Abstract
Background:
Wiskott-Aldrich syndrome (WAS) is an X-linked immunodeficiency characterized by eczema, infections, and susceptibility to autoimmunity and malignancies. Thrombocytopenia is a constant finding, but its pathogenesis remains elusive.
Objective:
To dissect the basis of the WAS platelet defect, we used a novel conditional mouse model (CoWas) lacking Wiskott-Aldrich syndrome protein (WASp) only in the megakaryocytic lineage in the presence of a normal immunologic environment, and in parallel we analyzed samples obtained from patients with WAS.
Methods:
Phenotypic and functional characterization of megakaryocytes and platelets in mutant CoWas mice and patients with WAS with and without autoantibodies was performed. Platelet antigen expression was examined through a protein expression profile and cluster proteomic interaction network. Platelet immunogenicity was tested by using ELISAs and B-cell and platelet cocultures.
Results:
CoWas mice showed increased megakaryocyte numbers and normal thrombopoiesis in vitro, but WASp-deficient platelets had short lifespan and high expression of activation markers. Proteomic analysis identified signatures compatible with defects in cytoskeletal reorganization and metabolism yet surprisingly increased antigen-processing capabilities. In addition, WASp-deficient platelets expressed high levels of surface and soluble CD40 ligand and were capable of inducing B-cell activation in vitro. WASp-deficient platelets were highly immunostimulatory in mice and triggered the generation of antibodies specific for WASp-deficient platelets, even in the context of a normal immune system. Patients with WAS also showed platelet hyperactivation and increased plasma soluble CD40 ligand levels correlating with the presence of autoantibodies.
Conclusion:
Overall, these findings suggest that intrinsic defects in WASp-deficient platelets decrease their lifespan and dysregulate immune responses, corroborating the role of platelets as modulators of inflammation and immunity. (J Allergy Clin Immunol.)
Keywords: Wiskott-Aldrich syndrome, platelet deficiency, CD40 ligand, autoantibodies, autoimmunity
GRAPHICAL ABSTRACT
Wiskott-Aldrich syndrome (WAS) is a severe X-linked immunodeficiency caused by mutations in the WAS gene encoding the Wiskott-Aldrich syndrome protein (WASp), which is specifically expressed in hematopoietic cells and involved in actin polymerization.1 Patients with WAS have low platelet numbers with reduced size, immunodeficiency, eczema, and high susceptibility to development of tumors and autoimmune manifestations.2,3 Thrombocytopenia in the absence of other clinical manifestations is referred to as X-linked thrombocytopenia (XLT).4–6
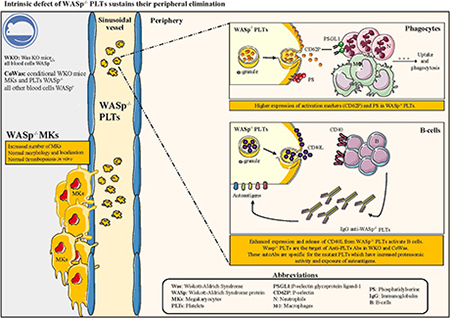
Although bleeding episodes occur in the majority of patients and can cause death in 4% to 10% of these patients,7–9 the pathogenesis of platelet defects remains elusive.10 Abnormal platelet shedding from megakaryocytes (MKs) and consequent premature bone marrow (BM) release in the presence of normal or increased megakaryopoiesis has been described as a cause of ineffective platelet production.2,11,12 In contrast, normal megakaryopoiesis and thrombopoiesis associated with increased peripheral elimination were reported by other authors.13–15 Several studies on platelets performed in Was−/− mouse models (WKO) led to contrasting results. The 2 mouse models of WAS16,17 both show reduced platelet counts but normal mean platelet volume in the absence of bleeding episodes. Studies carried out in these mutants demonstrated defective and premature thrombopoiesis18 and increased peripheral elimination of Was−/− platelets mediated by macrophages and anti-platelet autoantibodies (anti-PLT autoAbs).19–21 However, because all previous studies were performed in murine mutants or in patients lacking WASp in all hematopoietic lineages, it remains difficult to discriminate the contribution of the defective immune system to platelet defect. To this end, we generated a conditional Was−/− mouse model (CoWas) lacking WASp only in the megakaryocytic lineage. Studies performed in CoWas mice allowed us to identify novel intrinsic defects of Was−/− platelets, which were in part confirmed also in patients with WAS/XLT. In conclusion, studies in CoWas mice revealed platelet-intrinsic defects causing their increased peripheral elimination independently from other immune system abnormalities and contributing to abnormal modulation of immune responses.
METHODS
Mice
C57BL/6J Was−/− mice (WKO) were kindly provided by K. A. Siminovitch17; wild-type (WT) mice were purchased from Charles River Laboratories (Calco, Italy); Rag1−/−Was−/− mice were generated by breeding Rag1−/− mice (Jackson Laboratory, Bar Harbor, Me) with WKO mice. CoWas mice were generated by breeding Was-floxed female mice22 and Pf4-Cre male mice (Jackson Laboratory). All the mouse models (WT, WKO, WKO-Rag1−/−, and Rag1−/−) were on the C57BL/6J background. Mice were genotyped, as described in the Methods section in this article’s Online Repository at www.jacionline.org.
Flow cytometry of murine and human samples
Cell suspensions were stained in fluorescence-activated cell sorting (FACS) buffer (PBS, 0.3% BSA, and 0.1% NaN3) with antibodies specific for the following markers: anti-CD3 (145–2C11), anti-CD11b (M1/70), anti-B220 (RA3–6B2), anti-Sca1 (D7), anti-CD62P (RB40.34), anti-CD69 (H1.2F3), anti-CD36 (CRF D-2712), anti-CD47 (miap301), and anti-human PAC1 (PAC-1) from BD PharMingen (San Diego, Calif) and anti-CD117 (2B8), anti-CD150 (TC15–12F12.2), anti-CD105 (MJ7/18), anti-CD40L (MR1), anti-CD41 (MWReg30), anti-CD16/32 (93), anti-CD61 (2C9.G2), anti-CD38 (90), anti-CD34 (HM34), Lin− cocktail, anti-human CD61 (VI-PL2) from BioLegend (San Diego, Calif). Anti-CD42a (Xia.B4) was from Emfret Analytics (Eibelstadt, Germany). Anti-human CD62P (Psel.KO2.3) was from Invitrogen (Carlsbad, Calif). Intracellular WASp staining was performed, as described by Castiello et al.23 All the flow cytometric samples were acquired with a FACSCanto II system (BD, Franklin Lakes, NJ) and analyzed with FlowJo software (TreeStar, Ashland, Ore).
Platelet collection and surface staining
Blood collected in Citrate Phosphate Dextrose Solution (Sigma, St Louis, Mo) from the retro-orbital sinus was analyzed with a Sysmex KX-21N hemocytometer (Sysmex, Kobe, Japan). To collect platelet-rich plasma (PRP), blood was then diluted with the same volume of 1× Tyrode buffer (5 mmol/L HEPES, 137 mmol/L NaCl, 2.7 mmol/L KCl, 0.4 mmol/L NaH2PO4, and 2.8 mmol/L dextrose, pH 7.4) and centrifuged for 7 minutes at 700 rpm.
For analysis of δ-granules, 5 mL of whole blood was stained with 1 μL of anti-mouse CD61 and incubated for 10 minutes at room temperature. Next, samples were incubated for 30 minutes at 37°C in the dark with 500 μL of mepacrine staining solution (10 μmol/L) in PBS or 500 μL of PBS as negative control.
For reticulated platelet (RT-PLT) staining, 5 μL of whole blood was stained with 1 μL of anti-mouse CD61 and incubated for 10 minutes at room temperature. Next, 1 mL of Thiazole Orange solution (50 ng/mL; Sigma-Aldrich, St Louis, Mo) or PBS as a negative control was added and incubated for 30 minutes at room temperature in the dark. To analyze the staining, we set the gate on WT mice, considering the percentage of RT-PLTs in normal animals as 3% of total platelets.24
For Annexin V staining, 200,000 platelets are stained in 100 μL of 1× Binding Buffer with 5 μL of phycoerythrin–Annexin V (BD PharMingen) for 15 minutes at room temperature, followed by stopping reaction by 100 μL of 1× Binding Buffer addition.
To analyze CD40L surface expression, 5 μL of PRP in the presence of 5 μL of agonist was stained with anti-mouse CD61 and anti-mouse CD62P. PRP was incubated with thrombin (final concentration, 1 U/mL; Sigma-Aldrich) for 2 minutes at room temperature. The reaction was stopped by adding 200 μL of FACS buffer and 0.2% paraformaldehyde, and at the end, 1 μL of CD40L antibody per sample is added.
Transmission electron microscopic analysis
Platelet pellets were fixed in 4% paraformaldehyde and 2.5% glutaralde-hyde in 125 mmol/L cacodylate buffer at 4°C for 30 minutes and then in 2% OsO4 in 125 mmol/L cacodylate buffer for 1 hour. Samples were then washed, dehydrated, and embedded in Epon. Sections were examined on a LEO 912AB transmission electron microscope (Zeiss, Germany; 120 kVand CCD Camera 2048×2048 pixels).
