Abstract
Objectives
Baseline retinal examination has long been recommended at hydroxychloroquine (HCQ) initiation, but it is unknown how well this guideline is followed. We investigated baseline eye examinations among U.S. Medicaid SLE patients initiating HCQ.
Methods
Using billing codes, we identified SLE patients aged 18-65 enrolled in Medicaid, residing in the 29 most populated U.S. states from 2000-2010. New HCQ users were identified by filling a prescription, with none in the preceding 12 months. Baseline retinal exams were identified within 30 days before to one year after this index prescription. We examined proportions of patients receiving retinal exams over the study years and compared characteristics of those who did and did not receive exams using bivariable and multivariable logistic regression models.
Results
Of 12,755 SLE patients newly starting HCQ, 32.5% received baseline dilated eye exams. The proportions of individuals receiving baseline eye exams did not significantly change during these years (31.0% to 34.4%, p for trend 0.12). Factors associated with increased likelihood of examinations included female sex, Asian versus White race, and receiving a higher number of laboratory tests during the preceding year. Lower proportions of Black and Native American versus White SLE patients had baseline retinal exams.
Conclusion
Only one third of Medicaid SLE patients newly initiating HCQ received recommended baseline retinal examinations and this proportion did not significantly increase during these years. The sociodemographic variation in this indicated care has been observed for other recommended medical care for SLE and requires both further investigation and interventions to address it.
Keywords: Hydroxychloroquine, SLE, retinal examination, Medicaid
INTRODUCTION
Hydroxychloroquine (HCQ) is the anchor drug for systematic lupus erythematosus (SLE), prescribed for the majority of patients1. Its primary toxicity is retinal damage, binding to and accumulating in the retinal pigment epithelium melanin2. If undetected, patients may gradually lose central vision3. Patients who receive higher HCQ doses, ≥5 mg/kg of actual weight, > 5 years of exposure, and those who have renal, hepatic or retinal disease are at increased HCQ retinal toxicity risk2-4.
Baseline retinal examination has long been recommended to document any pre-existing ocular conditions, fundal appearance and visual fields, and to establish risk status for HCQ toxicity2-4. The American Academy of Ophthalmology (AAO) first published practice guidelines for screening for HCQ retinopathy in 2002, with updates in 2011 and 20162-4. These have consistently recommended that all patients should have a baseline retinal examination within the first year of HCQ use2-4. Additional guidelines put forth by the American College of Rheumatology and those developed specifically for SLE in 2009 have also included funduscopic exam within one year of starting HCQ1, 5.
Adherence with ophthalmologic exam recommendations for HCQ users has been sub-optimal6, 7. A study of HCQ users with rheumatoid arthritis (RA) and SLE in a commercially-insured population reported that 64% of those continuously prescribed HCQ for ≥ 4 years received indicated retinal examinations7. The U.S. Medicaid program provides medical insurance for a large segment of the U.S. population with low-income and disability, including many SLE patients8. In general, medically-necessary eye examinations are covered by Medicaid, although there is state-to-state variation including prior authorizations and limitations on visit frequency9. We aimed to investigate the proportion of Medicaid SLE patients who receive indicated baseline retinal examinations and to identify factors associated with having recommended eye exams.
METHODS
Data Source
The Medicaid Analytic eXtract (MAX) contained all billing claims and demographic data for Medicaid beneficiaries from the 29 most populated U.S. states from January 1, 2000 through December 31, 2010. Medicaid is the U.S. public health insurance program covering >60 million racially and ethnically diverse, low-income individuals nationwide. The Institutional Review Board at Brigham and Women’s Hospital approved this study. A data use agreement with the Centers for Medicare and Medicaid studies was approved for the study of healthcare access and outcomes among SLE Medicaid patients.
Study Cohort
Prevalent SLE patients were identified by ≥ 3 International Classification of Disease- 9th revision (ICD-9) codes for SLE (710.0), each ≥ 30 days apart, from hospital discharge diagnoses or physician visit claims. HCQ new users were identified by a HCQ prescription filled after the first SLE diagnosis code, with no HCQ use in the preceding 12 months. The first HCQ prescription fill was the index date. The year before the index date was the covariate assessment period. Each patient was followed for 1 year from the index date. Patients were excluded if they did not have continuous Medicaid enrollment for 1 year before and after the index date.
