To the editor
There is a paucity of inflammatory biomarkers for asthma exacerbation and current options require patient effort or discomfort and have not significantly impacted management1. Biologic production of carbon monoxide (CO) as a result of hemeoxygenase-1 (HO-1) upregulation in response to oxidative stress2 has consistently been associated with asthma activity3. Elevated levels of exhaled breath condensate CO and arterial carboxyhemoglobin are associated with loss of control and decrease with appropriate steroid therapy4–6.
In this context, we tested whether pulse CO-Oximetry (SpCO) is associated with asthma exacerbation in a prospective observational cohort7. Children aged 6–17 years with a history of physician-diagnosed asthma were enrolled during an emergency ward visit for acute asthma exacerbation and returned for a follow-up research visit six weeks later for a convalescent visit, when they had returned to symptomatic baseline (supplementary Figure E1). A comparison cohort of similar subjects with asthma, but not identified by exacerbation, was derived from the baseline assessment of the School Inner City Asthma intervention study (SICAS-2; ClinicalTrials.gov:NCT02291302). The study was approved by the Boston Children’s Hospital Institutional Review Board.
SpCO was measured by the Rad-57 device (Masimo corporation, Irvine, CA) at exacerbation and convalescent visits. Paired t-tests evaluated the mean difference between exacerbation and convalescence values for SpCO measurements in the primary analysis and by t-test for comparison to ambulatory visit measures (SICAS-2). Complete study methods are provided in this article’s Online Repository.
Fifty-nine subjects were included in the paired analysis. Subjects had a mean age of 11 years and were predominately black (37%) and Hispanic (39%). Fifteen percent of subjects were white. Eight subjects (14%) had frequent environmental tobacco smoke exposure (Table E1 in the Online Repository). Compared to the primary cohort, the SICAS-2 comparison group was younger (11.0 vs. 7.7 years old), was less aeroallergen sensitized (91% vs. 60% with any aeroallergen sensitization), and had lower FEV1 (109.6 vs. 98.6% predicted), but not level of obstruction based on FEV1/FVC ratio.
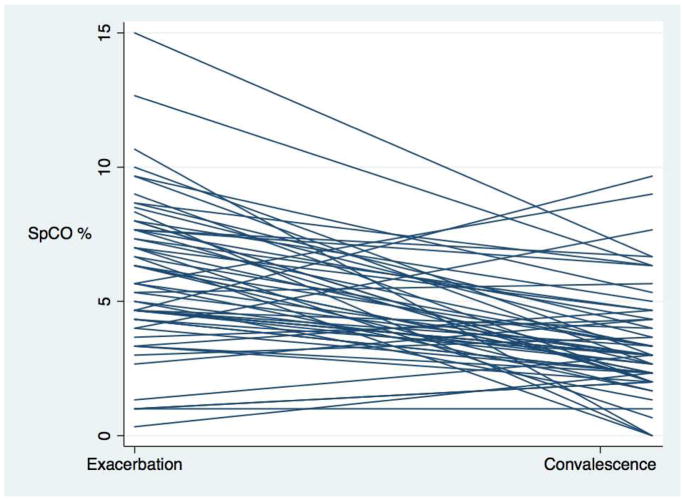
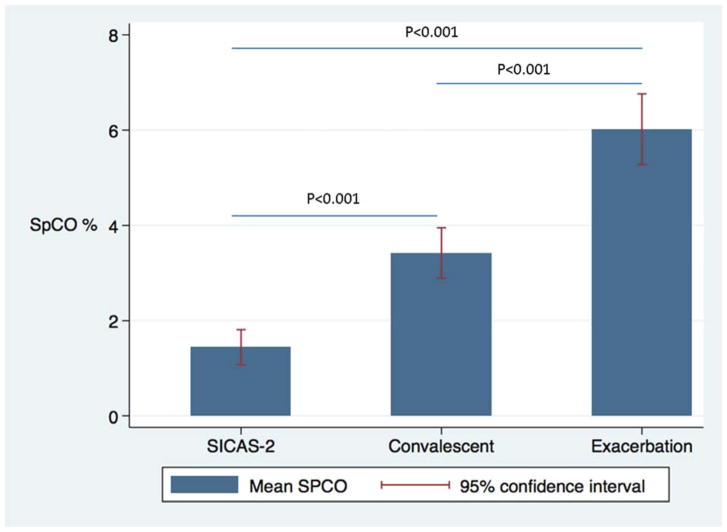
There was a significant difference between exacerbation and convalescent mean SpCO (6.02% ± 2.8% vs. 3.42% ± 2.0%, p <0.0001). Moreover, evaluation of each subject’s mean SpCO regression (Figure 1) demonstrated that nearly all subjects had a negative slope (lowering of SpCO) between exacerbation and convalescence. The ROC curve found SpCO determined exacerbation status with very good predictive value (area under the curve (AUC): 0.79, Figure E2 in the Online Repository). A level of ≥4% correctly classified exacerbation 74.6% of the time with sensitivity of 81.4% and specificity of 67.8% (Table E2 in the Online Repository). There were significant differences in SpCO between the mean exacerbation level, convalescent level, and interestingly, between the convalescent levels of children with prior exacerbation and the ambulatory SICAS-2 population (SICAS-2 SpCO mean=1.44, sd=1.96, p<0.001 for bivariate comparison to exacerbation and convalescent level; Figure 2).