In vivo experiments
Platelet depletion was performed by means of intravenous injection of 0.5 μg/g of rat purified anti-mouse GPIba (Emfret Analytics, Eibelstadt, Germany) in PBS. At different time points after injection, blood has been collected from the retro-orbital sinus to analyze platelet counts by using a hemogram and RT-PLTs by using Thiazole Orange staining. Platelet clearance was evaluated by mean of retro-orbital injection of 3 μg of DyLight 488–labeled or Alexa Fluor 647–labeled anti–GPIb-V-IX (Emfret Analytics) antibody diluted in PBS.25 To analyze the percentage of labeled platelets, 10 μL of whole blood was incubated with 2 μL of anti-CD61 for 15 minutes, followed by addition of 150 mL of PBS.
To perform the adoptive transfer experiment, Was−/− platelets were isolated form WKO or CoWas mice and stained with the CellTrace Violet Cell Proliferation Kit (Invitrogen) by using 1 μL of the dye every 5 × 106 of platelets. Platelets isolated from WT mice were labeled with the CellTrace FarRed Cell Proliferation Kit (Invitrogen) using 1 μL of the dye every 5 × 106 platelets. Platelets were mixed in a ratio of 30% WT and 70% Was−/− in PBS and injected intravenously into WKO mice (using Was−/− platelets isolated form WKO mice) or CoWas recipients (Was−/− platelets isolated form CoWas mice). We injected 33 × 106 labeled platelets into each recipient. Blood was collected 15 minutes later (time 0) and daily for 6 days; the percentage of Violet+ or FarRed+ platelets was monitored with a FACSCanto II (BD).
To deplete phagocytes, mice were injected intraperitoneally with 100 μL/10 g mouse weight of clodronate liposomes (concentration, 5 mg/mL) at days 0 and 426 or PBS liposomes. Blood was collected at day 0 (before injection) and day 7 to evaluate platelet counts.
ELISA
Anti–double-stranded DNA antibodies were evaluated by means of ELISA, as described by Bosticardo et al.27 The presence of anti-PLT autoAbs was assessed, as described by Brigida et al.28
ELISA kits to evaluate concentrations of murine soluble CD62P (sCD62P; eBioscience, San Diego, Calif) and plasma soluble CD40 ligand (sCD40L; eBioscience, San Diego, Calif) were used according to the manufacturer’s instructions.
MK analysis
BM cells were stained and MKs were identified as CD41+CD61+ cells by using flow cytometry to analyze the number and frequency of MKs. This analysis was performed in untreated animals 18 hours after treatment with anti-GPIba antibody. MK colony-forming units were analyzed with the MegaCult-C Complete Kit with Cytokines (STEMCELL Technologies, Vancouver, British Columbia, Canada), according to the manufacturer’s instructions. Visible MK colony-forming units with purple nuclei and brown granules were counted (4×) in a wide-field microscope. Ploidy distribution and RT-PLT formation have been evaluated as described in the Methods section in this article’s Online Repository.
B-cell and platelet culture system
CD43+ cells were depleted from the spleen by using anti-CD43 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) with the AutoMacs Pro Separator (Miltenyi Biotec). One hundred microliters of PRP containing at least 200,000 platelets/mL was incubated for 1 hour at 37°C in a thermomixer (300–350 rpm). Platelet-poor plasma (PPP) was collected on double centrifugation at 8000g for 3 minutes. We plated 150,000 CD43− cells (resting naive B cells) in the presence of 100 μL of PPP supernatants or complete RPMI as negative controls for 72 hours; CD69 expression was analyzed by using flow cytometry.
Human platelet activation analysis
PRP was collected by centrifuging blood for 10 minutes at 700 rpm. Two hundred thousand platelets per sample were stained for 10 minutes at room temperature in FACS buffer and then fixed with 0.2% paraformaldehyde. The ratio of CD62P/CD61 mean fluorescence intensity (MFI) or PAC1/CD61 MFI9 was used to normalize the expression level of activation markers for platelet volume, assigning to the healthy donor (HD) unstimulated sample a value of 100.
Sample preparation and proteomic analysis
PRP was centrifuged and lysed through 3 quick freeze/thaw cycles, and then protein extraction was performed by adding RapiGest SF at 0.2% (wt/wt), according to the manufacturer’s protocol (Waters, Milford, Mass). Detailed description of proteomic analysis, data processing, and interaction network reconstruction are described in the Methods section in this article’s Online Repository.
Statistical analysis
All results are expressed as means and SDs, if not stated otherwise. To assess significance, we used 1-way ANOVA with the Bonferroni postcorrection test or 1-way ANOVA when specified. We also used the 2-tailed Mann-Whitney test, where specified. P values of less than .05 were considered significant.
Study approval
Animal procedures were performed according to IACUC 557 and 741 approved by the Italian Institutional Animal Care and OSR Committee. Human studies were performed according to the Helsinki Declaration and the TIGET PERIBLOOD or TIGET08B, TIGET02, and TIGET06 clinical protocols approved by the OSR Ethical Committee. The study was also approved by the National Institutes of Health Institutional Review Board (protocol 16-I-N139).
RESULTS
CoWas mice selectively lack WASp in platelets
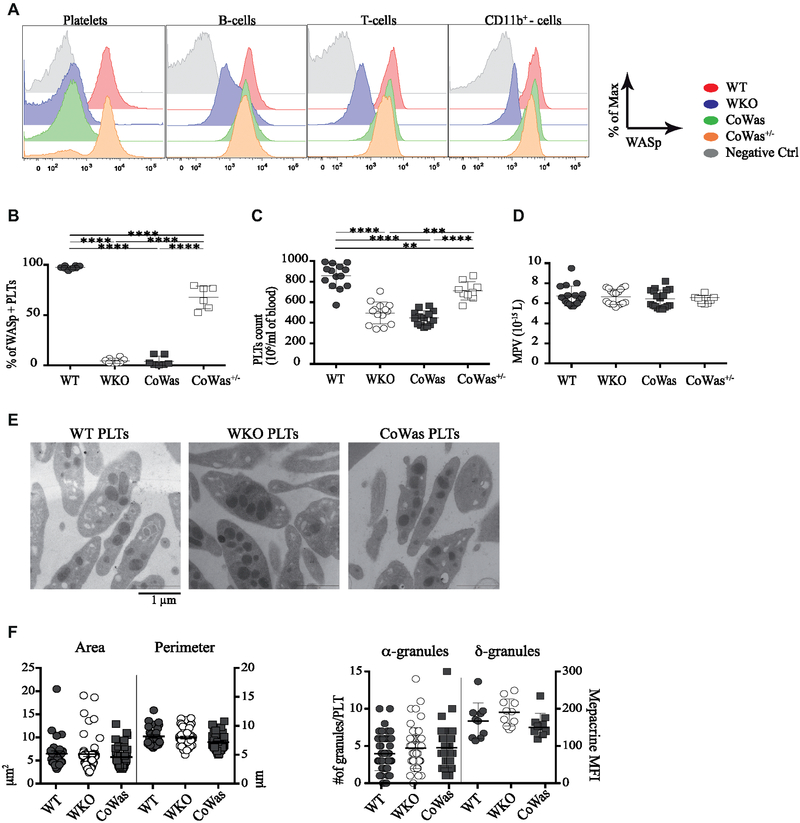
To understand the extent to which the lack of WASp expression affects platelet generation and survival, we generated mice lacking WASp only in the megakaryocytic lineage. We obtained compound heterozygous female (CoWas+/−) and CoWas mice, which were completely null for WASp expression in the megakaryocytic lineage (see Fig E1, A-C, in this article’s Online Repository at www.jacionline.org). Intracellular WASp expression was assessed by using flow cytometry on WBCs and platelets isolated from the peripheral blood of age-matched mice. As expected, WKO mice lacked WASp expression both in WBCs and in platelets, whereas CoWas mice lacked WASp selectively in platelets. CoWas+/− mice showed a double population of WASp+ and WASp− platelets in the presence of normal WASp expression in WBCs (Fig 1, A). CoWas+/− female mice showed more than 50% WASp+ platelets, suggesting a selective advantage for WASp+ platelets in the periphery (Fig 1, B). CoWas and WKO mice showed comparably low platelet counts (446.6 ± 69.62 × 106 platelets/mL and 495 ± 105.9 × 106 platelets/mL, respectively), whereas CoWas+/− mice showed intermediate platelet counts between WT and mutant mice (711.2 ± 89.23 × 106 platelets/mL; Fig 1, C). No differences in mean platelet volume, WBC counts, or hematocrit values were observed among mutants and control animals (Fig 1, D, and data not shown). Analysis of glycoproteins expressed on the platelet surface showed no alterations in mutant mice (see Fig E1, D).
FIG 1.
Characterization of CoWas mice. A, WASp expression was evaluated in different cell types in peripheral blood of6-week-old WT, WKO, CoWas, and CoWas+/− mice. B, Graph shows the percentage of WASp+ platelets in different mouse models at 8 to 12 weeks of age. C and D, Hemogram analysis of WT, WKO, CoWas, and CoWas+/− mice shows platelet counts (Fig 1, C) and mean platelet volume (MPV; Fig 1, D). E, Representative TEM images of platelets isolated from peripheral blood of WT, WKO, and CoWas mice. F, The perimeter and area were measured by using ImageJ Software (left panel), as was the number of α-granules (right panel). Dense granules were evaluated by using mepacrine and analyzed by using flow cytometry (right panel). In Fig 1, B-D, each dot represents a different mouse from 2 independent experiments; in Fig 1, F, each dot represents the analysis of single platelets within different TEM images. All the graphs report means ± SDs, and statistical analysis was performed with 1-way ANOVA and the Bonferroni postcorrection test. *P < .05, **P < .005, ***P < .001, and ****P < .0001. PLT, Platelets.
