Abstract
Objectives:
This study assessed the efficacy of oral consumption of N-acetyl cysteine (NAC) and melatonin (ML) in reducing early reperfusion injury and acute oxidative stress in patients undergoing coronary artery bypass grafting (CABG) with respect to the measurements of cardiac troponin I, lactate, malondealdehyde (MDA), and tumor necrosis factor-α (TNF-α) levels in the blood.
Methods:
This study was a randomized, open-label, placebo-controlled trial. Eighty eight patients, aged between 39 to 76 years and eligible for CABG, were recruited and randomly assigned into 3 intervention groups through a simple randomization method and underwent CABG surgery. Blood samples were withdrawn from arterial line, before the induction of anesthesia (before the start of surgery), after incision (before aortic cross-clamping), during global ischemia (during aortic cross-clamping), after aortic cross-clamping (on set of reperfusion), 15 minutes after reperfusion, and after recovery at the intense care unit. The blood samples were analyzed for troponin I, lactate, MDA and TNF-α levels.
Results:
There was no significant difference in influencing variables among the groups at the baseline. Overall mean troponin I, lactate, and TNF- α levels were significantly different between the intervention groups (all P < .001) at the recovery phase. Post-hoc pairwise comparisons showed that the differences of mean serum levels between ML and control groups were statistically significant for MDA, TNF- α, lactate, and troponin I (P < .001, P = .001, and P = .001, respectively). The differences between NAC and control groups and between ML and NAC groups were only significant for mean lactate level (P < .001).
Conclusion:
The current study revealed that ML and NAC are potent antioxidants with similar efficacy in terms of reducing CABG related cardiac injury and oxidative stress with the dosage employed for the intervention.
Keywords: coronary artery bypass grafting, melatonin, N-acetyl cysteine, reperfusion injury
1. Introduction
The reactive oxygen species are believed to be excessively elevated during coronary artery bypass surgery (CABG) due to compromised free radical scavenging mechanism in the myocardium that can make myocardium highly susceptible to oxidative stress and inflammation and result in reperfusion injury.[1] CABG associated global myocardial ischemic injury during aortic cross-clamping and subsequent reperfusion injury can adversely affect the prognosis.[1] Strategies to improve the CABG related complications are much needed to augment the positive outcomes in the post-operative surviving patients. Melatonin (N-acetyl-5-methoxytryptamine—ML) and N-acetyl-L-cysteine (NAC) are known to have antioxidant properties, and protect against ischemia–reperfusion (IR) injury.[2,3] ML is a hormone produced by pineal gland and possesses antioxidant properties such as the capacity to eliminate to deleterious free radicals[4–9] NAC consists of amino acid cysteine with an acetyl group linked to the nitrogen and has the ability to generate glutathione to help increase the endogenous antioxidant capacity; and its thiol moieties confer direct antioxidant properties.[10] Previous studies have shown the beneficial effect of NAC in animal models of heart disease and humans undergoing cardiac surgery mainly by reducing oxidative stress.[3,11,12] Recently, N-acetylcysteine in acute myocardial infarction (NACIAM) study reported that high dose intravenous NAC and low-dose nitroglycerin is safe and beneficial in reducing infarct size in patients undergoing primary percutaneous intervention in for ST segment elevation.[11]
In this study, we assessed the comparative efficiency of pre-operative oral consumption of ML and NAC as antioxidants on early cardiac reperfusion injury and related oxidative stress in patients with ischemic heart disease undergoing CABG, with respect to the measurements of malondealdehyde (MDA), cardiac troponin I, tumor necrosis factor (TNF- α), and lactate levels in the blood.
2. Methods and materials
2.1. Subjects and trial design
The study was approved by the Medical Ethics Committee of Bushehr University of Medical Sciences, Bushehr, Iran. The project was also registered with Clinical Trial Registry of Iran (Registration no: IRCT201304178129N3). The informed consent was obtained from each patient at the time of enrolment and continued as a process throughout the investigation. Also, the patients had free medical care and consultation during the study period, especially in the case of any adverse reaction or complications for 2 weeks after the operation.
Baseline characteristics and hemodynamic of the patients were recorded (Table 1). A total of 88 patients aged between 39 to 76 years were recruited and randomly assigned into 3 groups through a simple randomization method and were under medical observation from 1 week before undergoing CABG surgery (Fig. 1).
