Capsule Summary
GLP-1R signaling, an emerging anti-inflammatory therapeutic 59 target, 60 attenuated type 2-associated immunopathology in mice infected with a strain of RSV that was 61 isolated from a hospitalized infant with severe lower respiratory tract infection and bronchiolitis.
Keywords: glucagon-like peptide-1 (GLP-1), respiratory syncytial virus (RSV), group 2 innate lymphoid cells (ILC2), IL-13, IL-33, type 2 immunity (Th2), phenome-wide association study (PheWAS)
To the editor
Glucagon-like peptide-1 receptor (GLP-1R) agonists, which potentiate insulin and suppress glucagon secretion, are a well-accepted and safe treatment for Type II diabetes.1 Although GLP-1R agonists are currently used for their ability to potentiate insulin and suppress glucagon secretion, recent evidence suggests that GLP-1R signaling also has anti-inflammatory effects.2–4 Severe RSV-associated illness is in part caused by IL-13 production, which mediates the mucus production that directly contributes to airway obstruction and respiratory failure.5 We hypothesized that GLP-1R signaling inhibits IL-13-mediated immunopathology of RSV 12/12-6, a strain of RSV that was isolated from a hospitalized infant with severe lower respiratory tract infection and bronchiolitis.
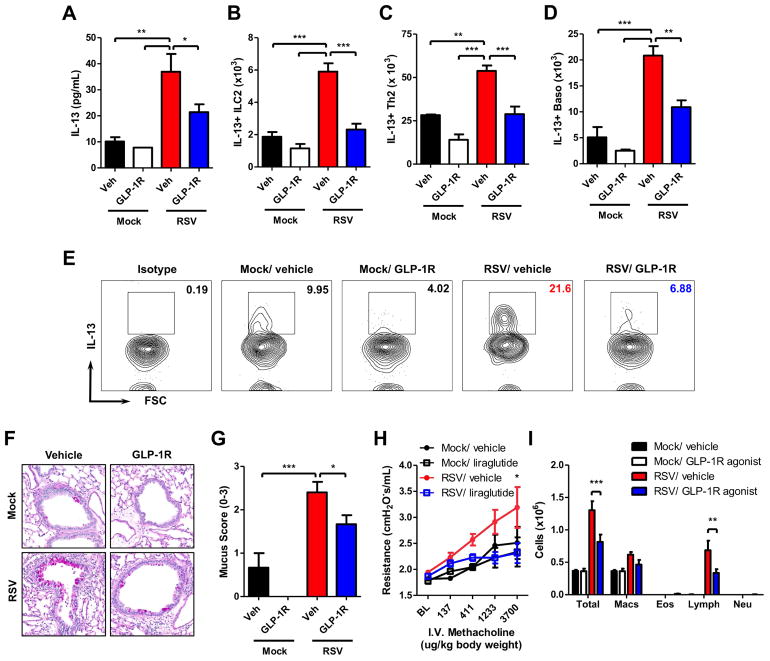
Eight week old mice were infected with 9 × 105 PFU of RSV. RSV 12/12-6 induced significant lung IL-13 and airway mucus, mimicking what is seen in patients with severe infection (Fig E1). We administered GLP-1R agonist or vehicle (0.1% BSA in PBS) twice daily beginning 2 days prior to RSV infection until all endpoints (Fig E2). GLP-1R agonist treatment significantly decreased lung IL-13 protein expression compared to vehicle treatment in RSV-infected mice (Fig 1, A). We identified the cellular sources of IL-13 that GLP-1R signaling was inhibiting. GLP-1R agonist treatment in RSV-infected mice significantly decreased the total number of cells in the lung, the total number of group 2 innate lymphoid cells (ILC2), and the percentage of ILC that were IL-13+ compared to RSV-infected vehicle-treated mice (Fig E3A, Fig E4A&F & Fig 1, B&E). There was significantly decreased MFI of IL-13 and CD127 on the ILC2 of RSV-infected GLP-1R agonist-treated mice compared to RSV-infected vehicle-treated mice, indicating decreased IL-13 production and CD127 expression on a per ILC2 basis with GLP-1R agonist treatment (Fig E4B–E). GLP-1R agonist treatment in RSV-infected mice significantly decreased the numbers of CD4+ T cells and basophils, as well as IL-13+ Th2 cells and basophils compared to RSV-infected vehicle-treated mice (Fig E3B–C, Fig E4G–H & Fig 1, C–D).
