Abstract
Choline acetyltransferase (ChAT) expressing retinal amacrine cells are present across vertebrates. These interneurons play important roles in the development of retinal projections to the brain and in motion detection, specifically in generating direction-selective responses to moving stimuli. ChAT amacrine cells typically comprise two spatially segregated populations that form circuits in the ‘ON’ or ‘OFF’ synaptic layers of the inner retina. This stereotypic arrangement is also found across the adult human retina, with the notable exception that ChAT expression is evident in the ON but not OFF layer of the fovea, a region specialized for high-acuity vision. We thus investigated whether the human fovea exhibits a developmental path for ON and OFF ChAT cells that is retinal location-specific. Our analysis shows that at each retinal location, human ON and OFF ChAT cells differentiate, form their separate synaptic layers, and establish non-random mosaics at about the same time. However, unlike in the adult fovea, ChAT immunostaining is initially robust in both ON and OFF population, up until at least mid-gestation. ChAT expression in the OFF layer in the fovea is therefore significantly reduced after mid-gestation. OFF ChAT cells in the human fovea and in the retinal periphery thus follow distinct maturational paths.
Keywords: human retinal development, primate foveal development, cholinergic neurons, topographic cell distributions. RRID: AB_2079751, RRID: AB_10000340, RRID: AB_10842165, RRID: AB_528315, RRID: IMSR_JAX: 000664, RRID: SCR_014305, RRID: SCR_007370, RRID: SCR_002285, RRID: SCR_001622, RRID:SCR_002798
INTRODUCTION
The retina is a region of the central nervous system that demonstrates a cellular organizational plan that is shared across vertebrates. All vertebrate retinas possess five major neuronal cell classes, each class comprising several cell types with their cell bodies and synaptic connections arranged in structurally and functionally discrete layers. Retinal cholinergic amacrine cells, in particular, exist in all vertebrates studied thus far; these cells are identified by their expression of choline acetyltransferase (ChAT) and their characteristic ‘mirror’ populations with somata on either side of the inner plexiform layer (IPL) (Brandon, 1991; Cuenca et al., 2003; Deng et al., 2001; Galli-Resta et al., 2000; Guiloff & Kolb, 1992; Hutchins & Hollyfield, 1987; Millar et al., 1985; Moritoh et al., 2013; Park et al., 2017; Reese et al., 2001; Rodieck, 1989; Sandmann et al., 1997; Schmidt et al., 1985; Vaney, 1984; Voigt, 1986; Zhang & Wu, 2001). Across species, ChAT amacrine cells with cell bodies in the ganglion cell layer (GCL) depolarize (i.e., are ON) in response to light, whereas those with cell bodies residing in the inner nuclear layer (INL) hyperpolarize (i.e., are OFF). ON and OFF ChAT amacrine cells each form a non-random mosaic, and their dendrites stratify in separate bands within the IPL.
ChAT amacrine cells play important roles during development of the visual system, generating spontaneous waves of activity that help shape retinal connections with the visual centers in the brain (Ford & Feller, 2012). In mouse and rabbit, these amacrine cells are critical for generating direction-selective light-evoked responses in subsets of adult retinal ganglion cells (Amthor et al., 2002; Mauss et al., 2017; Taylor & Smith, 2012; Yoshida et al., 2001). Release of GABA from the ChAT amacrine cells is necessary for generating direction-selective responses of their postsynaptic ganglion cells (reviewed by Mauss et al., 2017; Vaney et al., 2012). Further, cholinergic transmission from the ChAT cells is important for eliciting excitation in direction-selective ganglion cells in response to low-contrast stimulation or to natural scenes (Sethuramanujam et al., 2016).
Although direction-selective ganglion cells have not been identified in primates, it is clear that ChAT amacrine cells are present in humans and non-human primates (Hutchins & Hollyfield, 1987; Moritoh et al., 2013; Rodieck, 1989; Rodieck & Marshak, 1992). ON and OFF ChAT amacrine cells are found across the adult human retina. However, immunolabeling for ChAT suggests that OFF ChAT cells are absent or much reduced in the fovea, a circumscribed region in temporal retina that comprises specialized circuitry for high-acuity vision. The lack of ChAT-positive OFF cells in the fovea contrasts with the robust populations of this cell type in the human retinal periphery (Rodieck & Marshak, 1992) and in the retinas of many other species (Famiglietti, 1985; Kim et al., 2000; Moritoh et al., 2013; Reese et al., 2001; Schmidt et al., 1985; Whitney et al., 2008; Zhang & Wu, 2001). This raises the question of whether or not the difference in ON and OFF ChAT expression within and outside the fovea arises over time, or whether it is established from the onset of cell differentiation. The variability in the organization of ChAT amacrine cells across the human retina also presents an opportunity to compare the developmental scheme that establishes the arrangement of these amacrine cells both within the retina of an individual species, as well as across vertebrates.
Development of the human fovea is a protracted process, beginning the earliest in fetal life and continuing after birth (Hendrickson et al., 2012; Provis et al., 2013). An IPL is observed in the fovea by fetal week 8 (Fwk 8), and subsequently extends across the retina by Fwk 18 (Hendrickson, 2016). Differentiated amacrine cells with processes in the IPL are present in the fovea by Fwk 15 (van Driel et al., 1990). Expression of ionotropic glutamate receptors is observed in the IPL of the fovea as early as Fwk 13, suggesting the presence of synapses in this neuropil by this stage (Hendrickson and Zhang, 2017). These observations together indicate that amacrine cells are largely generated and undergo maturation during early- to mid-gestation in the human fovea. Thus, we focused on the development of the ChAT amacrine cells between Fwk 9 and 20. We then contrasted the development of the spatial distributions and morphologies of ON and OFF ChAT cells in the fovea with those in the human peripheral retina, and with ChAT cells of the retina of other species. We also examined the expression of transcription factors and a calcium-binding protein, calbindin, previously observed in ChAT cells in other species, and compared our findings in the human retina with that of other species. Together, our observations indicated that ChAT amacrine cells in the human retina share similarities in their developmental plan but also exhibit differences that appear to be specific to the fovea.
MATERIALS AND METHODS
Model and subject details
Human retina from early- to mid-gestation were studied. Age groups included Fwk 9–10 (Fetal day (Fd) 67–72; n=7 retinas), Fwk 11 (Fd 76–80; n=4), Fwk 13 (Fd 91; n=2), Fwk 14 (Fd 96–101; n=4), Fwk 17 (Fd 116–122; n=4), Fwk 18 (Fd 127; n=2), Fwk 19 (Fd 132; n=2), Fwk 20 (Fd 140; n=2). Non-identified male and female tissue samples were provided by the Birth Defects Research Lab at the University of Washington.
Female and male C57BL/6J mice were used in this study (Jackson Labs, RRID: IMSR_JAX: 000664). All procedures were conducted with the approval of the University of Washington Institutional Animal Care and Use Committee.
Antibody characterization
The goat polyclonal antibody to choline acetyltransferase recognizes a 68-70kDa band in Western blot on mouse brain lysates (manufacturer’s data sheet). This antibody has been applied to label cholinergic amacrine cells in the retina across species (Cuenca et al., 2003; Kim et al., 2000; Galli-Resta et al., 2000; Park et al., 2017; Reese et al., 2001; Whitney et al., 2008).
The rabbit polyclonal antibody to calbindin D-28K recognizes a 27–28 kDa band in Western blot on brain homogenate of various species including zebrafish, chicken mouse, rat, guinea pig, rabbit and macaca fascicularis (manufacturer’s data sheet). This antibody labels horizontal cells across species and other retinal cell types (photoreceptors, amacrine cells and bipolar cells with a species-specific pattern (Deng et al., 2001; Haverkamp & Wässle, 2000; Haverkamp et al., 2003; Nag & Wadhwa, 1999; Yan, 1997).
The mouse monoclonal antibody to L/M opsin (A12) was custom-made by H. Jing at the University of Washington. This antibody recognizes L/M opsin expressing cones, but not S cones in the fetal human retina. The labeling specificity was confirmed in fetal human retinal frozen sections and wholemount preparations, by using S opsin antibody and a rabbit polyclonal L/M opsin antibody reported previously (Xian & Hendrickson, 2000).
The specificity of the mouse monoclonal antibody to Sox2 (E-4) has been shown in embryonic human cerebral cortex with immunohistochemistry (Vinci et al., 2016).
