Abstract
It is conceivable that spermatid apico-basal polarity and spermatid planar cell polarity (PCP) are utmost important to support spermatogenesis. The orderly arrangement of developing germ cells in particular spermatids during spermiogenesis are essential to obtain structural and nutrient supports from the fixed number of Sertoli cells across the limited space of seminiferous epithelium in the tubules following Sertoli cell differentiation by ~17 day postpartum (dpp) in rodents and ~12 years of age after puberty in humans. Yet few studies are found in the literature to investigate the role of these proteins to support spermatogenesis. Herein, we briefly summarize recent findings in the field, in particular emerging evidence that supports the concept that apico-basal polarity and PCP are conferred by the corresponding polarity proteins through their effects on the actin- and microtubule (MT)-based cytoskeletons. While much research is needed to bridge our gaps of understanding cell polarity, cytoskeletal function, and signaling proteins, a critical evaluation of some latest findings in the field as summarized herein provides some important and also thought-provoking concepts to design better functional experiments to address this important, yet largely expored, research topic.
Introduction
During spermatogenesis in the mammalian testis, including both rodents and humans, developing spermatids display unusual polarity to support the packaging of millions of spermatids across the seminiferous epithelium. Thus, millions of spermatozoa can be produced daily in the limited space of the seminiferous tubules tightly packed inside the testes [1–3]. Studies have shown that there are two types of spermatid polarity during the epithelial cycle to support spermatogenesis. The first type is the apico-basal polarity in which the heads of elongating/elongated spermatids in the testis are orientated by pointing to the basement membrane (i.e., individual cell polarity), which is supported by the partitioning defective (Par)-[4], the Scribble- [5], and the Crumbs (Crb)- [6] based polarity protein complexes that are found in virtually all mammalian cells besides Sertoli and/or germ cells in the testis [7, 8]. The second type is the spermatid planar cell polarity (PCP) in which polarized elongating/elongated spermatids are aligned across the plane of the Sertoli cell epithelium in the tubules by orientated unidirectionally, supported by PCP proteins such as Vangl2 [9, 10]. Studies have shown that these polarity proteins and PCP proteins are working in concert with F-actin-based cytoskeleton to support spermatid polarity and PCP [2, 11]. However, emerging evidence based on published findings in the testis has shown that the microtubule (MT)-based cytoskeleton is also involved in polarity protein- and PCP protein-mediated spermatid polarity. A recent review in this Special Issue [11] has summarized recent findings regarding the role of the Par-, the Scribble- and the Crumbs homolog 3 (Crb3)-based polarity protein complexes in the adult rat testis by working closely with the F-actin-based cytoskeleton to modulate spermatid polarity. As such, we do not include such discussion and pertinent findings herein to avoid redundancy. Instead, we focus more on latest findings using different animal models to assess the relationship between spermatid polarity/spermatid PCP and changes in the organization of actin- and MT-based cytoskeletons. This information should provide insightful information regarding future experimental planning to better understand the integrated function of both cytoskeletons to support spermatid polarity and PCP.
Polarity proteins and planar cell polarity proteins
Studies in the rat testis have shown that, similar to other epithelial cells, the Par-based polarity complex is comprised of at least 4 proteins: Par3, Par6, aPKC, and Cdc42 which tightly associate with the integral membrane protein JAM-C (junctional adhesion molecule C, also known as JAM-1) (Table 1), predominantly expressed at the apical ES (ectoplasmic specialization) to modulate apico-basal spermatid polarity and adhesion [4] at the Sertoli cell-spermatid interface [4]. However, the Par-based proteins are also expressed at the basal ES at the Sertoli cell-cell interface near the basement membrane, consistent with its localization at the blood-testis barrier (BTB) [4]. In this context, it is of interest to note that the ES is a testis-specific adherens junction (AJ) type, restrictively expressed at the Sertoli-spermatid interface, limited to step 8–19 spermatids in the rat testis, whereas the basal ES is only found at the Sertoli cell-cell interface, coexisting with the tight junction (TJ) to create the Sertoli cell BTB [12–14]. The Par-based polarity complex is working closely with the Crb3-based polarity complex which is composed of Crb3 (an integral membrane protein), Pals1 and PatJ [6] to support apico-basal polarity as noted in other epithelia [15]. For instance, studies have shown that aPKC in the Par-based polarity protein can modulate Par3 or Crb3 function via phosphorylation, inducing the necessary cross-talk between these two polarity complex to modulate cell polarization [15–17]. On the other hand, the Scribble-based polarity complex that supports apico-basal polarity is composed of Scribble, Lgl2 (Lethal giant larvae 2) and Dlg1 (Discs large 1) in the rat testis [5], which is mutually exclusive regarding its function and also physical localization vs. the Par- and the Crb3-based polarity complexes [7, 15]. In the testis, Scribble is expressed predominantly at the basal ES in virtually all stages of the epithelial cycle, however, its expression at the apical ES is limited to stage VII–VIII tubules [5]. Interestingly, Crb3 is only expressed at the basal ES/BTB in stage I–VIII tubules [6]. As noted in Table 1, the function of each of these polarity protein complexes and their partner proteins has been evaluated based on studies of genetic models. Interestingly, their functional significance in the testis to support spermatid polarity and/or spermatogenesis remains largely unknown. Studies performed in the testis have noted that these polarity proteins that confer Sertoli and germ cell apico-basal polarity exert their effects through the actin-based cytoskeleton [4–6]. It is expected that MTs are involved in spermatid apico-basal polarity since the apical ES function is mutually supported by both the actin- and MST-based cytoskeletons [18–20] and both actin filaments and MT protofilaments are adjacent to each other at the apical ES [12, 21]. Nonetheless, the involvement of MT in spermatid apico-basal polarity in the testis remains to be investigated.
Table 1.
