Abstract
The dominant neural models of typical and atypical reading focus on the cerebral cortex. However, Nicolson and colleagues (2001) proposed a model, the cerebellar deficit hypothesis, in which the cerebellum plays an important role in reading. To evaluate the evidence in support of this model, we qualitatively review the current literature and employ meta-analytic tools examining patterns of functional connectivity between the cerebellum and the cerebral reading network. We find evidence for a phonological circuit with connectivity between the cerebellum and a dorsal fronto-parietal pathway, and a semantic circuit with cerebellar connectivity to a ventral fronto-temporal pathway. Furthermore, both cerebral pathways have functional connections with the mid-fusiform gyrus, a region implicated in orthographic processing. Consideration of these circuits within the context of the current literature suggests the cerebellum is positioned to influence both phonological and word-based decoding procedures for recognizing unfamiliar printed words. Overall, multiple lines of research provide support for the cerebellar deficit hypothesis, while also highlighting the need for further research to test mechanistic hypotheses.
Keywords: cerebellar deficit hypothesis, reading development, network, decoding, phonological, semantic
Graphical abstract
The predominant theories of developmental dyslexia view the underlying disorder as arising from dysfunction in the cerebral cortex (for review see Richlan, 2012). Similarly, efforts to understand the neural basis of reading development have focused largely upon the cerebral cortex. Central to the current review is the recognition that the predominant theories of dyslexia do not capture the full behavioral phenotype associated with dyslexia (Nicolson and Fawcett, 1990; Tallal, 1980). This has motivated continued consideration of alternative perspectives on the neural substrates of developmental dyslexia and reading. The current review evaluates one such alternative theory, the “cerebellar deficit hypothesis” proposed by Nicolson and colleagues (2001). Specifically, this review addresses three major questions: 1) Does a qualitative review of the literature provide compelling evidence linking abnormalities in the cerebellum with atypical reading development? 2) Is there meta-analytic evidence of functional connectivity between the cerebellum and cerebral regions involved in reading? 3) If so, does the pattern of functional connectivity yield insights into cerebellar involvement in reading development and dyslexia?
1. Current literature and open questions
1.1. The cerebellar deficit hypothesis
For decades, the predominant theories of developmental dyslexia have emphasized an underlying deficit in phonological (Liberman, 1973; Stanovich, 1988) and visual processing (Lovegrove et al., 1980; Stein, 1991), with a presumed locus of dysfunction in the cerebral cortex (Richlan, 2012). At the same time, there have been individuals who have raised questions about the completeness of this account. As one example, Nicholson and Fawcett (1995, 1990) proposed a cerebellar role that contributes to the acquisition and automatisation of reading procedures. Their alternative theory arose out of a series of investigations examining reading performance and motor skills in children with and without dyslexia. Importantly, in addition to reading impairments, they found that many of the children with dyslexia also exhibited behavioral impairments in sensorimotor function, such as poor balance and coordination, and slowed information processing (Nicolson et al., 1999; Nicolson and Fawcett, 1995, 1990). This observed pattern led Nicolson and colleagues (2001) to propose the “cerebellar deficit hypothesis” as an alternative account of developmental dyslexia.
The “cerebellar deficit hypothesis” (Nicolson et al., 2001) proposes that the cerebellum contributes to reading skills via two indirect processes: articulatory/phonological ability and skill automatisation. In the model, impairments of articulatory fluency lead to difficulties in engaging speech processes that contribute to reading development. This aspect of the model is consistent with the reviewed evidence that the cerebellum contributes to articulatory motor control (Marvel and Desmond, 2010), and that speech-based processes contribute to reading and phonological representation (Baddeley, 2003; Hickok and Poeppel, 2000). Importantly, Nicolson and colleagues’ consideration of skill automatisation draws a parallel beyond the reading and language literature, to theories of sensorimotor learning in which cerebellar error detection mechanisms help to establish automatic and accurate motoric actions (Llinas et al., 1973; Marr, 1969). Nicholson and colleagues reasoned that if this cerebellar procedural learning system was impaired, it could similarly lead to disadvantages in the automatic recognition of printed words.
The idea that reading development involves procedural learning systems has gained some traction in the literature. This has come through studies that have compared the procedural learning of individuals with or without dyslexia. One widely used task, the serial recall task (Nissen and Bullemer, 1987), measures unintentional motor learning by testing participants’ uninstructed improvements in accuracy and reaction time in response to a stimulus presented in a repeating sequence or a random order. A second widely used task, the artificial grammar learning paradigm (Reber, 1967), measures unintentional language learning by testing participants’ ability to learn the compositional structure of verbal or visual pattern sequences (i.e., grammatically legal versus illegal strings) without ever being explicitly informed about the rules of the grammar. The collective results are mixed for both tasks (for review, see Schmalz et al., 2017), with several studies reporting significant differences between individuals with or without dyslexia (e.g., Pavlidou et al., 2010; Pothos and Kirk, 2004; Stoodley et al., 2006; Vicari et al., 2005, 2003), and others reporting equivalent learning effects (e.g., Kelly et al., 2002; Laasonen et al., 2014; Nigro et al., 2016; Roodenrys and Dunn, 2008; Rüsseler et al., 2006; Waber et al., 2003). Overall, there is at least suggestive evidence that individuals with dyslexia have general difficulty with procedural learning tasks, as predicted by the cerebellar deficit hypothesis.
In summary, an important strength of Nicolson and colleagues’ (2001) theoretical framework is that it integrates ideas about cerebellar contributions to articulation and learning with cerebral systems implicated in reading and reading development. However, the authors concluded that the cerebellar deficit hypothesis remained in need of neuroanatomical evidence to directly link developmental dyslexia to the cerebellum (Nicolson and Fawcett, 2005). Below, we consider the available evidence to date and where there is a need for further research.
1.2. Neuroimaging evidence for a link between the cerebellum and dyslexia
Neuroimaging offers several approaches that can be used to test for links between the cerebellum and dyslexia. One approach is to search for evidence of aberrant functional engagement of the cerebellum during reading or reading-related tasks (e.g., implicit learning, lexical-decision) in individuals with or without dyslexia. This approach has yielded conflicting evidence. Specifically, functional neuroimaging investigations comparing individuals with or without dyslexia report both the absence (e.g., Cao et al., 2006; Georgiewa et al., 1999) and presence (e.g., Feng et al., 2017; Kronschnabel et al., 2013; Menghini et al., 2006; Yang et al., 2013) of aberrant cerebellar activation. In fact, the few meta-analytic studies of dyslexic versus normal reading do not report significant group differences within the cerebellum (Pollack et al., 2015; Richlan, 2012; Richlan et al., 2009). To this point, Richlan and colleagues (2009) note that the majority of reading studies do not provide coverage of the cerebellum, and therefore null results could be a sampling problem. Overall, comparisons of functional activation between individuals with or without dyslexia have not provided consistent information to merit a solid conclusion.
A second approach is to probe for links between neural tissue abnormalities within the cerebellum and reading ability (Eckert, 2004). A recent meta-analysis examined differences in cerebellar cortical volume between controls and individuals with three developmental disorders: autism spectrum disorder, attention deficit hyperactivity disorder, and dyslexia (Stoodley, 2014). This analysis revealed anatomic cerebellar sub-regions in which volumetric differences were specifically associated with only one of the developmental disorders. Notably, reduced cerebellar grey matter was found in clusters within bilateral lobule VI and right Crus2 that were specific to dyslexia. Partially convergent evidence for a link between cerebellar tissue abnormalities and reading comes from a study that examined the reading abilities of adults with acquired damage to the cerebellum (lesion group), as compared to a group of matched controls (Ben-Yehudah and Fiez, 2008). The authors did not find significant differences in reading ability between the lesion and control group. However, they did find that the lesion group performed more poorly on a rhyme judgment and a verbal working memory task. They concluded that cerebellar dysfunction might create a deficiency in phonological or articulatory monitoring, which could in turn impact reading development if it occurred in childhood.
Finally, a third approach is to test for differences in cerebro-cerebellar functional and structural connectivity in individuals with or without dyslexia. Stanberry and colleagues (2006) examined functional connectivity between these groups during a reading-related task (phoneme-mapping). While both groups exhibited synchronous signal changes between the left inferior frontal gyrus and right cerebellar lobules III-VI and Crus1, the individuals with dyslexia exhibited less synchrony than did controls. Fernandez and colleagues (2015) examined the integrity of microstructural white-matter tracts that connect the cerebellum and three reading-associated cerebral regions: the inferior frontal, temporoparietal, and occipitotemporal cortex. Relative to typical readers, children with dyslexia had aberrant white matter integrity in cerebellar tracts that connect to the inferior frontal and temporoparietal cortex. Furthermore, younger children with dyslexia exhibited decreased white matter integrity in an occipitotemporal-cerebellar tract, whereas older children with dyslexia had aberrant tract integrity. The authors speculated that the increase in older children’s inferior frontal and temporoparietal tracts could be a product of increased dependence on these tracts for the integration and automation of the motor, cognitive, and sensory processes needed to produce accurate and fluent word recognition.
Collectively, neuroimaging studies of cerebellar function, structure, and connectivity have provided a growing base of evidence that links differences in the cerebellum to differences in reading ability. Thus, while the field has yet to test the mechanistic details of the cerebellar deficit hypothesis, there is growing support for an association between cerebellar dysfunction and dyslexia.
1.3. Evidence for cerebellar contributions to normal reading
Thinking beyond dyslexia per se, the cerebellar deficit hypothesis posits that the contributions of the cerebellum are important for normal reading development. Therefore, another way to evaluate the cerebellar deficit hypothesis is to consider what is known about the cerebellum and typical reading development.