The specificity of the mouse monoclonal antibody to Islet-1 (2D6) has been tested in mouse cerebral cortex (Ivaniutsin et al., 2009). We have compared the staining pattern of this antibody to an Islet-1/2 mouse antibody (DSHB, Cat# 39.4D5) that has been used in rat and mouse retina to label ChAT cells (Elshatory et al., 2007; Galli-Resta et al., 1997). Both antibodies showed similar labeling of ChAT cells in human retina.
Immunohistochemistry
Whole eyes and wholemount retinas were fixed with 4% paraformaldehyde in 0.1 M phosphate buffered solution (PBS) for 30 minutes to 1 hour at room temperature. The eyes were cryoprotected, and embedded in OCT compound for serial sections at 14–20 μm thickness. The frozen sections or wholemount retinas were rinsed in PBS, then incubated in blocking solution (5% normal donkey serum and 0.5% Triton X-100 in PBS) for 1 hour at room temperature. Primary antibody incubation was performed at 4°C, overnight for frozen sections and 4-5 days for wholemount retinas. Retinal samples were then rinsed in PBS and incubated with secondary antibodies overnight at 4°C. Secondary antibodies used were anti-isotypic Alexa Fluor conjugates (1:1000, Invitrogen or Jackson ImmunoResearch). Subsequently, all the samples were rinsed in PBS and mounted in Vectashield (Vector lab) and coverslipped.
Biolistic transfection
Gold particles (1.6 μm diameter; 12.5 mg; Bio-Rad) were coated with DNA plasmids encoding tdTomato (24 μg DNA) under the control of the cytomegalovirus (CMV) promoter. The particles were propelled into wholemount retinas with the ganglion cell layer side up, using a Helios gene gun (60-80 psi; Bio-Rad) (Morgan et al., 2008). Transfected retinas were maintained for 1-2 days in a humid, oxygenated chamber at 33°C, in artificial cerebrospinal fluid (ACSF) containing the following in (mM): 119 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgCl2, 1 NaH2PO4, 11 glucose, and 20 HEPES, brought to pH 7.42 with NaOH. The morphology of ChAT cells from six retinas were analyzed using the biolistic labeling, including one Fwk14 retina (1 cell), two Fwk16 retinas (2 cells), two Fwk17 retinas (9 cells) and one Fwk19 retina (1 cell).
Image acquisition and processing
Images were acquired on a Leica TCS LSP8 confocal microscope, using a 0.75 numerical aperture (NA) 20× oil, or a 1.4 NA 63× oil objective lens. For measurement of cell density, ChAT cells were imaged within 78-180 regions of interest within a single retina (775 × 775 μm2 each), located at 0 to 12 mm from the foveal center in retinal wholemounts. Each image was acquired at a voxel size of 1.5×1.5×1 μm3. Images for all other analyses were acquired at a x-y resolution of 0.2–0.8 μm/pixel and z step of 0.4–1 μm.
Contrast, brightness and hue were adjusted using Amira (FEI, RRID: SCR_014305) or Imaris (Bitplane, RRID: SCR_007370). Maximum intensity projections and three-dimensional visualization of images were also performed using these software.
Morphological and cell distribution analysis
For the majority of eccentricity measurements on an individual retina, distances from the foveal center were measured directly on the wholemount. For some regions (~10%) where a relieving cut was made, measurements of distances to the foveal center were estimated after performing an algorithm to stitch the cut edges together and transforming the flatmount into the shape of an eyecup (Sterratt et al., 2013).
Measurements of cell somal sizes were performed on regions of interest selected at 3 different locations per retina: fovea (0–0.75 mm from the foveal center), peri-fovea (1.5–2.5mm) and nasal periphery (7–8mm). The maximum distances (diameters) across the cell bodies of about 200 ON and 200 OFF ChAT cells within each region were measured using Fiji (NIH, RRID: SCR_002285).
For cell density measurements, the numbers of ON and OFF ChAT cells within each region of interest were counted using PointPicker in Fiji (NIH). To map the cell density distribution, we created a two-dimensional grid (typically 25 × 25 tiles) that overlaid the entire sampled region of the retina. Cell density at the center of each grid tile was calculated upon triangulation of the nearest three measured density values, and performing a weighted average based on the distance of the sampled points. A heat map representing the cell density values was then generated using MATLAB (Mathworks, RRID: SCR_001622).
For each cell distribution, we measured the nearest neighbor distances (NNDs) and Voronoi domains within sampled areas using R 3.2.4 (R Foundation for Statistical Computing). Border effects were removed from the measurement by eliminating cells with Voronoi domains contacting the edges of the sampled area. Regularity indices of ON and OFF ChAT cell distributions for each area were calculated by dividing the mean of the NNDs by the standard deviation of NNDs as described previously (Wässle & Riemann, 1978). For each foveal region, NNDs were calculated from 1000 simulations of random cell distributions, based on cell densities within these regions and an exclusion zone defined by the somal size (Baddeley et al., 2015).
Quantification and statistical analysis
Statistical analyses were performed using Prism (GraphPad, RRID:SCR_002798). Distributions of each parameter were tested for normality using the D’Agostino and Pearson omnibus test and appropriate parametric or non-parametric statistical analyses were applied. Within the same retina, Kruskal-Wallis test was performed to compare somal diameters of ON or OFF ChAT cells across eccentricities and Dunn’s multiple comparison test was performed to compare somal diameters of ON and OFF at the same eccentricity. Two-way ANOVA with Sidak’s post-hoc test was performed to compare somal diameters of foveal ON or OFF ChAT cells across ages and ON vs. OFF at the same age. Statistical parameters including the value of n, mean ± S.E.M. and statistical significance are provided in the figure legends.
RESULTS
Emergence of ChAT expression in the human retina during fetal development
To determine when human ON and OFF ChAT cells are generated and in position during human retinal development, fetal retinas from early- to mid-gestational stages were immunostained for ChAT. Because of the changing expression of markers with age, several methods were used to identify the putative foveal region across ages. In Fwk 9–10 retina, the putative foveal center has a single-layer of cell nuclei in the outer nuclear layer (ONL) (Hendrickson, 2016) that is separated from the inner nuclear layer by a thin outer plexiform layer (OPL) (Fig. 1a, 1c, left panel). In contrast, the peri-foveal region (1.5–2.5 mm from the foveal center) only comprises a thick outer neuroblastic layer without an apparent OPL (Fig. 1b, 1d, left panel). By Fwk 11–12, a single layer of photoreceptors is also present in the foveal and peri-foveal regions, necessitating a different approach to identify the foveal center at these ages. At Fwk 11–12, calbindin-immunoreactive cells, with the morphology of developing horizontal cells (Nag & Wadhwa, 1999; Yan, 1997) that bear lateral processes, form a single layer within the putative foveal center (Fig. 1e); these cells have a very immature morphology outside the putative fovea (Fig. 1f, see also Fig. 2a). By Fwk 13, calbindin-positive horizontal cells are found both in the foveal and peri-foveal regions; thus these regions could not be readily distinguished based on horizontal cell distribution and morphology. Instead, Müller cells in the putative foveal center begin expressing calbindin at Fwk 13 (Fig. 2b, 2c) and expression of calbindin by Müller cells spreads more peripherally as the retina ages. Finally, at and after Fwk 15, cones in the putative foveal region begin expressing long and medium wavelength sensitive (L/M) Opsin (O’Brien et al., 2003; Xiao & Hendrickson, 2000), with the highest expression level in the foveal center (Fig. 2c).
Figure 1. ChAT expression patterns in the fetal human retina.