Table 1.
Baseline characteristics of the patients in the control, NAC, and the melatonin group and ICU and hospital stay.
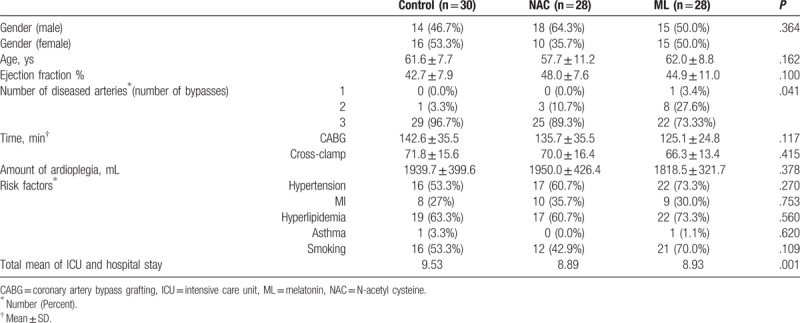
Figure 1.
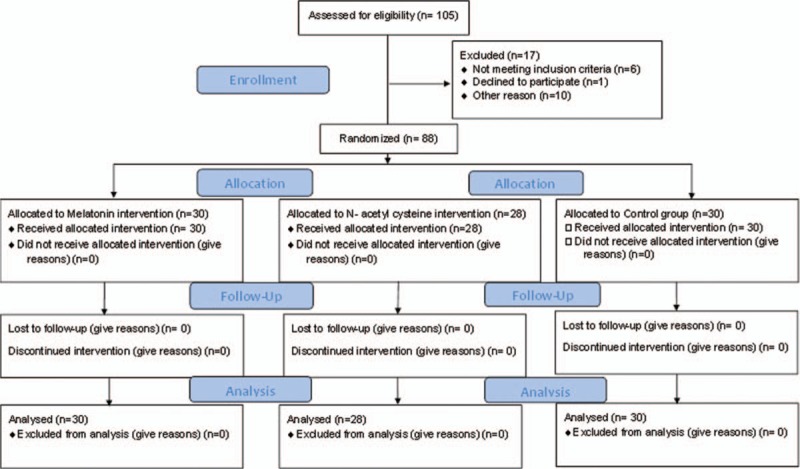
CONSORT 2010 Flow Diagram of the trial.
2.2. Treatment regime
The patients in the first group as the control (No = 30) received placebo (5% dextrose dissolved in water), the second group received melatonin (No = 30), 5 mg tablets (Nature made, CA) starting from 24 hours before the operation for 3 times and a single dose 1 hour before the operation with 200 cc water, the third group had N- acetyl cysteine (No = 28) orally (600 mg, effervescent tablets dissolved in 200 cc water) (Mucolytic, Zambon Svizzera, Cadempino, Ticino, Switzerland) for 2 days before the surgery for three times a day and a single dose in the morning of the CABG procedure.
2.3. Inclusion criteria
Ischemic heart disease patients having one or more arteries blocked, not having weak left ventricular function (normal LV function ejection fraction ≥ 40%), non-diabetics and non-emergency patients and the patients without history of endocrine, pulmonary, metabolic, and neurological diseases.
2.4. Exclusion criteria
Emergency patients, diabetic patients, patients having history of endocrine, pulmonary, metabolic and neurological diseases, smokers, patients having weak left ventricular function, patients consuming ML and NAC at least from 2 weeks before the operation.
2.5. Blood sampling and processing
Blood sample was drawn into vacutainer tubes from arterial line before the induction of anesthesia (before the start of surgery), after incision (before aortic cross-clamping), during global ischemia (during aortic cross-clamping), after aortic cross-clamping (on set of reperfusion), 15 minutes after reperfusion, and after recovery at the intensive care unit (ICU). These samples were immediately centrifuged and the serum was separated and kept frozen at −80°C until used.