To further characterize morphologic changes in Was−/− platelets, we analyzed the size and granule content using transmission electron microscopy (TEM; Fig 1, E). In contrast to human findings,2,8,29 platelets from WKO and CoWas mice did not show reduction in size (Fig 1, F, left panel), as previously reported in WKO models.16,18 Consistently, the quantity of α-granules and dense granules (δ-granules), both containing factors responsible for platelet activation and aggregation, was similar in all 3 groups of mutant mice (Fig 1, F, right panel).
Thrombopoiesis in the absence of WASp
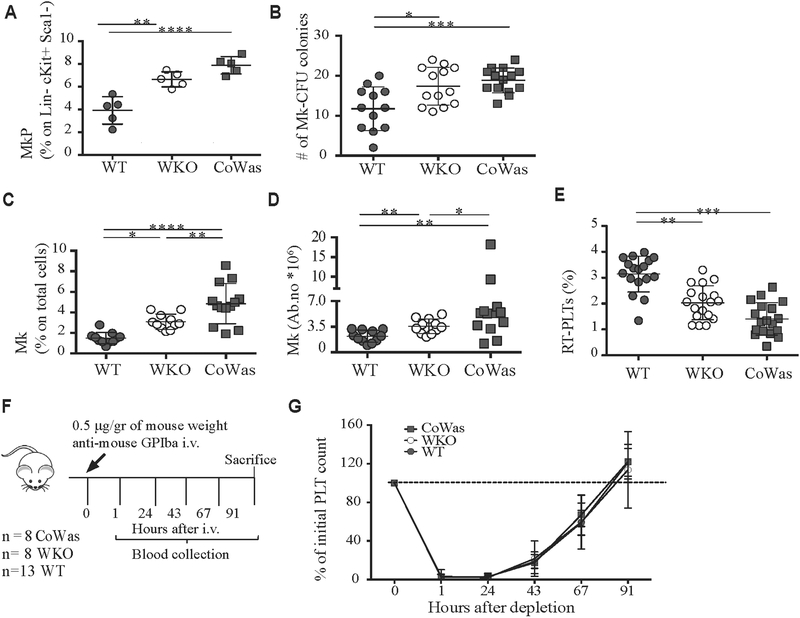
To test whether peripheral thrombocytopenia could be due to defective thrombopoiesis, we analyzed the megakaryocytic compartment. WKO and CoWas mice showed an increased percentage of MK progenitors (CD150+CD41+ on Lin−c-Kit+Sca1− cells Fig 2, A)30 and a greater number of megakaryocytic colony-forming units (Fig 2, B). Further analysis to better dissect MK development showed an increase in MK progenitors from the stage of promegakaryocytes (CD34− CD38+CD41+CD61+CD42a+/− cells; see Fig E2, A, in this article’s Online Repository at www.jacionline.org).31 Of note, no differences in ploidy distribution of MKs were observed in mutant compared with control animals (see Fig E2, B). Analysis of the percentage and absolute number of mature MKs (CD41+CD61+) revealed a significant increase in both CoWas and WKO mice, which is in line with data reported in the literature (Fig 2, C and D).18,19 All these findings suggest that peripheral thrombocytopenia stimulates megakaryopoiesis to compensate for the peripheral defect.
FIG 2.
Phenotypic and functional characterization of the megakaryocytic compartment. A, Evaluation of megakaryocyte progenitors (MkP) identified as CD41+CD150+ cells gated on the Lin−c-Kit+Sca1− compartment in WT, WKO, and CoWas mice is shown. B, The number of MKcolony-forming units was counted with the microscope by using a ×4 objective. C and D, MKs (CD61+CD41+ cells) are expressed as percentages on total BM cells (Fig 2, C) or absolute numbers (Fig 2, D) and analyzed in the bone marrow of age-matched WT, WKO, and CoWas mice. E, The percentage of RT-PLTs is assessed by using Thiazole Orange (TO). F, Platelet depletion was performed by using intravenous injection of anti-mouse GPIba (Emfret Analytics) according to the scheme indicated in the figure. G, Platelet counts were monitored daily by means of blood collection and expressed as percentages of initial platelet counts at time 0 at different time points. In all graphs each dot represents a different mouse from 1 (Fig 2, A), 2 (Fig 2, G), 4 (Fig 2, E), or 6 (Fig 2, B-D) independent experiments. All the graphs report means ± SDs, and statistical analysis was performed with 1-way ANOVA (Fig 2, A-E) or 2-way ANOVA (Fig 2, G) and the Bonferroni postcorrection test. *P < .05, **P < .005, ***P <.001, and ****P < .0001. PLT, Platelets.
To assess the efficiency of thrombopoiesis in vivo, we counted RT-PLTs24 using Thiazole Orange staining. WKO and CoWas mice showed a decreased percentage of RT-PLTs (Fig 2, E), which might suggest impaired thrombopoiesis. However, MK analysis in the BM of mutant mice assessed by using immunohistochemistry with anti-CD31 antibody (depicting sinusoids and MKs) and anti-Factor VIII antibody (von Willebrand factor, labeling MKs) did not reveal any mislocalization (data not shown). Also, Was−/− MKs showed in vitro normal proplatelet formation (see Fig E2, C-E).
To test in vivo thrombopoiesis, we depleted platelets by means of platelet injection of anti-GPIbα mAb (Fig 2, F). Daily blood collection demonstrated platelet recovery to predepletion values within 3 to 4 days in all mice (Fig 2, G). Of note, no effect of anti-GPIbα treatment on MK numbers has been found using WT animals (see Fig E2, F); however, we cannot exclude an effect of WASp deficiency on the recovery of the functional MK pool after GPIb treatment. Additionally, evaluating the percentage of RT-PLTs after anti-GPIba treatment, we found that RT-PLTs from mutant mice reach an equilibrium with the same kinetics of WT mice (see Fig E2, G). Although these in vivo data might suggest normal thrombopoiesis in WKO and CoWas mice, at least in stressed situations, because mutant animals have higher numbers of MKs in the BM and do not reach an equilibrium of RT-PLTs in a shorter time period, we cannot exclude that thrombopoiesis per MK could be affected.
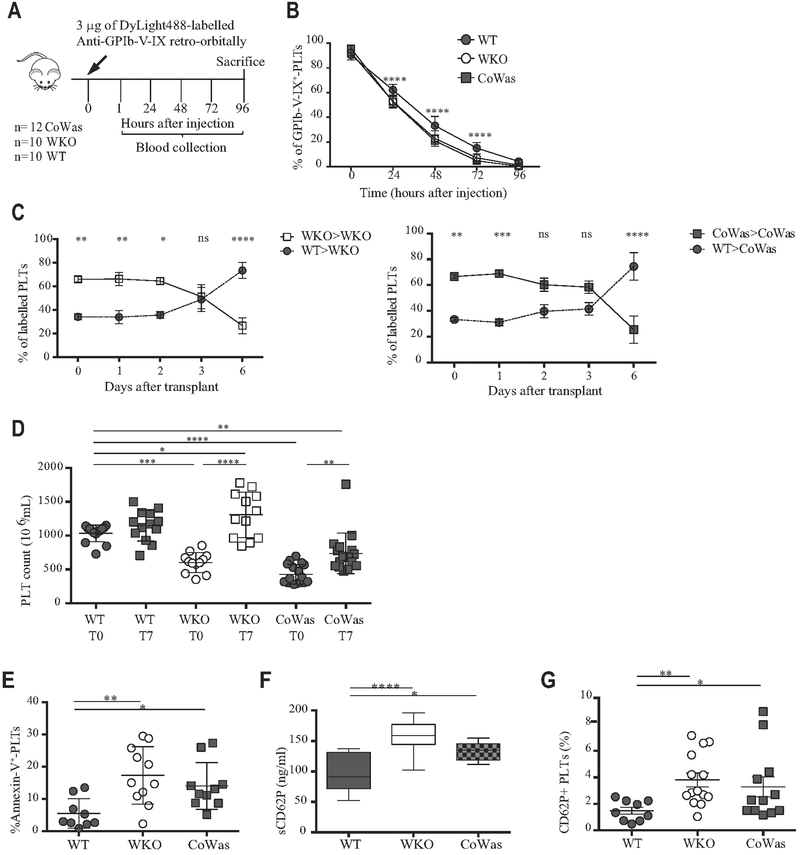
Increased peripheral elimination of hyperactived Was−/− platelets in CoWas mice
To test the increased peripheral platelet elimination hypothesis, we assessed platelet half-life by means of in vivo injection of Dy-Light 488-anti-GPIb-V-IX antibody selectively labeling circulating platelets25 and observed a shorter lifespan in WKO and CoWas mice (Fig 3, A and B), although we cannot exclude an effect of the antibody on platelet clearance. To further characterize this defect, we transferred a mixed population of differently labeled WASp+ (from WT) and WASp− (from WKO or CoWas) platelets into WKO and CoWas recipients. Specifically, labeled platelets were mixed at a ratio of 30% WT and 70% CoWas or 30% WT and 70% WKO and infused intravenously into CoWas and WKO mice, respectively. The proportion of WASp+ platelets increased over time, whereas proportions of WASp− platelets decreased in all recipients, indicating that WASp− platelet clearance is independent of the host environment (Fig 3, C) and suggesting an intrinsic platelet defect.