Covariates
Covariate data were collected during the one year covariate assessment period. We collected data on several demographic factors (age, sex, race and U.S. state of residence). Race was self-reported and categorized as Black, White, Asian, Hispanic, American Indian/Alaska Native or other. Lupus nephritis fulfilled our validated definition10. Other comorbid conditions and other covariates were identified by ≥ 1 ICD-9, National Drug Code (NDC) or Current Procedural Terminology (CPT) code (diabetes mellitus, chronic pulmonary disease, hematological disorders, cardiovascular disease, cataracts, and osteoporosis), laboratory and imaging examinations (SLE serologies, including antinuclear antibodies [ANA], anti-dsDNA, complement testing [C3 and C4], ESR, CRP, bone mineral density tests, chest radiographs, and liver function tests), and injection/infusion corticosteroid use, were included to investigate differences in characteristics of patients who did versus did not receive baseline retinal examinations. We also intended to test the relationships between receiving other types of indicated healthcare and receiving indicated retinal examinations. Lab values and imaging results are not available in MAX.
Outcomes
The primary outcome was having at least one retinal exam within the 30 days before to one year after the date of HCQ initiation4 (Supplemental Table 1). We included the 30-day period before the index date to include baseline retinal examinations occurring prior to first prescription filling. The secondary outcome was broadened to include optional eye exams such as visual field exams (Humphrey tests), fundus photography, fluorescein angiography, electroretinogram (ERG), electro-oculogram (EOG), extended color vision examination, and spectral domain optimal coherence tomography (SD-OCT). SD-OCT was introduced in the mid-2000s, but was not extensively used during the study years and became part of the AAO recommendations for HCQ retinal examinations in 20162. We performed three separate sensitivity analyses. First, we excluded those with diabetes mellitus, cataracts or glaucoma at baseline as they may have had other reasons for undergoing retinal examinations. Secondly, we repeated analyses excluding CPT and HCPCS codes for established patient ophthalmic visits, including only initial visits, to assess the proportion of patients newly referred for retinal examination at the time of HCQ initiation. Lastly, we expanded the time window for baseline retinal examination for the primary analysis to include the 6 months prior through 18 months after HCQ prescription index date.
Statistical Analysis
We examined differences in baseline covariates between patients with and without baseline eye exams using chi-squared tests for categorical variables and t-tests for continuous variables. We calculated the annual proportion of new HCQ users who received eye exams per year of index date prescription. Poisson regression models were used to generate confidence intervals and tests for linear trend in the proportion of individuals who had baseline dilated retinal exams across study years. We used multivariable logistic regression models to estimate the odds of receiving baseline eye exams. These models included age, sex, calendar year, race, U.S. state, and baseline factors including lupus nephritis, other comorbid conditions and laboratories and examinations, and injection/infusion corticosteroids. All covariates that were significant at p< 0.05 level in bivariable analyses were included in the multivariable analysis. All analyses were conducted using SAS version 9.4 (Cary, North Carolina, U.S.).
RESULTS
We identified 12,755 SLE patients who were HCQ users enrolled in Medicaid. Among these, in our primary analysis, 4,148 (32.5%) patients received a recommended eye exam in the 30 days before to 1 year after HCQ initiation (Table 1). This proportion increased to 40.0% when Humphrey visual field tests and other optional exams were included. Figure 1 illustrates the proportions of those who received recommended retinal examinations within one year of HCQ initiation. These proportions did not significantly increase from 31.0% of those who initiated HCQ in 2001 (allowing for the one year baseline period) to 34.4% in 2009 (allowing for one year of follow-up time for all, p for linear trend over time 0.12). In sensitivity analyses excluding those with claims for diabetes mellitus, cataracts, and glaucoma during the preceding 12 months who may have had their retinal examinations for other reasons, 31.3% received eye exams. In a separate sensitivity analysis, we excluded those with established patient codes as the retinal examination we captured may not have been a baseline screening for newly starting on HCQ, and the proportions were much lower, only 17.7% (1,846 of 10,453) for a recommended retinal examination and 26.8% when other optional retinal examinations were included. We also identified 798 (6.3%) patients with HCQ prescriptions preceding the baseline period, and of these 392 (49%) had ≥ 1 earlier retinal exam. When we expanded the time window surrounding HCQ prescription date to 6 months before through 18 months following index date, the proportion who received any retinal exam increased to 6,665 (52.3%).