Functions of different polarity proteins and PCP proteins in mammalian cells and tissues.
| Protein | Phenotype in rodents following deletion (knockout, KO) or knockdown (KD) in corresponding model | References | Mutation(s), deletion or changes in expression that lead to corresponding diseases in humans | References | |
|---|---|---|---|---|---|
| Par3-Complex | Par3 | Up-regulation in ovarian and prostate cancer, down-regulation in pancreatic cancer. | [40–42] | ||
| Par6 | Up-regulation in breast cancer and non-small-cell lung cancer, mutation inhibits heart development. | [43–45] | |||
| Cdc42 | Cdc42-deficiency causes forebrain malformation, failing to develop into two hemispheres, leading to holoprosencephaly. | [46] | Up-regulation in polycystic kidney disease, mutation leads to thrombocytopenia. | [47, 48] | |
| PKC | PKCι knockout leads to embryonic fatality, conditional deletion of aPKCλ in differentiated neurons causes polarity complex disruption. | [49, 50] | Mutation leads to Alzheimer’s disease; Down-regulation in B-cell chronic lymphocytic leukemia. |
[51, 52] | |
| Scribble-Complex | Scribble | Deletion leads to embryonic fatality, mutation leads to lung and prostate cancer. | [53–55] | Mutation leads to lung cancer, down-regulation in prostate cancer. | [54, 55] |
| Dlg1 | Deletion leads to embryo0nic fatality, requires for development of respiratory, cardiovascular and urogenital systems. | [56–58] | Mutation leads to Crohn’s disease, and schizophrenia. | [59, 60] | |
| Lgl2 | Mutation leads to Barrett gastric foveolar dysplasia, a congenital gastroesophageal reflux disease. | [61] | |||
| Crumbs-3-Complex | CRB3 | Deletion leads to embryonic fatality, requires for the development of kidney and lung. | [62] | ||
| PALS1 | PALS1 shRNA knockdown in developing brain leads to the presence of excessive neurons, and followed by massive apoptosis, causing abrogation of the entire cortical structure; conditional PALS1 knockdown in mouse E14 embryonic stem cells causes defects in retina. | [63, 64] | |||
| Vangl2 | Deletion in mice perturbs brain development, leading to embryonic fatality in some, but not all, mice. | [65] | Mutation leads to congenital heart defect and neural tube defects. | [66, 67] | |
| PCP Complex | Prickle1 | Deletion leads to embryonic fatality due to failure of distal visceral endoderm migration. | [68] | Mutation leads to neural tube defects and type 2 diabetes. | [69, 70] |
| Dvl3 | Deletion leads to embryonic fatality due to defects in heart formation. | [71] | Mutation leads to: (i) Robinow syndrome manifested by short-limbed dwarfism and abnormalities in the head, face and external genitalia; (ii) prostate cancer; (iii) leukemia; (iv) microcephaly; (v) depression; (vi) Hirschsprung’s disease; or (vii) lung cancer. | [72–78] | |
| Fzd3 | Embryonic fatality, essential for the brain development. | [79, 80] | Mutation leads to schizophrenia, and Hirschsprung disease (due to the absence of nerve cells in colon, leading to chronic constipoation); up-regulation in polycystic kidney disease. | [47, 81, 82] |
The NC1 (non-collagenous domain 1 of collagen α3 (IV) chain) domain model
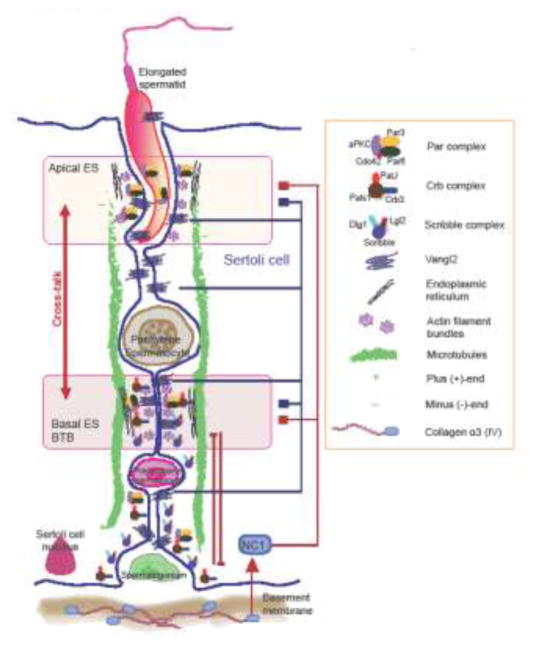
In the testis, the Sertoli and germ cells that constitute the seminiferous epithelium is considered to be an immune privilege site in particular the adluminal compartment behind the BTB [22–24]. This is due to the Sertoli cell-cell junctions, in particular the TJ and the basal ES, near the base of the epithelium, adjacent to the basement membrane, that create the BTB which deny the entry of other cells, such as macrophages and fibroblasts, and other biological and physiological substances including electrolytes, mineral salts and biomolecules into the adluminal (apical) compartment (Figure 1). However, recent studies have shown that one of the major building blocks of the basement membrane, the collagen α3 (IV) chain, is a regulator of the basal ES (i.e., BTB) and also the apical ES function. For instance, the inclusion of an anti-collagen type IV chain antibody obtained commercially in primary Sertoli cell cultures in vitro with an established TJ-barrier was found to perturb the Sertoli cell TJ-permeability barrier function [25]. This observation thus supports the notion that the collagen α3 (IV) chain in the basement membrane is playing a role in modulating the Sertoli cell barrier function at the BTB. This finding is also consistent with an earlier report that the use of antibodies prepared against the seminiferous tubule basement membrane induced extensive seminiferous epithelial damage including germ cell exfoliation, grossly disrupting spermatogenesis [26]. Subsequent studies have shown that such damages are mediated by the non-collagenous fraction of the basement membrane [27]. Additionally, studies from other epithelia have shown that the non-collagenous domains of collagen chains, in particular NC1 domain (non-collagenous domain 1) residing at the N-terminus when cleaved from collagen chains, act as biologically active peptides to modulate cell adhesion function and other biological activities (e.g., angiogenesis) in mammalian cells and tissues [28, 29]. We thus cloned the NC1 domain and obtained the recombinant protein against the NC1 domain at the N-terminal region of collagen α (IV) chain, a peptide of ~30 kDa, and noted that the purified recombinant protein was a potent biologically active peptide to induce Sertoli cell BTB restructuring [30]. Overexpression of the NC1 domain peptide using a mammalian expression vector pCI-neo using the Polyplus in