2.6. Surgical procedure
Anesthesia was induced using 5 μg/kg fentanyl, 0.2 mg/kg of etomidate, and muscle relaxant (0.5 mg/kg of Atracurium). The systolic and diastolic BP as well as mean arterial pressure was recorded as part of vital signs. Anesthesia was maintained by infusion of anesthetic drugs with syringe pump: sufentanil (0.01–0.02 μg/kg/min), midazolam (1 μg/kg/min) as amnestic agent, and the same relaxant atracurium (10 μg/kg /min) used in the induction stage, during the surgery, isoflurane was used for stable maintenance of anesthesia and hemodynamics. The patients were under controlled mechanical ventilation (CMV) with tidal volume of 8 to 10 mL/kg and a respiratory rate 10 to 12/min with dragger anesthesia machine, and had mechanical ventilation with %100 O2 during the study. Prior to the start of cannulation for cardiopulmonary bypass, heparin (300 unit/kg) was administered via central venous cannula. Prime solution consisted of crystalloid solution, gelatin, and heparin (5000 unit). The heart was arrested by delivering antegrade blood cardioplegia solution consisting of KCL (20 mq/L), magnesium sulfate (2 g/L), sodium bicarbonate (25 mq/L), lidocaine (20 mg/L), and later maintained by retrograde sinus blood cardioplegia delivery after each distal anastomosis.
2.7. Outcome measures
The primary study endpoint was change (from before CABG surgery) in troponin I assessed at recovery. Secondary endpoints included changes in MDA, TNF-α, and lactate from before surgery to recovery.
2.8. Biochemical measurements
The level of serum lactate from all the groups was measured by following manufacture's instruction (Bio Vision Research Products, CA). MDA, the byproduct of lipid peroxidation, was measured by using an improved thiobarbituric acid reactive substance (TBARS) based method.[13] TNF-α was determined by using EIA Kit (Enzo Life Sciences International INC, New York, NY). Troponin-I was determined by immunoenzyme assay Test Kit (DiaPlus Inc, Armonk, NY: IBM Corp, MA).
2.9. Statistical analysis
In order to achieve a power of 80% at the significance level of 0.05, 30 patients per group were recruited for the study. Normality of the data was checked by the Shapiro–Wilks test. As the data was not statistically significantly far from normality, comparison between the intervention groups at each time point was assessed by 1-way analysis of variance. Bonferroni correction was used for post-hoc pairwise comparisons between the groups. Repeated measure analysis of variance (ANOVA) was performed to evaluate the differences between the groups over time and for the interaction between time and the intervention in all groups. Mauchly's test of sphericity indicated that the assumption of sphericity had been violated for all the repeated measures analysis of variance (ANOVA) tests performed, and therefore, a Greenhouse–Geisser correction was used. Data were analyzed using GLM repeated measures analysis of variance menu of the SPSS computer package, version 21.0. Differences were considered statistically significant at P < .05.
3. Results
The baseline characteristics (before beginning treatment) of the patients in the 3 groups are shown in Table 1. There was no significant difference in influencing variables at the baseline among the 3 groups (Table 1). There was no significant effect of time on troponin I level (F [2.348, 199.574] = 1.906, P = .144] (Fig. 2 Fig. 2 A). However, the interaction between time and the intervention groups was significant [F (4.696, 199.574) = 3.872, P = .003] (Fig. 2 A). The result also showed that troponin I level was significantly different between the intervention groups (F [2, 85] = 10.449, P < .001) (Fig. 2 A). Further, the post-hoc comparison test showed that troponin I level in the ML group was significantly lower compared to placebo group (P = .001), but there was no significant difference in troponin level between NAC and placebo group (P = .062) or between the NAC and the ML groups (P = .108).Specifically, the troponin I levels in the ML group were significantly lower as compared with placebo group at the “baseline,” “during global ischemia,” and “recovery” time points (Fig. 2 A). Interestingly, troponin I level in the NAC group was significantly higher than ML group at the “onset of reperfusion” (Fig. 2 A).
Figure 2.