Table 1.
Proportions of SLE Medicaid Patients Newly Initiating Hydroxychloroquine in 29 U.S. States, 2000-2010, who received Baseline Ophthalmologic Examinations (30 days prior through one year after first prescription fill)
| Primary Analysis, n= 12,755 | Sensitivity Analysis excluding those with diabetes, cataracts or and glaucoma during the preceding 12 months, n= 10,836 | Sensitivity Analysis excluding those with established patient codes, n=10,453 | Sensitivity Analysis allowing 6 months prior through 18 months after first prescription fill, n= 12,755 | |
|---|---|---|---|---|
| Recommended Retinal Examinations*, % | 4,148 (32.5) | 3,387 (31.3) | 1,846 (17.7) | 5,680 (44.5) |
| Humphrey Visual Field Test and Other Additional Optional Examinations†, % | 3,093 (24.3) | 2,478 (22.9) | 1,764 (16.9) | 4,096 (32.1) |
| > 1 of above examinations, % | 5,099 (40.0) | 4,154 (38.3) | 2,797 (26.8) | 6,665 (52.3) |
Recommended dilated retinal examinations per American Academy of Ophthalmology guidelines 2002 and American College of Rheumatology 2009.
Optional eye exams include visual field exams (Humphrey tests), fundus photography, fluorescein angiography, electroretinogram (ERG), electro-oculogram (EOG), extended color vision examination, and spectral domain optimal coherence tomography (SD-OCT). Definitions of these examinations based on Current Procedural Terminology (CPT) and Healthcare Common Procedure Coding System (HCPCS) Codes found in Supplementary Table 1.
Figure 1. Proportions of Medicaid SLE Patients in 29 U.S. States newly-initiating Hydroxychloroquine who received Baseline Retinal Examinations from 2001-2009.
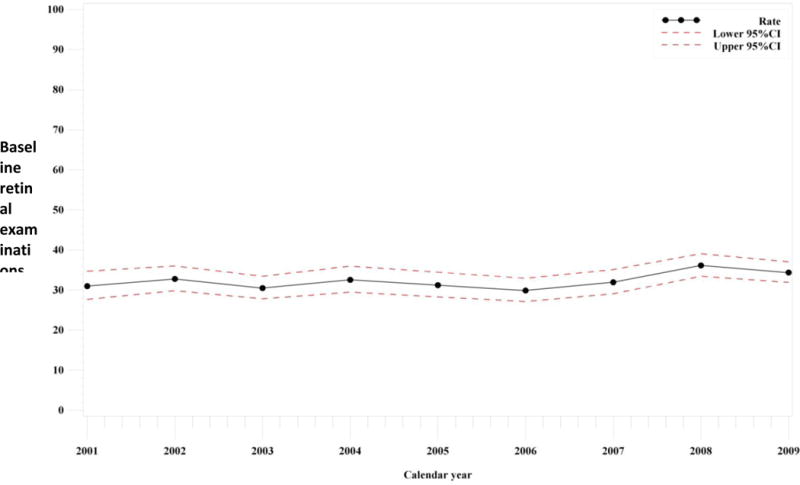
*Confidence intervals and trends were derived from Poisson regression. p linear trend = 0.12
In bivariable analyses, we observed that those who had exams with HCQ initiation were slightly older, more were White and fewer Black, and fewer had lupus nephritis, but a higher proportion had other chronic comorbidities, such as diabetes mellitus and osteoporosis, as well as cataracts and glaucoma (Table 2). The patients who had had recommended eye exams were more likely to have had baseline SLE laboratory tests, liver function tests, and chest radiographs and slightly more were receiving intravenous corticosteroids. However, of the 2,398 patients who had lupus nephritis, a lower proportion, only 30% (726), had baseline retinal examinations.