Figure 1.
Spaghetti plot of SpCO by subject
Figure 2.
Cross-sectional comparison of exacerbation and convalescent levels of SpCO compared to the SICAS-2 pediatric asthma cohort
Our study demonstrates that SpCO reliably differentiates asthma exacerbation versus convalescent state following recovery in children who present to the emergency ward and further discriminates convalescent states from well-controlled asthma without recent exacerbation. The prospective methodology of this study design with repeated measures suggests that transcutaneous carboxyhemoglobin is a potentially important biomarker of asthma exacerbations and may fill a void in personalized monitoring of asthma.
The biologic plausibility of carboxyhemoglobin as a biomarker for asthma control has been well established2, 3, 6. Transcutaneous CO-Oximetry has previously been association with increased Asthma Control Test (ACT) scores in a cross-sectional clinic cohort8. However, Naples, et al9 found that SpCO was not diagnostic for asthma in adults. Our results and those of Naples would suggest that the clinical utility of SpCO is as a measure of asthma activity during exacerbations but not as a diagnostic test for asthma. Potential clinical use will be limited to monitoring of patients at risk for exacerbation, but not in the diagnosis of asthma.
It is important to recognize that our study design identified subjects at the extremes of asthma control – emergency exacerbation and convalescence following intensive treatment. While we identified a clear distinction in the SpCO levels between these states, the design does not lend itself to determining if the SpCO measurement is a predictive marker in advance of exacerbations or if it can be generalized to ambulatory patients with asthma. Additionally, fundamental differences between the SICAS-2 comparison group and the primary cohort of children presenting with exacerbation may also contribute to differences in SpCO.
Transcutaneous CO-Oximetry is a reliable biomarker of asthma exacerbation in children with asthma. The non-invasive, effort-independent, simple-to-perform measurement makes its application to clinical practice appealing. Further investigation to determine its clinical utility in predicting loss of asthma control above currently available measures is needed.
Supplementary Material
Acknowledgments
Funding sources: This study was supported by NIH grants K23AI106945 (PI Gaffin), NIH T32 HD040128 (DBK); NIH K12 HD047349 (DBK); NIH K23 HL138162 (DBK); The American Medical Association Seed Grant (DBK); R01 AI 073964, R01AI 073964-02S1, K24 AI 106822, U01 AI 110397, U10HL098102 (PI: Phipatanakul), This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
We are thankful to the study staff, participants and families who contributed to this research. Thanks to Alicia Casey, MD, for intellectual contribution to the study.
Abbreviations
- SpCO
Peripheral blood carbon monoxide percent saturation of hemoglobin, measured by pulse CO-oximeter
- CO
Carbon monoxide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Szefler SJ, Mitchell H, Sorkness CA, Gergen PJ, O’Connor GT, Morgan WJ, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008;372:1065–72. doi: 10.1016/S0140-6736(08)61448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morse D, Choi AM. Heme oxygenase-1: from bench to bedside. Am J Respir Crit Care Med. 2005;172:660–70. doi: 10.1164/rccm.200404-465SO. [DOI] [PubMed] [Google Scholar]
- 3.Brussino L, Badiu I, Sciascia S, Bugiani M, Heffler E, Guida G, et al. Oxidative stress and airway inflammation after allergen challenge evaluated by exhaled breath condensate analysis. Clin Exp Allergy. 2010;40:1642–7. doi: 10.1111/j.1365-2222.2010.03604.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Yao X, Yu R, Bai J, Sun Y, Huang M, et al. Exhaled carbon monoxide in asthmatics: a meta-analysis. Respir Res. 2010;11:50. doi: 10.1186/1465-9921-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaoqing Y, Ruxin Z, Yingjian C, Jianqiu C, Yanshen W, Genhong L. A meta-analysis of the association of exhaled carbon monoxide on asthma and allergic rhinitis. Clin Rev Allergy Immunol. 2011;41:67–75. doi: 10.1007/s12016-009-8195-1. [DOI] [PubMed] [Google Scholar]
- 6.Yasuda H, Yamaya M, Yanai M, Ohrui T, Sasaki H. Increased blood carboxyhaemoglobin concentrations in inflammatory pulmonary diseases. Thorax. 2002;57:779–83. doi: 10.1136/thorax.57.9.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kantor DB, Stenquist N, McDonald MC, Schultz BJ, Hauptman M, Smallwood CD, et al. Rhinovirus and serum IgE are associated with acute asthma exacerbation severity in children. J Allergy Clin Immunol. 2016;138:1467–71e9. doi: 10.1016/j.jaci.2016.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurlandsky LE, Anbar RD, Soultan ZN. Elevated Carboxyhemoglobin in Active Asthma and Allergic Rhinitis as Measured by Pulse CO-Oximetry. Pediatric Allergy, Immunology & Pulmonology. 2013;26:35. doi: 10.1089/ped.2012.0201. [DOI] [PubMed] [Google Scholar]
- 9.Naples R, Laskowski D, McCarthy K, Mattox E, Comhair SA, Erzurum SC. Carboxyhemoglobin and methemoglobin in asthma. Lung. 2015;193:183–7. doi: 10.1007/s00408-015-9686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.