–20] There are indeed limitations in this study. We cannot confirm the association between the malaria and G6PD deficiency. In males, G6PD gene was in a nonmethylation status, while it showed partial methylation in females. The G6PD gene in males was in non-methylation status, while it showed partial methylation in females.[21] Males have only one X chromosome, while females have 2 X chromosomes. Random inactivation phenomenon namely “Lyon hypothesis” existed in the females. To ensure the equality of transcription levels between the sexes in mammalian, females will random inactivate one X chromosome, and maintain and transfer during cell proliferation and differentiation. Maintenance of X chromosome inactivation mainly depends on the methylation of the promoter. Range of G6PD enzyme activity fluctuates greatly in female heterozygote, which may be responsible for this.
For the comparison in the 6 most common G6PD mutations in eastern Fujian, statistical difference was observed between c.1376G > T and c.1388G > A. This may be related to the fact that c.1376G > T and c.1388G > A were located in exon 12 with a very close distance (only 12 bases), which resulted in the Arg in 459 and 463 sites were substituted by Leu and His, respectively. Arg of 459 and 463 sites are located in the regions containing more than 10 highly conserved amine acids. These 2 Arg are very close to the location of the first binding site of NADP (386–387) in space, which play an important role in the binding process between enzyme and NADP. Moreover, c.1376G > T is a conservative amino acid substitution (Arg-Leu) and it may affect the G6PD enzyme activity.[22] Additionally, c.487G > A and c.493A > G were located in exon 6, and their distance is only 6 bases. Gly in the 163 site was replaced by Ser, the Asp in the 165 site was replaced by aminosuccinic acid. The accuracy of the statistics may be hampered by the small case number with mutations. The mutations may cause different amino acid changes because of different location, which may lead to different changes of protein secondary structure and enzyme activity.
For the comparison of the G6PD mutation between eastern Fujian and other areas, there were no significant differences in the sum of c.1376G > T, c.1388G > A, and c.95A > G between Chinese Han population in eastern Fujian (72.67%) and other provinces including Taiwan (78.7%), Guangdong (81.5%), Chinese Han population in Guangxi (62%), Chinese Yao population in Guangxi (82.4%), Yunnan (78.6%), and Guizhou (79.4%). In contrast, there were differences in the proportion of c.1376G > T, c.1388G > A, and c.95A > G in different provinces.
G6PD mutation types and proportion varied in different geographical locations. For example, c.1376G > T was the main mutation type in Taiwan, Guangdong, Chinese Yao population in Guangxi, Chinese She population in Fujian Province, Chinese Han population in eastern Fujian. No statistical differences were noticed in the constituent ratio of c.1376G > T, c.1388G > A, and c.95A > G between Chinese Han population in Fujian and other areas including nonindigenous people in Taiwan, Guangdong, Chinese Han population in Guangxi, and Chinese Yao population in Guangxi. There were significant differences in the G6PD mutations between Chinese Han population in Fujian and other areas including Guizhou, Yunnan, and Tianjin. c.1388G > A was the main mutation type in Guizhou, Yunnan, and Tianjin, c.1376G > T and c.1388G > A have the same proportion in these areas.[10,11,15] For the Dong population, c.592C > T was the most common mutant which accounted for more than 50% of the G6PD gene mutation,[13] and the mutation type in other ethnic groups were relatively rare, which indicated that every region or nation had its own characteristics.
Mutation in G6PD deficiency was almost single nucleotide substitution. In this study, 17 G6PD mutation types were detected in Chinese Han population in Fujian. These mutations include 15 single point mutation and 7 compound mutations. These were all single nucleotide substitutions, and no frameshift mutation was found.
Among the 17 mutation types, c.1376G > T and c.1388G > A showed the largest enzyme activity fluctuation range, which indicated that there existed difference between phenotype and genotype. The same G6PD mutation would lead to different enzyme activity loss in different individuals. The most frequent mutation associated with c.1311C > T was T1365-13C in intron 11. The frequency of c.1311C > T was significantly higher in cases with abnormal G6PD enzyme activity than that in normal individuals, which demonstrated the presence of genetic linkage disequilibrium.
In conclusion, this study has confirmed that G6PD c.1376G > T was the most common G6PD deficiency mutation type, followed by G6PD c.1388G > A and c.95A > G in Chinese Han children in eastern Fujian. In this study, we did not find any novel G6PD deficiency mutation type. Further studies are required to identify novel G6PD mutation types, and functional trials are required to confirm the novel mutations.
Author contributions
Conceptualization: Wenlong Xiu.
Data curation: Wenlong Xiu.
Formal analysis: Yi Dong.
Funding acquisition: Yao Chen.
Investigation: Hong Zhao.
Methodology: Yueqing Su.
Project administration: Jinfu Zhou.
Resources: Yinglin Zeng, Hua Li.
Software: Hua Li.
Supervision: Jingzhi Wo, Feng Lin.
Validation: Honghua Zhang.
Visualization: Hanqiang Chen.
Writing – original draft: Yao Chen.
Writing – review & editing: Changyi Yang, Wenbin Zhu.
Footnotes
Abbreviations: G6PD = glucose-6-phosphate dehydrogenase, MMCA = multicolor melting curve analysis, PCR = polymerase chain reaction.