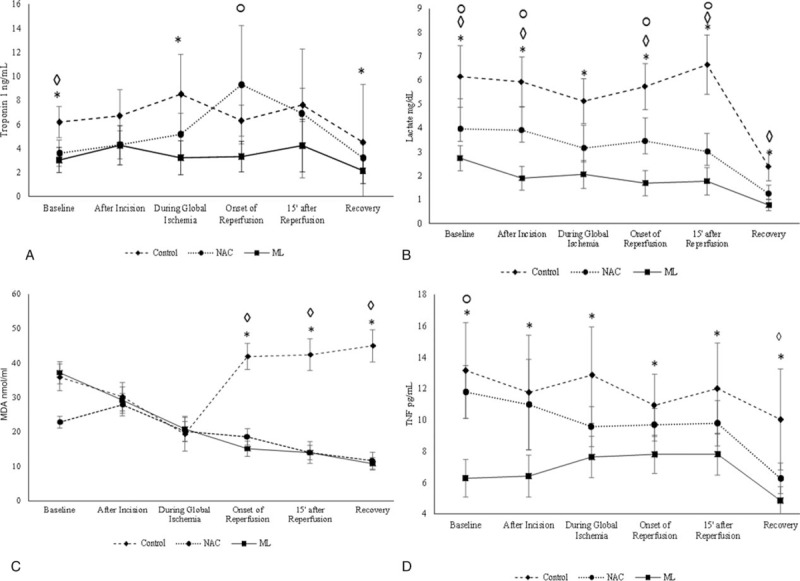
Effect of melatonin and N-acetyl cysteine on (A) and (B) cardiac injury markers, (C) oxidative stress and (D) inflammation. Means and 95% confidence intervals of four outcome measures over the study period from baseline (before surgery) to recovery stage), following signs on the top of each time-point are indicators of significant P values (P < .05) of pairwise comparisons, control versus ML = ∗; control versus NAC = ◊; ML versus NAC = ○; NAC = N-acetyl cysteine; ML = melatonin.
Repeated measures ANOVA showed that there was a significant effect of time on lactate level between intervention groups (P = .003). The serum concentration of lactate was significantly different during the time course of the surgery (F [2, 85] = 50.456, P < .001)(Fig. 2B). The overall lactate level differed significantly between the intervention groups(P < .001) (Fig. 2B). As per post-hoc comparison test, placebo group had significantly higher level of lactate compared with NAC and ML groups (P = .001). Repeated measures ANOVA showed that there was a significant effect of time on lactate level between intervention groups (P = .003). The results showed that the lactate level in the ML group was significantly lower than placebo group at all the time points. The lactate level in the NAC group at all the time points were significantly lower compared to placebo group except during global ischemia. Also, the lactate level in the NAC group was significantly higher compared to the ML group at all-time points except during global ischemia and at recovery time (Fig. 2B).
MDA level was significantly different between NAC and both placebo and ML groups (P < .001), but was not significantly different between control and ML groups before surgery (P = 1.00). There was a statistically significant effect of time on MDA level (F [3.838, 322.353)]= 43.457, P < .001) (Fig. 2C). The interaction between time and intervention was also statistically significant (F [7.675, 322.355] = 66.671, P < .001). The MDA level differed significantly between the intervention groups (F [2, 84] = 48.153, P < .001). Post-hoc comparisons showed that MDA level was significantly lower in NAC and ML intervention groups compared to placebo group (P < .001) at “recovery.” Specifically, the mean MDA level did not significantly differ between the placebo group and intervention groups at “after incision” and “during global ischemia” time points (P = 1.00). Whereas, MDA level was comparable between NAC treatment group and ML treatment group at “recovery” (P = .861). MDA level was also significantly higher in placebo group in comparison to both NAC and ML groups at the “onset of reperfusion,” “15 after reperfusion,” and “recovery” time points (P < .05).
The level of TNF-α was significantly lower in ML group in comparison to placebo and NAC groups before surgery. There was no significant difference in TNF-α level between control and NAC before surgery. Repeated measures ANOVA showed that there was a significant effect of time on TNF-α level (F [3.615, 307.238] = 9.425, P < .001). The interaction between time and the intervention was also significant (F [7.229, 307.238] = 2.131, P = .038). The level of TNF-α changed over the period of CABG to the time of recovery in all 3 groups (Fig. 2 D). Overall, TNF- α levels were significantly different between the intervention groups (P < .001). TNF-α level was significantly lower in ML group compared to placebo group (P < .001). Specifically, TNF-α level in the ML group was significantly lower than control group at all the time points, starting from before surgery to recovery. In contrast, TNF-α level in NAC group was lower than control group only at the “recovery” time point (P < .001), (Fig. 2 D).