Table 2.
Baseline Characteristics of SLE patients in Medicaid 2000-2010 newly initiating Hydroxychloroquine, Stratified by Receipt of Recommended Baseline Recommended Retinal Exams
| Total (N=12,755) |
Examined (N=4,148) |
Not Examined (N=8,607) |
Bivariable p value†* |
Multivariable Odds Ratio‡ (95%CI) | |
|---|---|---|---|---|---|
| Mean Age, (SD) | 38.3 (11.8) | 39.8 (11.9) | 37.5 (11.6) | <0.01 | 0.98 (0.82, 1.16) |
| Female, % | 94.8 | 95.7 | 94.4 | <0.01 | 1.30 (1.08, 1.55) |
| Race, % | <0.01 | ||||
| Black | 43.0 | 40.0 | 44.4 | 0.85 (0.77, 0.93) | |
| White | 31.6 | 33.2 | 30.9 | Ref. | |
| Hispanic | 18.3 | 18.7 | 18.1 | 0.95 (0.85, 1.08) | |
| Asian | 3.8 | 4.6 | 3.4 | 1.30 (1.06, 1.60) | |
| American Indian/Alaska Native | 1.2 | 0.9 | 1.4 | 0.69 (0.47, 1.02) | |
| Others | 2.1 | 2.6 | 1.9 | 1.14 (0.87, 1.47) | |
| Lupus Nephritis, % | 18.8 | 17.5 | 19.4 | <0.01 | 0.91 (0.82, 1.00) |
| Injection/Infusion Corticosteroids, % | 7.1 | 8.0 | 6.7 | <0.01 | 1.03 (0.89, 1.20) |
| Comorbid Conditions, % | |||||
| Chronic Pulmonary Disease | 20.9 | 21.5 | 20.6 | 0.22 | |
| Hematological Disorder | 18.3 | 18.3 | 18.4 | 0.91 | |
| Cardiovascular Disease | 7.8 | 8.3 | 7.5 | 0.13 | |
| Diabetes Mellitus | 11.5 | 13.1 | 10.8 | <0.01 | 1.16 (1.03, 1.30) |
| Osteoporosis | 4.3 | 5.4 | 3.8 | <0.01 | 1.21 (1.00, 1.46) |
| Cataract | 2.0 | 2.3 | 1.9 | <0.01 | 1.61 (1.24, 2.10) |
| Glaucoma | 3.2 | 5.4 | 2.1 | <0.01 | 2.37 (1.93, 2.91) |
| Examinations, % | |||||
| SLE-Related Laboratories§ | 81.0 | 86.1 | 78.5 | <0.01 | 1.33 (1.16, 1.52) |
| Bone Mineral Density Tests‖ | 2.3 | 3.2 | 1.9 | <0.01 | 1.26 (0.98, 1.62) |
| Chest Radiograph | 47.6 | 49.5 | 46.7 | <0.01 | 1.01 (0.93, 1.09) |
| Liver Function Tests | 64.9 | 70.1 | 62.4 | <0.01 | 1.16 (1.04, 1.29) |
Baseline characteristics assessed during the 12-month period before index new prescription for hydroxychloroquine and dilated retinal examinations (outcome) assessed during the 30 days prior to and 12 months following index new prescription date.
t test for continuous age and Chi squared tests for all categorical variables
Multivariable models including age, sex, calendar year, race, U.S. state of residence, lupus nephritis, comorbid conditions, examinations, SLE laboratories and injection/infusion corticosteroids.