FIG 3.
Peripheral elimination of platelets. A, Experimental plan of in vivo platelet half-life detection. B, Platelet half-life was analyzed as the percentage of GPIb-V-IX+ platelets 15 minutes after injection (time 0) and at different time points after injection. C, Differentially labeled WT and Was−/− platelets have been mixed in a 30%/70% ratio and injected into recipients. Was−/− platelets isolated from WKO mice have been transferred to WKO mice (left panels), whereas Was−/− platelets isolated form CoWas mice have beentransferred into CoWas mice(rightpanels; n = 5). D, Platelet counts havebeen evaluatedbefore clodronate liposome injection (T0) and after7 days (T7). E, Apoptotic platelets are expressed as Annexin V+ platelets. F, sCD62P (in nanograms per milliliter) plasma levels (n = 10). G, The graph shows CD62P+ platelets evaluated by means of flow cytometry and expressed as a percentage of the total platelet population. In all graphs each dot represents a different mouse from 1 (Fig 3, C), 2 (Fig 3, E and F), 3 (Fig 3, B and G), or 6 (Fig 3, D) independent experiments. All graphs report means ± SDs. Fig 3, C, reports means ± SEMs. Statistical analysis was performed with 1-way ANOVA(Fig 3, D-F) or 2-way ANOVA (Fig 3, B and C) and the Bonferroni postcorrection test. Fig 3, G, reports means ± SDs and has been analyzed with the Mann-Whitney test. *P < .05, **P < .005, ***P < .001, and ****P < .0001. ns, Not significant; PLT, platelets.
To test phagocyte contribution in mediating peripheral platelet elimination, we injected clodronate liposomes to deplete macrophages in vivo.26,32 Analysis of the frequency of F4/80+ cells in the spleens of clodronate liposome-treated mice confirmed macrophage depletion (data not shown). Seven days after administration, WKO and CoWas mice showed a significant increase in platelet numbers, indicating a critical contribution of phagocytes to peripheral elimination of WASp-deficient platelets (Fig 3, D). Platelet increase was more obvious in WKO mice than in CoWas mice. We speculate that the defective migratory ability of Was−/− macrophages in WKO mice could lead to increased platelet elimination within tissues, inducing a more substantial increase in platelet counts after phagocyte depletion. Rapid phosphatidylserine exposure occurring concomitantly to activation is one of the main mechanisms mediating platelet phagocytosis. In line with data from patients with WAS,15 platelets from both mutant strains showed higher frequencies of Annexin V+ platelets (Fig 3, E). Increased levels of sCD62P in plasma (Fig 3, F) and surface expression of CD62P (Fig 3, G) were also found in mutant compared with control platelets. In sum, our data indicate that cell-intrinsic mechanisms cause hyperactivation of WASp-deficient platelets, which are in turn rapidly removed from the periphery by phagocytic cells.
Proteomics of Was−/− platelets reveals perturbed metabolism and enhanced antigen processing
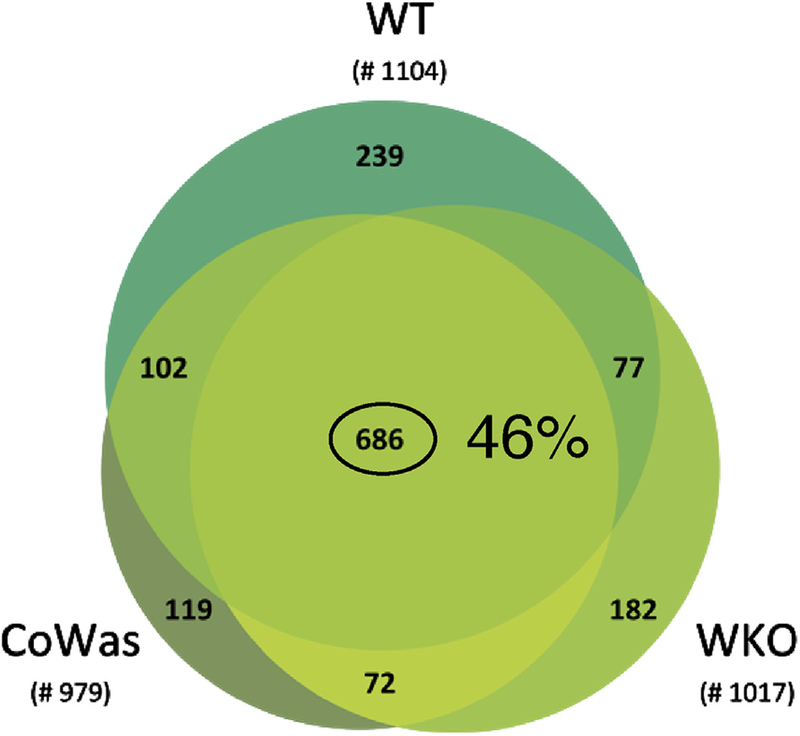
To investigate the intrinsic mechanisms underpinning the enhanced activation and removal of Was−/− platelets, we evaluated their proteomic profile. More than 1400 proteins were identified (see Table E1 in this article’s Online Repository at www.jacionline.org), and 686 proteins were shared among mutant and WT platelets (corresponding to >46% of total proteins; Fig 4 and see Fig E3, A-C, in this article’s Online Repository at www.jacionline.org).
FIG 4.
Proteins identified by using proteomic analysis. Venn diagram of proteins identified in different platelet conditions. The number of total proteins identified was 1104 in WT samples and 1017 and 979 in WKO and CoWas samples, respectively. Six hundred eighty-six proteins (corresponding to 46% of the total protein identified) are shared between mutant and WT animals.
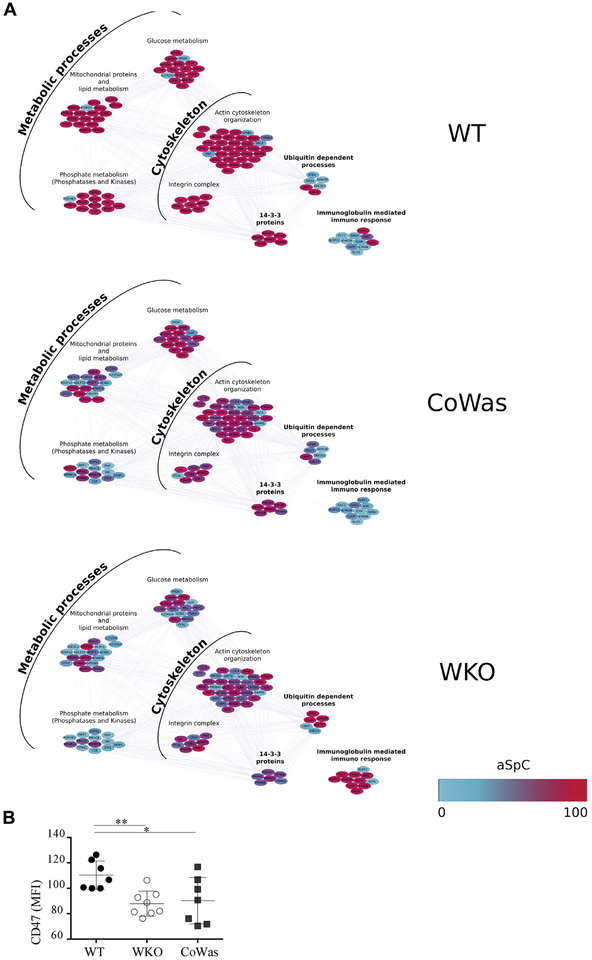
To identify differentially expressed proteins, linear discriminant analysis was applied, and 221 proteins presenting F ratios of 3.5 or greater and P values of .05 or less were extracted as descriptors (see Table E2 in this article’s Online Repository at www.jacionline.org). Hierarchical clustering obtained on processing descriptors showed that CoWas is an intermediate condition between WT and WKO (see Fig E3, D). Of note, the majority of extracted descriptors were confirmed by fold change and DAve analyses (see the Proteomics data processing section in the Methods section). In addition, the effects of WASp deletion were evaluated plotting the selected descriptors into a Mus musculus protein-protein interaction network by using Cytoscape and STRING db (see Fig E4 in this article’s Online Repository at www.jacionline.org). The network analysis (19 clusters) confirmed intermediate levels of expression of distinct biological processes/functional classes in CoWas platelets between WT and WKO platelets. This finding was more evident when focusing on a subnetwork of 8 clusters involving metabolic processes, cytoskeleton, ubiquitins, 14-3-3 proteins, and immunoglobulins (Fig 5, A). Specifically, we did not find any abnormalities in the total amount of actin-related proteins but relative changes in some proteins related to this pathway. Metabolic profile perturbation with a reduction in lipid, glucose, and phosphate metabolism-related proteins was also found. On the other hand, ubiquitin-related proteins were upregulated in line with reduced lifespan and increased antigen processing, which is frequently observed in patients with autoimmune disorders.33 The proteomic profile also revealed alterations in the immunoglobulin content, with a substantial increase in platelet-associated IgM, IgA, and IgG levels in WKO mice (Fig 5, A). Of note, the ‘‘don’t eat me’’ signal CD47 molecule decreases in CoWas and WKO mice, as confirmed by using flow cytometric analysis (Fig 5, B).