3.1. Clinical outcomes and complications
The mean value of total intensive care unit and hospital lengths of stay for the patients in the 3 groups: control, NAC, and ML were calculated to be 9.53, 8.89, and 8.93, respectively by using Kruskal–Wallis test. We found a significant difference between the control and the other 2 groups (Table 1). Inotropic agents’ doses (0.1–0.6 mg/kg/min) support weaning time of the patients postoperatively and blood gas parameters were the same in the 2 groups of patients (NAC and ML) as compared with the control.
4. Discussion
The CABG with cardiopulmonary bypass improves the myocardial perfusion in patients with severe coronary artery diseases and provides improved symptom relief and mortality benefits.[14–16] However, CABG is also associated with the risk of ischemia/reperfusion injury due to oxidative stress and activation of inflammatory pathways.[17] Cardiopulmonary bypass may also trigger oxidative stress and inflammatory response when the blood traverses through extracorporeal circuit devoid of endothelial surface under abnormal shear stress condition as opposed to the blood vessels, mainly due to the activation of polymorphonuclear leukocytes.[18–20] Even though cardioplegia is helpful in maintaining asystolic state during the procedure and limiting the myocardial damage, it does not provide optimal protection as evidenced by the post-operative myocardial injury and other complications.[21] Modulating the main contributing factors such as oxidative stress and inflammatory response has been recognized as valuable approach to tackle the postoperative complications and mortality associated with CABG.[22]
Our study was designed to investigate the efficacy of 2 promising candidates for improving the CABG related cardiac injury, oxidative stress, and associated inflammation in patients presented with similar characteristics. The doses for the interventions were based on the previous studies that reported the efficacious and tolerable administration of ML and NAC.[23,24] Our study showed that there was a significant reduction in troponin-I and lactate level in the treatment groups compared to placebo arm indicating that ML and NAC were able to prevent the post CABG cardiac injury. In contrast, a randomized, double-blind, placebo-controlled study, patients undergoing CABG who received NAC (600 mg orally the day before and the morning of the operation, a bolus of 150 mg/kg intravenously) did not show a reduction in cardiac injury related parameters including troponin T, creatinine, hemoglobin, and platelet levels.[23] The differences in the observations could be due to different dosages or level of cardiac injury or patient variability in responsiveness to interventions.
Previous studies showed that CABG results in an increase in the level of MDA suggesting the role of oxidative stress in reperfusion injury.[25,26] Our study also shows that during global ischemia and reperfusion during CABG, the MDA level is elevated compared to baseline and remains elevated at the recovery stage. Preoperative prophylactic treatment with ML and NAC resulted in a significant reduction in MDA level compared to the non-treatment group. This finding suggests that ML and NAC may be useful in preventing CABG related reperfusion injury. For the first time, we report that ML and NAC similar efficacy in preventing the CABG related oxidative stress during the procedure as we compared the antioxidant activity of both of them side by side. ML forms an endogenous antioxidant defense mechanism and its secretion is dependent on circadian rhythm.[27–29] ML is secreted by the pineal gland and the timing of the CABG surgery may influence the level of oxidative stress due to fluctuating level of ML secretion. ML can act as a direct and indirect free radical scavenging molecule when present endogenously in the body or given exogenously. Our study shows that preoperative treatment with ML may be a beneficial approach to limit the oxidative stress mediated reperfusion injury. Evidently, ML has also been shown to reduce lipid peroxidation products and improve membrane fluidity in erythrocytes from patients undergoing CABG.[30] ML has also been reported to positively modulate the antioxidant defense regulating Nrf2-ARE pathway in peripheral blood mononuclear cells of patients suggesting a possible mechanism of action for its indirect action as an antioxidant. ML has lipophilic properties which allow inter-membrane action on target molecules. NAC is also a well-established antioxidant that helps to prevent the depletion of glutathione.[31] NAC is used routinely against acetaminophen poisoning, as mucolytic agent and considered as a potential treatment option for psychiatric disorders.[32] The use of NAC is economical and proven as safe in similar dose that has been used in our study. Previous studies showed that NAC reduces oxidative stress and affords cardio-protective effects when incorporated in the cardioplegia solution or administered intravenously prior to the CABG procedure.[33–35] Consistent with previous studies, our study also shows that preoperative oral administration of 600 mg daily NAC reduces the level of well-established oxidative stress marker MDA in CABG patients.