vivo-jetPEI as a transfection medium (with a transfection efficiency at ~50–60%) in the testis in vivo was found to induce Sertoli BTB function disruption using a biotin-based BTB integrity assay in vivo [31], consistent with the findings in vitro that overexpression of NC1 domain in Sertoli cells [31] or inclusion of the recombinant NC1 domain peptide in Sertoli cell cultures [30] perturbed the Sertoli cell TJ-permeability barrier function. Additionally, it was of interest to note that overexpression of the NC1 domain in the testis in vivo also perturbed the apical ES function via a gross disruption on the organization of both actin- and MT-based cytoskeletons at the site [31]. Since the spatial expression and distribution of apical ES proteins β1-integrin [32–34] and laminin-γ3 chain [34, 35] were grossly disrupted [31], illustrating the apical ES function was perturbed. In fact, overexpression the NC1 domain in the testis in vivo led to extensive germ cell exfoliation, in particular elongating/elongated spermatids, such as within 7 days after NC1 domain overexpression when many tubules had contained only Sertoli cells and primitive germ cell types (e.g., spermatogonia, early spermatocytes) [31] since the loss of apical ES failed to support elongating and elongated spermatid adhesion onto the epithelium. In fact, over 50% of the tubules were devoid of elongating/elongated spermatids; the reason that not all tubules were affected is likely due to the transfection efficiency which was to be ~50–60% instead of >95% [31]. More important, numerous spermatids remained trapped deep inside the seminiferous epithelium and many of these elongated spermatids had defects in their polarity wherein their heads no longer aligned by pointing toward the basement membrane, but deviated by 90° to 180° from the intended orientation noted in control testes [31]. In short, even in the absence of functional apical ES, spermatids failed to be transported to the epithelium near the tubule lumen to undergo spermiation, and the non-funcitonal apical ES failed to support spermatid polarity. It is of important to note that the disruptive effects of NC1 domain on the basal ES/BTB and the apical ES function was reversible, since spermatogenesis gradually resumed and virtually all of the affected tubules repopulated with all germ cell types by day 45 [31].
Figure 1. A schematic drawing illustrating the functional relationship between the apical and basal ES in the seminiferous epithelium of adult rat testes.
As noted herein, the ES is supported by conspicuous actin filament bundles, either at the Sertoli-spermatid interface (step 1–8 spermatids in the rodent testis) or at the Sertoli cell-cell interface, known as the apical or the basal ES, respectively. The basal ES, which together with the TJ constitute the blood-testis barrier (BTB), which divides the epithelium into the apical (adluminal) and the basal compartments. The relative distribution of the Par-, the Scribble, and the Crb3-based polarity protein complexes and their corresponding partner proteins that confer the apico-basal polarity of individual spermatids in the seminiferous epithelium to support spermatogenesis are shown. Besides these polarity proteins, PCP proteins, such as Vangl2, are also present to support the alignment of polarized spermatids across the plane of the seminiferous epithelium, conferring PCP to the developing spermatids in the testis. Studies discussed in the text have shown that there are cross-talks between apical and basal ES, but more importantly that are also cross-talks between apical and basal ES through the action of Vangl2 but also NC1 domain peptide as noted in recent reports discussed in text. In short, both NC1 domain peptide generated from collagen α3 (IV) chain, a structural component of the basement membrane, and/or Vangl2 expressed by Sertoli and/or germ cells can modulate the actin- and/or MT-based cytoskeletons, thereby modulating the apical and/or basal ES function, modulating spermatid apico-basal and/or PCP polarity.
A detailed analysis on the status of spermatogenesis in the NC1 domain peptide affected tubules following its overexpression have noted that many of the step 19 spermatids remained trapped deep inside the seminiferous epithelium long after spermiation, even found in stage IX–XII tubules, and most of these spermatids had defects in polarity [31]. For instance, the heads of these elongated spermatids no longer pointed toward the basement membrane as noted in control and normal testes, but deviated by 90° to 180° from the intended orientation [31]. Additionally, the organization of F-actin and MT surrounding these elongated spermatids with defects in polarity were grossly disrupted, either missing altogether or diffusely localized, thereby failing to support spermatid polarity and adhesion [31]. Specifically, the track-like structures conferred by MTs that laid perpendicular to the basement membrane as noted in control testes were virtually non-detected in all the affected tubules that had defects in spermatogenesis following NC1 domain overexpression [31]. For instance, the MT-conferred tracks were extensively truncated and virtually no tracks were detected that ran from the basement membrane to the adluminal compartment near the tubule lumen across the entire epithelium in tubules following overexpression of NC1 domain as noted in control testes [31]. Collectively, these data thus support the notion that spermatid polarity (and also adhesion) are tightly associated with the integrity of actin- and MT-based cytoskeletons.