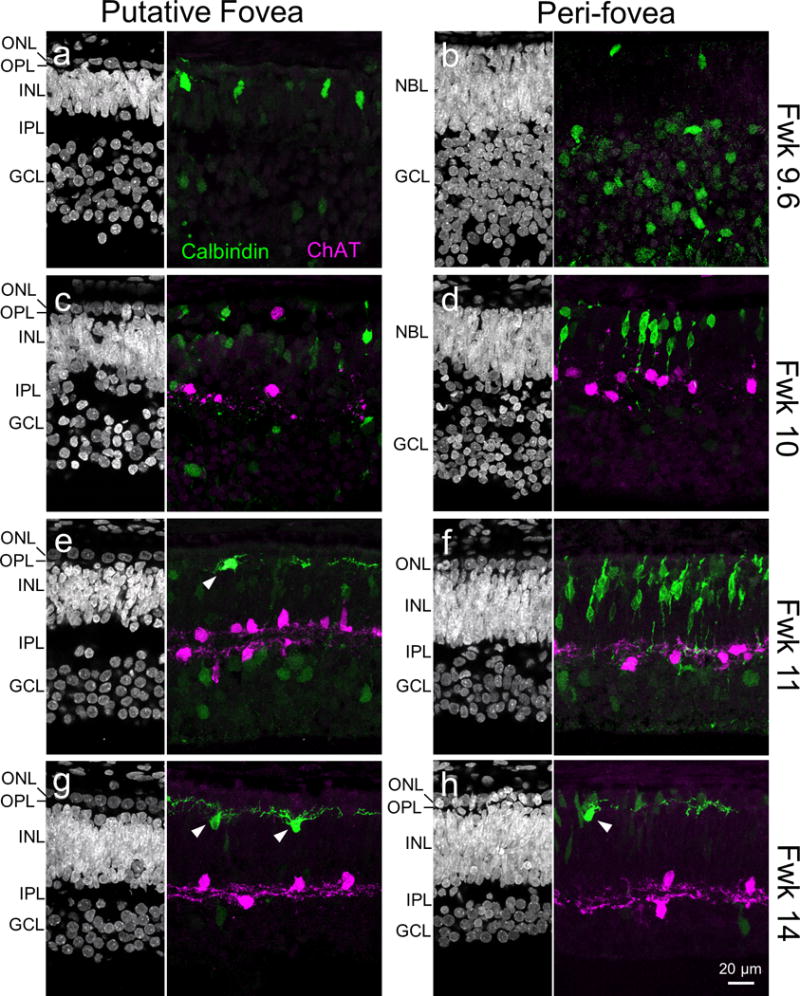
Representative images of the putative fovea and peri-foveal regions (1.5–2.5 mm from foveal center) of the human retina at different fetal weeks (Fwk). Cell nuclei in various retinal cell layers are stained by Hoechst (left, greyscale images). Calbindin (green) immunostaining patterns are used to locate the putative foveal center (see text, and also Fig 2). Arrowheads in (e, g and h) indicate horizontal cells in their final location, next to the OPL. Choline acetyltransferase (ChAT; magenta) immunostaining suggests that ChAT expression commences between Fd 67 and 72. GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer; OPL, outer plexiform layer; NBL, neuroblastic layer.
Figure 2. Identification of the putative fovea in midgestation human retina.
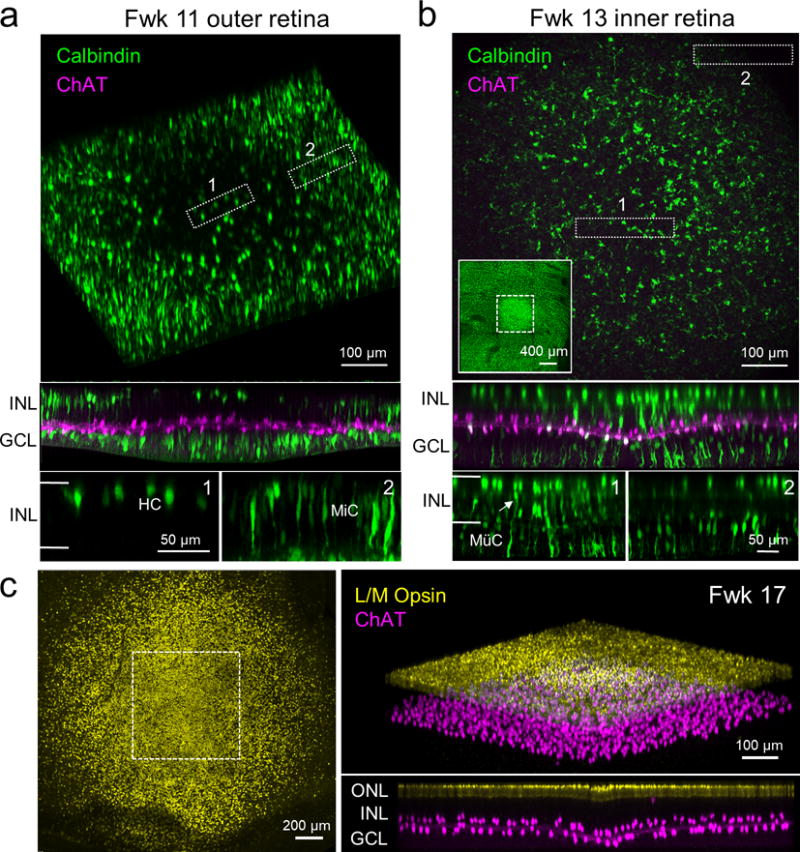
(a) Fwk 11 human retina stained for ChAT (magenta) and calbindin (green), showing that calbindin positive horizontal cells (HC) are detectable only in the putative foveal center. The top panel is a 3D reconstruction of the outer part of the inner nuclear layer (INL). The middle panel is a side view of this volume, and the bottom panels are side views of regions indicated by boxes 1 (fovea) and 2 (peri-fovea). The borders of the INL are indicated by the horizontal white lines in the higher magnified side views of the boxed regions. MiC, migrating cell. (b) Fwk 13 human retina stained for ChAT and calbindin that shows calbindin-positive Müller cell (MüC) endfeet only in the foveal center. The top panel is an en face view of the maximum intensity projection of the inner part of the ganglion cell layer (GCL) within a central region of intense labeling (within dotted square, see inset). Box 1 is within the foveal peak and box 2 is within the peri-fovea, where Müller cell endfeet do not express calbindin. The middle panel is a side view of the imaged region. The bottom panels are side views of the regions within boxes 1 and 2. The white arrow indicates a Müller cell body. (c) (Left) Fwk 17 retina stained for long and medium wavelength sensitive (L/M) Opsin (yellow) showing a zone of intense L/M Opsin labeling, the presumed fovea. Note that L/M Opsin staining drops off precipitously outside this zone. Right top panel is a 3D reconstruction of the L/M Opsin-positive and ChAT-positive layers in the region indicated by the dotted square shown in the left panel. Right bottom panel is the orthogonal rotation of this volume. ONL, outer nuclear layer.
ChAT expression was first detected at around Fwk 10, and localized to the putative fovea and adjacent peri-foveal regions (Fig 1a–d), covering about 20% of the retinal area. Most ChAT-positive cells resided in the inner retina and possessed sparse neurites (Fig 1c, 1d). By Fwk 11, ChAT expression was observed across the retina covering >90% of the retinal area. At this stage, foveal ChAT cell somata were localized to two separate layers, one in the INL and the other in the GCL (Fig 1e), suggesting the emergence of ON and OFF ChAT cells. By comparison, ON and OFF ChAT cell populations could not be readily distinguished in peri-foveal retina until Fwk 13–14 (Fig 1f, 1h). These observations suggest a centroperipheral sequence of ChAT cell emergence, whereby ChAT cells are found at the putative fovea around Fwk 10 and throughout most of the retina only another week later.
Similarities and differences in the maturational profiles of ON and OFF ChAT cells
ON and OFF stratification of ChAT cell neurites was first evident in the fovea around Fwk 11 (Fig. 1e), and was established across the retina by Fwk 17 (Fig. 3a). This centroperipheral gradient in the maturation of ON and OFF ChAT cells was also apparent when we labeled individual ChAT cells by biolistic transfection (Fig. 3b). At Fwk 17, ChAT cells closer to the fovea had a relatively more mature morphology in that they demonstrated a characteristic symmetric dendritic field (Fig. 3b). In contrast, ChAT cells that were located more peripherally had yet to elaborate an extensive arbor (Fig. 3b). Although ON and OFF ChAT cells stratified at a similar pace at any one region of the retina and both followed a centroperipheral developmental sequence, their somata exhibited distinct morphological maturation patterns. The mean somal size of ON ChAT cells was always larger than OFF ChAT cells at the same eccentricity across ages, except in the periphery at an early developmental stage (Fwk 14), or at a much later developmental stage (Fwk 20) in the putative fovea (Fig. 3c). These observations suggest differential growth rates for ON and OFF ChAT cells. Taken together, human ON and OFF ChAT cells follow a centroperipheral gradient in morphological development.
Figure 3. Morphological profiles of ON and OFF ChAT cells across different eccentricities and ages.