Our study also showed that ML and NAC reduce inflammatory marker, TNF-α, in comparison with patients who did not receive either of the two treatments. It is believed that oxidative stress and inflammatory response are intricately related to each other and exacerbate cardiac injury and remodeling. It should also be noted that 2 recent meta-analyses reported that NAC prevents postoperative atrial fibrillation and all-cause mortality in cardiac surgery patients.[36,37] In contrast, a previous meta-analysis showed that perioperative administration of NAC was not associated with any beneficial effect or risk in terms of measured outcomes in patients undergoing cardiac surgery.[38]
The current study revealed that ML and NAC have similar efficacy in reducing CABG related cardiac injury in conjunction with oxidative stress and inflammation markers. The results from the present study is well in agreement with the recent reports suggesting that administration of ML 5 days before the surgery reduces myocardial ischemia / reperfusion injury by inhibiting the oxidative stress, inflammation and apoptosis.[39] Importantly, it is worthwhile to study the combinatorial treatment with ML and NAC to understand whether this approach would be more beneficial in terms of rescuing the myocardium from CABG related injury. As discussed earlier, there are conflicting data regarding the efficacy of antioxidants such as NAC and ML for cardiovascular diseases. Our study also draws the attention to the fact that more clinical trials are needed to further evaluate the efficacy of NAC or ML with well-defined primary outcome measure and secondary outcome in CABG patients. Confirmation of the utility of NAC and ML may provide us with valuable new therapeutic option for CABG related complications.
4.1. Strengths and limitations of this study
This clinical trial aimed to investigate the efficacy of 2 well-known anti-oxidants to reducing reperfusion injury related biomarkers in Mediterranean population undergoing CABG. However, there were a few limitations, one of which was the baseline values of the biomarkers reported here are from just before the induction of anesthesia as the blood samples were not collected at least 1 day before the surgery. This may be explained as the study was designed to assess the effects of ML and NAC just during the CABG operation. This was single center, open labeled study, not a blinded study. Moreover, there was no follow-up for the patients including recorded hemodynamics, especially the percentage of ejection fraction, IC staying, and general conditions of heart functions after the surgery to ascertain the short term and long-term effects of ML and NAC.
Acknowledgments
The authors wish to thank the ethics and research committee members and staffs for processing the approval of the clinical trial.
Author contributions
AM and MB conceived the idea for this human trial. AM and RT were involved in the planning and study design. AM prepared the study protocol. AM was involved in recruiting and conducting the human trial. AO analyzed all the data from the study and helped in writing and editing the results. TN and PR participated in writing and editing of the full article. SA helped in carrying out the biochemical analysis in the laboratory. ES and AA collected the blood samples from the arterial line during the surgery and recorded the hemodynamic. MB, DI, HS, and AA were involved in anesthesia and surgery.
Conceptualization: Mehrzad Bahtoei, Ali Movahed.
Formal analysis: Afshin Ostovar, Ali Movahed.
Investigation: Rahim Tahmasebi.
Methodology: Ebrahim Shafiei, Daryoush Iranpour, Samad Akbarzadeh, Hooshang Shahryari, Abdorasoul Anvaripour.
Visualization: Pema Raj.
Writing – original draft: Ali Movahed.
Writing – review & editing: Pema Raj, Afshin Ostovar, Thomas Netticadan.
Footnotes
Abbreviations: ANOVA = analysis of variance, CABG = coronary artery bypass grafting, CMV = controlled mechanical ventilation, ELISA = enzyme-linked immunosorbent assay, ICU = intense care unit, IR = ischemic reperfusion, KCL = Potassium Chloride, LV = left ventricle, MDA = malondialdehyde, ML = melatonin, NAC = N-acetyl cysteine, NACIAM = N-acetylcysteine in acute myocardial infarction, TBARS = thiobarbituric acid reactive substance, TNF-α = tumor necrosis factor-α.