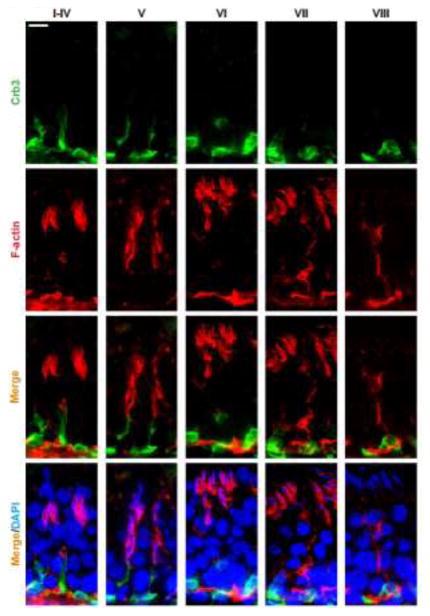
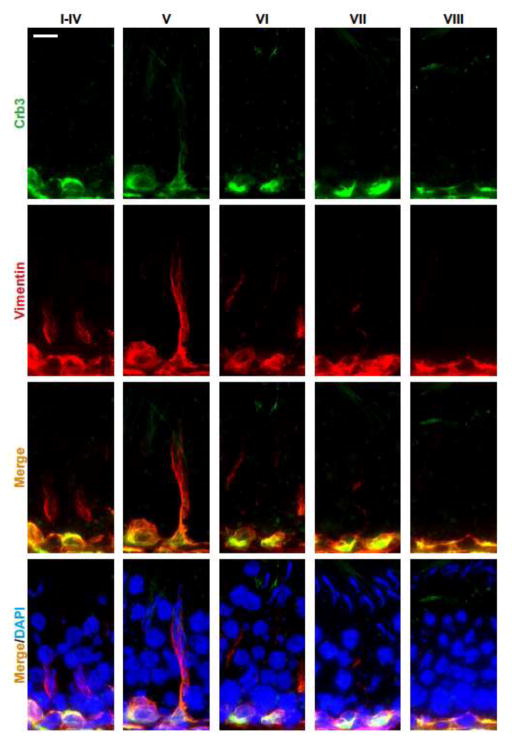
In this context, it is of interest to note that while defects of spermatid polarity noted in this NC1 model is likely contributed by the three polarity protein complexes involving the actin- and MT-based cytoskeletons, some recent observations suggest that the third cytoskeleton, namely the vimentin-based intermediate filament, may also be involved. For instance, as noted in Figure 2, the polarity protein Crb3 was only partially co-localized with F-actin in the seminiferous epithelium, however, Crb3 almost localized superimposable with vimentin (Figure 3). While much work is needed to define the role and involvement of intermediate filament-based cytoskeleton on spermatid polarity, the observations noted in Figures 2 and 3 have shown that such an expanded is much needed in future investigation.
Figure 2. A study that illustrates co-localization of polarity protein Crb3 and F-actin in the seminiferous epithelium of adult rat testes.
Crb3 (green fluorescence) and F-actin (red fluorescence) were visualized in the seminiferous epithelium of adult rat testes using corresponding specific antibody and/or reagents as earlier described [6, 39]. It was noted that Crb3 only partially co-localized with F-actin in the epithelium during the epithelial cycle of spermatogenesis. Cell nuclei were stained by DAPI (4′,6-diamidino-2-phenylindole). Scale bar, 40 μm, which applies to all other micrographs.
Figure 3. A study that illustrates co-localization of polarity protein Crb3 and vimentin in the seminiferous epithelium of adult rat testes.
Crb3 (green fluorescence) and vimentin (red fluorescence) were visualized in the seminiferous epithelium of adult rat testes using corresponding specific antibody and/or reagents as described [6, 39]. It was noted that Crb3 co-localized with F-actin, almost superimposable, in the epithelium during the epithelial cycle of spermatogenesis. Cell nuclei were stained by DAPI (4′,6-diamidino-2-phenylindole). Scale bar, 40 μm, which applies to all other micrographs.
The Vangl2 (Van Gogh-like 2) model
Vangl2 (also known as Strabismus 1 or Loop-tail protein 1 homolog) is a small integral membrane protein of ~60 kDa. It works in concert with prickle to modulate PCP polarity in flies, rodents and humans. Vangl2 exerts its regulatory effects by modulating effects on the actin-based cytoskeleton, particularly involved in the embryo implantation and embryogenesis during development, and also PCP orientation of different tissues and/or organs in adult animals such as stereociliary bundles in the cochlea of the inner ear in rodents and humans [36–38]. Indeed, Vangl2 (and also Vangl1) are expressed by Sertoli and germ cells in the testis [10]. Vangl2 is expressed in the seminiferous epithelium at virtually all stages of the epithelial cycle in the rat testis, co-localizing with F-actin at the apical and the basal ES, the ultrastructures that are involved in apico-basal polarity and PCP. When Vangl2 was knockdown in the testis in vivo by transfecting the testis with Vangl2-specific siRNA duplexes using the Polyplus in vivo-jetPEI transfection medium vs. non-targeting negative control siRNA duplexes, the status of spermatogenesis was grossly disrupted [10]. First, following knockdown of Vangl2 by RNAi, spermatids displayed signs of defects of polarity in which their heads no longer pointed toward the basement membrane but deviated by 90° to 180° of their intended orientation [10]. More important, the spatial expression of F-actin at the apical ES was grossly disrupted as F-actin appeared as bulb-like structures located predominantly at the concave side of spermatid heads in control testes [10]. However, following Vangl2 knockdown, F-actin moved away from spermatids, which was the result of changes in spatial expression of actin barbed-end capping and bundling protein Eps8 and branched actin polymerization inducing protein Arp3 [10], such that F-actin no longer prominently expressed at the apical ES to support its function. This mis-localization of F-actin thus impeded apical ES adhesion protein function since the apical ES proteins β-integrin, nectin 3 and laminin-γ3 chain all utilized F-actin for their attachment. In short, these three apical ES proteins no longer tightly associated with apical ES surrounding the spermatid heads, but mis-localized and considerably down-regulated at the site [10]. Second, the organization of F-actin across the Sertoli cell cytosol after Vangl2 knockdown was grossly disrupted since they no longer stretched across the entire Sertoli cell to support cell adhesion function, actin filaments became extensively truncated and mis-aligned [10]. Third, it was noted that following Vnagl2 knockdown in the testis in vivo, the frequency of meiosis I/II across the seminiferous epithelium in stage XIV tubules was considerably reduced, by as much as ~60–70%, due to the disruption of F-actin organization. Since the meiotic bundles that support chromosomal segregation during meiosis also modulated by MTs, we speculated that Vangl2 and other PCP proteins might also modulate MT dynamics. Indeed, a recent report has shown that a knockdown of Vangl2 in the testis in vivo perturbed spermatid PCP when visualized by confocal microscopy [9]. This disruption effect on MT organization following Vanlg2 known appeared to be mediated by changes in the spatial expression of MARK2 in Sertoli cells [9], a MT regulatory protein known to be involved in MT dynamics by promoting MT catastrophe [21]. Taking collectively, these findings thus support the notion unequivocally that Vangl2 that supports spermatid polarity is mediated through changes in F-actin and MT% organization.