Funding: This project was supported by the Fujian Provincial Health and Family Planning Commission Youth Program (No. 2016-2-9) and the Fujian Provincial Maternity and Children's Hospital, Affiliated Hospital of Fujian Medical University (No. 16-20).
Conflict of interest: The author(s) declare(s) that there is no conflict of interest regarding the publication of this paper.
References
- [1].Howell RR. Advisory committee on heritable disorders and genetic diseases in newborns and children. Ment Retard Dev Disabil Res Rev 2006;12:313–5. [DOI] [PubMed] [Google Scholar]
- [2].Jiang W, Yu G, Liu P, et al. Structure and function of glucose-6-phosphate dehydrogenase-deficient variants in Chinese population. Hum Genet 2006;119:463. [DOI] [PubMed] [Google Scholar]
- [3].Yan JB, Xu HP, Xiong C, et al. Rapid and reliable detection of glucose-6-phosphate dehydrogenase (G6PD) gene mutations in Han Chinese using high-resolution melting analysis. J Mol Diagn 2010;12:305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pan M, Cai Y. Development of a new method for genotype of G6PD deficiency and its molecular characterization. J Mol Diagn Ther 2012;4:222–6. [Google Scholar]
- [5].Lui P. Glucose-6-phosphate dehydrogenase gene mutations in She Nationality, Fujian province. Zhonghua Xue Ye Xue Za Zhi 2005;26:612–5. [PubMed] [Google Scholar]
- [6].Tang D, Ma X, Song C. Glucose-6-phosphate dehydrogenase mutations among Cantonese revealed by polymerase chain reaction using dried blood spots. Zhonghua Xue Ye Xue Za Zhi 1998;19:189. [PubMed] [Google Scholar]
- [7].Xie J, Long G, Tang Z, et al. G6PD gene mutations in Guangxi, China. Zhonghua Yixue Yichuanxue Zazhi 1998;15:151. [PubMed] [Google Scholar]
- [8].Liu J, Ren X, Chen Q, et al. Comparative study of three common G6PD gene mutations in Yao and Han People in Guangxi. Zhonghua Xue Ye Xue Za Zhi 2000;21:190. [PubMed] [Google Scholar]
- [9].Chang JG, Chiou SS, Perng LI, et al. Molecular characterization of glucose-6-phosphate dehydrogenase (G6PD) deficiency by natural and amplification created restriction sites: five mutations account for most G6PD deficiency cases in Taiwan. Blood 1992;80:1079. [PubMed] [Google Scholar]
- [10].Yang M, Du CS. Gene mutations of glucose-6-phosphate dehydrogenase found in Qianxi, Guizhou. Zhonghua Xue Ye Xue Za Zhi 1996;17:188. [Google Scholar]
- [11].He Y, Chen Q, Zhang Z. Identification of three common G6PD gene mutations in Yi minority in Yunnan province. Zhonghua Xue Ye Xue Za Zhi 1999;20:194. [PubMed] [Google Scholar]
- [12].Cai W, Filosa S, Martini G, et al. Molecular characterization of glucose-6-phosphate dehydrogenase deficiency in the Han and Li nationalities in Hainan, China and identification of a new mutation in human G6PD gene. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2001;18:105. [PubMed] [Google Scholar]
- [13].Qi X, Shan K, Yuan X, et al. Study on the mutations of G6PD gene in Dong ethnic group in Guizhou Congjiang. Zhongguo Di Fang Bing Xue Za Zhi 2006;25:283–5. [Google Scholar]
- [14].Ren XL, Xiu J, Yan HE. Study on common mutations of G6PD gene in Miao, Shui and Yao ethnic groups. Zhongguo Gong Gong Wei Sheng 2004;20:672–3. [Google Scholar]
- [15].Liu JM. Study on G6PD gene mutation of Chinese minority nationalities. Acta Medicinae Sinica 2001;3:68–9. [Google Scholar]
- [16].Pan S. G6PD Deficiency: Distribution in East and Southeast Asia and Positive Selection by Malaria. Commun Contemp Anthropol 2007;13:42–51. [Google Scholar]
- [17].Malaria Genomic Epidemiology Network. Reappraisal of known malaria resistance loci in a large multicenter study. Nat Genet 2014;46:1197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Luzzatto L. G6PD deficiency: a polymorphism balanced by heterozygote advantage against malaria. Lancet Haematol 2015;2:e400–1. [DOI] [PubMed] [Google Scholar]
- [19].Uyoga S, Carolyne M, Ndila CM, et al. Glucose-6-phosphate dehydrogenase deficiency and the risk of malaria and other diseases in children on the coast of Kenya: a case-control and a cohort study. Lancet Haematol 2015;2:e437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Luzzatto L, Arese P. Favism and glucose-6-phosphate dehydrogenase deficiency. N Engl J Med 2018;378:60–71. [DOI] [PubMed] [Google Scholar]
- [21].Gendrel AV, Heard E. Fifty years of X-inactivation research. Development 2011;138:5049–55. [DOI] [PubMed] [Google Scholar]
- [22].Scopes DA, Bautista JM, Naylor CE, et al. Amino acid substitutions at the dimer interface of human glucose-6-phosphate dehydrogenase that increase thermostability and reduce the stabilising effect of NADP. Eur J Biochem 2010;251:382–8. [DOI] [PubMed] [Google Scholar]