SLE-Related Laboratories:1 of ANA, anti-dsDNA, C3, C4, ESR, CRP
Bone mineral density tests: CPT codes 77078-77083
Liver function tests: CPT codes 80053, 80076, 84450, 84460
Multivariable logistic regression models revealed that factors associated with increased likelihood of having baseline retinal examinations included female vs. male sex (adjusted OR 1.30 [95%CI 1.08,1.55]), Asians vs. White race (adjusted OR 1.30 [1.06,1.60]), and having other eye conditions, such as cataracts, glaucoma and diabetes. On the other hand, it appeared that Blacks and American Indian/Alaska Natives were less likely than Whites to receive this indicated care (OR 0.85 [0.77, 0.93] and OR 0.69 [0.47, 1.02]). Those who had received increased numbers of SLE-related laboratories and liver function tests had higher odds for receiving eye exams within HCQ initiation (Table 2). However, we observed no relationships between the presence of lupus nephritis, the use of injection or infusion corticosteroids, or chest radiographs during the period prior to HCQ initiation, and likelihood of having retinal examinations. In the sensitivity analysis excluding those with baseline diabetes mellitus, cataracts and glaucoma, the same patterns were found: females vs. males OR 1.22 [1.01, 1.49]), Asians OR 1.26 [1.01, 1.58]), Blacks OR 0.85 [0.76, 0.94] and American Indian/Alaska Natives OR 0.63 [0.41, 0.98] vs. Whites; and OR 1.38 [1.19, 1.60] and 1.14 [1.01, 1.27]) with more SLE laboratories and liver function testing at baseline. Similarly, in the sensitivity analysis excluding established patient billing codes visits, although the sample size was smaller, results were similar, e.g. OR for females vs. males 1.65 (1.26, 2.16) and for Blacks vs. Whites 0.89 (0.78, 1.01).
DISCUSSION
Guidelines consistently recommend baseline dilated retinal examinations within one year of HCQ initiation to establish fundal appearance and document abnormalities, allowing for future toxicity screening2, 4. In this Medicaid SLE cohort, retinal exams took place in only 32.5% in the first year of HCQ. The proportion increased only slightly from 31.0% in 2001 to 34.4% of those who initiated this treatment in 2009. Lupus nephritis patients, at higher retinal toxicity risk, did not receive more indicated baseline exams. Female vs. male and Asian vs. White SLE patients, and patients receiving more frequent SLE laboratory exams and liver function testing during the baseline period had higher likelihood of having indicated dilated retinal examinations with HCQ initiation. In contrast, Blacks and Native American/Alaska Natives compared to Whites had reduced odds of having this recommended screening exam. We have previously observed that these sociodemographic factors have been associated with differences in both access to care and adherence in the SLE Medicaid population for indicated care, including infection prevention, adherence to indicated medications including HCQ, treatments for lupus nephritis, and choices in renal replacement therapies, and use of erythropoetin-stimulating agents among SLE end-stage renal disease patients11-14.
Receiving a baseline HCQ retinal exam was associated with increased receipt of other SLE care and laboratory testing. Both patient and physician factors determining adherence with indicated preventive medical care are likely involved. Additionally, as HCQ is associated with increased toxicity in the setting of retinal or hepatic disease, the increased odds of having baseline retinal examination in the presence of other eye disease and increased numbers of liver function tests could reflect increased vigilance for retinal toxicity2-4. However, HCQ retinal toxicity risk is also elevated in lupus nephritis patients, who had a lower proportion (30%) receiving baseline screening2-4. When we liberalized the screening time period to 6 months before through 18 months after initial prescription, the proportion of patients receiving any eye examination increased to 52%; patients and providers may be slow to obtain retinal exams or insurance may restrict access in some states.