Figure 1. GLP-1R agonist decreases RSV-induced type 2 responses and immunopathology.
(A) ELISA for IL-13 in whole lung homogenate (right lung only). (B) Total number of IL-13+ ILC2, (C) Th2 cells, and (D) basophils. (E) Representative IL-13 expression measured by flow cytometry in ILC2. (F) Representative PAS-stained section of mucus-containing airways in the lungs (40x magnification); arrowhead denotes intraluminal mucus strand. (G) Quantification of airway mucus from the experiment in A. (H) Airway responsiveness and (I) BAL cell counts. Data plotted as mean + SEM. n = 3–6 mice per group representative of 3 (A) or 2 (B–G & I) independent experiments. n = 6–12 mice per group combined from 2 independent experiments (H). *p < 0.05, **p < 0.01, ***p < 0.001 by one-way (B & E–H) or two-way (C–D) ANOVA. BL = baseline.
Moreover, there were significant decreases in methacholine-induced airway responsiveness and mucus severity scores in RSV-infected GLP-1R agonist-treated mice compared to RSV-infected vehicle-treated mice (Fig 1, F–H). RSV-infected GLP-1R agonist-treated mice had significantly decreased numbers of total bronchoalveolar lavage (BAL) cells and lymphocytes compared to RSV-infected vehicle-treated mice (Fig 1, I). Administration of the GLP-1R agonist beginning 2 days after RSV infection also significantly decreased lung IL-13 and there was a trend towards decreased airway mucus (Fig E5). Collectively, these data demonstrate that GLP-1R signaling attenuates IL-13-mediated immunopathology during RSV infection.
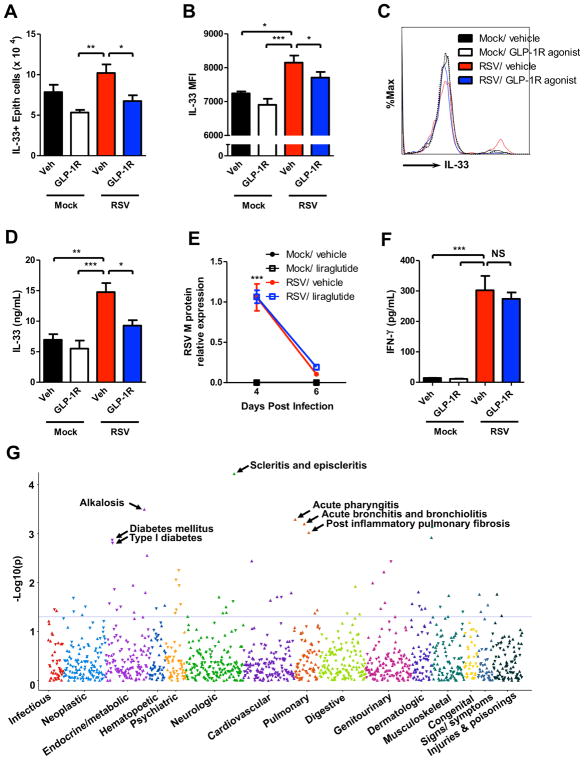
IL-33 activates type 2 cytokine-producing immune cells including ILC2, Th2, and basophils. We used Il33Citrine/+ reporter mice to examine the effect of GLP-1R signaling on IL-33 expression on a per epithelial cell basis. GLP-1R agonist treatment in RSV-infected mice significantly decreased the total number of cells in the lung, the total number of IL-33-expressing epithelial cells, and the percentage of epithelial cells that were IL-33+ compared to RSV-infected vehicle-treated mice (Fig E6A–B & Fig 2, A). There was significantly decreased MFI of IL-33 in the epithelial cells of RSV-infected GLP-1R agonist-treated mice compared to RSV-infected vehicle-treated mice, indicating decreased IL-33 expression on a per epithelial cell basis with GLP-1R agonist treatment (Fig 2, B–C). RSV-infected GLP-1R agonist-treated mice had significantly decreased lung IL-33 protein expression compared to RSV-infected vehicle-treated mice (Fig 2, D). These data indicate that GLP-1R agonist treatment inhibits the expression of IL-33 by epithelial cells during RSV infection.