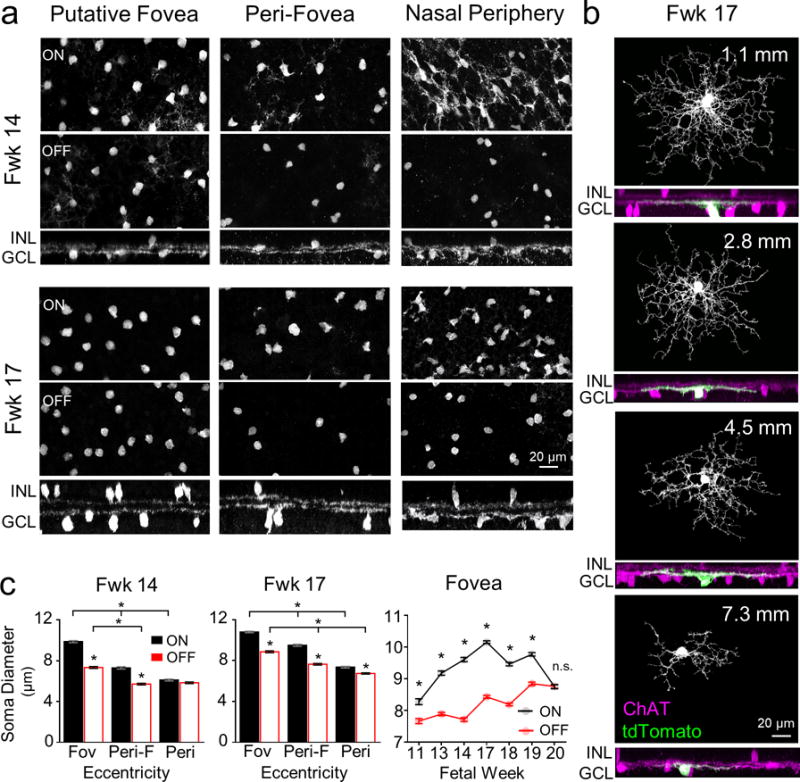
(a) Representative images of human fetal week (Fwk) 14 and 17 ON and OFF ChAT cells. En face (top, middle panels) and side views (bottom panels) at three different eccentricities (putative fovea, 0–0.5 mm; peri-fovea, 1.5–2.5 mm; nasal periphery, 7–8 mm) are shown. (b) Morphology of individual ON ChAT cells labeled by biolistic transfection (tdTomato fluorescent protein) at Fwk 17, across eccentricities. For each cell, en face views (top) display the dendritic morphology and side views (bottom) display the colocalization of the labeled cell’s dendrites (green) with the ON ChAT band (magenta). (c) Comparisons of the mean somal diameters of ON and OFF ChAT cells across eccentricities for Fwk 14 and 17 retinas (n=200 cells, 1 retina each) for each dataset; Kruskal-Wallis test with Dunn’s multiple comparison test. * p<0.0001). Mean somal diameters of foveal ON and OFF ChAT cells across ages are plotted in the far right panel (n≥300 cells for each age, n = 2-3 retinas per age; two-way ANOVA with Sidak post-hoc test, * p<0.0001 for ON versus OFF at each age; n.s. = not significant). Data are plotted as mean ± S.E.M. INL, inner nuclear layer; GCL, ganglion cell layer.
Topographical distributions of developing human ON and OFF ChAT cells
In the adult human and macaque retina, at any one location, ON ChAT cell density is higher than that of the OFF ChAT cells (Rodieck & Marshak, 1992). Both ON and OFF ChAT cells exhibit a centroperipheral density gradient, except that compared to the retinal periphery, there are fewer ChAT-expressing cells and/or a lower expression of ChAT in the INL of the fovea (Rodieck & Marshak, 1992; Yamada et al., 2003). During early development, however, both human ON and OFF ChAT cells appear numerous in the foveal and peripheral retina (Figs. 1, 3). Thus, we quantified and mapped the topographic distributions of ON and OFF ChAT cells beginning from Fwk 14 when ON and OFF populations can be readily separated across the retina. We excluded ChAT cells that appeared to be migrating, that is cells with somata in the IPL (Fig. 4a). For ON, and to a lesser degree OFF ChAT cells, a high cell density ‘wavefront’ spreads across the retina from the putative foveal region between Fwk 14 and 20 (Fig. 4b, 4c). At all stages examined, OFF ChAT cell density outside the fovea was lower than that of the ON ChAT cells (Fig. 4c), but both populations had higher densities compared to their respective adult levels (Rodieck & Marshak, 1992). This is not surprising because of further expansion of the retinal area with maturation. In the fetal fovea, however, the densities of ON and OFF ChAT cells were approximately equal (Fig. 4d), unlike at adulthood (Rodieck & Marshak, 1992). Thus, OFF ChAT cells transiently form a significant cell population in the fovea during fetal development. In contrast, ON ChAT cells in the fovea have already reached their adult density level (Rodieck & Marshak, 1992) at or before Fwk 20. Therefore, ON and OFF ChAT cell densities are likely regulated by independent mechanisms at the same retinal location. However, ON and OFF ChAT cells may share a common mechanism in regulating their mosaic organization because both populations show a regularity index above that expected for a random distribution, once these populations can be distinguished (Fig. 4e and Fig. 5).
Figure 4. Topography and regularity of ON and OFF ChAT cell distribution across ages.

(a) Pseudo-colored ON (cyan), OFF (magenta) and presumed migrating (yellow) ChAT cells at three different eccentricities in a Fwk 17 retina. The migrating ChAT cells have their somata located in between the two ChAT positive bands in the inner plexiform layer (IPL). GCL, ganglion cell layer; INL, inner nuclear layer. (b) Heat maps show ON and OFF ChAT cell density distribution from Fwk 14 to Fwk 20. The putative fovea (dotted circle) and optic disc (black spot) of each retina are indicated. T, temporal; N, nasal. (c) Scatter plots of the density of ON and OFF ChAT cell densities across the horizontal meridian for three age groups. For each age group, the closed circles represent the younger of the two ages within that group. 0 mm=foveal center. The locations of the optic disc of the retinas from each group are indicated by the grey bars. (d) Ratio of foveal ON and OFF ChAT cell density across ages (n=2 retinas for each age). Black and grey filled circles show the ratios of two separate retinas. Grey dotted line plots the mean across retinas at each age. (e) Plots of the regular index (RI) of ON and OFF ChAT cells across ages and eccentricities. Regression lines for the ON and OFF RI distributions are provided, and the theoretical random RI distribution (blue line) is indicated for each plot.
Figure 5. Regularity of ON and OFF ChAT cell body mosaics across ages.
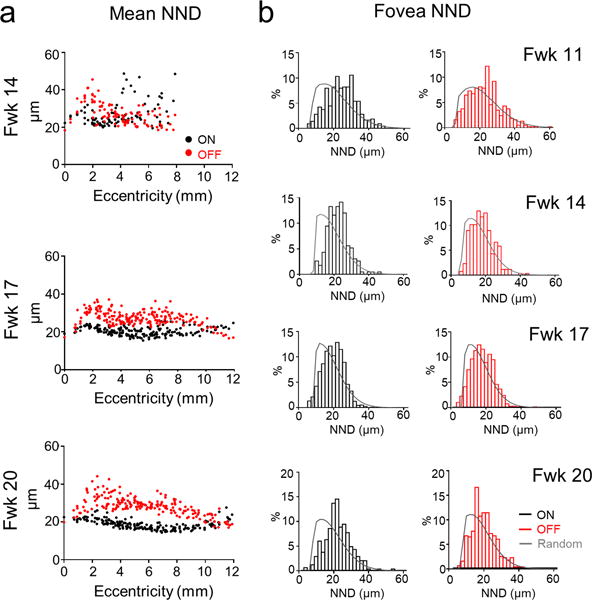
(a) Histograms of the mean nearest neighbor distance (NND) of ON and OFF ChAT cells across ages. (b) The frequency distributions of NNDs of foveal ON and OFF ChAT cells and simulations of random distributions (grey) across ages. Foveal ON and OFF ChAT cells already attain a non-random distribution by Fwk 11.
Molecular expression patterns of developing ON and OFF ChAT cells
In mice, the transcription factor, sex determining region Y box 2 (Sox2) is expressed in both developing and adult ON and OFF ChAT amacrine cells. Sox2 is crucial for the positioning of ON and OFF ChAT cell bodies within their respective nuclear layers (Whitney et al., 2014). The LIM-homeodomain transcription factor, Islet-1, also regulates the development of rodent ChAT amacrine cells, and is an early marker of both ON and OFF ChAT populations (Elshatory et al., 2007; Galli-Resta et al., 1997). We found that Sox2 and Islet-1 are also expressed by ON and OFF ChAT cells in the human retina (Figs. 6a, 6b). These transcription factors may play similar roles in the development of ChAT cells in humans and in rodents.
