Abstract
Preoperative assessment of nodal stage is of importance in breast cancer treatment decision-making. This study was done to determine the power of combined mammography and ultrasonography in differentiating N0–N1 from N2–N3 breast cancer.
We retrospectively reviewed clinical data of 3944 female patients with invasive breast cancer by preoperative mammography and ultrasonography between January 2006 and December 2013 at our hospital. Pathological diagnosis was available for each patient. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of mammography alone, ultrasonography alone, and combination of them for assessment of axillary lymph node (ALN) status were calculated, using definitive histological results as the baseline.
The sensitivity, specificity, PPV, NPV, and accuracy was 90.4%, 68.2%, 36.5%, 97.2%, and 71.9% for ultrasonography; was 66.9%, 80.8%, 41.3%, 92.3%, and 78.4% for mammography; and was 94.9%, 62.4%, 33.8%, and 98.4% for combined mammography and ultrasonography. For combination, accuracy and the area under the receiver operating characteristic curve was 67.9% and 0.85, respectively.
In conclusion, combining ultrasonography and mammography improves the sensitivity in differentiating N0–N1 breast cancer from N2–N3 breast cancer, but leading to a reduced specificity. Addition of mammography to ultrasonography seems not to provide significant benefits in predicting ALN status in breast cancer patients.
Keywords: breast cancer, diagnosis, lymph node metastasis, mammography, prognosis, ultrasonography
1. Introduction
Breast cancer is the most common malignancy among females in the world, with over 1 million new cases every year.[1,2] Effective treatment options for breast cancer include surgery, local radiation, and systemic chemotherapy. Due to its radical nature, surgery has now been reduced to a minimum by the use of breast-conserving procedures[3,4] and axillary lymph node (ALN) dissection.[5] Lymph node status is an important prognostic factor for breast cancer.[6] Nodal stage also affects the selection of adjuvant therapeutic modalites.[7] Patients with N2 or greater disease have an increased risk of locoregional recurrence and postmastectomy radiotherapy should be considered for those patients.[8] By contrast, N1 patients have been found to receive few benefits from adjuvant radiotherapy.[9] Therefore, preoperative characterization of nodal stage is of clinical significance in breast cancer treatment decision-making.
Several imaging techniques are currently available for preoperative assessment of lymph node status, including mammography, ultrasonography, magnetic resonance imaging (MRI), and positron emission tomography/computed tomography (PET/CT).[10,11] Axillary ultrasonography is useful in excluding N2 and N3 invasive ductal metastases.[12] However, an axillary ultrasound fails to accurately differentiate between pN1 and pN2–pN3 breast cancer.[13]
The aim of this study is to determine the power of combined mammography and ultrasonography in differentiating N0 (negative axillary nodes) and N1 disease from N2 and N3 disease.
2. Materials and methods
2.1. Patients
From January 2006 to December 2013, a total of 3944 female patients were diagnosed as invasive breast cancer by preoperative mammography and ultrasonography and then received sentinel lymph node biopsy or modified radical mastectomy in the First Hospital of China Medical University (Shenyang, China). Pathological diagnosis was available for each patient. Clinical data including age, body mass index (BMI), tumor stage, and tumor molecular subtype were retrieved from medical records. Patients with previous surgery, neochemotherapy, multicentric lesions, recurrent breast cancer, or palpable lymph nodes were excluded. This study was approved by the Ethics Committee of the First Hospital of China Medical University.
2.2. Assessment of ALNs by mammography and ultrasonography
For mammography, standard craniocaudal (CC) and mediolateral oblique (MLO) images were obtained with further magnifications or additional views taken as required. Bilateral whole-breast ultrasonography was performed by experienced breast sonographers using 7- to 14-MHz linear probes (Hitachi Preirus, Tokyo, Japan). Mammographic and ultrasonographic findings were classed as normal/probably benign, equivocal, and suspicious of metastasis. On mammograms, a normal (probably benign) lymph node had an oval shape (long/short diameter ratio >2) with a clear, smooth border and a lymph node suspected of having metastasis showed a thickened cortex and evident calcification. On ultrasonograms, a lymph node with oval-shaped structures with an echogenic hilum was defined as normal or probably benign and one with an irregular morphology, cortical thickening, and compressed or displaced hilum was defined as suspicious metastasis. All mammography and ultrasonography examinations were confirmed by histological studies.