Concluding remarks and future perspectives
While the role of actin- and/or MT-based cytoskeletons to support spermatid polarity and spermatid PCP is a rapidly developing field, there are emerging evidence, as briefly discussed herein, to support the involvement of cytoskeletons and cell polarity in the testis. However, much work is needed in the years to come. For instance, the role of Par-, Crb3- and Scribble-based apico-basal polarity protein complexes in modulating MT-organization remains largely unexplored. Also, besides Vangl2, the roles of Prickles, Dishevelled, and Frizzled proteins, in particular how Frizzled/Dishevelled complex vs. Vangl2/Prickle are working in concert with each other to modulate F-actin- and MT-based cytoskeletons including the downstream signaling molecules are not known. It is anticipated many of these questions will be answered in the near future. This information will provide a better picture to relate these findings to the biology of spermatogenesis in particular the functional cross-talk between polarity proteins and cytoskeletons in the testis to support germ cell differentiation and development.
Acknowledgments
This work was supported by grants from the National Institutes of Health, NIDCHD (R01 HD056034 to C.Y.C. and U54 HD029990 Project 5 to C.Y.C.)
Footnotes
Conflicts of Interest: Nothing to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Kretser DM, Kerr JB. The cytology of the testis. In: Knobil E, Neill JB, Ewing LL, Greenwald GS, Markert CL, Pfaff DW, editors. The Physiology of Reproduction. Vol. 1. New York: Raven Press; 1988. pp. 837–932. [Google Scholar]
- 2.Chen H, Mruk DD, Lui WY, Wong CKC, Lee WM, Cheng CY. Cell polarity and planar cell polarity (PCP) in spermatogenesis. Semin Cell Dev Biol. 2017 doi: 10.1016/j.semcdb.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen H, Mruk DD, Xiao X, Cheng CY. Human spermatogenesis and its regulation. In: Ed Winters SJ, Huhtaniemi IT, editors. Male Hypogonadism, Contemporary Endocrinology. New York: Springer International Publishing AG; 2017. pp. 49–72. doi:101007/978-3-319-53298-1_3. [Google Scholar]
- 4.Wong EWP, Mruk DD, Lee WM, Cheng CY. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:9657–62. doi: 10.1073/pnas.0801527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su WH, Wong EWP, Mruk DD, Cheng CY. The Scribble/Lgl/Dlg polarity protein complex is a regulator of blood-testis barrier dynamics and spermatid polarity during spermatogenesis. Endocrinology. 2012;153:6041–53. doi: 10.1210/en.2012-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao Y, Lui WY, Lee WM, Cheng CY. Polarity protein Crumbs homolog-3 (CRB3) regulates ectoplasmic specialization dynamics through its action on F-actin organization in Sertoli cells. Scientific reports. 2016;6:28589. doi: 10.1038/srep28589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim Biophys Acta. 2008;1778:614–30. doi: 10.1016/j.bbamem.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 8.Lang CF, Munro E. The PAR proteins: from molecular circuits to dynamic self-stabilizing cell polarity. Development. 2017;144:3405–16. doi: 10.1242/dev.139063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H, Xiao X, Lui WY, Lee WM, Cheng CY. Vangl2 regulates spermatid planar cell polarity through microtubule (MT)-based cytoekelton in the rat testis. Cell Death & Dis. 2018 doi: 10.1038/s41419-018-0339-x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H, Mruk DD, Lee WM, Cheng CY. Planar cell polarity (PCP) protein Vangl2 regulates ectoplasmic specialization dynamics via its effects on actin microfilaments in the testes of male rats. Endocrinology. 2016;157:2140–59. doi: 10.1210/en.2015-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen Q, Mruk D, Tang EI, Wong CKC, Lui WY, Lee WM, et al. Cell polarity and cytoskeletons-lesson from the testis. Semin Cell Dev Biol. 2017 doi: 10.1016/j.semcdb.2017.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol. 2008;636:186–211. doi: 10.1007/978-0-387-09597-4_11. [DOI] [PubMed] [Google Scholar]
- 13.Wong EWP, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochem Biophys Acta. 2008;1778:692–708. doi: 10.1016/j.bbamem.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan HHN, Mruk DD, Lee WM, Cheng CY. Ectoplasmic specialization: a friend or a foe of spermatogenesis? BioEssays. 2007;29:36–48. doi: 10.1002/bies.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iden S, Collard JG. Crosstalk between small GTPases and polarity proteins in cell polarization. Nature Rev Mol Cell Biol. 2008;9:846–59. doi: 10.1038/nrm2521. [DOI] [PubMed] [Google Scholar]
- 16.Bazellieres E, Aksenova V, Barthelemy-Requin M, Massey-Harroche D, Le Bivic A. Role of the crumbs proteins in ciliogenesis, cell migration and actin organization. Semin Cell Dev Biol. 2017 doi: 10.1016/j.semcdb.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Mack NA, Georgiou M. The interdependence of the Rho GTPases and apicobasal cell polarity. Small GTPases. 2014;5:10. doi: 10.4161/21541248.2014/973768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell LD, Goh JC, Rashed RMA, Vogl AW. The consequences of actin disruption at Sertoli ectoplasmic specialization sites facing spermatids after in vivo exposure of rat testis to cytochalasin D. Biol Reprod. 1988;39:105–18. doi: 10.1095/biolreprod39.1.105. [DOI] [PubMed] [Google Scholar]
- 19.Russell LD, Malone JP, MacCurdy DS. Effect of the microtubule disrupting agents, colchicine and vinblastine, on seminiferous tubule structure in the rat. Tissue Cell. 1981;13:349–67. doi: 10.1016/0040-8166(81)90010-0. [DOI] [PubMed] [Google Scholar]