Figure 6. Molecular expression patterns of ON and OFF ChAT cells.
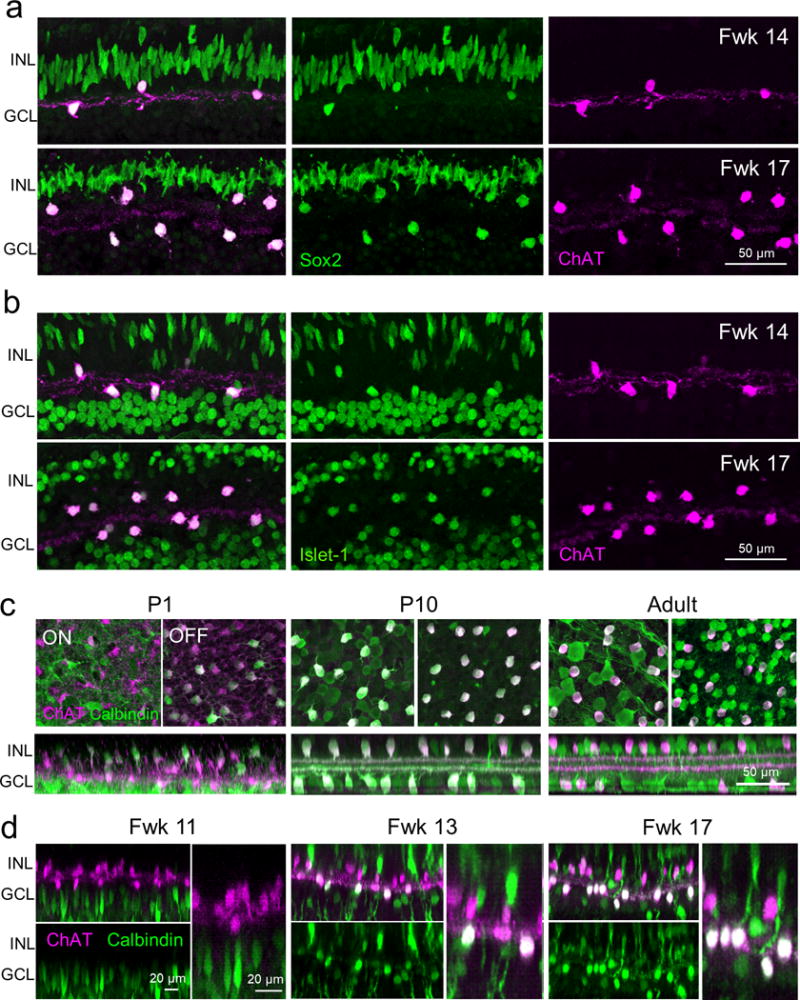
ChAT immunostaining together with immunolabeling for (a) Sox2 and (b) Islet-1 in the peri-fovea of the human retina at fetal weeks (Fwks) 14 and 17. (c) Calbindin and ChAT immunostaining in the postnatal day (P) 1, 9 and adult mouse retina. (d) Calbindin and ChAT immunostaining in the putative human fovea at different fetal ages. For each age, representative ON and OFF ChAT cells are displayed at higher magnification in the right panels. INL, inner nuclear layer; GCL, ganglion cell layer.
However, although mouse ON and OFF ChAT cells do express some proteins in common, human ON and OFF cells can demonstrate differential expression of such proteins. For example, both ON and OFF mouse ChAT cells strongly express calbindin by postnatal day 9 and maintain expression in the adult retina (Fig. 6c). In contrast, we found that whereas human ON ChAT cells strongly expressed calbindin, with expression first appearing in the fovea at Fwk 13, only a few foveal OFF ChAT cells expressed calbindin. Calbindin expression in the few foveal OFF cells appeared at a relatively low level compared to the ON cells (Fig. 6d). Thus, human ON and OFF ChAT cells can develop distinct expressions of some proteins (calbindin), which do not show differential expression in mice.
ChAT cells that deviate from conventional cellular arrangements
Our analysis of the human fetal retina also revealed ChAT cells that do not bear the expected morphological arrangements that are consistent across species. Although the majority of ON and OFF ChAT cells in the human fetal retina had their somata and neurites in their appropriate layers, there were also ChAT cells with arbors stratifying within the IPL at a depth (ON or OFF) that was incompatible with their somal locations (Fig. 7a). We also discovered a population of ChAT cells with somata in the INL but next to the OPL and neurites stratifying within the OPL, as early as Fwk 10 when ChAT cells were first observed in the inner retina (Fig. 7b). These outer ChAT cells were found only in the foveal center after Fwk13, intermingled with calbindin-positive/ChAT-negative horizontal cells (Fig. 7c). Some outer ChAT cells were also calbindin-positive whereas some were not (Fig. 7c) suggesting that the outer ChAT population may comprise cells that are counterparts of conventional ON and OFF ChAT cells. Many of the outer ChAT cells bore radial dendritic morphologies that resembled that of the conventionally placed ChAT cells (Figs. 3b and 7d). Outer ChAT cells were always found within the fetal fovea although their numbers varied from 1 to 12 cells per retina, without any correlation with age. In fact, outer ChAT cells have been found previously in adult human retina (Rodieck & Marshak, 1992), suggesting that this sparse population in the fetal retina is not transient.
Figure 7. ChAT positive cells with unconventional cell arrangements.
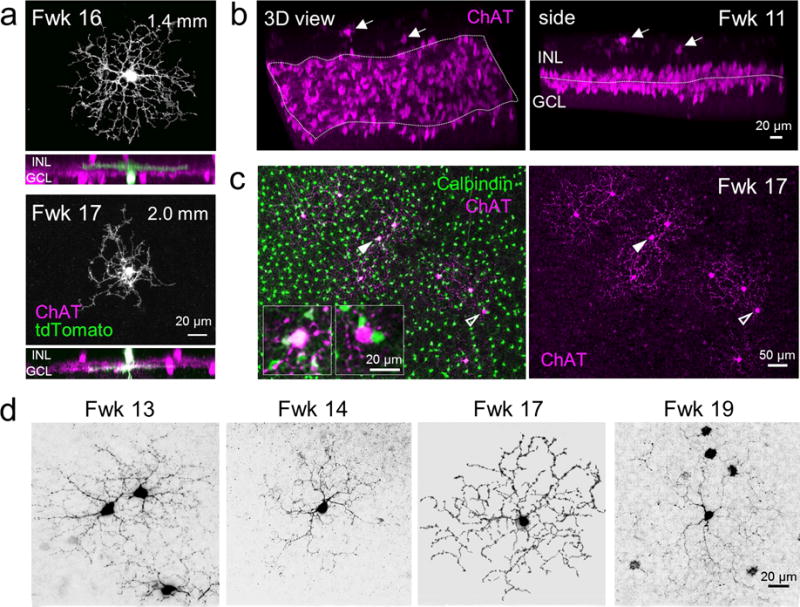
(a) Morphology of ChAT cells labeled by biolistic transfection (tdTomato expression) that have their dendrites stratifying within an inner plexiform layer (IPL) depth that is incompatible with their somal locations. The top cell has its cell body in the GCL but an arbor in the OFF ChAT band, whereas the bottom cell has its soma in the INL and dendrites within the ON ChAT band. GCL, ganglion cell layer; INL, inner nuclear layer. (b) Three-dimensional reconstruction (left) and side view (right) of a Fwk 11 putative fovea stained for ChAT showing conventionally place ON and OFF populations, and some ChAT-positive cells (arrows) with somata in the INL and processes in the OPL (outer plexiform layer). (c) En face view of a Fwk 17 fovea showing cells at the the outer retina stained for ChAT (magenta) and calbindin (green). A ChAT/calbindin double positive cell (solid arrowheads) and a ChAT-positive/calbindin-negative cell (open arrowheads) are indicated. Insets show higher magnification of the indicated cells. (d) Dendritic morphology of outer retinal ChAT cells in the fovea at different ages.