Unfortunately, theoretical perspectives on the neural basis of reading rarely consider the cerebellum (e.g., Taylor et al., 2012; Turkeltaub et al., 2002). Nonetheless, cerebellar activation is frequently observed during reading or reading-related tasks (for review, see Stoodley and Stein, 2011; Vlachos et al., 2007). The challenge has been to understand the significance of this functional engagement. This difficulty is summarized in an excellent consensus paper by Mariën and colleagues (2014), wherein the authors tried to gather and integrate various theoretical conceptualizations of cerebellar function. The authors particularly focused on linking potential aspects of cerebellar function to processes that might drive reading development. Their graphical summary, reprinted in Figure 1, illustrates the breadth of speculated cerebellar contributions to the development of skilled reading. The authors proposed that any (or even all) of the proposed cerebellar functions might help to scaffold skilled reading. They concluded that “the insights of developmental cognitive neuroscience—especially the role of the cerebellum and cerebellar networks in reading—are crucial to the understanding and acceleration of reading development” (Mariën et al., 2014, p. 399). The reviews of Mariën et al. and others (Stoodley, 2012; Vlachos et al., 2007) make clear that the field has progressed as far to believe that there is a cerebellar contribution to reading, but the specific nature of that contribution remains an enigma.
Figure 1.
Proposed cerebellar components that contribute to the development of reading according to a consensus paper. Image reprinted from “Consensus Paper: Language and the Cerebellum: An Ongoing Enigma” by P. Mariën et al., 2014, Cerebellum, 13, p. 399. Permission to be obtained.
In considering how to gain traction on this issue, it is worth noting that theories about the cerebellum and reading have tended to focus more on articulatory and phonological than semantic processes (Ackermann, 2008; Ben-Yehudah and Fiez, 2008; Chen and Desmond, 2005; Peeva et al., 2010). This is interesting because neural models of reading often posit the existence of separable neural correlates of phonological and semantic processing (e.g., Fiez, 1997; Gold et al., 2005; Price et al., 1997). Typically, the phonological stream is thought to comprise dorsal brain regions in the parietal and frontal cortex, and to play a critical role in orthographic to phonologic mapping (i.e., decoding the pronunciation of the printed word). The semantic stream is thought to involve ventral brain regions in the occipital and temporal cortex, and to contribute to mapping visual word-forms to their meaning and corresponding conceptual and lexical-level phonological knowledge. This sets up the expectation that if the cerebellum does indeed interface with the reading network, it may predominantly interconnect with cerebral regions implicated in phonological as compared to semantic aspects of reading.
To our knowledge, this prediction has yet to be tested. Indeed, only a few studies have specifically examined the connectivity of the cerebellum with cerebral regions implicated in reading, and no systematic review of this topic is available. Fortunately, shared datasets and meta-analytic software provide the ability to comprehensively tackle this question. In the next section, we take advantage of these resources to examine the patterns of functional connectivity between the cerebral reading network and the cerebellum.
2. Linking the cerebellum into the reading network
Functional connectivity analyses involve identifying one or more seed-regions and identifying those voxels throughout the rest of the brain volume that exhibit correlated patterns of blood-oxygen-level dependent (BOLD) signal. When performed using resting-state data, it is thought that the degree of signal correlation between two regions provides a measure of their connectivity (Biswal, 2012; Buckner et al., 2013). In the context of the current study, the intent is to use cerebral seed-regions that are clearly established constituents of the reading network, and to then test whether any of these regions are functionally connected with regions within the cerebellum.
2.1. Characterizing the cerebral reading network
Because the neural constituents of the reading network are not fully specified within the literature, we first used a meta-analytic approach to identify a set of reliable seed-regions for our planned functional connectivity analysis. More specifically, we identified our reading-related seed-regions using two popular online meta-analytic tools in the following manner: (1) Neurosynth– we extracted the “term-based” reverse inference imaging map for the term reading1, (2) GingerALE (ALE) – we conducted a secondary meta-analytical contrast of previously published meta-analytic results that specifically focused on phonological versus semantic processing in word recognition2. Table 1 provides details about the studies and foci included in this ALE analysis.
Table 1.
List of meta-analyses included in the GingerALE process
| Number of foci (left hemisphere)
|
|||||
|---|---|---|---|---|---|
| Meta-analysis | Cluster procedure | Contrast | # of articles | Phon | Sem |
| Cattinelli et al. (2013) | Binomial test | Item | 35 | 1 | 8 |
| Jobard et al. (2003) | Hierarchical clustering | Item | 35 | 5 | 3 |
| Maisog et al. (2008) | ALE | Ability | 9 | 7 | 0 |
| Martin et al. (2015) | ES-SDM | Stage | 20 | 1 | 4 |
| McNorgan et al. (2015) | ALE | Item | 33 | 3 | 4 |
| Paulesu et al. (2014) | Hierarchical clustering & ALE | Ability | 53 | 9 | 2 |
| Richlan et al. (2009) | ALE | Ability | 17 | 9 | 5 |
| Taylor et al. (2012) | ALE | Item | 37 | 22 | 20 |
Note. In-house analysis began by identifying published reviews of the neural basis of reading indexed within either Pubmed (http://www.ncbi.nlm.nih.gov/pubmed/) or PsychINFO (http://search.proquest.com/) databases, filtering the results to papers that reported coordinates in standard stereotactic space (MNI or Talairach) from a quantitative meta-analysis examining functional MRI or PET studies of reading-related tasks (e.g., lexical decision, rhyme judgment). The search was further narrowed to eight papers, chosen because they report meta-analytic results from one of three contrasts theoretically associated with phonological processing (Phon) versus semantic processing (Sem) weighting within the reading network: (1) item-level – nonword (Phon) vs. word (Sem) single-word reading in adults (n = 4), (2) stage-level – children (Phon) vs. adults (Sem) reading (n = 1), and (3) ability-level – control (Phon) vs. dyslexic (Sem) reading (n = 3). ES-SDM = Effect-size signed differential mapping.
Because the reading network is predominantly lateralized within the left hemisphere, we considered only left-hemisphere clusters within the statistical images that resulted from our two meta-analytic tools. Within each map, we identified those activation clusters that surpassed our significance criteria, and extracted their centroid voxel loci to serve as seeds for our subsequent functional connectivity analysis. As detailed in Table 2, we identified 14 regions associated with reading-related processing: eight derived from the Neurosynth inference map for the term reading, and six from our ALE analysis contrasting differential phonological versus semantic processing. Somewhat surprisingly, the majority of the ALE phonological versus semantic clusters localized to the periphery of the Neurosynth reading map (Figure 2a), suggesting that the Neurosynth reading regions are less biased towards phonological or semantic processing than the neighboring regions identified through the ALE analysis. To test this interpretation, we inspected the patterns of functional activation at each of 14 regions. This was accomplished by placing a 3 mm sphere around each seed-voxel locus and extracting each sphere’s average z-value within Neurosynth’s reverse inference maps for the terms phonological, semantic, and reading (Figure 2b). As anticipated, for the ALE phonological > semantic spheres the highest z-values were found for the phonological term map, and for the ALE semantic > phonological spheres they were found for the semantic term map. The Neurosynth reading spheres had a generally impartial weighting for any of the three reading-related inference maps. Thus, while we do not claim to have identified every neural constituent of reading processing, our 14 identified regions seem to be clearly part of the cerebral network for reading and reading-related processes.
Table 2.
Peak centroid clusters of the direct ALE contrast for phonological vs. semantic processing, and the Neurosynth reverse inference map for the term “reading”
| MNI coordinates
|
||||||
|---|---|---|---|---|---|---|
| Meta-analytic approach | Cerebral-cortical region | BA | mm3 | x | y | z |
| ALE (phonological > semantic) | ||||||
| IPL* | 40 | 10136 | −42 | −44 | 42 | |
| mFG | 37 | 8296 | −46 | −58 | −12 | |
| STG | 22 | 4784 | −60 | −24 | 4 | |
| IFGop | 44/6 | 1504 | −52 | 6 | 18 | |
| ALE (semantic > phonological) | ||||||
| AG | 39 | 1880 | −48 | −66 | 22 | |
| MTG* | 21/20 | 168 | −62 | −44 | −8 | |
| Neurosynth (reading) | ||||||
| mFG | 37 | 5624 | −44 | −52 | −14 | |
| MTG/STG | 22 | 3144 | −56 | −40 | 4 | |
| IFJ* | 9 | 1096 | −46 | 12 | 30 | |
| vIFG | 46 | 944 | −46 | 32 | 4 | |
| IFGtr | 45 | 776 | −52 | 22 | 16 | |
| SFG | 6 | 392 | −6 | 12 | 60 | |
| PreCG | 6 | 280 | −52 | 0 | 48 | |
| PostCG | 3/4 | 184 | −52 | −10 | 46 | |
Note.
Indicates a region with significant functional connectivity to the cerebellum in subsequent analysis
MNI = Montreal Neurological Institute, BA = Brodmann’s Area (nearest), mm3 = cluster volume, IPL = inferior parietal lobe, mFG = mid-fusiform gyrus, STG = superior temporal gyrus, IFGop = inferior frontal gyrus (opercularis), AG = angular gyrus, MTG = middle temporal gyrus, IFJ = inferior frontal junction, vIFG = inferior frontal gyrus (ventral), IFGtr = inferior frontal gyrus (triangularis), SFG = superior frontal gyrus, PreCG = precentral gyrus, PostCG = postcentral gyrus. Cluster centroids were localized utilizing Brainmap.org’s recommended Multi-Image Analysis Program Mango; http://ric.uthscsa.edu/mango/.
Figure 2.