A few past studies have examined retinal screening among SLE patients starting HCQ. Nika et al employed the OptumInsight administrative database to examine ophthalmologic testing rates among 6,339 commercially-insured RA and SLE HCQ users from 2001-2011. The testing items included perimetry, multifocal electroretinography, fluorescein angiography, and fundus photography7. They found the eye exam rate was 48.8% overall and 63.9% among patients continuously exposed to HCQ for at least 4 years. For less frequent HCQ users, 44.5% received ophthalmologic examinations during the 5-year period7. A smaller 2003 study of the Montreal General Hospital SLE population reported that only half of the 52 patients receiving antimalarials for ≥ 5 years were adherent with ACR retinal examination guidelines, but did not report baseline examinations separately. Thus, adherence with guidelines for retinal examinations for patients starting HCQ may be poor in a variety of academic, private insurance and public insurance settings, and a target for improvement. The prevalence of additional optional testing (fundus photography, fluorescein angiography, ERG, EOG, and extended color vision examination, SD-OCT) recommended by 2002 AAO guideline at baseline was approximately 24% in this Medicaid cohort. Low use of SD-OCT and ERG was also found in the commercially-insured RA or SLE population7. These findings were expected before 2010, because these procedures were not prevalent then and optional per the 2002 AAO guidelines. In the 2011 AAO revised guidelines, recommended retinal exams included one subjective test and one objective test (SD-OCT, multifocal ERD or fundus autofluorescence)3. HCQ screening entirely consistent with the later 2011 AAO guidelines was reported to be 54% in a study of 2011-2014 HCQ users seen in a large ophthalmologic clinic in Cleveland15.
The current study cohort is comprised of over 12,000 SLE HCQ new users and has an extended study period. It is thus capable of representing practice patterns in Medicaid over this recent time span. However, there were limitations due to the nature of administrative data. First, the positive predictive value of codes for eye exams is unknown and it is possible that we over-estimated the true exam rates. We excluded the procedure codes for follow-up ophthalmic visits in sensitivity analyses to increase specificity for baseline HCQ eye exams. We cannot confirm the eye exams were scheduled for HCQ new use, although we investigated the period of 30 days before through 1 year after HCQ initiation to approximate exams to new HCQ prescriptions. Physician specialty is not available within MAX. Ophthalmologic sub-specialty preferential practice patterns have been reported for HCQ eye exams: retinal specialists may perform more retinal imaging, while comprehensive ophthalmologists rely upon Amsler grid testing15.
From 2001-2010, the proportion of Medicaid SLE patients initiating HCQ having recommended baseline retinal examinations was low despite multiple guidelines2-4. We found substantial sociodemographic variation in this care, likely reflecting problems with both healthcare access and adherence. As in our past studies of quality of care for SLE in the Medicaid population, we found that, compared to Whites, a lower proportion of Blacks had recommended baseline examinations, while a higher proportion of Asians did13,14. While adherence to daily dosing, by the latest recommendations < 5 mg/kg actual weight, is a more important factor determining retinal toxicity risk, baseline examinations are necessary to avoid treating patients with pre-existing retinal disease and to allow early detection of changes from baseline. Quality improvement interventions should include efforts to increase retinal examinations among SLE patients starting HCQ, particularly in the Medicaid population.
Supplementary Material
SIGNIFICANCE AND INNOVATION.
As HCQ retinal toxicity risk can be minimized with baseline retinal examination that allows early detection of changes, multiple past clinical guidelines have recommended baseline retinal examination for all patients initiating HCQ therapy. However, it was not known how well these recommendations are followed in clinical practice. We employed Medicaid data from the 29 most populated U.S. states from 2000-2010 to examine the proportions of patients with SLE newly starting HCQ who received an indicated baseline retinal examination.
Only 32.5% of patients received recommended retinal examinations in the 30 days before through one year after starting HCQ, and this proportion increased to 40% when other optional ophthalmologic examinations were included.
We found substantial sociodemographic variation in receipt of these examinations, likely reflecting problems with both healthcare access and adherence to care. Compared to Whites, a lower proportion of Blacks, but a higher proportion of Asians had recommended baseline examinations.
While the proportions of patients with diabetes, glaucoma and cataracts who underwent retinal examinations were slightly higher than the overall proportion, the proportion who had exams was lower among patients with lupus nephritis, who are also at increased risk of HCQ retinal toxicity.
Acknowledgments
Grant support: This work was supported Rheumatology Research Foundation Awards (Feldman and Barbhaiya), NIH K23 AR071500 (Feldman), NIH K24 AR066109 (Costenbader), and NIH R01 AR057327 (Costenbader).