DISCUSSION
Tracking the development of ChAT amacrine cells in the human retina from early- to mid-gestation revealed that human ChAT cells share a basic developmental plan with that of other vertebrates. Like in other species, the maturation of ON and OFF ChAT cells in the human retina follows a centroperipheral gradient. ChAT expression and separation into ON and OFF populations were first apparent in the putative fovea. Moreover, the dendritic morphology of foveal ChAT cells was more developed compared to non-foveal counterparts across the fetal ages examined. Despite the developmental gradient across eccentricity, human ON and OFF ChAT cells at the same eccentricity simultaneously position their somata in the INL and GCL, attain quasi-regular somal distributions and stratify their processes within their respective IPL sublayers. Thus, at any one retinal location, ON and OFF ChAT cells mature at about the same time during development, as found in other species. Our findings support the notion that a common developmental plan is likely ‘conserved’ to replicate the general cellular arrangements of ON and OFF ChAT amacrine cells across vertebrates (Dann, 1989; Famiglietti & Tumosa, 1987; Galli-Resta et al., 1997; Kim et al., 2000; Knabe et al., 2007; Nguyen et al., 2000; Reese et al., 2001; Schmidt et al., 1985; Spira et al., 1987; Vardi et al., 1989; Wong & Collin, 1989).
Although human ON and OFF ChAT cells share similarities in their overall developmental progression, they also showed differences in their developmental paths. First, the somal size of ON and OFF ChAT cells develop at different rates regardless of eccentricity; ON cell bodies increase in size more rapidly than OFF cells within a given region. More intriguingly, within the fovea, ChAT expression in the INL but not the GCL showed a dramatic loss between fetal life and maturity (Rodieck & Marshak, 1992; Yamada et al., 2003). The loss of ChAT staining in the OFF layer in the human fovea may be due to a loss of ChAT expression, migration of ChAT cells away from the fovea, or cell death. Although overall, there is very little cell death observed in the fovea after Fwk 20 (Georges et al., 1999, Provis & van Driel, 1985), loss of OFF ChAT cells may be difficult to detect because of the relatively small numbers. The existence of a large but transient population of OFF ChAT cells during development has not been observed before in other species. Like other species, human OFF and ON ChAT cells remain in the adult peripheral retina. Thus, OFF ChAT cells in the human fovea and retinal periphery appear to follow distinct maturational paths.
It is perhaps not surprising that there are differences in ChAT cell development between foveal and non-foveal regions, given the unique structure (avascular), cell arrangement (cone-rich), circuitry (midget pathway-dominating), and function (color vision and high spatial acuity) of the fovea (Dacey & Packer, 2003; Provis et al., 2013; Sinha et al., 2017). However, the developmental loss of ChAT-labeled cells in the INL of the fovea is not a universal phenomenon of primate fovea. In the adult marmoset fovea, OFF ChAT cell densities are even higher than those of the ON cells (Moritoh et al., 2013). Interestingly, like the human retina, ChAT expression is also very low in the INL of the adult Macaque monkey fovea. This raises the possibility that like humans, there is downregulation of ChAT expression in the INL during maturation of the Macaque fovea. If so, this would suggest that there may be a common set of developmental mechanisms that shape the foveal organization of Macaques and humans in ways that are distinct from Marmosets. Indeed, the sequence of short and long wavelength opsin expression in the fovea during development of the Macaque and human retinas differs from that of Marmosets (Hendrickson et al., 2009, Xiao & Hendrickson, 2000).
Rodent ON and OFF ChAT cells express some transcription factors in common, such as Sox2 and IsIet-1 (Galli-Resta et al., 1997; Whitney et al., 2014). We also observed expression of Sox2 and Islet-1 in fetal human ON and OFF ChAT cells. Thus, ChAT cells share some transcription factors in common between human and rodents. On the other hand, ON and OFF ChAT cells have also been observed to differ in their expression of some molecules. For instance, mouse ON, but not OFF, ChAT cells contain glycine receptor α4 subunits (Heinze et al., 2007); OFF, but not ON, ChAT cells largely express P2X2 purinoceptors (Kaneda et al., 2004); and NMDA receptor-mediated excitation is observed at the proximal dendrites of OFF, but not ON, ChAT cells (Fransen and Borghuis, 2017). Here, we found a difference in the pattern of calbindin expression in ON and OFF ChAT cells in the developing human retina. ON ChAT cells first express calbindin in the fovea. In contrast, very few OFF ChAT cells across the retina express calbindin at levels comparable to the ON cells. This differential expression pattern in ON and OFF ChAT cells contrasts with that found in other species in which ChAT cells express calbindin. Both ON and OFF ChAT cells express calbindin at comparable levels in the salamander, mudpuppy (Deng et al., 2001), mouse (Fig. 6c) and ground squirrel (Cuenca et al., 2003). There is no calbindin expression in either ON or OFF cells in rabbits (Lee & Jeon, 2013). Although the function of calbindin in ON ChAT cells is not yet known, our observations suggest that ON and OFF ChAT cells in the human retina diverge early in the developmental path that creates their subtype specific properties.
ON and OFF subtype specific properties are unlikely to be dictated by developmental mechanisms that localize these subtypes to their respective positions in the inner retina. This is because we encountered a group of ChAT-positive cells in the developing human fovea that has their somata at the same level as horizontal cells and elaborate their processes within the OPL. Some of these ‘outer’ ChAT cells are calbindin-positive (like ON conventionally placed ChAT cells) and others are not, suggesting a heterogeneous composition of the outer ChAT cells. So far, outer ChAT cells have been discovered also in the adult Macaque retina (Rodieck & Marshak, 1992), C57/B6 mice (Kang et al., 2004) and bats (Park et al., 2017), indicating that this population is not unique to humans, but it is uncommon across species. It remains to be determined whether the outer ChAT cells are those ChAT cells that failed to migrate to their respective ON and OFF layers, or whether these cells have a specific function in the adult.
In summary, our study on human retinal development here supports the notion that vertebrate retinas share a basic developmental scheme. However, our findings also indicate that there are fine-scale differences in developmental schemes that might reflect specific demands on each species, or each region of the retina, to uniquely tailor its detailed structural motifs to best suit the required functions.
Table 1.
Primary antibodies used
| Antibody | Immunogen | Species/Type | Dilution | Source, RRID |
|---|---|---|---|---|
| Anti-Choline Acetyltransferase | Human placental enzyme | goat, polyclonal | 1:500 | Millipore, Billerica, MA, Cat# AB144P, RRID: AB_2079751 |
| Anti-Calbindin D-28k | Rat calbindin D-28k | rabbit, polyclonal | 1:1,000 | Swant, Marly, Switzerland, Cat# CB 38, RRID: AB_10000340 |
| Anti-red/green (L/M) Opsin A12 | Synthesized peptide (CAQQWSLQRLAGRHPQDSYEDS) mapping to human green/red cone opsin | mouse, monoclonal | 1:10 | Jing Huang, University of Washington, Seattle, WA |
| Anti-Sox2 E-4 | Epitope mapping between amino acids 170-201 within an internal region of human Sox2 | mouse, monoclonal | 1:50 | Santa Cruz, Dallas, TX, Cat# sc-365823, RRID: AB_10842165 |
| Anti-Islet-1 2D6 | Rat Islet-1 C-terminal (aa 178-349) | mouse, monoclonal | 1:100 | DSHB, University of Iowa, IA, Cat# 40.2D6, RRID: AB_528315 |
Acknowledgments
Supported by an Allen Distinguished Investigator award to T.A. Reh, R.O. Wong and F. Rieke, NIH grant 1F32 EY025117-01A1 to A. Hoshino, and the Vision Core grant EY01730.
Footnotes
AUTHOR CONTRIBUTIONS
Conceptualization, C.Z. and R.O.W.; Methodology and Investigation, C.Z., A.H. and F.R.; Antibody Production, J.H.; Data Analysis and Curation, C.Z. and W.-Q.Y.; Writing – Original Draft, C.Z. and R.O.W.; Writing – Review and Editing, C.Z., W.-Q.Y., A.H., F.R., T.A.R. and R.O.W.