FIG 5.
Different proteomic profile between WT and Was−/− platelets. A, Focus on the main biological processes identified during proteomic analysis. Proposed clusters were selected by using the BINGO Cytoscape plugin (P ≤ .05), and color gradients correspond to the normalized average spectral count (aSpC; 0–100), where red indicates upregulated proteins, blue indicates downregulated proteins, and the shade between red and blue indicate intermediate level of expression. B, The graph shows the CD47 MFI on the CD61+ platelet subpopulation. Statistical analysis has been performed with the unpaired t test. Each dot represents a mouse. *P < .05 and **P < .005.
The proteomic profile suggests a scenario in which absence of WASp (1) decreases the platelet lifespan through inhibition of several metabolic pathways and enhancement of ubiquitination and proteasomal activity that (2) increases antigen processing, contributing to triggering of autoimmunity. In addition, our data show that WASp-deficient platelets present intrinsic defects that become more severe in WKO mice, likely because of the aggravating role of platelet-extrinsic immune dysfunction.
Was−/− platelets trigger autoimmune responses in CoWas mice
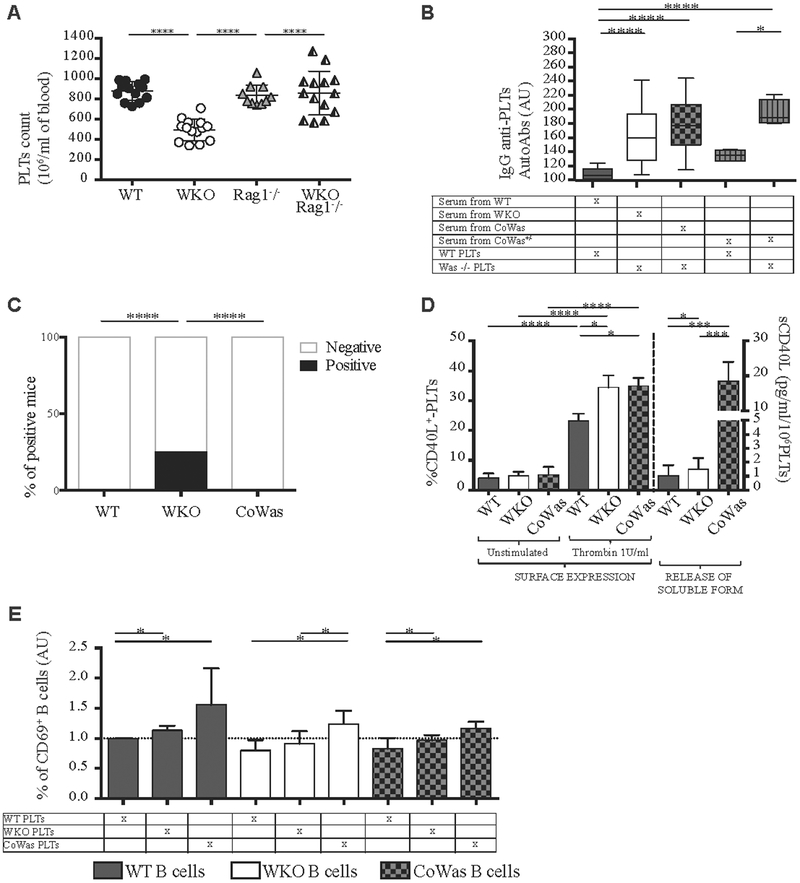
Because of the presence of anti-PLT autoAbs in patients34 and WKO mice,20 we hypothesized that WASp-deficient B cells can react against platelets by producing neutralizing antibodies. In line with this, Was−/−Rag1−/− double-knockout mice (WKO-Rag1−/−), which lack T and B cells, showed platelet counts comparable with those of WT mice, thus supporting a partial involvement of adaptive immunity in the peripheral elimination of Was−/− platelets (Fig 6, A) and further supporting data observed in the μMT−/− x Was−/− model.20
FIG 6.
Interaction between platelets (PLTs) and B cells mediated by CD40L. A, Platelet counts from age-matched WT, WKO, Rag1−/−, and WKO- Rag1−/− mice are shown. B, Evaluation of IgG antiplatelet autoantibodies. ELISA plates were coated with total protein lysates from WT or Was−/− platelets and incubated with sera according to the grid presented (n = 23). C, The graph represents the percentage of mice positive for the anti-double-stranded DNA autoantibodies. D, Percentage of CD40L+ platelets was evaluated by using flow cytometry before (n = 10) and after (n = 12) stimulation with thrombin (1 U/mL; left); sCD40L was measured in PPP from unwashed platelets isolated from WT (n = 16), WKO (n = 16), and CoWas (n = 5) mice (right). E, Resting B cells (CD43− cells) isolated from the spleens of WT, WKO, and CoWas mice were kept in culture for 72 hours with PPP and analyzed by using flow cytometry for CD69 expression as an activation marker. In Fig 6, A, each dot represents a different mouse, and means ± SDs are reported. In Fig 6, B, D, and E, the mean ± SD reported is the result of 2 (Fig 6, B) and 4 (Fig 6, D) independent experiments. Statistical analysis was performed with 1-way ANOVA and the Bonferroni postcorrection test (Fig 6, A, B, and D, left panel). In Fig 6, D, sCD40L levels (rightpanel) are analyzed with the Mann-Whitney test, as well as Fig 6, D. Fig 6, C, has been analyzed statistically with the χ2test. *P <.05, **P < .005, ***P < .001, and ****P < .0001.
To test whether production of anti-PLT autoAbs could be related to B-cell dysregulation23,35–39 or triggered by the intrinsic immunogenicity of Was−/− platelets, we measured anti-PLT autoAbs in CoWas mice. To do this, we used an ELISA detecting serum anti-PLT IgG that was more sensitive than the proteomics approach used above. No autoantibodies against WT platelets were detected in WT mice, whereas autoantibodies against Was−/− platelets were present in sera of WKO and CoWas mice. Very importantly, CoWas+/− mice had autoantibodies specifically against Was−/− platelets but not against WT platelets (Fig 6, B). Of note, CoWas mice do not show any signs of autoimmunity and the analysis of serum anti-double-stranded DNA autoantibodies did not detect any positivity (Fig 6, C).
Because of the presence of autoantibodies in CoWas mice despite the normal B-cell compartment, we hypothesized that Was−/− platelets could sustain humoral autoimmunity by producing immunomodulatory factors that trigger B-cell responses. In this context platelets have been demonstrated to mediate inflammatory and immune responses by releasing chemokines and cytokines, including CD40L, which is highly expressed and released on activation. Platelet-derived CD40L can modulate adaptive immune mechanisms influencing B-cell homeostasis by inducing isotype switching.40–43 Thus we evaluated platelet CD40L surface expression on in vitro activation, finding a significant increase in WKO and CoWas mice (Fig 6, D, left). Consistently higher release of sCD40L was retrieved in mutant PPP obtained from unwashed platelets, possibly reflecting higher activation of Was−/− B cells, which can consume sCD40L (Fig 6, D, right).
Next, we tested whether the greater expression and release of CD40L could directly modulate B-cell activation. We cultured B cells isolated from spleens of WT, WKO, and CoWas mice in the presence of PPP from WT, WKO, or CoWas mice and analyzed B-cell activation. B cells cultured in the presence of supernatants obtained from platelets lacking WASp showed a higher proportion of the activation marker CD69 (Fig 6, E). Overall, these findings suggest that, in the absence of WASp, platelets can contribute to sustaining immune dysregulation by expressing and releasing higher levels of CD40L, which can induce B-cell activation, even in a normal immune environment.
Activated platelets and increased sCD40L in patients with WAS/XLT
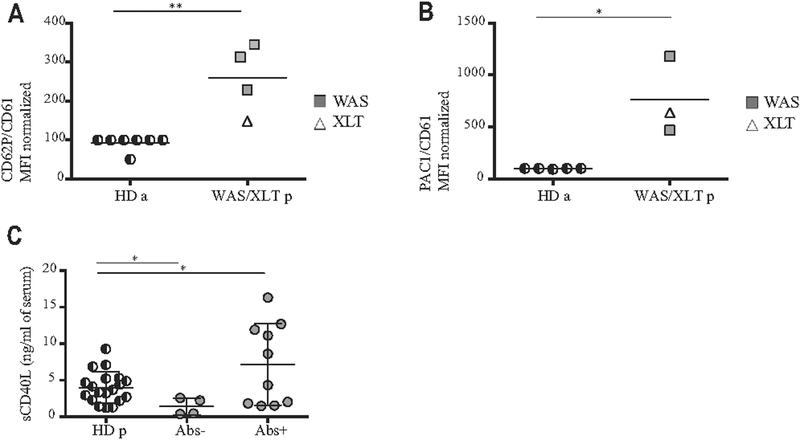
To corroborate these findings in human subjects, we evaluated platelet activation and analyzed levels of serum sCD40L in a cohort of patients with WAS with various clinical manifestations and the presence or absence of autoantibodies (see Table E3 in this article’s Online Repository at www.jacionline.org).37,44 Human platelet activation was evaluated in adult HDs and patients by analyzing the expression of CD62P and PAC1, the activated form of integrin αIIbβ3 (GPIIbIIIa).45 We found that the MFIs of both CD62P and PAC1 were significantly increased in patients (Fig 7, A and B). To support the immunomodulatory role of platelets, different autoimmune conditions have been associated with increased serum levels of sCD40L, of which platelets are the major source.46–48 We tested levels of sCD40L in the plasma of 14 patients with WAS who were also at risk of autoimmune complications and in 19 pediatric age-matched HDs. Higher sCD40L concentrations were found in patients with circulating autoantibodies than in patients with no autoantibodies (Fig 7, C, and see Table E3).