2.3. Data analysis
The associations between clinicopathological parameters and ALN metastasis were examined using multiple logistic regression analysis. Sensitivity (percentage of patients with true lymph node metastases who were diagnosed with ultrasound or mammography or both), specificity (the percentage of patients with pathologically proven node-negative patients who were truly no lymph node metastases in ultrasound or mammography or both), positive predictive value (PPV) (percentage of patients with pathologically confirmed metastases in patients with lymph node metastasis in ultrasound or mammography or both), negative predictive value (NPV) (percentage of patients with pathologically confirmed no metastases in patients with no lymph node metastasis in ultrasound or mammography or both), and accuracy of mammography alone, ultrasonography alone, and combination of them for detection of ALN metastasis were calculated. Receiver operating characteristic (ROC) curves were plotted and the area under the ROC curve (AUC) was calculated to compare the diagnostic power of ultrasonography and mammography alone or in combination. All statistical analyses were conducted using SPSS version 16.0 (SPSS Inc, Chicago, IL). When the P value was <.05, difference was considered statistically significant.
3. Results
3.1. Clinicopathological characteristics
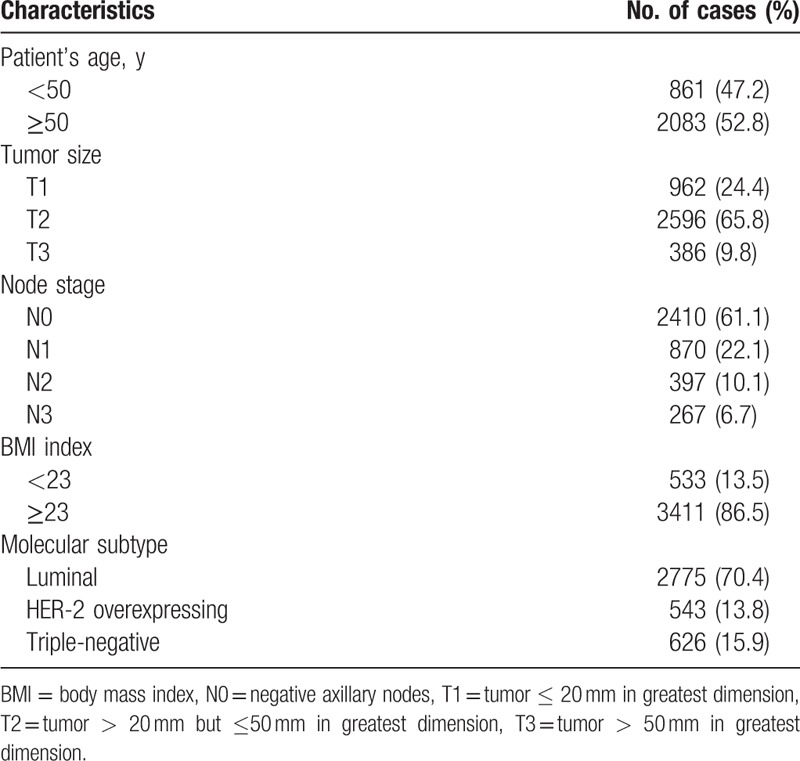
The clinicopathological characteristics of the patients studied are shown in Table 1. About half of the patients (47.2%) were aged <50 years; 52.8% of the patients were aged ≥50 years. T1 tumors (tumor ≤20 mm in greatest dimension) were detected in 962 patients, T2 tumors (tumor >20 mm but ≤50 mm in greatest dimension) in 2596 patients, and T3 tumors (tumor >50 mm in greatest dimension) in 386 patients; 2410 patients (61.1%) showed N0 disease, 870 patients (22.1%) N1, 397 patients (10.1%) N2, and 267 patients (0.00057%) N3. The mean BMI was 24.8 kg/m2 (range 15.2–40.5), with 3411 patients (86.5%) having a BMI of ≥23 kg/m2. The majority of these patients had luminal tumors (70.4%), 13.8% HER-2-overexpressing tumors, and 15.9% triple-negative tumors.
Table 1.
The clinicopathological characteristics of patients.