Figure 2. GLP-1R signaling decreases IL-33, does not increase viral titer or decrease IFN-γ production, and associates with acute bronchiolitis in humans.
(A) Total number of IL-33+ epithelial cells, (B) MFI of IL-33 expression in epithelial cells, (C) representative IL-33 expression measured by flow cytometry in epithelial cells, and (D) ELISA for IL-33 in whole lung homogenate (left lung only). (E) Lung mRNA RSV M protein expression normalized to GAPDH. (F) ELISA for IFN-γ in whole lung homogenate (right lung only). (G) Phenome-wide association study (PheWAS) plot for THADA rs7578597 using logistic regression assuming an additive genetic model adjusted for age, sex, study site, and the first 3 principal components. rs7578597 associated with acute bronchiolitis (OR = 1.24, P = 6.3 × 10−3). Data plotted as mean + SEM. n = 3–6 mice per group representative of 2 independent experiments (A–F). *p < 0.05, **p < 0.01, ***p < 0.001 by one-way (A–B, D, & F) or two-way (E) ANOVA. NS = not significant.
To determine whether GLP-1R agonist treatment had a deleterious effect on viral-associated disease severity parameters, we evaluated viral load, an indicator of RSV disease severity.6 There were no significant differences in the viral load between RSV-infected GLP-1R agonist and vehicle-treated mice (Fig 2, E). Consistent with these data, we did not observe any significant differences in lung interferon-γ (IFN-γ) expression or IFN-γ+ Th1 and natural killer (NK) cells between RSV-infected GLP-1R agonist and vehicle-treated mice 6 days post-infection (Fig E4B&D, Fig 2, F & Fig E7A–B). Further, there were no significant differences in lung IFN-α, IFN-β, or IL-27 protein expression between RSV-infected GLP-1R agonist and vehicle-treated mice (Fig E7C–E). We also found that there were no statistically significant changes in plasma glucose or insulin levels with GLP-1R treatment compared to vehicle (Fig E7F & G)
To determine whether GLP-1R agonist treatment during primary infection has an impact on the immune response to a later secondary infection, we infected mice with RSV a second time following primary RSV infection (Fig E8). GLP-1R agonist treatment during primary infection significantly decreased the number of RSV-induced total BAL cells and lymphocytes compared to vehicle treatment after secondary infection (Fig E9A). The mice treated with GLP-1R agonist during primary infection did not exhibit altered lung IFN-γ expression nor RSV F-protein-specific antibody responses compared to vehicle-treated mice during secondary RSV infection (Fig E9B–E). These data demonstrate that GLP-1R agonist treatment does not exacerbate disease or impede anti-viral responses.
We next sought to identify associations between GLP-1 signaling and human RSV disease. The phenome-wide association study (PheWAS) is a new, validated reverse genetics approach that associates genetic variants of interest with phenotypes by linking a database of de-identified genotyping to a broad range of electronic medical record (EMR)-derived clinical phenotypes. The EMR phenotypes are derived from cluster of common International Classification of Diseases, Ninth Revision, codes. The loss-of-function rs7578597 variant of THADA, encoding thyroid adenoma-associated protein, is associated with lower beta-cell response to GLP-1.7 A PheWAS on the single nucleotide polymorphism (SNP) rs7578597 (missense, T1187A) and 1,000 phenoyptes from the Vanderbilt BioVU biobank of 29,713 individuals of European ancestry (EA)8 revealed a highly significant association of rs7578597 with acute bronchitis and bronchiolitis (OR = 1.24, P = 6.3 × 10−3; Fig 2, G and Table E1).
This study is the first investigation of GLP-1R signaling during viral infection. We show that administration of a GLP-1R agonist attenuates type 2-associated immunopathology during RSV infection. This is also the first report of an FDA-approved pharmacologic agent inhibiting lung IL-33 protein expression, and this finding has significant implications as it may provide an alternative to biologic therapies such as monoclonal antibodies or receptor antagonists that target IL-33-mediated diseases.9 Together, these data highlight a novel potential therapeutic for RSV infection, a disease for which there currently is no treatment after infection has occurred.
Supplementary Material
Acknowledgments
Funding:
R01 AI 124456 – R. S. P.