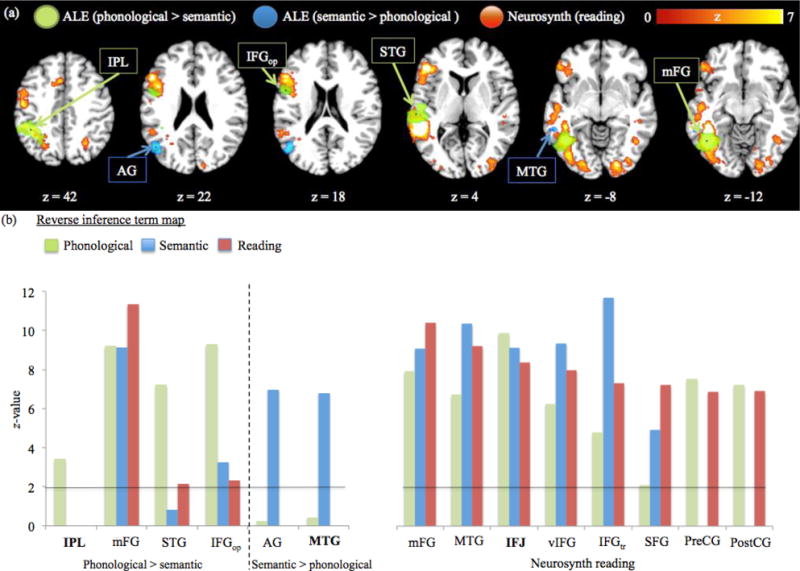
Comparison of the three reading-related networks as determined by the ALE and Neurosynth map. (A) Activation comparison of the fractionated reading network regions for the six ALE phonological > semantic (green) and semantic > phonological (blue) clusters with the Neurosynth reverse inference map for the term reading (red). Spheres indicate each localized ALE cluster’s centroid voxel. Images were analyzed in MNI space and overlaid onto a 2×2×2 anatomical template provided by Brainmap.org (http://brainmap.org/). (B) Bar graph of the ALE phonological > semantic, semantic > phonological, and Neurosynth reading seed-voxels’ preferential bias towards functional terms. A 3 mm sphere was centered on each seed-voxel and placed onto the Neurosynth reverse inference maps for the terms phonological (green bars), semantic (blue bars), and reading (red bars). For each sphere, the average z-values were extracted and compared across the three reverse inference maps. Horizontal line indicates the significance threshold (z > 1.96). Regions in boldface font indicate functional connectivity with the cerebellum in subsequent analysis.
2.2. Functional attributions of cerebro-cerebellar circuits
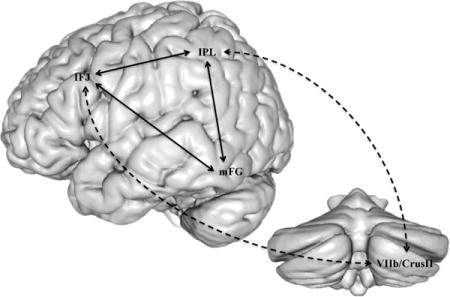
Having identified reliable cerebral constituents of the reading network, as well as their bias towards phonological or semantic processing, we next used Neurosynth’s “location based analysis” feature3 to determine whether there is evidence in support of cerebellar engagement with any of our 14 reading-related cerebral regions. More specifically, the centroid coordinates reported in Table 2 were entered into Neurosynth’s functional connectivity analysis as seed-voxels to search for cerebellar connectivity. For the purpose of this review’s main aim – crosstalk between the cerebral reading network and the cerebellum – we chose to focus on positive correlations within the right hemisphere of the cerebellum, which is anatomically connected to the left cerebral hemisphere. The centroids of cerebellar clusters with significant functional connectivity to our seed coordinates, as well as further methodological details, are provided in Table 3. Overall, three reading-related cerebral seed-voxels revealed significant functional connectivity to the cerebellum: 1) the inferior frontal junction (IFJ), 2) inferior parietal lobule (IPL), and 3) the middle temporal gyrus (MTG). Interestingly, these cerebral regions also displayed significant functional connectivity to some of the same cerebellar regions. To further test for functional sets of connectivity, we examined the functional connections between those cerebral regions that also revealed connectivity convergence to the same cerebellar region. As illustrated in Figure 3, this analysis revealed three distinct sets of functionally connected regions. For the first set, we found functional connections between our IFJ and IPL regions, which converged to a region in the right lateral posterior inferior cerebellum, at or near lobule HVIIb/CrusII (Figure 3a). For the second set, we found functional connectivity between our IFJ and MTG regions, which converged to a region in the right posterior superior cerebellum, at or near lobule CrusI/CrusII (Figure 3b). For the third set, our MTG region also exhibited functional connectivity with the right lateral anterior inferior region of the cerebellum, at or near lobule CrusI (Figure 3c).
Table 3.
Reading-related seed-regions revealing significant functional connectivity to clusters within the right cerebellar hemisphere
| Coordinate
|
|||||
|---|---|---|---|---|---|
| Cerebral seed-region | Correlated cerebellar region | mm3 | x | y | z |
| IFJ | R VIIb/Crus2 | 152 | 30 | −68 | −50 |
| R Crus1/Crus2 | 224 | 10 | −76 | −28 | |
| IPL | R VIIb/Crus2 | 56 | 28 | −70 | −50 |
| MTG | R Crus1 | 560 | 42 | −66 | −42 |
| R Crus1/Crus2 | 320 | 10 | −82 | −28 | |
Note. The cerebellar coordinate overlap in IFJ and IPL functional connectivity to right VIIb/Crus2, and IFJ and MTG’s connectivity to Crus1/Crus2 are shown in Figure 3. The identified cerebellar regions were structurally normalized and localized using a hi-resolution Spatially Unbiased Atlas Template (SUIT; Diedrichsen et al., 2011, 2009). Time-series correlation for peak centroid coordinates to the regions within the cerebellum was set at a voxel-wise threshold of r > .25, and minimum cluster volume of 50 mm3.
Figure 3.
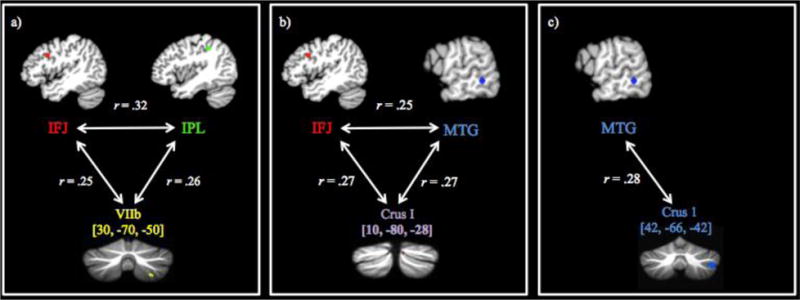
Functional connectivity results between the cerebral regions within the reading network and the cerebellum. (A) Functional connections between the inferior frontal junction from the Neurosynth reading map and the inferior parietal lobule from the ALE phonological > semantic map showed functional connectivity convergence with a cerebellar region localized in lobule HVIIb/Crus2 (cerebellar coordinate is the mean centroid voxel from the IFJ and IPL connectivity analyses). (B) Functional connectivity between the inferior frontal junction and the middle temporal gyrus from the ALE semantic > phonological map showed functional connectivity convergence to a medial section of the cerebellum localized around Crus1/Crus2 (cerebellar coordinate is the mean centroid voxel from the IFJ and MTG connectivity analyses). (C) The middle temporal gyrus also revealed a direct connection to a more posterior-lateral cerebellar region of lobule Crus1. The identified cerebellar regions were structurally normalized and localized using a hi-resolution Spatially Unbiased Atlas Template (SUIT; J. Diedrichsen et al., 2011; Jörn Diedrichsen, Balsters, Flavell, Cussans, & Ramnani, 2009). Time-series correlation for peak centroids was set at a voxel-wise threshold of r > .25, and cluster volume of 50 mm3.
We next attempted to characterize the functional roles of our three sets of interconnected cerebro-cerebellar regions. To do so, we made use Neurosynth’s “word association” metrics to identify overlapping cognitive terms within each set of regions. Anatomical terms associated with a coordinate were excluded (e.g., fusiform, FG, frontal). As shown in Figure 4, this process revealed evidence of terminology consistent with a phonological role for the first set of regions, a semantic role for the second set of regions, and no common terminology for the third set of regions4.
Figure 4.
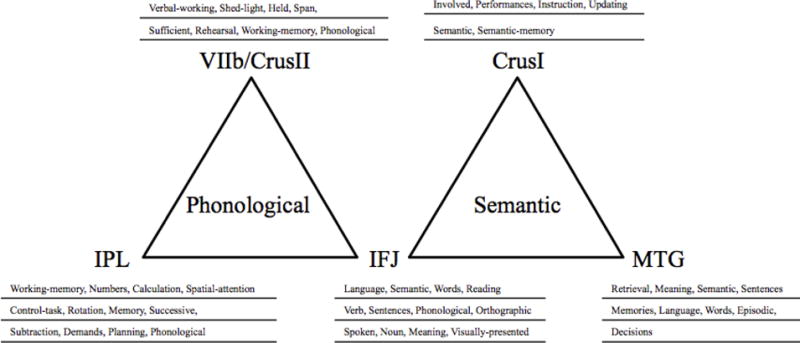
Top 12 terms (highest z-value) corresponding to the seed-voxel for the functionally connect cerebro-cerebellar regions, as determined by Neurosynth’s term association metrics. Neuroanatomical and redundant terms were excluded and replaced with the next greatest associated term.
2.3. Interconnectivity to mid-fusiform gyrus
As a final point of exploration, we considered the interconnections between all of our identified seed-regions to the mid-fusiform gyrus (mFG). The mFG territory has received considerable attention for its role in reading. Functional neuroimaging studies have reported greater mFG activity for word reading as compared to pseudowords (Cohen et al., 2002b; McCandliss et al., 2003), non-words, digits (Polk et al., 2002), or false-fonts (Brunswick et al., 1999; Cohen et al., 2002a). Neuroanatomical investigations show that individuals who sustain damage to this territory exhibit letter-by-letter reading strategies, a hallmark of acquired alexia (Cohen et al., 2003; Gaillard et al., 2006; Hirshorn et al., 2016). These findings implicate the mFG territory in the representation and processing of orthographic knowledge, leading some to argue for its specialization as a visual word-form area (e.g., Cohen and Dehaene, 2004; Dehaene and Cohen, 2011). We therefore reasoned that if the cerebellum influences reading development, it should have direct or indirect functional connectivity with the mFG.