3.2. Associations of clinicopathological factors with ALN metastasis
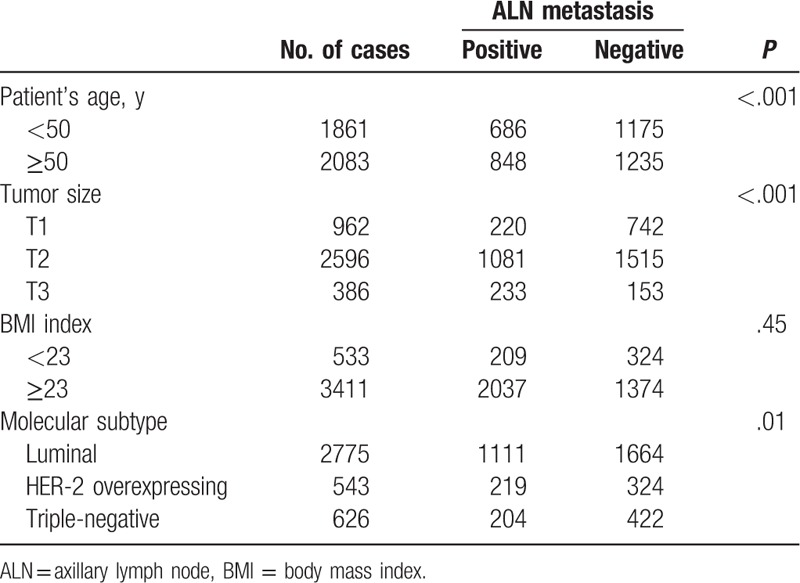
We analyzed the associations of the patient clinicopathological features with ALN metastasis status (Table 2). Patient's age (P < .001), tumor size (P < .001), and tumor molecular subtype (P = .01) were significantly associated with ALN metastasis. No correlation was observed between patient's BMI and ALN metastasis (P = .45).
Table 2.
Associations of ALN metastasis with clinicopathological parameters.
3.3. Diagnostic performance of ultrasonography and mammography alone or in combination
We explored the diagnostic performance of ultrasonography and mammography, alone or in combination, for prediction of ALN metastasis. A positive diagnosis of ALN metastasis was defined as the presence of indeterminate or malignant nodes on ultrasonography or mammography. Of the 2300 normal/probably benign lesions based on preoperative ultrasonography, 1962 cases (85.3%) were identified as pN0, 274 (11.9%) pN1, 49 (2.1%) pN2, and 15 (0.7%) pN3 (Table 3). Of the 1644 patients who were suspected of having ALN metastasis based on preoperative ultrasonography, 448 (27.3%) did not actually have ALN metastasis and 596 (36.3%) had pN1 disease and 600 (36.4%) had pN2 or pN3 disease based on pathological examination. Figure 1 demonstrates the AUCs for ultrasonography, mammography, and combination of them. The sensitivity, specificity, PPV, NPV, accuracy, and AUC of ultrasonography for distinguishing between N0–N1 and N2–N3 disease was 90.4%, 68.2%, 36.5%, 97.2%, 71.9%, and 0.83%, respectively (Table 4).
Table 3.
Assessment of axillary lymph node status by imaging tests.
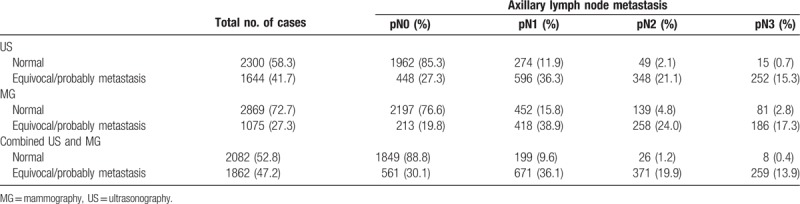
Figure 1.