U19 AI 095227 – R. S. P.
R01 AI 111820 – R. S. P.
2I01BX000624 – R. S. P.
T32 GM07347 – Vanderbilt MSTP
F30 AI118376– M. H. B.
We thank Anne Hotard for isolation of RSV clinical isolate 12/2-6; Janey Wang for managing the rs7578597 PheWAS study; John T. Bates and James E. Crowe Jr. for RSV F protein; Kevin P. Weller, David K. Flaherty, and Brittany Matlock in the VUMC Flow Cytometry Shared Resource for technical assistance; and Andrew N. J. McKenzie for Il33Citrine/Citrine mice. The VUMC Flow Cytometry Shared Resource is supported by the Vanderbilt Ingram Cancer Center (P30 CA68485) and the Vanderbilt Digestive Disease Research Center (DK058404). The PheWAS study was supported by the National Center for Research Resources, Grant UL1 RR024975-01, and is now at the National Center for Advancing Translational Sciences, Grant 2 UL1 TR000445-06. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations used
- ILC2
group 2 innate lymphoid cells
- RSV
respiratory syncytial virus
- Th2
T helper 2
- GLP-1
glucagon-like peptide-1
- WT
wild type
- MFI
mean fluorescence intensity
- PFU
plaque forming unit
- PheWAS
phenome-wide association study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17(6):819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB LEADER Steering Committee, LEADER Trial Investigators. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahern T, Tobin A-M, Corrigan M, Hogan A, Sweeney C, Kirby B, O’Shea D. Glucagon-like peptide-1 analogue therapy for psoriasis patients with obesity and type 2 diabetes: a prospective cohort study. J Eur Acad Dermatol Venereol. 2013;27(11):1440–1443. doi: 10.1111/j.1468-3083.2012.04609.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhu T, Wu X-L, Zhang W, Xiao M. Glucagon Like Peptide-1 (GLP-1) Modulates OVA-Induced Airway Inflammation and Mucus Secretion Involving a Protein Kinase A (PKA)-Dependent Nuclear Factor-κB (NF-κB) Signaling Pathway in Mice. Int J Mol Sci. 2015;16(9):20195–20211. doi: 10.3390/ijms160920195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol. 2007;20(1):108–119. doi: 10.1038/modpathol.3800725. [DOI] [PubMed] [Google Scholar]
- 6.DeVincenzo JP, El Saleeby CM, Bush AJ. Respiratory syncytial virus load predicts disease severity in previously healthy infants. J Infect Dis. 2005;191(11):1861–1868. doi: 10.1086/430008. [DOI] [PubMed] [Google Scholar]
- 7.Simonis-Bik AM, Nijpels G, van Haeften TW, Houwing-Duistermaat JJ, Boomsma DI, Reiling E, van Hove EC, Diamant M, Kramer MHH, Heine RJ, Maassen JA, Slagboom PE, Willemsen G, Dekker JM, Eekhoff EM, de Geus EJ, ‘t Hart LM. Gene Variants in the Novel Type 2 Diabetes Loci CDC123/CAMK1D, THADA, ADAMTS9, BCL11A, and MTNR1B Affect Different Aspects of Pancreatic -Cell Function. Diabetes. 2010;59(1):293–301. doi: 10.2337/db09-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denny JC, Bastarache L, Ritchie MD, Carroll RJ, Zink R, Mosley JD, Field JR, Pulley JM, Ramirez AH, Bowton E, Basford MA, Carrell DS, Peissig PL, Kho AN, Pacheco JA, Rasmussen LV, Crosslin DR, Crane PK, Pathak J, Bielinski SJ, Pendergrass SA, Xu H, Hindorff LA, Li R, Manolio TA, Chute CG, Chisholm RL, Larson EB, Jarvik GP, Brilliant MH, McCarty CA, Kullo IJ, Haines JL, Crawford DC, Masys DR, Roden DM. Systematic comparison of phenome-wide association study of electronic medical record data and genome-wide association study data. Nat Biotechnol. 2013;31(12):1102–1111. doi: 10.1038/nbt.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borish L. The immunology of asthma: Asthma phenotypes and their implications for personalized treatment. Ann Allergy Asthma Immunol. 2016;117(2):108–114. doi: 10.1016/j.anai.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.