The Neurosynth functional connectivity analysis for the mFG seed-voxel [−46, −58, −12] revealed functional connections with both our IFJ and the IPL regions. None of the remaining 12 seed-regions were found to have significant connectivity with the mFG seed-voxel, and no voxels with significant connectivity to the mFG seed voxel were found in the cerebellum.
3. Theoretical implications of cerebro-cerebellar connectivity
Overall, our meta-analytic results support the cerebellar deficit hypothesis (Nicolson et al., 2001) by providing evidence that specific regions of the cerebellum are functionally connected with the cerebral reading network. Our most coherent evidence identified two functionally connected sets of cerebro-cerebellar regions, both of which also possess functional connections with the mFG. The first set is a cerebro-cerebellar dorsal circuit comprising IFJ and IPL regions that are functionally connected with the mFG, and with the lateral posterior inferior cerebellum (Figure 4). The second set is a cerebro-cerebellar ventral circuit, comprising IFJ and MTG regions that have partial interconnectivity with the mFG, and with the posterior superior cerebellum. In the following sections, we consider these circuits in greater detail and evaluate them within the context of the current literature.
3.1. Cerebro-cerebellar dorsal circuit
Turning first to the dorsal circuit, it is noteworthy that our meta-analytic review identified only one common term (phonological) across all four regions of this circuit (this includes the mFG). The observed cerebral regions are consistent with previous work implicating the IFJ and IPL in orthographic to phonological decoding (Taylor et al., 2012; Vigneau et al., 2006). Specifically, these regions have been posited as components of a fronto-parietal pathway that supports visual-verbal conversions for unfamiliar words via phonological decoding of letter-sound spelling patterns.
This decoding interpretation is supported by the considerable amount of evidence linking the fronto-parietal pathway to phonological processing. For example, functional neuroimaging investigations have reported evidence of stronger IFJ activation for multiple task contrasts indicative of phonological processing, such as reading rhymes versus synonyms (Roskies et al., 2001), pseudowords versus real words (Herbster et al., 1997), and syllable versus semantic judgment of words (Poldrack et al., 1999). The IFJ territory has also been implicated in resolving phonological output demands (Cao et al., 2006; Taylor et al., 2011; Thompson-Schill et al., 1998). Finally, this territory is recruited during tongue movements and laryngeal articulation of sounds (Braun et al., 1997; Riecker et al., 2000), supporting the notion of its involvement in phonology within the subvocal rehearsal system (Chen and Desmond, 2005; Paulesu et al., 1993). In all of these cases, the reported frontal coordinate of activation fell within 20 mm of our IFJ seed-voxel.
Research addressing the role of IPL in reading has frequently implicated its involvement in associating written word-forms with their spoken constituents (Taylor et al., 2012). Engagement of IPL has been found for cross-modal word-judgment tasks that specifically involve judging whether acoustically presented words have the same spelling patterns or judging whether visually presented words rhymed (Booth et al., 2003). Greater activation has also been reported following training on novel symbol-phoneme versus novel symbol-nonspeech associations, further implicating this region in the acquisition of visual-verbal knowledge (Hashimoto and Sakai, 2004). In all of these cases, the reported parietal coordinate of activation also fell within 20 mm of our IPL seed-voxel.
More recently, investigations have gone beyond thinking about the IFJ and IPL regions separately to considering how they work together to support or transform orthographic representations. This interest has been driven by an expanding body of evidence demonstrating that the IFJ and IPL territories both interact with the mFG, a critical site for orthographic processing (Thiebaut De Schotten et al., 2014; Zachariou et al., 2014). Consistent with these past findings, not only did we replicate functional connectivity between the mFG and our IFJ and IPL seed-voxels, these were the only two of our 14 seed-voxels that met our significance threshold for connectivity with the mFG.
If fronto-parietal connectivity with the mFG influences orthographic processing, then individual differences in this interconnectivity should be associated with individual differences in visual word recognition. Recent studies involving individuals with and without dyslexia have provided evidence in support of this prediction (Cao et al., 2008; Koyama et al., 2013; Schurz et al., 2015; van der Mark et al., 2011; Vogel et al., 2012a). One noteworthy study demarcated the left occipitotemporal cortex into five non-overlapping seed-regions (posterior to anterior), which includes the mFG, to examine functional connectivity differences in children with or without dyslexia (van der Mark et al., 2011). Children without dyslexia exhibited mFG connectivity to frontal and parietal regions comparable to the localized clusters in our dorsal circuit (within 20 mm), but significant connectivity was not found for children with dyslexia. No significant group differences were found for IFJ and IPL connectivity from any of the other demarcated occipitotemporal seed regions. Based on these results, the authors concluded that a functionally connected fronto-parietal-mFG system plays a special role in visual print processing.
Relatedly, Cao and colleagues (2008) had children with and without reading difficulties perform a rhyme judgment task. Pairs of words were viewed under a difficult conflicting condition (e.g., similar/different orthography and different/similar phonology) and easier non-conflicting condition (e.g., matching phonology and orthography or different phonology and orthography). Their effective connectivity analysis found three main modulatory effects relevant to the current review. First, children without reading difficulties exhibited a stronger modulatory effect from both the mFG and the IPL to the IFJ for the conflicting versus non-conflicting condition. Second, there was a modulatory effect from mFG to the IPL that was stronger in children without reading difficulties than children with reading difficulties for conflicting trials only. Finally, a top-down modulatory effect from the IFJ to the IPL was positively correlated with reading skills (as assessed by the word-attack task) for non-conflicting trials. Findings such as these are indicative of a sophisticated fronto-parietal-mFG cerebral system that is relevant for skilled reading.
Overall, the existing literature supports ideas about a fronto-parietal route that contributes in some fashion to phonological processing. Moreover, phonological influences on visual word recognition might be represented by the interactivity between this frontal-parietal pathway and the mFG. Having reviewed the idea of an interactive network comprising frontal, parietal and mFG regions, we next focus on how the cerebellum might interface with network.
As noted in the introduction, cerebellar connectivity to the cerebral reading network has received relatively little attention. However, most theories about a cerebellar contribution to reading have emphasized a role in phonological and articulatory processing (Ackermann, 2008; Ben-Yehudah and Fiez, 2008; Chen and Desmond, 2005). Therefore, we hypothesized that cerebellar functional connectivity would be observed to a dorsal phonological route for reading. Our results, which show functional connectivity between the cerebellum and our IFJ and IPL regions, support this general prediction.
The next question pertains to how the cerebellum may interface with this dorsal pathway. As previously discussed, the cerebellar deficit hypothesis describes an indirect association with reading that stems from cerebro-cerebellar loops that support phonological and articulatory skill (Nicolson et al., 2001; Nicolson and Fawcett, 2011). We provide anatomical support for this theoretical claim, with evidence that the cerebellum is functionally connected with a dorsal phonological pathway, but no evidence of direct cerebellar connectivity to mFG. This suggests that the cerebellum supports phonological decoding by exerting a modulatory role on phonological processing, which can then indirectly influence orthographic processing (Figure 5). For instance, cerebellar fine-tuning and coordination of phonological information held within IFJ and IPL, such as sensorimotor representations of speech content (Peeva et al., 2010), could contribute to more accurate and rapid phonological processing.
Figure 5.
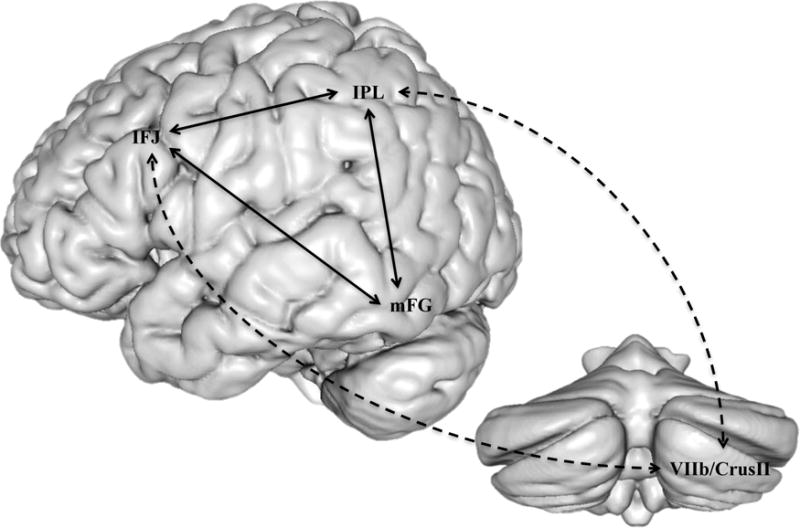
Cerebro-cerebellar dorsal circuit. Dashed line indicates a proposed indirect cerebellar support in developing orthographic knowledge stored in the mFG via direct modulatory connections with the fronto-parietal pathway for phonological processing.
Improving the performance of the dorsal pathway could improve the accuracy of decoding, which could in turn bootstrap skilled visual word recognition. This link is explained by Share’s (1995) model of reading development, which posits that each successful decoding experience leads to the accrual of word-specific orthographic information, so that eventually a familiar word can be recognized “on sight” without effortful decoding. This idea is consistent with the broad claims of the cerebellar deficit hypothesis (Nicolson et al., 2001; Nicolson and Fawcett, 1995), which posits that the cerebellum contributes to the automatisation of visual word recognition. However, whereas the cerebellar deficit hypothesis conceptualizes articulatory fluency and automatisation as separable mechanisms of reading development, our account suggests that they are intertwined through the common process of decoding, and the modulatory influence of the cerebellum on decoding performance.
3.2. Cerebro-cerebellar ventral circuit
Our meta-analytic review also identified a cerebro-cerebellar ventral circuit. The common term (semantic) across the four regions of the ventral circuit suggests a semantic interpretation. Interestingly, the frontal region of this circuit involves the same IFJ centroid that is also a component of our cerebro-cerebellar dorsal circuit. We will return to this subject in the section.