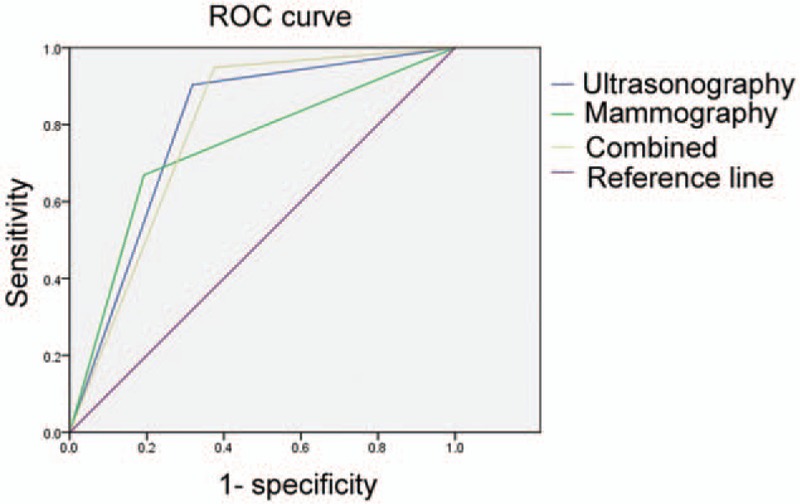
The area under the receiver operating characteristic curve of combined mammography and ultrasonography.
Table 4.
Performance of different imaging modalities in differentiating N0–N1 breast cancer from N2–N3 disease.
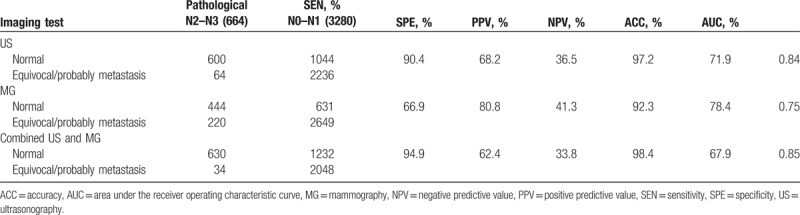
As shown in Table 3, 2197 (76.6%) of the 2869 patients who were identified to have normal/probably benign lesions by mammography had no evidence of ALN metastasis on pathological diagnosis. A total of 1075 patients were diagnosed as suspicious of metastasis by mammography. Of them, 213 patients (19.8%) were identified as pN0, 418 (38.9%) pN1, and 444 (41.3%) pN2 or greater. The sensitivity, specificity, PPV, NPV, accuracy, and AUC were 66.9%, 80.8%, 41.3%, 92.3%, 78.4%, and 0.74%, respectively (Table 4).
Based on the combination of ultrasonography and mammography, 2082 cases were diagnosed as normal or benign (Table 3). Among them, 1849 cases (88.8%) were pN0, 199 (9.6%) pN1, and 34 (1.6%) pN2 or pN3. Of the 1862 cases who presented suspicious metastasis on combined ultrasonography and mammography, 561 cases (30.1%) were pN0, 371 (36.1%) pN1, and 630 (33.8%) pN2 or pN3. The sensitivity, specificity, PPV, NPV, accuracy, and AUC were 94.9%, 62.4%, 33.8%, 98.4%, 67.9%, and 0.85%, respectively (Table 4).
4. Discussion
Preoperative axillary staging is critical for determining prognosis and for making treatment decision in patients with invasive breast cancer. Ultrasonography is the primary imaging modality for assessment of axillary nodes. An et al[14] compared the diagnostic performance of 18F-fluorodeoxyglucose (FDG) PET/CT, ultrasonography, and MRI in detecting ALN metastasis in 215 breast cancer patients. They found no significant difference in diagnostic ability among the imaging modalities, with the sensitivity of 72.3%, specificity of 77.3%, and accuracy of 75.3% for ultrasonography. However, the sensitivity of preoperative imaging modalities in identifying lymph node metastasis still needs to be improved. Seok et al[15] retrospectively reviewed 104 breast cancer patients with clinically negative ALN using ultrasonography, FDG PET, and MRI and found that 21 patients of them (20.2%) were proven to have ALN metastasis. Combined ultrasonography and MRI has been documented to improve the accuracy of ALN staging in breast cancer patients, compared with ultrasonography or MRI alone.[16] In several other types of malignancies, combination of different imaging techniques also improves diagnostic performance in assessment of lymph node metastasis.[17] Mammographic screening is of clinical significance in identifying individuals with high-risk breast cancer. A combination of mammography and breast ultrasound has been found to offer additional benefits of breast cancer screening.[18] These findings encourage us to evaluate the ability of combined mammography and ultrasonography to predict ALN status in invasive breast cancer patients.