FIG 7.
Activation profile of human platelets. A and B, Activation profile of human platelets expressed as the MFI ratio of activation markers and CD61 MFI (Fig 7, A, for CD62P and Fig 7, B, for PAC1). We assigned to the unstimulated adult HD (HD a) a value of 100, and we normalized the patients accordingly. In Fig 7, A, we analyzed 4 patients (3 with WAS and 1 with XLT) and 7 HDs; in Fig 7, B, we analyzed 3 patients (2 with WAS and 1 with XLT) and 5 HDs. C, The level of sCD40L in the plasma of patients with WAS/XLT was analyzed by means of ELISA. Patients with WAS/XLT were divided according to the presence or absence of autoantibodies or other clinical signs related to autoimmunity (see Table E3). Pediatric HDs (HD p) were tested as control subjects. Fig 7, A and B, show means analyzed with the Mann-Whitney test. Fig 7, C, reports means ± SDs and shows analysis with 1-way ANOVA and the Bonferroni postcorrection test. *P < .05 and **P < .005.
Overall, in patients with WAS, platelets are hyperactivated, and in turn, this activation can contribute to their peripheral elimination and further sustain inflammation and B-cell activation, predisposing to autoimmune complications.
DISCUSSION
Thrombocytopenia is a common feature present in both patients with XLT and those with WAS.2–6 Controversial data on platelet defects have been reported, but all these studies were performed in patients and murine models lacking WASp in all hematopoietic cells, thus preventing a clear dissection of the contribution of intrinsic versus extrinsic factors. To overcome this issue, we generated CoWas mice lacking WASp only in the megakaryocytic lineage. Although no gross morphologic changes were detected in platelets from CoWas mice, the lower absolute count in a normal immune environment strongly suggested an intrinsic defect.
One of the more controversial issues in the setting of WAS-related thrombocytopenia is analysis of the thrombopoietic activity of deficient MKs, and indeed, observations in our mutant models do not completely clarify this complex issue. BM analysis in WKO and CoWas mice showed an increased percentage of MK progenitors and MKs, suggesting that peripheral thrombocytopenia triggers megakaryopoiesis. In vitro proplatelet production tests showed that Was−/− MKs are able to produce proplatelets at a normal rate compared with WT counterparts. Additionally, after in vivo platelet depletion, mutant animals treated with anti-GPIb antibody restored platelet counts with kinetics comparable with those in WT mice. To corroborate our findings, it has been demonstrated recently that human proplatelet formation in vitro depends on N-WASp, whereas WASp is dispensable.49
Despite this evidence, RT-PLT numbers and percentages in peripheral blood were reduced in both mutants to a level similar to those seen in patients with WAS20,50; these data contrast with the work published by Prislovsky et al,19 who showed a normal frequency of RT-PLTs in WKO mice. This discrepancy might be due to the different genetic background and effect of genetic modifiers present in various mouse models.16,17 The reduced frequencies and counts of RT-PLTs present in WKO and CoWas mice could also suggest a defective thrombopoiesis, although in contrast with our in vitro data. However, a reduced half-life of Was−/− RT-PLTs compared with WT RT-PLTs based on the in vivo data of mature platelets could be hypothesized. Accelerated kinetics of RT-PLT maturation to compensate for the faster mature platelet elimination might be an additional factor. Moreover, we cannot exclude the hypothesis that in the absence of WASp, an important fraction of platelets could be generated as nonreticulated.19 Finally, because WKO and CoWas mice have increased MK numbers, thrombopoiesis per MK could still be affected. Overall, because the data reported here are obtained in a stress condition (after severe depletion), defective thrombopoiesis at steady state cannot be excluded.
We speculated that despite the normal immune environment, peripheral platelet destruction in CoWas mice could be the result of an intrinsic platelet defect. In line with this hypothesis, a shorter half-life of Was−/− platelets was observed in CoWas and WKO mice. Moreover, mutant mice showed increased sCD62P levels in plasma with no increase in sCD62P mRNA expression (data not shown), indicating that the increased protein expression and serum levels might reflect enhanced a-granule release because of the perturbation of actin polymerization inside the cytosol consistently with the role of WASp in regulation of granule release. Thus WASp deficiency causes dysregulation in the expression and release of CD62P, which in turn might induce increased uptake of activated platelets by phagocytes through CD62P/PSGL1 interactions.51–55 Platelet activation also results in rapid exposure of phosphatidylserine, a well-known ‘‘eat- me’’ signal. In line with this, in vivo elimination of phagocytes increased platelet counts in WKO and CoWas mice. Additionally, B cells can produce anti-PLT autoAbs, which are frequently detected in patients and WKO mice.20,34 Remarkably, CoWas mice and even CoWas+/− mice, produce autoantibodies selectively against WASp-deficient platelets. The increased expression and release of CD40L can contribute to tolerance breakdown by promoting B-cell activation and inducing immunotype switching.23,40,56–59 Indeed, the difference between sCD40L levels retrieved in PPP from WKO and CoWas mice might reflect the altered distribution of B-cell subpopulations. In fact, with similar release of sCD40L by Was−/− platelets, CoWas mice have no defects in the B-cell compartment and require a lower amount of sCD40L produced by platelets. On the contrary, WKO mice have hyperactivated B cells that produce higher levels of circulating IgM and IgG at steady state, thus suggesting that higher consumption of sCD40L occurs to sustain B-cell activation and class-switch recombination.22,59–61
These data were corroborated further in a small cohort of patients with WAS. We observed greater expression of activation markers in patients; our observations are in contrast with data reported by Gerrits et al,9 who evaluated platelet activation on whole-blood samples, finding no significant difference between patients and HDs.
We performed analyses of platelet activation on platelets isolated from peripheral blood. The different type of samples analyzed could explain this discrepancy because in whole blood activated platelets could bind to leukocytes, thus underestimating the P-selectin expression. Patients with detectable autoantibodies also showed increased plasma levels of sCD40L, which are likely derived from in vivo activation of platelets. In turn, sCD40L increase can sustain inflammation and induce B-cell isotype switching. The proteomic profile of Was−/− platelets isolated from WKO but not CoWas mice revealed relevant changes in immunoglobulin content (IgA, IgG2b, IgG3, and IgM), reflecting perturbation of the B-cell compartment.23,56–59 In parallel, the proteomic analysis demonstrated that both platelet-intrinsic and platelet-extrinsic mechanisms cooperate in the pathogenesis of WAS-related thrombocytopenia. Proteomic alterations shared between WKO and CoWas mice indicate intrinsic platelet defects because of the absence of WASp, whereas proteins differentially expressed between WKO and CoWas mice reflect the direct influence of the immune system. Was−/− platelets from both murine models show metabolic dysregulation, with lower expression of proteins involved in lipid metabolism processes, such as those involved in the electron transport chain or in fatty acid β-oxidation, as well as SLC25A24, a mitochondrial calcium-dependent carrier of Mg-ATP/Mg-ADP that protects against oxidative stress.62,63 Conversely, WKO platelets upregulated stomatin, a lipid raft component of a-granules involved in platelet activation.64 Glucose metabolism, protein kinases, phosphates, and relative cascade pathways were downregulated in the absence of WASp.
Interestingly, levels of CD47, a molecule that, when absent, leads to mild spontaneous thrombocytopenia,65 progressively decrease from CoWas to WKO mice, suggesting an additional mechanism contributing to the induction of phagocytosis. Consistent with the frequent bleeding episodes occurring in patients irrespective of clinical severity, integrin β3, integrin IIb/IIIa, and some 14-3-3 family proteins were downregulated.66 On the other hand, upregulation of ubiquitin-related proteins was found. All these findings support the intrinsic defect that renders platelets more prone to apoptosis and concomitantly more capable of processing antigens for presentation to the immune system, a phenomenon that can contribute to the development of autoimmunity.33
In conclusion, our study provides novel insights into the pathogenesis of WAS, highlighting the intrinsic role of WASp in platelet turnover and peripheral homeostasis. We shed new light on the mechanisms underlying WAS immune dysregulation, showing that in the absence of WASp, platelets secrete a high amount of CD40L, sustaining inflammation and contributing to the altered B-cell function. Overall, our study identifies platelet defects as contributing to both impaired hemostasis and dysregulated immunity, extending the complex scenario of defective cellular processes occurring in patients with WAS.
Supplementary Material
Key messages.
Selective WAS absence is dispensable for MK function and thrombopoiesis.
Conditional WAS inactivation in platelets affects peripheral survival, leading to phagocytosis and autoantibody elimination through CD40L.
Was−/− platelets have decreased metabolic activity and increased ubiquitination pathways.
Increased platelet activation and CD40L plasma levels are observed in patients with WAS.
Acknowledgments
Supported by the Italian Telethon foundation (Telethon Core Grant) and supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. L.S. has conducted this study as fulfillment of her PhD in Molecular Medicine, Program in Basic and Applied Immunology, San Raffaele University, Milan, Italy. GlaxoSmithKline is the financial sponsor of an ongoing Wiskott-Aldrich syndrome gene therapy program in which some of the patients listed in this study are participating. This study was also supported in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. This work was also supported by PNR–CNR Aging Program 2012–2014 to A.V.