- 20.Russell LD, Saxena NK, Turner TT. Cytoskeletal involvement in spermiation and sperm transport. Tissue Cell. 1989;21:361–79. doi: 10.1016/0040-8166(89)90051-7. [DOI] [PubMed] [Google Scholar]
- 21.Tang EI, Mruk DD, Cheng CY. Regulation of microtubule (MT)-based cytoskeleton in the seminiferous epithelium during spermatogenesis. Semin Cell Dev Biol. 2016;59:35–45. doi: 10.1016/j.semcdb.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hedger MP. Immunophysiology and pathology of inflammation in the testis and epididymis. J Androl. 2011;32:625–40. doi: 10.2164/jandrol.111.012989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaur G, Thompson LA, Dufour JM. Sertoli cells - immunological sentinels of spermatogenesis. Semin Cell Dev Biol. 2014;30:36–44. doi: 10.1016/j.semcdb.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meinhardt A, Hedger MP. Immunological, paracrine and endocrine aspects of testicular immune privilege. Mol Cell Endocrinol. 2011;335:60–8. doi: 10.1016/j.mce.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 25.Siu MKY, Lee WM, Cheng CY. The interplay of collagen IV, tumor necrosis factor-α, gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloprotease-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology. 2003;144:371–87. doi: 10.1210/en.2002-220786. [DOI] [PubMed] [Google Scholar]
- 26.Lustig L, Denduchis B, Gonzalez NN, Puig RP. Experimental orchitis induced in rats by passive transfer of an antiserum to seminiferous tubule basement membrane. Arch Androl. 1978;1:333–43. doi: 10.3109/01485017808988354. [DOI] [PubMed] [Google Scholar]
- 27.Denduchis B, Satz ML, Sztein MB, Puig RP. Multifocal damage to the testis induced in rats by passive transfer of antibodies prepared against non-collagenous fraction of basement membrane. J Reprod Immunol. 1985;7:59–75. doi: 10.1016/0165-0378(85)90021-x. [DOI] [PubMed] [Google Scholar]
- 28.Marneros AG, Olsen BR. The role of collagen-derived proteolytic fragments in angiogenesis. Matrix Biol. 2001;20:337–45. doi: 10.1016/s0945-053x(01)00151-2. [DOI] [PubMed] [Google Scholar]
- 29.Gaggar A, Weathington N. Bioactive extracellular matrix fragments in lung health and disease. J Clin Invest. 2016;126:3176–84. doi: 10.1172/JCI83147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong EW, Cheng CY. NC1 domain of collagen alpha3(IV) derived from the basement membrane regulates Sertoli cell blood-testis barrier dynamics. Spermatogenesis. 2013;3:e25465. doi: 10.4161/spmg.25465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen H, Mruk DD, Lee WM, Cheng CY. Regulation of spermatogenesis by a local functional axis in the testis: role of the basement membrane-derived noncollagenous 1 domain peptide. FASEB J. 2017;31:3587–607. doi: 10.1096/fj.201700052R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palombi F, Salanova M, Tarone G, Farini D, Stefanini M. Distribution of β1 integrin subunit in rat seminiferous epithelium. Biol Reprod. 1992;47:1173–82. doi: 10.1095/biolreprod47.6.1173. [DOI] [PubMed] [Google Scholar]
- 33.Salanova M, Ricci G, Boitani C, Stefanini M, De Grossi S, Palombi F. Junctional contacts between Sertoli cells in normal and aspermatogenic rat seminiferous epithelium contain α6β1 integrins, and their formation is controlled by follicle-stimulating hormone. Biol Reprod. 1998;58:371–8. doi: 10.1095/biolreprod58.2.371. [DOI] [PubMed] [Google Scholar]
- 34.Siu MKY, Cheng CY. Interactions of proteases, protease inhibitors, and the β1 integrin/laminin γ3 protein complex in the regulation of ectoplasmic specialization dynamics in the rat testis. Biol Reprod. 2004;70:945–64. doi: 10.1095/biolreprod.103.023606. [DOI] [PubMed] [Google Scholar]
- 35.Koch M, Olson PF, Albus A, Jin W, Hunter DD, Brunken WJ, et al. Characterization and expression of the laminin γ3 chain: a novel, non-basement membrane-associated, laminin chain. J Cell Biol. 1999;145:605–18. doi: 10.1083/jcb.145.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torban E, Iliescu A, Gros P. An expanding role of Vangl proteins in embryonic development. Curr Top Dev Biol. 2012;101:237–61. doi: 10.1016/B978-0-12-394592-1.00005-3. [DOI] [PubMed] [Google Scholar]
- 37.Torban E, Patenaude AM, Leclerc S, Rakowiecki S, Gauthier S, Andelfinger G, et al. Genetic interaction between members of the Vangl family causes neural tube defects in mice. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3449–54. doi: 10.1073/pnas.0712126105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bailly E, Walton A, Borg JP. The planar cell polarity Vangl2 protein: from genetics to cellular and molecular functions. Semin Cell Dev Biol. 2017 doi: 10.1016/j.semcdb.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 39.Li L, Tang EI, Chen H, Lian Q, Ge R, Silvestrini B, et al. Sperm release at spermiation is regulated by changes in the organization of actin- and microtubule-based cytoskeletons at the apical ectoplasmic specialization - a study using the adjudin model. Endocrinology. 2017;158:4300–16. doi: 10.1210/en.2017-00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakamura H, Nagasaka K, Kawana K, Taguchi A, Uehara Y, Yoshida M, et al. Expression of Par3 polarity protein correlates with poor prognosis in ovarian cancer. BMC Cancer. 2016;16:897. doi: 10.1186/s12885-016-2929-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou PJ, Xue W, Peng J, Wang Y, Wei L, Yang Z, et al. Elevated expression of Par3 promotes prostate cancer metastasis by forming a Par3/aPKC/KIBRA complex and inactivating the hippo pathway. J Exp Clin Cancer Res. 2017;36:139. doi: 10.1186/s13046-017-0609-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo X, Wang M, Zhao Y, Wang X, Shen M, Zhu F, et al. Par3 regulates invasion of pancreatic cancer cells via interaction with Tiam1. Clinical and experimental medicine. 2016;16:357–65. doi: 10.1007/s10238-015-0365-2. [DOI] [PubMed] [Google Scholar]