Breast positioning is the key factor affecting the image quality of mammography.[19] Optimal positioning for CC and MLO views is important to improve the diagnostic performance of mammography. Despite these efforts, mammography has a limited capacity to identify abnormal ALNs.[20] Our data revealed that the sensitivity of mammography for detection of ALN metastasis was only 66.9%; but, the specificity was as high as 80.8%. This finding is consistent with a previous study.[11] Due to relatively high specificity, mammography is still commonly used for preoperative evaluation of ALN status. In comparison with mammography, ultrasonography had a higher sensitivity (90.4%) but lower specificity (68.2%) for predicting ALN metastasis in our patients. Ultrasound examination combined with fine-needle aspiration has been reported to increase the specificity to 100% but decrease the sensitivity to 53%.[21] Another study reported similar findings that ultrasonography-guided fine-needle aspiration had a low sensitivity (39.5%) and high specificity (95.7%) for detecting ALN metastasis.[22] Our data showed that combination of mammography and ultrasonography resulted in an increased sensitivity (94.9% vs 90.4%) and modestly decreased specificity (62.4% vs 68.2%), compared to ultrasonography alone. The accuracy for differentiating N0 and N1 disease from N2 and N3 disease was similar between ultrasonography alone and mammography/ultrasonography combination.
Although combination of ultrasonography and mammography modestly increased the sensitivity for evaluating ALN status, the specificity was not elevated, but undesirably decreased. Therefore, addition of mammography seems not to offer significant benefits in preoperative ALN staging. However, this is a retrospective study based on clinical data from a signal center. The presence of inherent selection bias may compromise our conclusions.
5. Conclusion
In conclusion, our data show that combining ultrasonography and mammography would be favorable to improve the sensitivity in differentiating N0–N1 breast cancer from N2–N3 breast cancer, but leading to a reduced specificity. The potential benefit of mammography as an adjuvant to preoperative ultrasonography needs to be further explored.
Author contributions
Conceptualization: Qun Liu, Peng Xing, Feng Jin.
Data curation: Qun Liu, Peng Xing.
Formal analysis: Qun Liu, Peng Xing, Huiting Dong, Tingting Zhao, Feng Jin.
Investigation: Qun Liu, Peng Xing, Huiting Dong, Tingting Zhao, Feng Jin.
Methodology: Qun Liu, Peng Xing, Huiting Dong, Tingting Zhao, Feng Jin.
Project administration: Qun Liu, Peng Xing, Huiting Dong.
Resources: Qun Liu, Peng Xing, Huiting Dong.
Software: Qun Liu, Peng Xing, Huiting Dong.
Supervision: Qun Liu, Peng Xing, Huiting Dong, Tingting Zhao, Feng Jin.
Validation: Qun Liu, Peng Xing, Huiting Dong, Tingting Zhao, Feng Jin.
Visualization: Qun Liu, Peng Xing, Huiting Dong, Tingting Zhao, Feng Jin.
Writing – original draft: Qun Liu.
Writing – review and editing: Feng Jin.
Footnotes
Abbreviations: ALN = axillary lymph node, AUC = the area under the ROC curve, BMI = body mass index, FDG = 18F-fluorodeoxyglucose, MRI = magnetic resonance imaging, NPV = negative predictive value, PET/CT = positron emission tomography/computed tomography, PPV = positive predictive value, ROC = receiver operating characteristic.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Tao Z, Shi A, Lu C, et al. Breast cancer: epidemiology etiology. Cell Biochem Biophys 2015;72:333–8. [DOI] [PubMed] [Google Scholar]
- [2].Bonafede MM, Kalra VB, Miller JD, et al. Value analysis of digital breast tomosynthesis for breast cancer screening in a commercially-insured US population. Clinicoecon Outcomes Res 2015;7:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Maughan KL, Lutterbie MA, Ham PS. Treatment of breast cancer. Am Fam Physician 2010;81:1339–46. [PubMed] [Google Scholar]
- [4].Warren LE, Punglia RS, Wong JS, et al. Management of the regional lymph nodes following breast-conservation therapy for early-stage breast cancer: an evolving paradigm. Int J Radiat Oncol Biol Phys 2014;90:772–7. [DOI] [PubMed] [Google Scholar]