Footnotes
DR. TZU-CHIEH LIN (Orcid ID : 0000-0002-8333-7666)
DR. SARAH CHEN (Orcid ID : 0000-0002-8206-597X)
References
- 1.Yazdany J, Panopalis P, Gillis JZ, Schmajuk G, MacLean CH, Wofsy D, Yelin E. A quality indicator set for systemic lupus erythematosus. Arthritis Rheum. 2009;61(3):370–7. doi: 10.1002/art.24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF, American Academy of O Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy (2016 Revision) Ophthalmology. 2016;123(6):1386–94. doi: 10.1016/j.ophtha.2016.01.058. [DOI] [PubMed] [Google Scholar]
- 3.Marmor MF, Kellner U, Lai TY, Lyons JS, Mieler WF, American Academy of O Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011;118(2):415–22. doi: 10.1016/j.ophtha.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 4.Marmor MF, Carr RE, Easterbrook M, Farjo AA, Mieler WF. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology. 2002;109(7):1377–82. doi: 10.1016/s0161-6420(02)01168-5. [DOI] [PubMed] [Google Scholar]
- 5.Guidelines for monitoring drug therapy in rheumatoid arthritis. American College of Rheumatology Ad Hoc Committee on Clinical Guidelines. Arthritis Rheum. 1996;39(5):723–31. [PubMed] [Google Scholar]
- 6.Bernatsky S, Pineau C, Joseph L, Clarke A. Adherence to ophthalmologic monitoring for antimalarial toxicity in a lupus cohort. J Rheumatol. 2003;30(8):1756–60. [PubMed] [Google Scholar]
- 7.Nika M, Blachley TS, Edwards P, Lee PP, Stein JD. Regular examinations for toxic maculopathy in long-term chloroquine or hydroxychloroquine users. JAMA Ophthalmol. 2014;132(10):1199–208. doi: 10.1001/jamaophthalmol.2014.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldman CH, Hiraki LT, Liu J, Fischer MA, Solomon DH, Alarcon GS, Winkelmayer WC, Costenbader KH. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000-2004. Arthritis Rheum. 2013;65(3):753–63. doi: 10.1002/art.37795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson P. Medicaid and indigent care issue brief: Medicaid: benefits and services: year end report-2003. Issue Brief Health Policy Track Serv. 2003:1–33. [PubMed] [Google Scholar]
- 10.Chibnik LB, Massarotti EM, Costenbader KH. Identification and validation of lupus nephritis cases using administrative data. Lupus. 2010;19(6):741–3. doi: 10.1177/0961203309356289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devlin A, Waikar SS, Solomon DH, Lu B, Shaykevich T, Alarcon GS, Winkelmayer WC, Costenbader KH. Variation in initial kidney replacement therapy for end-stage renal disease due to lupus nephritis in the United States. Arthritis Care Res (Hoboken) 2011;63(12):1642–53. doi: 10.1002/acr.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez-Puerta JA, Waikar SS, Solomon DH, Liu J, Alarcon GS, Winkelmayer WC, Costenbader KH. Erythropoiesis-stimulating Agent Use among Patients with Lupus Nephritis Approaching End-stage Renal Disease. J Clin Cell Immunol. 2013;4(6):179. doi: 10.4172/2155-9899.1000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yazdany J, Feldman CH, Liu J, Ward MM, Fischer MA, Costenbader KH. Quality of care for incident lupus nephritis among Medicaid beneficiaries in the United States. Arthritis Care Res (Hoboken) 2014;66(4):617–24. doi: 10.1002/acr.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feldman CH, Hiraki LT, Winkelmayer WC, Marty FM, Franklin JM, Kim SC, Costenbader KH. Serious infections among adult Medicaid beneficiaries with systemic lupus erythematosus and lupus nephritis. Arthritis Rheumatol. 2015;67(6):1577–85. doi: 10.1002/art.39070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Au A, Parikh V, Modi YS, Ehlers JP, Schachat AP, Singh RP. Hydroxychloroquine screening practice patterns within a large multispecialty ophthalmic practice. American Journal of Ophthalmology. 2015;160(3):561–8 e2. doi: 10.1016/j.ajo.2015.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