- 43.Nolan ME, Aranda V, Lee S, Lakshmi B, Basu S, Allred DC, et al. The polarity protein Par6 induces cell proliferation and is overexpressed in breast cancer. Cancer Res. 2008;68:8201–9. doi: 10.1158/0008-5472.CAN-07-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al-Saad S, Al-Shibli K, Donnem T, Persson M, Bremnes RM, Busund LT. The prognostic impact of NF-kappaB p105, vimentin, E-cadherin and Par6 expression in epithelial and stromal compartment in non-small-cell lung cancer. Br J Cancer. 2008;99:1476–83. doi: 10.1038/sj.bjc.6604713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Townsend TA, Wrana JL, Davis GE, Barnett JV. Transforming growth factor-beta-stimulated endocardial cell transformation is dependent on Par6c regulation of RhoA. J Biol Chem. 2008;283:13834–41. doi: 10.1074/jbc.M710607200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen L, Liao G, Yang L, Campbell K, Nakafuku M, Kuan CY, et al. Cdc42 deficiency causes Sonic hedgehog-independent holoprosencephaly. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:16520–5. doi: 10.1073/pnas.0603533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luyten A, Su X, Gondela S, Chen Y, Rompani S, Takakura A, et al. Aberrant regulation of planar cell polarity in polycystic kidney disease. Journal of the American Society of Nephrology : JASN. 2010;21:1521–32. doi: 10.1681/ASN.2010010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takenouchi T, Kosaki R, Niizuma T, Hata K, Kosaki K. Macrothrombocytopenia and developmental delay with a de novo CDC42 mutation: Yet another locus for thrombocytopenia and developmental delay. Am J Med Genet A. 2015;167A:2822–5. doi: 10.1002/ajmg.a.37275. [DOI] [PubMed] [Google Scholar]
- 49.Bandyopadhyay G, Standaert ML, Sajan MP, Kanoh Y, Miura A, Braun U, et al. Protein kinase C-lambda knockout in embryonic stem cells and adipocytes impairs insulin-stimulated glucose transport. Mol Endocrinol. 2004;18:373–83. doi: 10.1210/me.2003-0087. [DOI] [PubMed] [Google Scholar]
- 50.Yamanaka T, Tosaki A, Kurosawa M, Akimoto K, Hirose T, Ohno S, et al. Loss of aPKClambda in differentiated neurons disrupts the polarity complex but does not induce obvious neuronal loss or disorientation in mouse brains. PLoS One. 2013;8:e84036. doi: 10.1371/journal.pone.0084036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alfonso SI, Callender JA, Hooli B, Antal CE, Mullin K, Sherman MA, et al. Gain-of-function mutations in protein kinase Calpha (PKCalpha) may promote synaptic defects in Alzheimer’s disease. Sci Signal. 2016;9:ra47. doi: 10.1126/scisignal.aaf6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diez P, Lorenzo S, Degano RM, Ibarrola N, Gonzalez-Gonzalez M, Nieto W, et al. Multipronged functional proteomics approaches for global identification of altered cell signalling pathways in B-cell chronic lymphocytic leukaemia. Proteomics. 2016;16:1193–203. doi: 10.1002/pmic.201500372. [DOI] [PubMed] [Google Scholar]
- 53.Hartleben B, Widmeier E, Wanner N, Schmidts M, Kim ST, Schneider L, et al. Role of the polarity protein Scribble for podocyte differentiation and maintenance. PLoS One. 2012;7:e36705. doi: 10.1371/journal.pone.0036705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elsum IA, Yates LL, Pearson HB, Phesse TJ, Long F, O’Donoghue R, et al. Scrib heterozygosity predisposes to lung cancer and cooperates with KRas hyperactivation to accelerate lung cancer progression in vivo. Oncogene. 2014;33:5523–33. doi: 10.1038/onc.2013.498. [DOI] [PubMed] [Google Scholar]
- 55.Pearson HB, Perez-Mancera PA, Dow LE, Ryan A, Tennstedt P, Bogani D, et al. SCRIB expression is deregulated in human prostate cancer, and its deficiency in mice promotes prostate neoplasia. J Clin Invest. 2011;121:4257–67. doi: 10.1172/JCI58509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iizuka-Kogo A, Senda T, Akiyama T, Shimomura A, Nomura R, Hasegawa Y, et al. Requirement of DLG1 for cardiovascular development and tissue elongation during cochlear, enteric, and skeletal development: possible role in convergent extension. PLoS One. 2015;10:e0123965. doi: 10.1371/journal.pone.0123965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iizuka-Kogo A, Akiyama T, Senda T. Decreased apoptosis and persistence of the common nephric duct during the development of an aberrant vesicoureteral junction in Dlg1 gene-targeted mice. Anat Rec (Hoboken) 2013;296:1936–42. doi: 10.1002/ar.22814. [DOI] [PubMed] [Google Scholar]
- 58.Mahoney ZX, Sammut B, Xavier RJ, Cunningham J, Go G, Brim KL, et al. Discs-large homolog 1 regulates smooth muscle orientation in the mouse ureter. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19872–7. doi: 10.1073/pnas.0609326103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu S, Zhou F, Tao J, Song L, Ng SC, Wang X, et al. Exome sequencing identifies DLG1 as a novel gene for potential susceptibility to Crohn’s disease in a Chinese family study. PLoS One. 2014;9:e99807. doi: 10.1371/journal.pone.0099807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carroll LS, Williams HJ, Walters J, Kirov G, O’Donovan MC, Owen MJ. Mutation screening of the 3q29 microdeletion syndrome candidate genes DLG1 and PAK2 in schizophrenia. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2011;156B:844–9. doi: 10.1002/ajmg.b.31231. [DOI] [PubMed] [Google Scholar]