References
- Amthor FR, Keyser KT, Dmitrieva NA. Effects of the destruction of starburst-cholinergic amacrine cells by the toxin AF64A on rabbit retinal directional selectivity. Visual Neuroscience. 2002;19(4):495–509. doi: 10.1017/s0952523802194119. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Rubak E, Turner R. Spatial Point Patterns: Methodology and Applications with R. Chapman and Hall/CRC Press; 2015. [Google Scholar]
- Brandon C. Cholinergic amacrine neurons of the dogfish retina. Visual Neuroscience. 1991;6(6):553–562. doi: 10.1017/s0952523800002534. [DOI] [PubMed] [Google Scholar]
- Cuenca N, Deng P, Linberg KA, Fisher SK, Kolb H. Choline acetyltransferase is expressed by non-starburst amacrine cells in the ground squirrel retina. Brain Research. 2003;964(1):21–30. doi: 10.1016/s0006-8993(02)04049-0. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Packer OS. Colour coding in the primate retina: diverse cell types and cone-specific circuitry. Current Opinion in Neurobiology. 2003;13(4):421–427. doi: 10.1016/s0959-4388(03)00103-x. [DOI] [PubMed] [Google Scholar]
- Dann JF. Cholinergic amacrine cells in the developing cat retina. The Journal of Comparative Neurology. 1989;289(1):143–155. doi: 10.1002/cne.902890112. [DOI] [PubMed] [Google Scholar]
- Deng P, Cuenca N, Doerr T, Pow DV, Miller R, Kolb H. Localization of neurotransmitters and calcium binding proteins to neurons of salamander and mudpuppy retinas. Vision Research. 2001;41(14):1771–1783. doi: 10.1016/s0042-6989(01)00060-8. [DOI] [PubMed] [Google Scholar]
- Elshatory Y, Everhart D, Deng M, Xie X, Barlow RB, Gan L. Islet-1 controls the differentiation of retinal bipolar and cholinergic amacrine cells. The Journal of Neuroscience. 2007;27(46):12707–12720. doi: 10.1523/JNEUROSCI.3951-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti EV. Starburst amacrine cells: morphological constancy and systematic variation in the anisotropic field of rabbit retinal neurons. The Journal of Neuroscience. 1985;5(2):562–577. doi: 10.1523/JNEUROSCI.05-02-00562.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti EV, Tumosa N. Immunocytochemical staining of cholinergic amacrine cells in rabbit retina. Brain Research. 1987;413(2):398–403. doi: 10.1016/0006-8993(87)91037-7. [DOI] [PubMed] [Google Scholar]
- Ford KJ, Feller MB. Assembly and disassembly of a retinal cholinergic network. Visual Neuroscience. 2012;29(1):61–71. doi: 10.1017/S0952523811000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransen JW, Borghuis BG. Temporally Diverse Excitation Generates Direction-Selective Responses in ON- and OFF-Type Retinal Starburst Amacrine Cells. Cell Reports. 2017;18(6):1356–1365. doi: 10.1016/j.celrep.2017.01.026. [DOI] [PubMed] [Google Scholar]
- Galli-Resta L, Novelli E, Volpini M, Strettoi E. The spatial organization of cholinergic mosaics in the adult mouse retina. The European Journal of Neuroscience. 2000;12(10):3819–3822. doi: 10.1046/j.1460-9568.2000.00280.x. [DOI] [PubMed] [Google Scholar]
- Galli-Resta L, Resta G, Tan SS, Reese BE. Mosaics of islet-1-expressing amacrine cells assembled by short-range cellular interactions. The Journal of Neuroscience. 1997;17(20):7831–7838. doi: 10.1523/JNEUROSCI.17-20-07831.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges P, Madigan MC, Provis JM. Apoptosis during development of the human retina: relationship to foveal development and retinal synaptogenesis. The Journal of Comparative Neurology. 1999;413(2):198–208. [PubMed] [Google Scholar]
- Guiloff GD, Kolb H. Neurons immunoreactive to choline acetyltransferase in the turtle retina. Vision Research. 1992;32(11):2023–2030. doi: 10.1016/0042-6989(92)90063-o. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Haeseleer F, Hendrickson A. A comparison of immunocytochemical markers to identify bipolar cell types in human and monkey retina. Visual Neuroscience. 2003;20(6):589–600. doi: 10.1017/s0952523803206015. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Wässle H. Immunocytochemical analysis of the mouse retina. The Journal of Comparative Neurology. 2000;424(1):1–23. [PubMed] [Google Scholar]
- Heinze L, Harvey RJ, Haverkamp S, Wässle H. Diversity of glycine receptors in the mouse retina: localization of the alpha4 subunit. The Journal of Comparative Neurology. 2007;500(4):693–707. doi: 10.1002/cne.21201. [DOI] [PubMed] [Google Scholar]
- Hendrickson A. Development of Retinal Layers in Prenatal Human Retina. American Journal of Ophthalmology. 2016;161:29–35 e21. doi: 10.1016/j.ajo.2015.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson A, Possin D, Vajzovic L, Toth CA. Histologic development of the human fovea from midgestation to maturity. American Journal of Ophthalmology. 2012;154(5):767–778 e762. doi: 10.1016/j.ajo.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson A, Troilo D, Djajadi H, Possin D, Springer A. Expression of synaptic and phototransduction markers during photoreceptor development in the marmoset monkey Callithrix jacchus. The Journal of Comparative Neurology. 2009;512(2):218–231. doi: 10.1002/cne.21893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson A, Zhang C. Development of cone photoreceptors and their synapses in the human and monkey fovea. The Journal of Comparative Neurology. 2017 doi: 10.1002/cne.24170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A, Ratnapriya R, Brooks MJ, Chaitankar V, Wilken MS, Zhang C, Starostik MR, Gieser L, La Torre A, Nishio M, Bates O, Walton A, Bermingham-McDonogh O, Glass IA, Wong ROL, Swaroop A, Reh TA. Molecular Anatomy of the Developing Human Retina. Developmental cell. 2017;43(6):763–779 e764. doi: 10.1016/j.devcel.2017.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins JB, Hollyfield JG. Cholinergic neurons in the human retina. Experimental Eye Research. 1987;44(3):363–375. doi: 10.1016/s0014-4835(87)80171-9. [DOI] [PubMed] [Google Scholar]
- Ivaniutsin U, Chen Y, Mason JO, Price DJ, Pratt T. Adenomatous polyposis coli is required for early events in the normal growth and differentiation of the developing cerebral cortex. Neural Development. 2009;4:3. doi: 10.1186/1749-8104-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda M, Ishii K, Morishima Y, Akagi T, Yamazaki Y, Nakanishi S, Hashikawa T. OFF-cholinergic-pathway-selective localization of P2X2 purinoceptors in the mouse retina. The Journal of Comparative Neurology. 2004;476(1):103–111. doi: 10.1002/cne.20208. [DOI] [PubMed] [Google Scholar]
- Kang TH, Ryu YH, Kim IB, Oh GT, Chun MH. Comparative study of cholinergic cells in retinas of various mouse strains. Cell and Tissue Research. 2004;317(2):109–115. doi: 10.1007/s00441-004-0907-5. [DOI] [PubMed] [Google Scholar]
- Kim IB, Lee EJ, Kim MK, Park DK, Chun MH. Choline acetyltransferase-immunoreactive neurons in the developing rat retina. The Journal of Comparative Neurology. 2000;427(4):604–616. doi: 10.1002/1096-9861(20001127)427:4<604::aid-cne8>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Knabe W, Washausen S, Happel N, Kuhn HJ. Development of starburst cholinergic amacrine cells in the retina of Tupaia belangeri. The Journal of Comparative Neurology. 2007;502(4):584–597. doi: 10.1002/cne.21324. [DOI] [PubMed] [Google Scholar]
- Lee ES, Jeon CJ. Starburst amacrine cells express parvalbumin but not calbindin and calretinin in rabbit retina. Neuroreport. 2013;24(16):918–923. doi: 10.1097/WNR.0000000000000026. [DOI] [PubMed] [Google Scholar]
- Mauss AS, Vlasits A, Borst A, Feller M. Visual Circuits for Direction Selectivity. Annual Review of Neuroscience. 2017 doi: 10.1146/annurev-neuro-072116-031335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar T, Ishimoto I, Johnson CD, Epstein ML, Chubb IW, Morgan IG. Cholinergic and acetylcholinesterase-containing neurons of the chicken retina. Neuroscience Letters. 1985;61(3):311–316. doi: 10.1016/0304-3940(85)90482-3. [DOI] [PubMed] [Google Scholar]