Within the current literature, most discussions of prefrontal cortex and semantic processing have focused on the contributions of the ventral territory of the inferior frontal gyrus (Vigneau et al., 2006), as opposed to the more dorsal IFJ territory. However, there have been some proposals that IJF plays a role in the selection and retrieval of semantic information (Thompson-Schill et al., 1998; Wagner et al., 2001). Similarly, proposals have been made about the involvement of the MTG in verbal knowledge and generation (Binder et al., 2000; Fiez et al., 1996b). Unlike the previously discussed IFJ-IPL pathway, however, there has been little investigation of an interactive IFJ-MTG ventral pathway. Thus, while prior work supports a semantic interpretation for our ventral pathway, it provides comparatively little evidence about how this pathway may function and interact with the mFG to support reading development.
Similarly, there is a sparse literature about how the cerebellum might contribute to semantic processes that support reading development. For instance, Nicholson and Fawcett’s (1995) cerebellar model of reading development does not include any terminology or consideration of semantic influence. For this reason, we did not predict cerebellar interconnectivity into a ventral semantic route. Therefore, our results are somewhat surprising, and it is not clear how elements of semantic processing might fit into the cerebellar deficit model.
Some clues may come from considering evidence that the cerebellum contributes to cued word-level retrieval tasks. For instance, early neuroimaging and neuropsychological research implicated the posterior cerebellum in the performance of verb generation tasks, in which participants are given a noun that serves as a cue to produce an appropriately related verb (Fiez et al., 1992; Petersen et al., 1989). Interestingly, the verb generation task is also associated with MTG activation (Fiez et al., 1996a). As another example, Desmond and colleauges (1998) found significant cerebellar activation when subjects performed a word-stem retrieval task, especially for items in which there are few words that complete the stem (e.g., produce a word that begins with the letters PSA___). More recently, Mariën and colleagues (2014) reviewed a compelling literature associating the cerebellum with verbal fluency tasks, in which a letter or semantic category label serves as a cue for the retrieval of a semantically related word. Although the form of these tasks varies, as does the degree to which the cue emphasizes semantic, orthographic, and phonological features, they share the common the need to produce an appropriate word-level response.
This common task demand brings to the fore perspectives on the dorsal and ventral routes that have emphasized their respective involvement in sub-lexical versus lexical processing (Mechelli et al., 2003; Simos et al., 2002). Most germane for our purposes is the idea that the dorsal pathway may support visual word recognition through sub-word phonological analysis, while the ventral pathway may support visual word recognition through word-level semantic analysis (Patterson and Lambon Ralph, 1999; Wilson et al., 2009). Thus, through its interconnectivity with the ventral route, the cerebellum may be modulating word-level semantic processing, and thereby indirectly influencing the representation and processing of the corresponding orthographic forms in the mFG.
Interestingly, Share’s (1995) model of reading development argues that decoding can transition from a primarily sub-lexical phonological process to one in which unfamiliar words are recognized by analogy to a previously learned and visually similar word (i.e., lexicalized decoding). This shift towards lexicalized decoding might be a function of the ventral pathway, and could involve the use of the cerebellum to fine-tune word-level selection and retrieval, and thereby improve the performance of lexicalized decoding.
4. Alternative perspectives, limitations, and directions for future work
4.1. Considering an attentional perspective
In speculating about the functional roles of our two identified cerebro-cerebellar circuits, we have emphasized associations with phonological and semantic processing. However, given that IFJ emerged as a common node across the dorsal and ventral circuits, and that furthermore the IFJ is functionally interconnectivity with mFG, our findings can also be viewed from a broader attentional perspective. Proponents of this perspective have posited that the IFJ territory serves as a source of attentional top-down constraints on the selection of visual representations (Vogel et al., 2012a, 2012b). This framework aligns well with alternative perspectives on the mFG, which have highlighted its broad engagement in linguistic tasks (Price and Devlin, 2011, 2003), and evidence of activation in response to both orthographic and non-orthographic stimuli (Ben-shachar et al., 2007; Kherif et al., 2011; Price and Devlin, 2003; Vogel et al., 2012b).
An attention-based account of the IFJ raises alternative perspectives on the modulatory influence of the cerebellum. For instance, a timing perspective reasons that the cerebellum may function as part of an internal monitoring network that participates in the sequencing and organization of visual and articulatory codes (Kujala et al., 2007). Drawing from this idea, it is possible that the cerebellum could modulate the cerebral reading network by fine-tuning selective attention to properties that are relevant for reading. For instance, through its interconnectivity with the IFJ, the cerebellum may help to attentionally constrain lower-level properties, such as the detection of spatial features and locations, and minimize non-pertinent spatial features and locations (Corbetta and Shulman, 2002).
It considering future work on this topic, it is important to recognize that alternative accounts of IFJ function may not be mutually exclusive. For instance, some have suggested that the IFJ territory can be subdivided into different sectors with specific patterns of connection to the occipitotemporal gyrus (Ben Shalom and Poeppel, 2008; Bookheimer, 2002). Therefore, it is possible that our work has blurred together what are separable streams for phonological, semantic, or attentional control. Alternatively, IFJ might have the capacity for broad contributions to cognition, but become more specific when dynamically engaged in tandem with specific posterior cerebral regions. Future work will be needed to disentangle competing perspectives, and the implications they have for how the cerebellum influences the cerebral reading network.
Another issue for future work concerns the relationship between articulation and our dorsal phonological circuit. We were intrigued to find that for some of the functionally connected cerebro-cerebellar regions the terms “rehearsal” (VIIB/Crus2) and “working-memory” (VIIB/Crus2 and IPL) were found in the Neurosynth’s term association metrics. This suggests that speech-based or articulatory coding and maintenance may be important in the function of the dorsal circuit, as predicted by the cerebellar deficit hypothesis (Nicolson et al., 2001) and empirical studies of orthographic learning (Kyte and Johnson, 2006; Tree et al., 2011). It is also consistent with past reviews of cerebellar contributions to covert speech associated with reading and verbal working-memory tasks (Desmond and Fiez, 1998; Strick et al., 2009). The absence of a full term overlap for “rehearsal” or “working-memory,” as was found for the term “phonological,” may reflect proposed ideas about topographic distinctions between cerebellar support for distinct articulatory functions, such as the production of novel versus familiar articulatory sequences (Ben-Yehudah and Fiez, 2008), or the integration of pre versus post articulatory verbal codes (Ackermann, 2008). Alternatively, it could reflect biases in the terminology used by authors to describe their studies and findings, such as a propensity to use the general term “phonological”.
Similarly, as noted earlier, the dorsal and ventral routes have sometimes been described as sub-lexical versus lexical routes, respectively, and we draw upon this distinction in speculating about their functional roles in reading development. However, these terms did not appear in any of our term lists for any of our seed-regions. To confirm this surprising result, we examined the Neurosynth reverse inference map for the term “lexical.” We observed z-values that exceeded our statistical threshold in over half of our regions, albeit less robustly than observed for our other terms. Most notably, the z-values were well below our threshold for the IPL seed-voxel in the dorsal pathway, and the MTG seed-voxel in the ventral pathway (z = 0.00 and 0.62, respectively). Thus, our data-driven approach does not provide evidence in support of a dorsal/sub-lexical versus ventral/lexical pathway distinction. This could once again reflect terminology biases that impact Neurosynth results, or perhaps the association of these terms with regions outside of our identified dorsal and ventral pathways (Graves et al., 2010).
Finally, it is important to acknowledge that we focused on the left cerebral hemisphere and the right cerebellum. This stems from our focus on investigating cerebellar connectivity to the cerebral reading network, which is predominantly considered left-lateralized. This means that our data do not provide a window into what might be happening in the right cerebral network and left cerebellar hemisphere, and how these structures might provide an additional causal or compensatory role. Recent results suggest this is a fruitful avenue for further investigation (Feng et al., 2017). Specifically, as compared to typical readers, individuals with dyslexia have displayed overactivation of the bilateral cerebellum (lobules VI) during both an orthographic and phonological task. Interestingly, greater activity was also found to be negatively correlated with measures of literacy. Individuals with dyslexia also revealed atypically stronger functional connectivity between the left Fusiform Gyrus and left cerebellar VI in the orthographic task, and left supramarginal gyrus and right VI in the phonological task. These results are suggestive of a potential compensatory role for the cerebellum and reading-related processes. As the field continues to offer more work that examines this possibility, a future review will be warranted to provide an overview of the collective research.
4. Summary and conclusion
The current review made systematic attempts to evaluate the cerebellar deficit hypothesis, which claims that the cerebellum contributes to dyslexia and normal reading development. We first reviewed neurobiological evidence implicating the cerebellum as a region associated with both normal and dyslexic reading. Based upon the accumulated findings, we concluded that there is compelling evidence linking individual differences in cerebellar structure and function with individual differences in reading ability. However, considerable speculation remains as to the specific nature of cerebellar contributions to reading development.
To address this knowledge gap, we used meta-analytical tools to examine interconnectivity between the cerebellum and cerebral regions involved in reading. From this effort emerged evidence for two circuits in which there is functional connectivity between specific sectors of the cerebellum, and dorsal and ventral cerebral reading pathways. Based upon the term overlap across the regions within the circuits and the extent literature, we propose that the dorsal circuit supports orthographic-phonological decoding at the sub-lexical level, with the cerebellum playing a modulatory role that improves phonological processing and thus decoding performance. We also propose that the ventral circuit supports lexicalized decoding, in which the pronunciation of unfamiliar words is based upon analogy to previously learned words. Although sparse, a literature on cued-word retrieval tasks suggests the cerebellum may play a modulatory role that improves the retrieval of word knowledge from semantic memory, and thus decoding performance. Because successful decoding is linked to the acquisition of orthographic knowledge, the interconnection of the cerebellum with dorsal and ventral reading pathways may thus indirectly support the automatisation of visual word recognition.