Disclosure of potential conflict of interest: A. J. Thrasher received board membership and consultancy fees from Mallory, Torus, and Rocket Pharmaceuticals and stock options from Orchard Therapeutics and Torus. A. Aiuti’s institution received a grant from GlaxoSmithKline for this work and grants from the European Research Council for other works. P. Mauri’s institution received grants from the Italian CNR InterOmics Project funded by the MIUR and the Amanda Project funded by Regione Lombardia for this work. L. D. Notarangelo received board membership from Frontiers in Immunology, the Journal of Clinical Immunology, and Clinical Immunology and receives royalties from UpToDate. The rest of the authors declare that they have no relevant conflicts of interest.
We thank Professor H. Ochs for the anti-WASp antibody. We thank Dr M. C. Panzeri from the San Raffaele Alembic Facility for the Electron Microscopy studies and Dr F. Ficara from CNR-IRGB Humanitas Research Hospital. We acknowledge Dr M. P. Cicalese, Dr F. Ferrua, Dr M. E. Bernardo, Dr M. Migliavacca, Mrs M. Casiraghi, and all the medical and nursing personnel of the San Raffaele Pediatric Immunohematology Unit and SR-Tiget Clinical Trial Office personnel for clinical trial management and support. We thank Dr Nico van Rooijen for providing clodronated liposomes.
Abbreviations used
- Anti-PLT autoAb
Anti-platelet autoantibody
- FACS
Fluorescence-activated cell sorting
- HD
Healthy donor
- MFI
Mean fluorescence intensity
- MK
Megakaryocyte
- PPP
Platelet-poor plasma
- PRP
Platelet-rich plasma
- RT-PLT
Reticulated platelet
- sCD40L
Soluble CD40 ligand
- sCD62P
Soluble CD62P
- WAS
Wiskott-Aldrich syndrome
- WASp
Wiskott-Aldrich syndrome protein
- WT
Wild-type
- XLT
X-linked thrombocytopenia
REFERENCES
- 1.Moulding DA, Record J, Malinova D, Thrasher AJ. Actin cytoskeletal defects in immunodeficiency. Immunol Rev 2013;256:282–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ochs HD, Slichter SJ, Harker LA, Von Behrens WE, Clark RA, Updated RJW, et al. The Wiskott-Aldrich syndrome: studies of lymphocytes, granulocytes and platelets. Blood 1980;55:243–52. [PubMed] [Google Scholar]
- 3.Bosticardo M, Ferrua F, Cavazzana M, Aiuti A. Gene therapy for Wiskott-Aldrich syndrome. Curr Gene Ther 2014;14:413–21. [DOI] [PubMed] [Google Scholar]
- 4.Villa A, Notarangelo L, Macchi P, Mantuano E, Cavagni G, Brugnoni D, et al. X-linked thrombocytopenia and Wiskott-Aldrich syndrome are allelic diseases with mutations in the WASP gene. Nat Genet 1995;9:414–7. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Q, Zhang M, Blaese RM, Derry JM, Junker A, Francke U,et al. The Wiskott-Aldrich syndrome and X-linked congenital thrombocytopenia are caused by mutations of the same gene. Blood 1995;86:3797–804. [PubMed] [Google Scholar]
- 6.Mahlaoui N, Pellier I, Mignot C, Jais J-P, Bilhou-Nabéra C, Moshous D, et al. Characteristics and outcome of early-onset, severe forms of Wiskott-Aldrich syndrome. Blood 2013;121:1510–6. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan KE, Mullen CA, Blaese RM, Winkelstein JA. A multiinstitutional survey of the Wiskott-Aldrich syndrome. J Pediatr 1994;125:876–85. [DOI] [PubMed] [Google Scholar]
- 8.Imai K, Morio T, Zhu Y, Jin Y, Itoh S, Kajiwara M, et al. Clinical course of patients with WASP gene mutations. Blood 2004;103:456–64. [DOI] [PubMed] [Google Scholar]
- 9.Gerrits AJ, Leven E, Frelinger AL III, Brigstocke SL, Berny-lang M, Mitchell WB, et al. Effects of eltrombopag on platelet count and platelet activation in Wiskott-Aldrich syndrome/X-linked thrombocytopenia. Blood 2015;126: 1367–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sereni L, Castiello MC, Villa A. Platelets in Wiskott-Aldrich syndrome: Victims or executioners? J Leukoc Biol 2017;1–14. [DOI] [PubMed] [Google Scholar]
- 11.Kajiwara M, Nonoyama S, Ecuchi M, Morio T, Imai K, Okawa H, et al. WASP is involved in proliferation and differentiation of human haemopoietic progenitors in vitro. Br J Haematol 1999;107:254–62. [DOI] [PubMed] [Google Scholar]
- 12.Eto K, Kunishima S. Linkage between the mechanisms of thrombocytopenia and thrombopoiesis. Blood 2016;127:1234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mullen BCA, Anderson KD, Blaese RM. Splenectomy and/or bone marrow transplantation in the management of the Wiskott-Aldrich syndrome: long-term follow-up of 62 cases. Blood 1993;82:2961–6. [PubMed] [Google Scholar]
- 14.Haddad E, Cramer E, Rivière C, Rameau P, Louache F, Guichard J, et al. The thrombocytopenia of Wiskott Aldrich syndrome is not related to a defect in proplatelet formation. Blood 1999;94:509–18. [PubMed] [Google Scholar]
- 15.Shcherbina A, Rosen FS, Remold-O’Donnell E. Pathological events in platelets of Wiskott-Aldrich syndrome patients. Br J Haematol 1999;106:875–83. [DOI] [PubMed] [Google Scholar]
- 16.Snapper SB, Rosen FS, Mizoguchi E, Cohen P, Khan W, Liu CH, et al. Wiskott- Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity 1998;9:81–91. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Shehabeldin A, da Cruz LA, Butler J, Somani AK, McGavin M, et al. Antigen receptor-induced activation and cytoskeletal rearrangement are impaired in Wiskott-Aldrich syndrome protein-deficient lymphocytes. J Exp Med 1999; 190:1329–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabri S, Foudi A, Boukour S, Franc B, Charrier S, Jandrot-Perrus M, et al. Deficiency in the Wiskott-Aldrich protein induces premature proplatelet formation and platelet production in the bone marrow compartment. Blood 2006;108:134–40. [DOI] [PubMed] [Google Scholar]
- 19.Prislovsky A, Marathe B, Hosni A, Bolen AL, Nimmerjahn F, Jackson CW, et al. Rapid platelet turnover in WASP(−) mice correlates with increased ex vivo phagocytosis of opsonized WASP(−) platelets. Exp Hematol 2008;36:609–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marathe BM, Prislovsky A, Astrakhan A, Rawlings DJ, Wan JY, Strom TS. Antiplatelet antibodies in WASP(−) mice correlate with evidence of increased in vivo platelet consumption. Exp Hematol 2009;37:1353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prislovsky A, Strom TS. Increased uptake by splenic red pulp macrophages contributes to rapid platelet turnover in WASP(−) mice. Exp Hematol 2013;41:789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Recher M, Burns SO, De La Fuente M, Volpi S, Dahlberg C, Walter JE, et al. B cell-intrinsic deficiency of the Wiskott-Aldrich syndrome protein (WASp) causes severe abnormalities of the peripheral B-cell compartment in mice. Blood 2012; 119:2819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castiello MC, Bosticardo M, Pala F, Catucci M, Chamberlain N, van Zelm MC, et al. Wiskott-Aldrich Syndrome protein deficiency perturbs the homeostasis of B-cell compartment in humans. J Autoimmun 2014;50:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ault KA, Rinder HM, Mitchell J, Carmody MB, Vary CPH, Hillman RS. The significance of platelets with increased RNA content (reticulated platelets): a measure of the rate of thrombopoiesis. Am J Clin Pathol 1992;98:637–46. [DOI] [PubMed] [Google Scholar]
- 25.Bender M, Stritt S, Nurden P, van Eeuwijk JMM, Zieger B, Kentouche K, et al. Megakaryocyte-specific profilin1-deficiency alters microtubule stability and causes a Wiskott-Aldrich syndrome-like platelet defect. Nat Commun 2014;5: 4746. [DOI] [PubMed] [Google Scholar]
- 26.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods 1994;174:83–93. [DOI] [PubMed] [Google Scholar]
- 27.Bosticardo M, Draghici E, Schena F, Sauer AV, Fontana E, Castiello MC, et al. Lentiviral-mediated gene therapy leads to improvement of B-cell functionality in a murine model of Wiskott-Aldrich syndrome. J Allergy Clin Immunol 2011;127:1376–84. [DOI] [PubMed] [Google Scholar]
- 28.Brigida I, Sauer AV, Ferrua F, Giannelli S, Scaramuzza S, Pistoia V, et al. B-cell development and functions and therapeutic options in adenosine deaminase-deficient patients. J Allergy Clin Immunol 2014;133:799–806.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosticardo M, Marangoni F, Aiuti A, Villa A, Grazia Roncarolo M. Recent advances in understanding the pathophysiology of Wiskott-Aldrich syndrome. Blood 2009;113:6288–95. [DOI] [PubMed] [Google Scholar]
- 30.Pronk CJH, Rossi DJ, Månsson R, Attema JL, Norddahl GL, Chan CKF, et al. Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell 2007;1:428–42. [DOI] [PubMed] [Google Scholar]