- Morgan JL, Schubert T, Wong RO. Developmental patterning of glutamatergic synapses onto retinal ganglion cells. Neural Development. 2008;3:8. doi: 10.1186/1749-8104-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritoh S, Komatsu Y, Yamamori T, Koizumi A. Diversity of retinal ganglion cells identified by transient GFP transfection in organotypic tissue culture of adult marmoset monkey retina. PloS One. 2013;8(1):e54667. doi: 10.1371/journal.pone.0054667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag TC, Wadhwa S. Developmental expression of calretinin immunoreactivity in the human retina and a comparison with two other EF-hand calcium binding proteins. Neuroscience. 1999;91(1):41–50. doi: 10.1016/s0306-4522(98)00654-x. [DOI] [PubMed] [Google Scholar]
- Nguyen LT, De Juan J, Mejia M, Grzywacz NM. Localization of choline acetyltransferase in the developing and adult turtle retinas. The Journal of Comparative Neurology. 2000;420(4):512–526. doi: 10.1002/(sici)1096-9861(20000515)420:4<512::aid-cne8>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- O’Brien KM, Schulte D, Hendrickson AE. Expression of photoreceptor-associated molecules during human fetal eye development. Molecular Vision. 2003;9:401–409. [PubMed] [Google Scholar]
- Park EB, Gu YN, Jeon CJ. Immunocytochemical localization of cholinergic amacrine cells in the bat retina. Acta Histochemica. 2017;119(4):428–437. doi: 10.1016/j.acthis.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Provis JM, Dubis AM, Maddess T, Carroll J. Adaptation of the central retina for high acuity vision: cones, the fovea and the avascular zone. Progress in Retinal and Eye Research. 2013;35:63–81. doi: 10.1016/j.preteyeres.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provis JM, van Driel D. Retinal development in humans: the roles of differential growth rates, cell migration and naturally occurring cell death. Australian and New Zealand Journal of Ophthalmology. 1985;13(2):125–133. doi: 10.1111/j.1442-9071.1985.tb00413.x. [DOI] [PubMed] [Google Scholar]
- Reese BE, Raven MA, Giannotti KA, Johnson PT. Development of cholinergic amacrine cell stratification in the ferret retina and the effects of early excitotoxic ablation. Visual Neuroscience. 2001;18(4):559–570. doi: 10.1017/s0952523801184063. [DOI] [PubMed] [Google Scholar]
- Rodieck RW. Starburst amacrine cells of the primate retina. The Journal of Comparative Neurology. 1989;285(1):18–37. doi: 10.1002/cne.902850104. [DOI] [PubMed] [Google Scholar]
- Rodieck RW, Marshak DW. Spatial density and distribution of choline acetyltransferase immunoreactive cells in human, macaque, and baboon retinas. The Journal of Comparative Neurology. 1992;321(1):46–64. doi: 10.1002/cne.903210106. [DOI] [PubMed] [Google Scholar]
- Sandmann D, Engelmann R, Peichl L. Starburst cholinergic amacrine cells in the tree shrew retina. The Journal of Comparative Neurology. 1997;389(1):161–176. [PubMed] [Google Scholar]
- Schmidt M, Wassle H, Humphrey M. Number and distribution of putative cholinergic neurons in the cat retina. Neuroscience Letters. 1985;59(3):235–240. doi: 10.1016/0304-3940(85)90137-5. [DOI] [PubMed] [Google Scholar]
- Sethuramanujam S, McLaughlin AJ, deRosenroll G, Hoggarth A, Schwab DJ, Awatramani GB. A Central Role for Mixed Acetylcholine/GABA Transmission in Direction Coding in the Retina. Neuron. 2016;90(6):1243–1256. doi: 10.1016/j.neuron.2016.04.041. [DOI] [PubMed] [Google Scholar]
- Sinha R, Hoon M, Baudin J, Okawa H, Wong RO, Rieke F. Cellular and Circuit Mechanisms Shaping the Perceptual Properties of the Primate Fovea. Cell. 2017;168(3):413–426 e412. doi: 10.1016/j.cell.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira AW, Millar TJ, Ishimoto I, Epstein ML, Johnson CD, Dahl JL, Morgan IG. Localization of choline acetyltransferase-like immunoreactivity in the embryonic chick retina. The Journal of Comparative Neurology. 1987;260(4):526–538. doi: 10.1002/cne.902600406. [DOI] [PubMed] [Google Scholar]
- Sterratt DC, Lyngholm D, Willshaw DJ, Thompson ID. Standard anatomical and visual space for the mouse retina: computational reconstruction and transformation of flattened retinae with the Retistruct package. PLoS Computational Biology. 2013;9(2):e1002921. doi: 10.1371/journal.pcbi.1002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WR, Smith RG. The role of starburst amacrine cells in visual signal processing. Visual Neuroscience. 2012;29(1):73–81. doi: 10.1017/S0952523811000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Driel D, Provis JM, Billson FA. Early differentiation of ganglion, amacrine, bipolar, and Muller cells in the developing fovea of human retina. The Journal of Comparative Neurology. 1990;291(2):203–219. doi: 10.1002/cne.902910205. [DOI] [PubMed] [Google Scholar]
- Vaney DI. ‘Coronate’ amacrine cells in the rabbit retina have the ‘starburst’ dendritic morphology. Proceedings of the Royal Society of London Series B, Biological Sciences. 1984;220(1221):501–508. doi: 10.1098/rspb.1984.0016. [DOI] [PubMed] [Google Scholar]
- Vaney DI, Sivyer B, Taylor WR. Direction selectivity in the retina: symmetry and asymmetry in structure and function. Nature Reviews Neuroscience. 2012;13(3):194–208. doi: 10.1038/nrn3165. [DOI] [PubMed] [Google Scholar]
- Vardi N, Masarachia PJ, Sterling P. Structure of the starburst amacrine network in the cat retina and its association with alpha ganglion cells. The Journal of Comparative Neurology. 1989;288(4):601–611. doi: 10.1002/cne.902880407. [DOI] [PubMed] [Google Scholar]
- Vinci L, Ravarino A, Fanos V, Naccarato AG, Senes G, Gerosa C, Bevilacqua G, Faa G, Ambu R. Immunohistochemical markers of neural progenitor cells in the early embryonic human cerebral cortex. European Journal of Histochemistry: EJH. 2016;60(1):2563. doi: 10.4081/ejh.2016.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt T. Cholinergic amacrine cells in the rat retina. The Journal of Comparative Neurology. 1986;248(1):19–35. doi: 10.1002/cne.902480103. [DOI] [PubMed] [Google Scholar]
- Wassle H, Riemann HJ. The mosaic of nerve cells in the mammalian retina. Proceedings of the Royal Society of London Series B, Biological Sciences. 1978;200(1141):441–461. doi: 10.1098/rspb.1978.0026. [DOI] [PubMed] [Google Scholar]
- Whitney IE, Keeley PW, Raven MA, Reese BE. Spatial patterning of cholinergic amacrine cells in the mouse retina. The Journal of Comparative Neurology. 2008;508(1):1–12. doi: 10.1002/cne.21630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney IE, Keeley PW, St John AJ, Kautzman AG, Kay JN, Reese BE. Sox2 regulates cholinergic amacrine cell positioning and dendritic stratification in the retina. The Journal of Neuroscience. 2014;34(30):10109–10121. doi: 10.1523/JNEUROSCI.0415-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RO, Collin SP. Dendritic maturation of displaced putative cholinergic amacrine cells in the rabbit retina. The Journal of Comparative Neurology. 1989;287(2):164–178. doi: 10.1002/cne.902870203. [DOI] [PubMed] [Google Scholar]
- Xiao M, Hendrickson A. Spatial and temporal expression of short, long/medium, or both opsins in human fetal cones. The Journal of Comparative Neurology. 2000;425(4):545–559. [PubMed] [Google Scholar]
- Yamada ES, Dmitrieva N, Keyser KT, Lindstrom JM, Hersh LB, Marshak DW. Synaptic connections of starburst amacrine cells and localization of acetylcholine receptors in primate retinas. The Journal of Comparative Neurology. 2003;461(1):76–90. doi: 10.1002/cne.10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XX. Prenatal development of calbindin D-28K and parvalbumin immunoreactivities in the human retina. The Journal of Comparative Neurology. 1997;377(4):565–576. [PubMed] [Google Scholar]
- Yoshida K, Watanabe D, Ishikane H, Tachibana M, Pastan I, Nakanishi S. A key role of starburst amacrine cells in originating retinal directional selectivity and optokinetic eye movement. Neuron. 2001;30(3):771–780. doi: 10.1016/s0896-6273(01)00316-6. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wu SM. Immunocytochemical analysis of cholinergic amacrine cells in the tiger salamander retina. Neuroreport. 2001;12(7):1371–1375. doi: 10.1097/00001756-200105250-00017. [DOI] [PubMed] [Google Scholar]