- [5].Williams PA, Suggs J, Mangana SH. Axillary lymph node treatment in breast cancer: an update. J Miss State Med Assoc 2014;55:145–7. [PubMed] [Google Scholar]
- [6].Hernandez-Aya LF, Chavez-Macgregor M, Lei X, et al. Nodal status and clinical outcomes in a large cohort of patients with triple-negative breast cancer. J Clin Oncol 2011;29:2628–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang CW, Kuo WH, Chang KJ, et al. Should adjuvant radiotherapy to the supraclavicular fossa be routinely given in patients with breast conservative treatment? J Surg Oncol 2007;96:144–50. [DOI] [PubMed] [Google Scholar]
- [8].Chen X, Yu X, Chen J, et al. Analysis in early stage triple-negative breast cancer treated with mastectomy without adjuvant radiotherapy: patterns of failure and prognostic factors. Cancer 2013;119:2366–74. [DOI] [PubMed] [Google Scholar]
- [9].Viani GA, Godoi da Silva LB, Viana BS. Patients with N1 breast cancer: who could benefit from supraclavicular fossa radiotherapy? Breast 2014;23:749–53. [DOI] [PubMed] [Google Scholar]
- [10].Choi YJ, Shin YD, Kang YH, et al. The effects of preoperative (18)F-FDG PET/CT in breast cancer patients in comparison to the conventional imaging study. J Breast Cancer 2012;15:441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Valente SA, Levine GM, Silverstein MJ, et al. Accuracy of predicting axillary lymph node positivity by physical examination, mammography, ultrasonography, and magnetic resonance imaging. Ann Surg Oncol 2012;19:1825–30. [DOI] [PubMed] [Google Scholar]
- [12].Neal CH, Daly CP, Nees AV, et al. Can preoperative axillary US help exclude N2 and N3 metastatic breast cancer? Radiology 2010;257:335–41. [DOI] [PubMed] [Google Scholar]
- [13].Schipper RJ, van Roozendaal LM, de Vries B, et al. Axillary ultrasound for preoperative nodal staging in breast cancer patients: is it of added value? Breast 2013;22:1108–13. [DOI] [PubMed] [Google Scholar]
- [14].An YS, Lee DH, Yoon JK, et al. Diagnostic performance of 18F-FDG PET/CT, ultrasonography and MRI. Detection of axillary lymph node metastasis in breast cancer patients. Nuklearmedizin 2014;53:89–94. [DOI] [PubMed] [Google Scholar]
- [15].Seok JW, Kim Y, An YS, et al. The clinical value of tumor FDG uptake for predicting axillary lymph node metastasis in breast cancer with clinically negative axillary lymph nodes. Ann Nucl Med 2013;27:546–53. [DOI] [PubMed] [Google Scholar]
- [16].Abe H, Schacht D, Kulkarni K, et al. Accuracy of axillary lymph node staging in breast cancer patients: an observer-performance study comparison of MRI and ultrasound. Acad Radiol 2013;20:1399–404. [DOI] [PubMed] [Google Scholar]
- [17].Yoon DY, Hwang HS, Chang SK, et al. MR, US, 18F-FDG PET/CT, and their combined use for the assessment of cervical lymph node metastases in squamous cell carcinoma of the head and neck. Eur Radiol 2009;19:634–42. [DOI] [PubMed] [Google Scholar]
- [18].Corsetti V, Houssami N, Ghirardi M, et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: interval breast cancers at 1 year follow-up. Eur J Cancer 2011;47:1021–6. [DOI] [PubMed] [Google Scholar]
- [19].Popli MB, Teotia R, Narang M, et al. Breast positioning during mammography: mistakes to be avoided. Breast Cancer (Auckl) 2014;8:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shetty MK, Carpenter WS. Sonographic evaluation of isolated abnormal axillary lymph nodes identified on mammograms. J Ultrasound Med 2004;23:63–71. [DOI] [PubMed] [Google Scholar]
- [21].García Fernández A, Fraile M, Giménez N, et al. Use of axillary ultrasound, ultrasound-fine needle aspiration biopsy and magnetic resonance imaging in the preoperative triage of breast cancer patients considered for sentinel node biopsy. Ultrasound Med Biol 2011;37:16–22. [DOI] [PubMed] [Google Scholar]
- [22].Park SH, Kim MJ, Park BW, et al. Impact of preoperative ultrasonography and fine-needle aspiration of axillary lymph nodes on surgical management of primary breast cancer. Ann Surg Oncol 2011;18:738–44. [DOI] [PubMed] [Google Scholar]