The project was registered with Clinical Trial Registry of Iran (Registration no: IRCT201304178129N3). Ali Movahed received a grant from Research Deputy of Bushehr University of Medical Science, Iran, to support this study.
The authors declare no conflicts of interest.
References
- [1].Verma S, Fedak PW, Weisel RD, et al. Fundamentals of reperfusion injury for the clinical cardiologist. Circulation 2002;105:2332–6. [DOI] [PubMed] [Google Scholar]
- [2].Reiter RJ, Tan DX, Sainz RM, et al. Melatonin protects the heart against both ischemia/reperfusion injury and chemotherapeutic drugs. Cardiovasc Drugs Ther 2002;16:5–6. [DOI] [PubMed] [Google Scholar]
- [3].Sochman J. N-acetylcysteine in acute cardiology: 10 years later: what do we know and what would we like to know?!. J Am Coll Cardiol 2002;39:1422–8. [DOI] [PubMed] [Google Scholar]
- [4].Varoni EM, Soru C, Pluchino R, et al. The impact of melatonin in research. Molecules 2016;21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Emamgholipour S, Hossein-Nezhad A, Ansari M. Can melatonin act as an antioxidant in hydrogen peroxide-induced oxidative stress model in human peripheral blood mononuclear cells? Biochem Res Int 2016;2016:5857940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pal R, Gulati K, Banerjee BD, et al. Pharmacological and biochemical studies on the protective effects of melatonin during stress-induced behavioral and immunological changes in relation to oxidative stress in rats. Can J Physiol Pharmacol 2016;94:296–301. [DOI] [PubMed] [Google Scholar]
- [7].Madhu P, Reddy KP, Reddy PS. Melatonin reduces oxidative stress and restores mitochondrial function in the liver of rats exposed to chemotherapeutics. J Exp Zool A Ecol Genet Physiol 2015;323:301–8. [DOI] [PubMed] [Google Scholar]
- [8].Zavodnik IB, Domanski AV, Lapshina EA, et al. Melatonin directly scavenges free radicals generated in red blood cells and a cell-free system: chemiluminescence measurements and theoretical calculations. Life Sci 2006;79:391–400. [DOI] [PubMed] [Google Scholar]
- [9].Reiter RJ. Interactions of the pineal hormone melatonin with oxygen-centered free radicals: a brief review. Braz J Med Biol Res 1993;26:1141–55. [PubMed] [Google Scholar]
- [10].Dhouib IE, Annabi A, Jallouli M, et al. A minireview on N-acetylcysteine: an old drug with new approaches. Life Sci 2016;151:359–63. [DOI] [PubMed] [Google Scholar]
- [11].Pasupathy S, Tavella R, Grover S, et al. early use of N-acetylcysteine with nitrate therapy in patients undergoing primary percutaneous coronary intervention for ST-segment-elevation myocardial infarction reduces myocardial infarct size (the NACIAM Trial [N-acetylcysteine in acute myocardial infarction]). Circulation 2017;136:894–903. [DOI] [PubMed] [Google Scholar]
- [12].Thiele H, Hildebrand L, Schirdewahn C, et al. Impact of high-dose N-acetylcysteine versus placebo on contrast-induced nephropathy and myocardial reperfusion injury in unselected patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. The LIPSIA-N-ACC (Prospective, Single-Blind, Placebo-Controlled, Randomized Leipzig Immediate PercutaneouS Coronary Intervention Acute Myocardial Infarction N-ACC) Trial. J Am Coll Cardiol 2010;55:2201–9. [DOI] [PubMed] [Google Scholar]
- [13].Armstrong D, Browne R. The analysis of free radicals, lipid peroxides, antioxidant enzymes and compounds related to oxidative stress as applied to the clinical chemistry laboratory. Adv Exp Med Biol 1994;366:43–58. [DOI] [PubMed] [Google Scholar]
- [14].Kim JB, Yun SC, Lim JW, et al. Long-term survival following coronary artery bypass grafting: off-pump versus on-pump strategies. J Am Coll Cardiol 2014;63:2280–8. [DOI] [PubMed] [Google Scholar]