Collectively, our results provide support for the broad claims of the cerebellar deficit hypothesis. Specifically, they link specific ideas within the model, such as articulatory fluency and automatisation, to particular cerebro-cerebellar circuits. Our findings are also consistent with the idea that the cerebellum does not need to be directly connected with the mFG for it to contribute to the development of orthographic knowledge. Thus, dysfunction in the cerebellum could put an individual at risk for a developmental reading disorder. Continued efforts to integrate the cerebellum – a brain structure with an enormous computation power that drives learning (Marr, 1969) – into neuroanatomical models of reading and dyslexia is important for understanding the neural mechanisms that underlie reading development and which contribute to individual differences in reading ability.
Highlights.
Current literature offers compelling evidence of cerebellar engagement in reading.
Meta-analytic approach identified a cerebro-cerebellar dorsal and ventral circuit.
Circuits characterized by specific attributes associated with reading development.
Cerebellum indirectly supports the development of fluent visual word recognition.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) Grants R01HD60388, T32 GM 81760, and the National Science Foundation (NSF) Grant BCS-1125719.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The popular online platform Neurosynth can automatically synthesize and generate information (e.g., coordinates, term frequency) from extensive amount of collected neuroimaging studies, allowing for an efficient systematic review practice (Yarkoni et al., 2011). The Neurosynth reading map was generated using this program’s reverse inference “term-based” feature. This meta-analysis provided a statistical map (i.e., z-values for each voxel) comparing coordinates for studies that contain the term “reading” with studies that do not (false discovery rate corrected at p < 0.01, localization was set at a voxel-wise threshold of z > 6.5, and minimum cluster volume of 50 mm3).
The GingerALE meta-analytic program computes and extracts voxel-by-voxel activation likelihood estimates that represent the convergence of article coordinates, which allow for a subsequent analysis to identify brain regions more strongly associated with a contrast of interest (Eickhoff et al., 2009; Turkeltaub et al., 2012). The ALE phonological > semantic and semantic > phonological maps were determined by first assigning coordinates from previously published meta-analyses as weighted towards phonological or semantic demands, based upon the comparison in which they were identified (see Table 1), and then entering each group of coordinates into a first level ALE analysis. This process established significant peak voxel overlap (i.e., probability-of-convergence) of phonological-demand clusters and semantic-demand clusters (thresholded at a cluster-level inference of p < .05 over 1000 iterations, corrected for multiple comparisons). These extracted probability-of-convergence maps were then used in a second level contrast analysis to identify specific cortical regions more strongly associated with components of phonological processing (phonological > semantic) than with components of semantic processing (semantic > phonological). Localization for this contrast analysis was thresholded at p < .05, uncorrected, and a minimum cluster volume of 50 mm3.
Neurosynth’s “location-based analyses” uses meta-analytic image mapping to detect information (e.g., correlation, co-activation) about the relationship between the indicated coordinate-of-interest and other regions in the website’s large database. Although uncorrected for multiple comparisons, the resting-state fMRI time series’ effects are relatively reliable with the remarkably large sample size acquired (N = 1000).
Not only was there no term convergence for the temporal-cerebellar pathway, the word metric report for this cerebellar coordinate generated broad terms: heat, consecutive, chronic, pain, progressive, executive, and painful.
References
- Ackermann H. Cerebellar contributions to speech production and speech perception: psycholinguistic and neurobiological perspectives. Trends Neurosci. 2008;31:265–272. doi: 10.1016/j.tins.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: Looking back and looking forward. Nat Rev Neurosci. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Ben-shachar M, Dougherty RF, Wandell BA. White matter pathways in reading. Sci Direct. 2007;17:258–270. doi: 10.1016/j.conb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Ben-Yehudah G, Fiez Ja. Impact of cerebellar lesions on reading and phonological processing. Ann N Y Acad Sci. 2008;1145:260–274. doi: 10.1196/annals.1416.015. [DOI] [PubMed] [Google Scholar]
- Ben Shalom D, Poeppel D. Functional Anatomic Models of Language: Assembling the Pieces. Neurosci. 2008;14:119–127. doi: 10.1177/1073858407305726. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost Ja, Hammeke Ta, Bellgowan PS, Springer Ja, Kaufman JN, Possing ET. Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex. 2000;10:512–528. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- Biswal BB. Resting state fMRI: A personal history. Neuroimage. 2012;62:938–944. doi: 10.1016/j.neuroimage.2012.01.090. [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: New approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Relation between brain activation and lexical performance. Hum Brain Mapp. 2003;19:155–169. doi: 10.1002/hbm.10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun AR, Varga M, Stager S, Schulz G, Selbie S, Maisog JM, Carson RE, Ludlow CL. Altered patterns of cerebral activity during speech and language production in developmental stuttering. An H215O positron emission tomography study. Brain. 1997;120:761–784. doi: 10.1093/brain/120.5.761. [DOI] [PubMed] [Google Scholar]
- Brunswick N, McCrory E, Price CJ, Frith CD, Frith U. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics. A search for Wernicke’s Wortschatz? Brain. 1999;122:1901–1917. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Yeo BTT. Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci. 2013;16:832–837. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- Cao F, Bitan T, Booth JR. Effective brain connectivity in children with reading difficulties during phonological processing. Brain Lang. 2008;107:91–101. doi: 10.1016/j.bandl.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F, Bitan T, Chou T, Burman DD, Booth JR. Deficient orthographic and phonological representations in children with dyslexia revealed by brain activation patterns. J Child Psychol Psychiatry. 2006;47:1041–1050. doi: 10.1111/j.1469-7610.2006.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattinelli I, Borghese NA, Gallucci M, Paulesu E. Reading the reading brain: A new meta-analysis of functional imaging data on reading. J Neurolinguistics. 2013;26:214–238. doi: 10.1016/j.jneuroling.2012.08.001. [DOI] [Google Scholar]
- Chen SHA, Desmond JE. Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. Neuroimage. 2005;24:332–338. doi: 10.1016/j.neuroimage.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S. Specialization within the ventral stream: The case for the visual word form area. Neuroimage. 2004;22:466–476. doi: 10.1016/j.neuroimage.2003.12.049. [DOI] [PubMed] [Google Scholar]
- Cohen L, Leheiricy S, Chocho L, Lemer C, Sophie R, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain. 2002a;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehéricy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain. 2002b;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Cohen L, Martinaud O, Lemer C, Lehéricy S, Samson Y, Obadia M, Slachevsky A, Dehaene S. Visual Word Recognition in the Left and Right Hemispheres: Anatomical and Functional Correlates of Peripheral Alexias. Cereb Cortex. 2003;13:1313–1333. doi: 10.1093/cercor/bhg079. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of Goal-Directed and Stimulus-Driven Attention in the Brain. Nat Rev Neurosci. 2002;3:215–229. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L. The unique role of the visual word form area in reading. Trends Cogn Sci. 2011;15:254–262. doi: 10.1016/j.tics.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Fiez Ja. Neuroimaging studies of the cerebellum: Language, learning and memory. Trends Cogn Sci. 1998;2:355–362. doi: 10.1016/S1364-6613(98)01211-X. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JD, Glover GH. Dissociation of frontal and cerebellar activity in a cognitive task: evidence for a distinction between selection and search. Neuroimage. 1998;7:368–376. doi: 10.1006/nimg.1998.0340. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46:39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Maderwald S, Küper M, Thürling M, Rabe K, Gizewski ER, Ladd ME, Timmann D. Imaging the deep cerebellar nuclei: A probabilistic atlas and normalization procedure. Neuroimage. 2011;54:1786–1794. doi: 10.1016/j.neuroimage.2010.10.035. [DOI] [PubMed] [Google Scholar]
- Eckert M. Neuroanatomical Markers for Dyslexia: A Review of Dyslexia Structural Imaging Studies. Neurosci. 2004;10:362–371. doi: 10.1177/1073858404263596. [DOI] [PubMed] [Google Scholar]
- Eickhoff S, Laird A, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based ALE meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718.Coordinate-based. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Li L, Zhang M, Yang X, Tian M, Xie W, Lu Y, Liu L, Bélanger NN, Meng X, Ding G. Dyslexic Children Show Atypical Cerebellar Activation and Cerebro-Cerebellar Functional Connectivity in Orthographic and Phonological Processing. Cerebellum. 2017;16:496–507. doi: 10.1007/s12311-016-0829-2. [DOI] [PubMed] [Google Scholar]
- Fernandez VG, Juranek J, Romanowska-pawliczek A, Stuebing K, Williams VJ, Fletcher JM. Brain & Language White matter integrity of cerebellar-cortical tracts in reading impaired children : A probabilistic tractography study. Brain Lang. 2015 doi: 10.1016/j.bandl.2015.07.006. [DOI] [PMC free article] [PubMed]