- 61.Patil DT, Bennett AE, Mahajan D, Bronner MP. Distinguishing Barrett gastric foveolar dysplasia from reactive cardiac mucosa in gastroesophageal reflux disease. Hum Pathol. 2013;44:1146–53. doi: 10.1016/j.humpath.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 62.Whiteman EL, Fan S, Harder JL, Walton KD, Liu CJ, Soofi A, et al. Crumbs3 is essential for proper epithelial development and viability. Mol Cell Biol. 2014;34:43–56. doi: 10.1128/MCB.00999-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim S, Lehtinen MK, Sessa A, Zappaterra MW, Cho SH, Gonzalez D, et al. The apical complex couples cell fate and cell survival to cerebral cortical development. Neuron. 2010;66:69–84. doi: 10.1016/j.neuron.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park B, Alves CH, Lundvig DM, Tanimoto N, Beck SC, Huber G, et al. PALS1 is essential for retinal pigment epithelium structure and neural retina stratification. J Neurosci. 2011;31:17230–41. doi: 10.1523/JNEUROSCI.4430-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Okerlund ND, Stanley RE, Cheyette BN. The Planar Cell Polarity Transmembrane Protein Vangl2 Promotes Dendrite, Spine and Glutamatergic Synapse Formation in the Mammalian Forebrain. Molecular neuropsychiatry. 2016;2:107–14. doi: 10.1159/000446778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yuan Y, Gao Y, Wang H, Ma X, Ma D, Huang G. Promoter methylation and expression of the VANGL2 gene in the myocardium of pediatric patients with tetralogy of fallot. Birth Defects Res A Clin Mol Teratol. 2014;100:973–84. doi: 10.1002/bdra.23291. [DOI] [PubMed] [Google Scholar]
- 67.Kibar Z, Salem S, Bosoi CM, Pauwels E, De Marco P, Merello E, et al. Contribution of VANGL2 mutations to isolated neural tube defects. Clin Genet. 2011;80:76–82. doi: 10.1111/j.1399-0004.2010.01515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tao H, Suzuki M, Kiyonari H, Abe T, Sasaoka T, Ueno N. Mouse prickle1, the homolog of a PCP gene, is essential for epiblast apical-basal polarity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14426–31. doi: 10.1073/pnas.0901332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bosoi CM, Capra V, Allache R, Trinh VQ, De Marco P, Merello E, et al. Identification and characterization of novel rare mutations in the planar cell polarity gene PRICKLE1 in human neural tube defects. Hum Mutat. 2011;32:1371–5. doi: 10.1002/humu.21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perry JR, McCarthy MI, Hattersley AT, Zeggini E, Weedon MN, et al. Wellcome Trust Case Control C. Interrogating type 2 diabetes genome-wide association data using a biological pathway-based approach. Diabetes. 2009;58:1463–7. doi: 10.2337/db08-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Etheridge SL, Ray S, Li SD, Hamblet NS, Lijam N, Tsang M, et al. Murine Dishevelled 3 Functions in Redundant Pathways with Dishevelled 1 and 2 in Normal Cardiac Outflow Tract, Cochlea, and Neural Tube Development. PLoS genetics. 2008:4. doi: 10.1371/journal.pgen.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.White JJ, Mazzeu JF, Coban-Akdemir Z, Bayram Y, Bahrambeigi V, Hoischen A, et al. WNT Signaling Perturbations Underlie the Genetic Heterogeneity of Robinow Syndrome. Am J Hum Genet. 2018;102:27–43. doi: 10.1016/j.ajhg.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim PJ, Park JY, Kim HG, Cho YM, Go H. Dishevelled segment polarity protein 3 (DVL3): a novel and easily applicable recurrence predictor in localised prostate adenocarcinoma. BJU Int. 2017;120:343–50. doi: 10.1111/bju.13783. [DOI] [PubMed] [Google Scholar]
- 74.Khan AS, Hojjat-Farsangi M, Daneshmanesh AH, Hansson L, Kokhaei P, Osterborg A, et al. Dishevelled proteins are significantly upregulated in chronic lymphocytic leukaemia. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2016;37:11947–57. doi: 10.1007/s13277-016-5039-5. [DOI] [PubMed] [Google Scholar]
- 75.Kadir R, Harel T, Markus B, Perez Y, Bakhrat A, Cohen I, et al. ALFY-Controlled DVL3 Autophagy Regulates Wnt Signaling, Determining Human Brain Size. PLoS genetics. 2016;12:e1005919. doi: 10.1371/journal.pgen.1005919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jansen R, Penninx BW, Madar V, Xia K, Milaneschi Y, Hottenga JJ, et al. Gene expression in major depressive disorder. Molecular psychiatry. 2016;21:339–47. doi: 10.1038/mp.2015.57. [DOI] [PubMed] [Google Scholar]
- 77.Chen D, Mi J, Wu M, Wang W, Gao H. Expression of dishevelled gene in Hirschsprung’s disease. Int J Clin Exp Pathol. 2013;6:1791–8. [PMC free article] [PubMed] [Google Scholar]
- 78.Li XY, Liu SL, Cha N, Zhao YJ, Wang SC, Li WN, et al. Transcription expression and clinical significance of dishevelled-3 mRNA and delta-catenin mRNA in pleural effusions from patients with lung cancer. Clin Dev Immunol. 2012;2012:904946. doi: 10.1155/2012/904946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hua ZL, Jeon S, Caterina MJ, Nathans J. Frizzled3 is required for the development of multiple axon tracts in the mouse central nervous system. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E3005–14. doi: 10.1073/pnas.1406399111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hua ZL, Smallwood PM, Nathans J. Frizzled3 controls axonal development in distinct populations of cranial and spinal motor neurons. eLife. 2013;2:e01482. doi: 10.7554/eLife.01482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pantavou KG, Braliou GG, Kontou PI, Dimou NL, Bagos PG. A meta-analysis of FZD3 gene polymorphisms and their association with schizophrenia. Psychiatric genetics. 2016;26:272–80. doi: 10.1097/YPG.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 82.Su L, Zhang Z, Gan L, Jiang Q, Xiao P, Zou J, et al. Deregulation of the planar cell polarity genes CELSR3 and FZD3 in Hirschsprung disease. Exp Mol Pathol. 2016;101:241–8. doi: 10.1016/j.yexmp.2016.09.003. [DOI] [PubMed] [Google Scholar]