- [15].Duarte JH. Coronary artery disease. Drug-eluting stents or CABG? Nat Rev Cardiol 2015;12:259. [DOI] [PubMed] [Google Scholar]
- [16].Sipahi I, Akay MH, Dagdelen S, et al. Coronary artery bypass grafting vs percutaneous coronary intervention and long-term mortality and morbidity in multivessel disease: meta-analysis of randomized clinical trials of the arterial grafting and stenting era. JAMA Intern Med 2014;174:223–30. [DOI] [PubMed] [Google Scholar]
- [17].Iqbal J, Ghaffar A, Shahbaz A, et al. Postoperative arrhythmias after coronary artery bypass grafting: a comparison between ’off pump’ and ’on pump’ CABG. J Ayub Med Coll Abbottabad 2010;22:48–53. [PubMed] [Google Scholar]
- [18].Warren OJ, Smith AJ, Alexiou C, et al. The inflammatory response to cardiopulmonary bypass: part 1—mechanisms of pathogenesis. J Cardiothorac Vasc Anesth 2009;23:223–31. [DOI] [PubMed] [Google Scholar]
- [19].Caplan LR. On-pump versus off-pump CABG. N Engl J Med 2010;362:852–3. [PubMed] [Google Scholar]
- [20].Zakkar M, Guida G, Suleiman MS, et al. Cardiopulmonary bypass and oxidative stress. Oxid Med Cell Longev 2015;2015:189863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Turer AT, Hill JA. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am J Cardiol 2010;106:360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Abacilar F, Dogan OF, Duman U, et al. The changes and effects of the plasma levels of tumor necrosis factor after coronary artery bypass surgery with cardiopulmonary bypass. Heart Surg Forum 2006;9:E703–709. [DOI] [PubMed] [Google Scholar]
- [23].El-Hamamsy I, Stevens LM, Carrier M, et al. Effect of intravenous N-acetylcysteine on outcomes after coronary artery bypass surgery: a randomized, double-blind, placebo-controlled clinical trial. J Thorac Cardiovasc Surg 2007;133:7–12. [DOI] [PubMed] [Google Scholar]
- [24].Andersen LP, Werner MU, Rosenberg J, et al. A systematic review of peri-operative melatonin. Anesthesia 2014;69:1163–71. [DOI] [PubMed] [Google Scholar]
- [25].Cavalca V, Sisillo E, Veglia F, et al. Isoprostanes and oxidative stress in off-pump and on-pump coronary bypass surgery. Ann Thorac Surg 2006;81:562–7. [DOI] [PubMed] [Google Scholar]
- [26].van Boven WJ, Gerritsen WB, Driessen AH, et al. Myocardial oxidative stress, and cell injury comparing three different techniques for coronary artery bypass grafting. Eur J Cardiothorac Surg 2008;34:969–75. [DOI] [PubMed] [Google Scholar]
- [27].Sokullu O, Sanioglu S, Kurc E, et al. Does the circadian rhythm of melatonin affect ischemia-reperfusion injury after coronary artery bypass grafting? Heart Surg Forum 2009;12:E95–99. [DOI] [PubMed] [Google Scholar]
- [28].Guo X, Kuzumi E, Charman SC, et al. Perioperative melatonin secretion in patients undergoing coronary artery bypass grafting. Anesth Analg 2002;94:1085–91. [DOI] [PubMed] [Google Scholar]
- [29].Yin YQ, Luo AL, Guo XY, et al. Perioperative melatonin circadian secretion in patients undergoing coronary artery bypass grafting surgery. Zhonghua Yi Xue Za Zhi 2004;84:456–9. [PubMed] [Google Scholar]
- [30].Ochoa JJ, Vilchez MJ, Palacios MA, et al. Melatonin protects against lipid peroxidation and membrane rigidity in erythrocytes from patients undergoing cardiopulmonary bypass surgery. J Pineal Res 2003;35:104–8. [DOI] [PubMed] [Google Scholar]
- [31].Haghjooy Javanmard S, Ziaei A, Ziaei S, et al. The effect of preoperative melatonin on nuclear erythroid 2-related factor 2 activation in patients undergoing coronary artery bypass grafting surgery. Oxid Med Cell Longev 2013;2013: 676829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dean O, Giorlando F, Berk M. N-acetylcysteine in psychiatry: current therapeutic evidence and potential mechanisms of action. J Psychiatry Neurosci 2011;36:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]