- Fiez JA. Phonology, semantics, and the role of the left inferior prefrontal cortex. Hum Brain Mapp. 1997;5:79–83. doi: 10.1002/(SICI)1097-0193(1997)5:2<79∷AIDHBM1>3.0.CO;2-J. [pii] [DOI] [PubMed] [Google Scholar]
- Fiez JA, Petersen SE, Cheney MK, Raichle ME. Impaired non-motor learning and error detection associated with cerebellar damage. A single case study. Brain. 1992;115:155–178. doi: 10.1093/brain/115.1.155. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Raichle ME, Balota DA, Tallal P, Petersen SE. PET Activation of Posterior Temporal Regions during Auditory Word Presentation and Verb Generation. Cereb Cortex. 1996a;6:1–10. doi: 10.1093/cercor/6.1.1. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Raife EA, Balota DA, Schwarz JP, Raichle ME, Petersen SE. A positron emission tomography study of the short-term maintenance of verbal information. J Neurosci. 1996b;16:808–822. doi: 10.1146/annurev.ne.06.030183.001341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard R, Naccache L, Pinel P, Clémenceau S, Volle E, Hasboun D, Dupont S, Baulac M, Dehaene S, Adam C, Cohen L. Direct Intracranial, fMRI, and Lesion Evidence for the Causal Role of Left Inferotemporal Cortex in Reading. Neuron. 2006;50:191–204. doi: 10.1016/j.neuron.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Georgiewa P, Rzanny R, Hopf JM, Knab R, Glauche V, Kaiser WA, Blanz B. fMRI during word processing in dyslexic and normal reading children. Neuroreport. 1999;10:3459–3465. doi: 10.1097/00001756-199911080-00036. [DOI] [PubMed] [Google Scholar]
- Gold BT, Balota DA, Kirchhoff BA, Buckner RL. Common and dissociable activation patterns associated with controlled semantic and phonological processing: Evidence from fMRI adaptation. Cereb Cortex. 2005;15:1438–1450. doi: 10.1093/cercor/bhi024. [DOI] [PubMed] [Google Scholar]
- Graves WW, Desai R, Humphries C, Seidenberg MS, Binder JR. Neural systems for reading aloud: A multiparametric approach. Cereb Cortex. 2010;20:1799–1815. doi: 10.1093/cercor/bhp245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R, Sakai KL. Learning letters in adulthood: Direct visualization of cortical plasticity for forming a new link between orthography and phonology. Neuron. 2004;42:311–322. doi: 10.1016/S0896-6273(04)00196-5. [DOI] [PubMed] [Google Scholar]
- Herbster AN, Mintun Ma, Nebes RD, Becker JT. Regional Cerebral Blood Flow During Word and. Hum Brain Mapp. 1997;92:84–92. doi: 10.1002/(sici)1097-0193(1997)5:2<84::aid-hbm2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel Towards a functional neuroanatomy of speech perception. Trends Cogn Sci. 2000;4:131–138. doi: 10.1016/s1364-6613(00)01463-7. https://doi.org/10740277. [DOI] [PubMed] [Google Scholar]
- Hirshorn EA, Li Y, Ward MJ, Richardson RM, Fiez JA, Ghuman AS. Decoding and disrupting left midfusiform gyrus activity during word reading. Proc Natl Acad Sci. 2016;113:8162–8167. doi: 10.1073/pnas.1604126113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: A metanalysis of 35 neuroimaging studies. Neuroimage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Kelly SW, Griffiths S, Frith U. Evidence for implicit sequence learning in dyslexia. Dyslexia. 2002;8:43–52. doi: 10.1002/dys.208. [DOI] [PubMed] [Google Scholar]
- Kherif F, Josse G, Price CJ. Automatic top-down processing explains common left occipito-temporal responses to visual words and objects. Cereb Cortex. 2011;21:103–114. doi: 10.1093/cercor/bhq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama MS, Di Martino A, Kelly C, Jutagir DR, Sunshine J, Schwartz SJ, Castellanos FX, Milham MP. Cortical Signatures of Dyslexia and Remediation: An Intrinsic Functional Connectivity Approach. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronschnabel J, Schmid R, Maurer U, Brandeis D. Visual print tuning deficits in dyslexic adolescents under minimized phonological demands. Neuroimage. 2013;74:58–69. doi: 10.1016/j.neuroimage.2013.02.014. [DOI] [PubMed] [Google Scholar]
- Kujala J, Pammer K, Cornelissen P, Roebroeck A, Formisano E, Salmelin R. Phase Coupling in a Cerebro-Cerebellar Network at 8-13 Hz during Reading. Cereb Cortex. 2007;17:1476–1485. doi: 10.1093/cercor/bhl059. [DOI] [PubMed] [Google Scholar]
- Kyte CS, Johnson CJ. The role of phonological recoding in orthographic learning. J Exp Child Psychol. 2006;93:166–185. doi: 10.1016/j.jecp.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Laasonen M, Väre J, Oksanen-Hennah H, Leppämäki S, Tani P, Harno H, Hokkanen L, Pothos E, Cleeremans A. Project DyAdd: Implicit learning in adult dyslexia and ADHD. Ann Dyslexia. 2014;64:1–33. doi: 10.1007/s11881-013-0083-y. [DOI] [PubMed] [Google Scholar]
- Liberman I. Segmentation of the spoken word and reading acquisition. Bull Ort Soc. 1973;23:65–77. [Google Scholar]
- Llinas R, Hillman DE, Precht W. Neuronal circuit reorganization in mammalian agranular cerebellar cortex. J Neurobiol. 1973;4:69–94. doi: 10.1002/neu.480040106. [DOI] [PubMed] [Google Scholar]
- Lovegrove WJ, Bowling A, Badcock D, Blackwood M. Specific reading disability: differences in contrast sensitivity as a function of spatial frequency. Science (80-) 1980;210:439–440. doi: 10.1126/science.7433985. [DOI] [PubMed] [Google Scholar]
- Maisog JM, Einbinder ER, Flowers DL, Turkeltaub PE, Eden GF. A meta-analysis of functional neuroimaging studies of dyslexia. Ann N Y Acad Sci. 2008;1145:237–59. doi: 10.1196/annals.1416.024. [DOI] [PubMed] [Google Scholar]
- Mariën P, Ackermann H, Adamaszek M, Barwood CHS, Beaton A, Desmond J, De Witte E, Fawcett AJ, Hertrich I, Küper M, Leggio M, Marvel C, Molinari M, Murdoch BE, Nicolson RI, Schmahmann JD, Stoodley CJ, Thürling M, Timmann D, Wouters E, Ziegler W. Consensus paper: Language and the cerebellum: An ongoing enigma. Cerebellum. 2014;13:386–410. doi: 10.1007/s12311-013-0540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol. 1969;202:437–470. doi: 10.2307/1776957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Schurz M, Kronbichler M, Richlan F. Reading in the brain of children and adults : A meta-analysis of 40 functional magnetic resonance imaging studies. Hum Brain Mapp. 2015;1981:1963–1981. doi: 10.1002/hbm.22749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvel CL, Desmond JE. Functional Topography of the Cerebellum in Verbal Working Memory. Neuropsychol Rev. 2010;20:271–279. doi: 10.1007/s11065-010-9137-7.Functional. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: Expertise for reading in the fusiform gyrus. Trends Cogn Sci. 2003;7:293–299. doi: 10.1016/S1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- McNorgan C, Chabal S, O’Young D, Lukic S, Booth JR. Task dependent lexicality effects support interactive models of reading: A meta-analytic neuroimaging review. Neuropsychologia. 2015;67:148–158. doi: 10.1016/j.neuropsychologia.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Gorno-Tempini ML, Price CJ. Neuroimaging studies of word and pseudoword reading: consistencies, inconsistencies, and limitations. J Cogn Neurosci. 2003;15:260–271. doi: 10.1162/089892903321208196. [DOI] [PubMed] [Google Scholar]
- Menghini D, Hagberg GE, Caltagirone C, Petrosini L, Vicari S. Implicit learning deficits in dyslexic adults: An fMRI study. Neuroimage. 2006;33:1218–1226. doi: 10.1016/j.neuroimage.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ. Dyslexia, dysgraphia, procedural learning and the cerebellum. Cortex. 2011;47:117–127. doi: 10.1016/j.cortex.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ. Developmental dyslexia, learning and the cerebellum, in: Neurodevelopmental Disorders. Springer-Verlag; Vienna: 2005. pp. 19–36. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ. Dyslexia is more than a phonological disability Roderick I Nicolson and Angela J. Fawcett University of Sheffield Dyslexia: An international journal of research and practice (in press) DYSLEXIA-CHICHESTER. 1995;1:19–36. [Google Scholar]
- Nicolson RI, Fawcett AJ. Automaticity: A new framework for dyslexia research? Cognition. 1990;35:159–182. doi: 10.1016/0010-0277(90)90013-a. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ, Berry EL, Jenkins IH, Dean P, Brooks DJ. Association of abnormal cerebellar activation with motor learning difficulties in dyslexic adults. Lancet. 1999;353:1662–1667. doi: 10.1016/S0140-6736(98)09165-X. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ, Dean P. Developmental dyslexia: The cerebellar deficit hypothesis. Trends Neurosci. 2001;24:508–511. doi: 10.1016/S0166-2236(00)01896-8. [DOI] [PubMed] [Google Scholar]
- Nigro L, Jiménez-Fernández G, Simpson IC, Defior S. Implicit learning of non- linguistic and linguistic regularities in children with dyslexia. Ann Dyslexia. 2016;66:202–218. doi: 10.1007/s11881-015-0116-9. [DOI] [PubMed] [Google Scholar]
- Nissen MJ, Bullemer P. Attentional requirements of learning: Evidence from performance measures. Cogn Psychol. 1987;19:1–32. doi: 10.1016/0010-0285(87)90002-8. [DOI] [Google Scholar]
- Patterson K, Lambon Ralph MA. Selective disorders of reading? Curr Opin Neurobiol. 1999;9:235–239. doi: 10.1016/S0959-4388(99)80033-6. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Danelli L, Berlingeri M. Reading the dyslexic brain: multiple dysfunctional routes revealed by a new meta-analysis of PET and fMRI activation studies. Front Hum Neurosci. 2014;8:830. doi: 10.3389/fnhum.2014.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RSJ. The neural correlates of the verbal component of working memory. Nat Lett. 1993;362 doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Pavlidou EV, Louise Kelly M, Williams JM. Do children with developmental dyslexia have impairments in implicit learning? Dyslexia. 2010;16:143–161. doi: 10.1002/dys.400. [DOI] [PubMed] [Google Scholar]