- 31.Attar A Changes in the cell surface markers during normal hematopoiesis: a guide to cell isolation. Glob J Hematol Blood Transfus 2014;1:20–8. [Google Scholar]
- 32.Jordan MB, Van Rooijen N, Izui S, Kappler J, Marrack P. Liposomal clodronate as a novel agent for treating autoimmune hemolytic anemia in a mouse model. Blood 2003;101:594–601. [DOI] [PubMed] [Google Scholar]
- 33.Feist E, Burmester GR, Krüger E. The proteasome—victim or culprit in autoimmunity. Clin Immunol 2016;172:83–9. [DOI] [PubMed] [Google Scholar]
- 34.Dupuis-Girod S, Medioni J, Haddad E, Quartier P, Cavazzana-Calvo M, Le Deist F, et al. Autoimmunity in Wiskott-Aldrich syndrome: risk factors, clinical features, and outcome in a single-center cohort of 55 patients. Pediatrics 2003;111:e622–7. [DOI] [PubMed] [Google Scholar]
- 35.Catucci M, Castiello MC, Pala F, Bosticardo M, Villa A. Autoimmunity in wiskott-Aldrich syndrome: an unsolved enigma. Front Immunol 2012;3:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simon KL, Anderson SM, Garabedian EK, Moratto D, Sokolic RA, Candotti F. Molecular and phenotypic abnormalities of B lymphocytes in patients with Wiskott-Aldrich syndrome. J Allergy Clin Immunol 2014;133:896–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crestani E, Volpi S, Candotti F, Giliani S, Notarangelo LD, Chu J, et al. Broad spectrum of autoantibodies in patients with Wiskott-Aldrich syndrome and X-linked thrombocytopenia. J Allergy Clin Immunol 2015;136:1401–4, e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kolhatkar NS, Brahmandam A, Thouvenel CD, Becker-Herman S, Jacobs HM, Schwartz M, et al. Altered BCR and TLR signals promote enhanced positive selection of autoreactive transitional B cells in Wiskott-Aldrich syndrome. J Exp Med 2015;212:1663–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pala F, Morbach H, Castiello MC, Schickel J, Scaramuzza S, Chamberlain N, et al. Lentiviral-mediated gene therapy restores B cell tolerance in Wiskott- Aldrich syndrome patients. J Clin Invest 2015;125:3941–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elzey BD, Tian J, Jensen RJ, Swanson AK, Lees JR, Lentz SR, et al. Platelet-mediated modulation of adaptive immunity: a communication link between innate and adaptive immune compartments. Immunity 2003;19:9–19. [DOI] [PubMed] [Google Scholar]
- 41.Elzey BD, Sprague DL, Ratliff TL. The emerging role of platelets in adaptive immunity. Cell Immunol 2005;238:1–9. [DOI] [PubMed] [Google Scholar]
- 42.Sprague DL, Sowa JM, Elzey BD, Ratliff TL. The role of platelet CD154 in the modulation in adaptive immunity. Immunol Res 2007;39:185–93. [DOI] [PubMed] [Google Scholar]
- 43.Elzey BD, Ratliff TL, Sowa JM, Crist S. Platelet CD40L at the interface of adaptive immunity. Thromb Res 2011;127:180–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marangoni F, Trifari S, Scaramuzza S, Panaroni C, Martino S, Notarangelo LD, et al. WASP regulates suppressor activity of human and murine CD4(+)CD25(+) FOXP3(+) natural regulatory T cells. J Exp Med 2007;204:369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abrams C, Shattil SJ. Immunological detection of activated platelets in clinical disorders. Thromb Haemost 1991;65:467–73. [PubMed] [Google Scholar]
- 46.Vakkalanka RK, Woo C, Kirou KA, Koshy M, Berger D, Crow MK. Elevated levels and functional capacity of soluble CD40 ligand in systemic lupus erythematosus sera. Arthritis Rheum 1999;42:871–81. [DOI] [PubMed] [Google Scholar]
- 47.Aukrust P, Müller F, Ueland T, Berget T, Aaser E, Brunsvig A, et al. Enhanced levels of soluble and membrane-bound CD40 ligand in patients with unstable angina. Circulation 1999;100:614–20. [DOI] [PubMed] [Google Scholar]
- 48.Nagahama M, Nomura S, Kanazawa S, Ozaki Y, Kagawa H, Fukuhara S. Significance of chemokines and soluble CD40 ligand in patients with autoimmune thrombocytopenic purpura. Eur J Haematol 2002;69:303–8. [DOI] [PubMed] [Google Scholar]
- 49.Palazzo A, Bluteau O, Messaoudi K, Marangoni F, Chang Y, Souquere S, et al. The cell division control protein 42/Src family kinase/Neural Wiskott-Aldrich syndrome protein pathway regulates human proplatelet formation. J Thromb Haemost 2016;14:2524–35. [DOI] [PubMed] [Google Scholar]
- 50.Sokolic R, Oden N, Candotti F. Assessment of immature platelet fraction in the diagnosis of Wiskott-Aldrich syndrome. Front Pediatr 2015;3:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maugeri N, Rovere-Querini P, Evangelista V, Covino C, Capobianco A, Bertilaccio MTS, et al. Neutrophils phagocytose activated platelets in vivo: a phosphati-dylserine, P-selectin, and {beta}2 integrin-dependent cell clearance program. Blood 2009;113:5254–65. [DOI] [PubMed] [Google Scholar]
- 52.Manfredi A, Rovere-Querini P, Maugeri N. Dangerous connections: neutrophils and the phagocytic clearance of activated platelets. Curr Opin Hematol 2010;17:3–8. [DOI] [PubMed] [Google Scholar]
- 53.Maugeri N, Malato S, Femia E, Pugliano M, Campana L, Lunghi F, et al. Clearance of circulating activated platelets in polycythemia vera and essential thrombocythemia. Blood 2011;118:3359–66. [DOI] [PubMed] [Google Scholar]
- 54.Passacquale G, Vamadevan P, Pereira L, Hamid C, Corrigall V, Ferro A. Monocyte-platelet interaction induces a pro-inflammatory phenotype in circulating monocytes. PLoS One 2011;6:e25595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lam FW, Vijayan KV, Rumbaut RE. Platelets and their interactions with other immune cells. Compr Physiol 2015;5:1265–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Westerberg L, Larsson M, Hardy SJ, Fernández C, Thrasher AJ, Severinson E. Wiskott-Aldrich syndrome protein deficiency leads to reduced B-cell adhesion, migration, and homing, and a delayed humoral immune response. Blood 2005; 105:1144–52. [DOI] [PubMed] [Google Scholar]
- 57.Meyer-Bahlburg A, Becker-Herman S, Humblet-Baron S, Khim S, Weber M, Bouma G, et al. Wiskott-Aldrich syndrome protein deficiency in B cells results in impaired peripheral homeostasis. Blood 2008;112:4158–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Westerberg LS, De La Fuente M, Wermeling F, Ochs HD, Karlsson MCI, Snapper SB, et al. WASP confers selective advantage for specific hematopoietic cell populations and serves a unique role in marginal zone B-cell homeostasis and function. Blood 2008;112:4139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Becker-Herman S, Meyer-Bahlburg A, Schwartz MA, Jackson SW, Hudkins KL, Liu C, et al. WASp-deficient B cells play a critical, cell-intrinsic role in triggering autoimmunity. J Exp Med 2011;208:2033–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Castiello MC, Pala F, Sereni L, Draghici E, Inverso D, Sauer AV, et al. In vivo chronic stimulation unveils autoreactive potential of Wiskott-Aldrich syndrome protein-deficient B cells. Front Immunol 2017;8:490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Humblet-Baron S, Sather B, Anover S, Becker-Herman S, Kasprowicz DJ, Khim S, et al. Wiskott-Aldrich syndrome protein is required for regulatory T cell homeostasis. J Clin Invest 2007;117:407–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fiermonte G, De Leonardis F, Todisco S, Palmieri L, Lasorsa FM, Palmieri F. Identification of the mitochondrial ATP-Mg/Pi transporter. Bacterial expression, reconstitution, functional characterization, and tissue distribution. J Biol Chem 2004;279:30722–30. [DOI] [PubMed] [Google Scholar]
- 63.Traba J, Del Arco A, Duchen MR, Szabadkai G, Satrústegui J. SCaMC-1 promotes cancer cell survival by desensitizing mitochondrial permeability transition via ATP/ADP-mediated matrix Ca(2+) buffering. Cell Death Differ 2012;19:650–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoylaerts MF. Do lipid rafts contribute to platelet activation? J Thromb Haemost 2003;1:1140–1. [DOI] [PubMed] [Google Scholar]
- 65.Olsson M, Bruhns P, Frazier WA, Ravetch JV, Oldenborg P-A. Platelet homeostasis is regulated by platelet expression of CD47 under normal conditions and in passive immune thrombocytopenia. Blood 2005;105:3577–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gu M, Xi X, Englund GD, Berndt MC, Du X. Analysis of the roles of 14-3-3 in the platelet glycoprotein Ib-IX-mediated activation of integrin alpha(IIb)beta(3) using a reconstituted mammalian cell expression model. J Cell Biol 1999;147:1085–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.