- Peeva MG, Guenther FH, Tourville Ja, Nieto-castanon A, Anton L, Nazarian B, Alario F. Syllabic Sequences in the Speech Production Network. Neuroimage. 2010;50:626–638. doi: 10.1016/j.neuroimage.2009.12.065.Distinct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron Emission Tomographic Studies of the Processing of Singe Words. J Cogn Neurosci. 1989;1:153–170. doi: 10.1162/jocn.1989.1.2.153. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JDEE. Functional Specialization for Semantic and Phonological Processing in the Left Inferior Prefrontal Cortex 1. Neuroimage. 1999;35:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Polk TA, Stallcup M, Aguirre GK, Alsop DC, D’Esposito M, Detre JA, Farah MJ. Neural specialization for letter recognition. J Cogn Neurosci. 2002;14:145–159. doi: 10.1162/089892902317236803. [DOI] [PubMed] [Google Scholar]
- Pollack C, Luk G, Christodoulou JA. A meta-analysis of functional reading systems in typically developing and struggling readers across different alphabetic languages. Front Psychol. 2015;6:1–10. doi: 10.3389/fpsyg.2015.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothos EM, Kirk J. Investigating learning deficits associated with dyslexia. Dyslexia. 2004;10:61–76. doi: 10.1002/dys.266. [DOI] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The Interactive Account of ventral occipitotemporal contributions to reading. Trends Cogn Sci. 2011;15:246–253. doi: 10.1016/j.tics.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The myth of the visual word form area. Neuroimage. 2003;19:473–481. doi: 10.1016/S1053-8119(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Humphreys GW, Wise RJS. Segregating Semantic from Phonological Processes during Reading. J Cogn Neurosci. 1997;9:727–733. doi: 10.1162/jocn.1997.9.6.727. [DOI] [PubMed] [Google Scholar]
- Reber AS. Implicit learning of artificial grammars. J Verbal Learning Verbal Behav. 1967;6:855–863. [Google Scholar]
- Richlan F. Developmental dyslexia: dysfunction of a left hemisphere reading network. Front Hum Neurosci. 2012;6:1–5. doi: 10.3389/fnhum.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Functional abnormalities in the dyslexic brain: A quantitative meta-analysis of neuroimaging studies. Hum Brain Mapp. 2009;30:3299–3308. doi: 10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecker A, Ackermann H, Wildgruber D, Meyer J, Dogil G, Haider H, Grodd W. Articulatory/Phonetic Sequencing at the Level of the Anterior Perisylvian Cortex: A Functional Magnetic Resonance Imaging (fMRI) Study. Brain Lang. 2000;75:259–276. doi: 10.1006/brln.2000.2356. [DOI] [PubMed] [Google Scholar]
- Roodenrys S, Dunn N. Unimpaired implicit learning in children with developmental dyslexia. Dyslexia. 2008;14:1–15. doi: 10.1002/dys.340. [DOI] [PubMed] [Google Scholar]
- Roskies AL, Fiez JA, Balota DA, Raichle ME, Petersen SE. Task-Dependent Modulation of Regions in the Left Inferior Frontal Cortex during Semantic Processing. J Cogn Neurosci. 2001;13:829–843. doi: 10.1162/08989290152541485. [DOI] [PubMed] [Google Scholar]
- Rüsseler J, Gerth I, Münte T. Implicit learning is intact in adult developmental dyslexic readers: Evidence from the serial reaction time task and artificial grammar learning. J Clin Exp Neuropsychol. 2006;28:808–827. doi: 10.1080/13803390591001007. [DOI] [PubMed] [Google Scholar]
- Schmalz X, Altoè G, Mulatti C. Statistical learning and dyslexia: a systematic review. Ann Dyslexia. 2017;67:147–162. doi: 10.1007/s11881-016-0136-0. [DOI] [PubMed] [Google Scholar]
- Schurz M, Wimmer H, Richlan F, Ludersdorfer P, Klackl J, Kronbichler M. Resting-state and task-based functional brain connectivity in developmental dyslexia. Cereb Cortex. 2015;25:3502–3514. doi: 10.1093/cercor/bhu184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Share DL. Phonological recoding and self-teaching: Sine qua non of reading acquisition. Cognition. 1995;55:151–218. doi: 10.1016/0010-0277(94)00645-2. [DOI] [PubMed] [Google Scholar]
- Simos PGPG, Breier JI, Fletcher JM, Foorman BR, Castillo EM, Papanicolaou AC. Brain mechanisms for reading words and pseudowords: an integrated approach. Cereb Cortex. 2002;12:297–305. doi: 10.1093/cercor/12.3.297. [DOI] [PubMed] [Google Scholar]
- Stanberry LI, Richards TL, Berninger VW, Nandy RR, Aylward EH, Maravilla KR, Stock PS, Cordes D. Low-frequency signal changes reflect differences in functional connectivity between good readers and dyslexics during continuous phoneme mapping. Magn Reson Imaging. 2006;24:217–229. doi: 10.1016/j.mri.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Stanovich KE. Explaining the differences between the dyslexic and the garden-variety poor reader The phonological-core variable-difference model. J Learn Disabil. 1988;21:590–604. doi: 10.1177/002221948802101003. [DOI] [PubMed] [Google Scholar]
- Stein JF. Vision and visual dyslexia. CRC Pr I Llc 1991 [Google Scholar]
- Stoodley CJ. Distinct regions of the cerebellum show gray matter decreases in autism, ADHD, and developmental dyslexia. Front Syst Neurosci. 2014;8:1–17. doi: 10.3389/fnsys.2014.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ. The cerebellum and cognition: Evidence from functional imaging studies. Cerebellum. 2012;11:352–365. doi: 10.1007/s12311-011-0260-7. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Harrison EPD, Stein JF. Implicit motor learning deficits in dyslexic adults. Neuropsychologia. 2006;44:795–798. doi: 10.1016/j.neuropsychologia.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Stein JF. The cerebellum and dyslexia. Cortex. 2011;47:101–116. doi: 10.1016/j.cortex.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Tallal P. Auditory temporal perception, phonics, and reading disabilities in children. Brain Lang. 1980;9:182–198. doi: 10.1016/0093-934x(80)90139-x. [DOI] [PubMed] [Google Scholar]
- Taylor JSH, Plunkett K, Nation K. The influence of consistency, frequency, and semantics on learning to read: an artificial orthography paradigm. J Exp Psychol Learn Mem Cogn. 2011;37:60–76. doi: 10.1037/a0020126. [DOI] [PubMed] [Google Scholar]
- Taylor JSH, Rastle K, Davis MH. Can Cognitive Models Explain Brain Activation During Word and Pseudoword Reading? A Meta-Analysis of 36 Neuroimaging Studies. Psychol Bull. 2012;139:766–791. doi: 10.1037/a0030266. [DOI] [PubMed] [Google Scholar]
- Thiebaut De Schotten M, Cohen L, Amemiya E, Braga LW, Dehaene S. Learning to read improves the structure of the arcuate fasciculus. Cereb Cortex. 2014;24:989–995. doi: 10.1093/cercor/bhs383. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Swick D, Farah MJ, D’Esposito M, Kan IP, Knight RT. Verb generation in patients with focal frontal lesions: a neuropsychological test of neuroimaging findings. Proc Natl Acad Sci U S A. 1998;95:15855–15860. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tree JJ, Longmore C, Besner D. Orthography, phonology, short-term memory and the effects of concurrent articulation on rhyme and homophony judgements. Acta Psychol (Amst) 2011;136:11–19. doi: 10.1016/j.actpsy.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro Ta. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum Brain Mapp. 2012;33:1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Mark S, Klaver P, Bucher K, Maurer U, Schulz E, Brem S, Martin E, Brandeis D. The left occipitotemporal system in reading: Disruption of focal fMRI connectivity to left inferior frontal and inferior parietal language areas in children with dyslexia. Neuroimage. 2011;54:2426–2436. doi: 10.1016/j.neuroimage.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Vicari S, Finzi A, Menghini D, Marotta L, Baldi S, Petrosini L. Do children with developmental dyslexia have an implicit learning deficit? J Neurol Neurosurg Psychiatry. 2005;76:1392–1397. doi: 10.1136/jnnp.2004.061093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari S, Marotta L, Menghini D, Molinari M, Petrosini L. Implicit learning deficit in children with developmental dyslexia. Neuropsychologia. 2003;41:108–114. doi: 10.1016/S0028-3932(02)00082-9. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé PY, Duffau H, Crivello F, Houde O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Vlachos F, Papathanasiou I, Andreou G. Cerebellum and reading. Folia Phoniatr Logop. 2007;59:177–183. doi: 10.1159/000102929. [DOI] [PubMed] [Google Scholar]
- Vogel AC, Miezin FM, Petersen SE, Schlaggar BL. The putative visual word form area is functionally connected to the dorsal attention network. Cereb Cortex. 2012a;22:537–549. doi: 10.1093/cercor/bhr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel AC, Petersen SE, Schlaggar BL. The left occipitotemporal cortex does not show preferential activity for words. Cereb Cortex. 2012b;22:2715–2732. doi: 10.1093/cercor/bhr295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waber DP, Marcus DJ, Forbes PW, Bellinger DC, Weiler MD, Sorensen LG, Curran T. Motor sequence learning and reading ability: Is poor reading associated with sequencing deficits? J Exp Child Psychol. 2003;84:338–354. doi: 10.1016/S0022-0965(03)00030-4. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Paré-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: Left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–338. doi: 10.1016/S0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Brambati SM, Henry RG, Handwerker DA, Agosta F, Miller BL, Wilkins DP, Ogar JM, Gorno-Tempini ML. The neural basis of surface dyslexia in semantic dementia. Brain. 2009;132:71–86. doi: 10.1093/brain/awn300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Bi HY, Long ZY, Tao S. Evidence for cerebellar dysfunction in Chinese children with developmental dyslexia: An fMRI study. Int J Neurosci. 2013;123:300–310. doi: 10.3109/00207454.2012.756484. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack R, Nichols T. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635.Large-scale. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Klatzky R, Behrmann M. Ventral and Dorsal Visual Stream Contributions to the Perception of Object Shape and Object Location. J Cogn Neurosci. 2014;26:189–209. doi: 10.1162/jocn_a_00475. [DOI] [PMC free article] [PubMed] [Google Scholar]


