Abstract
Neurotensin (NT) is a 13 amino acid neuropeptide that is expressed throughout the central nervous system and is implicated in the etiology of multiple diseases and disorders. Many primary investigations of NT-induced modulation of neuronal excitability at the level of the synapse have been conducted, but they have not been summarized in review form in nearly 30 years. Therefore, the goal of this review is to discuss the many actions of NT on neuronal excitability across brain regions as well as NT circuit architecture. In the basal ganglia as well as other brain nuclei NT can act through diverse intracellular signaling cascades to enhance or depress neuronal activity by modulating activity of ion channels, ionotropic and metabotropic neurotransmitter receptors, and presynaptic release of neurotransmitters. Further, NT can produce indirect effects by evoking endocannabinoid release, and recently has itself been identified as a putative retrograde messenger. In the basal ganglia the diverse actions and circuit architecture of NT signaling allow for input-specific control of reward-related behaviors.
Keywords: neuropeptide, endocannabinoids, methamphetamine, VTA, substantia nigra
Graphical abstract
Neurotensin-induced modulation of neuronal excitability is reviewed here, revealing diverse actions across many brain regions. In midbrain dopamine neurons, neurotensin induces plasticity at glutamatergic, GABAergic, and dopaminergic synapses, leading to complex effects on motivated behavior. The diverse signaling mechanisms and actions of this neuromodulatory peptide will require further investigation but could provide promising treatments for mood and motivation-related disorders.
Introduction
Neurotensin (NT) is a signaling molecule with remarkably diverse actions. Since the initial discovery of NT by Carraway & Leeman (1973), the wide range of NT actions throughout the body has led to its study in the context of cancer, inflammation, pain, Alzheimer’s disease, obesity, and anxiety (Qiu et al., 2017; Black, 2002; Kleczkowska & Lipkowski, 2013; Xiao et al., 2014; Li et al., 2016; Shilling & Feifel, 2008, Normandeau et al., 2017). The role of NT in ethanol and other drug use disorders has also been heavily studied (Erwin & Su, 1989; Erwin et al., 1995, 2001; Ferraro et al., 2016) and has led to an extensive accumulation of literature centered around NT action as a neuromodulator in the basal ganglia, although NT functions have been widely observed throughout the brain. Jennes and colleagues (1982) strikingly noted in their initial investigation into central NT expression that, “areas with low [NT] immunoreactivity, or lack of it, stand out.” The extensive presence of NT throughout the brain is matched by its numerous reported interactions with neurons. However, there have been no recent reviews that focus on the action of NT at the level of the synapse or the expanding literature describing the role of NT circuit architecture in physiology and behavior. The current review delineates findings from rodent models regarding NT physiology including modulation of neuronal excitability and the role of NT circuitry in behavior, with emphasis on NT action in the basal ganglia and related nuclei. The diverse actions of NT, circuit-specificity of NT signaling, and modulation of a range of behaviors may allow for the development of NT-based pharmacotherapeutic treatments for mood and motivation-related disorders.
Receptor physiology
There are four known subtypes of NT receptor. The NT type-1 (NTS1) and type-2 (NTS2) receptors are G protein-coupled receptors, and the type-3 (NTS3, also known as sortilin) and type-4 (NTS4, also known as SORL1) receptors are sorting receptors, with domain structures similar to the vacuolar protein sorting 10 protein (Vps10p) domain receptor family (Tanaka et al., 1990; Marcusson et al., 1994; Chalon et al., 1996; Mazella et al., 1996; Mazella et al., 1998). While NT exhibits high affinity for all four receptor subtypes, NT has higher affinity at NTS1 and NTS3 (0.1–0.3 nM) compared to NTS2 (1–5 nM) and NTS4 (30 nM) (Mazella et al., 1988; Jacobsen et al., 2001; Pelaprat, 2006). All four NT receptors are expressed (to varying extents) in almost all brain regions (Elde et al., 1990; Nicot et al., 1994; Nicot et al., 1995; Boudin et al., 1996; Mazella et al., 1996; Alexander & Leeman, 1998; Sarret et al., 1998; Walker et al., 1998; Motoi et al., 1999; Fassio et al., 2000; Sarret et al., 2003a; Sarret et al., 2003b; Geisler et al., 2006). An exhaustive description of the precise location and expression levels of NT and NT receptors is beyond the scope of this review, but has been provided previously (Geisler et al., 2006). NT receptor function proceeds through several different intracellular signaling pathways and varies by the specific cell-type on which the receptors are expressed. NTS1 has been reported to couple to Gαi/o, Gαs, and Gαq/11 G proteins (Shi & Bunney, 1992b; Jiang et al., 1994; Wu & Wang, 1995; Gailly et al., 2000; Pelaprat, 2006). The intracellular signaling mechanisms of NTS2 are not as well understood and may differ between human and mouse, but potentially involve Gαi/o, Gαq/11, and/or Gα12/13 (Vita et al., 1998; Holst et al., 2004; Mazella & Vincent, 2006). There are currently no pharmacological tools available to isolate NTS3 or NTS4 activity, and as such little is known of their specific physiological function in the context of neuromodulation. Regardless, the diversity of receptors and second messenger cascades activated by NT contributes to the modulation of neuronal excitability across many cell types and brain regions.
Direct effects on neuronal excitability
NT-induced depolarization of neurons has been extensively reported in the basal ganglia and elsewhere, and usually occurs via NTS1. This has been observed in the diagonal band of Broca (Jassar et al., 1999), dorsal raphe nucleus (Jolas & Aghajanian, 1996), entorhinal cortex (Xiao et al., 2014), globus pallidus (Chen et al., 2004), substantia nigra pars compacta (SNc; Wu et al., 1995, Wu & Wang, 1995), and the ventral tegmental area (VTA; Jiang et al., 1994) (Table 1). Other direct excitatory effects of NT have also been reported, but those experiments were carried out before a NTS1 selective antagonist was available (see Shi & Bunney 1992a for review). The specific ion channels that are responsible for NTS1-induced depolarization vary by brain region. In the entorhinal cortex, NT depolarizes pyramidal cells via inhibition of the TWIK-related K+ (TREK)-2 channel (Xiao et al., 2014). Similarly, in the dentate gyrus, NT depolarizes granule cells via inhibition of the TWIK-related acid-sensitive K+ (TASK)-3 channel (Zhang et al., 2016). In the diagonal band of Broca, NT depolarizes neurons by decreasing a K+ conductance through voltage-sensitive Ca2+-activated K+ channels, in part by modulating Ca2+ entry through T- and/or N-type Ca2+ channels (Jassar et al., 1999). NT-induced depolarization of dopamine neurons in the SNc proceeds through Gαq/11 activation, increasing intracellular Ca2+ release via inositol triphosphate (IP3) receptors (Wu et al., 1995). Single-channel analysis of this unidentified cation channel indicates that it is capable of opening in bursts and exhibits permeability to both Na+ and Cs+ (Chien et al., 1996). It is possible that transient receptor potential (TRP) non-selective cation channels also play a role in NT effects on dopamine neurons, as application of Zn2+ or SKF96365 (both capable of blocking TRP channels) partially block NT-induced excitation (St-Gelais et al., 2004; Stuhrman & Roseberry, 2015). Thus, NT-induced neuronal depolarization by NTS1 proceeds through different intracellular signaling cascades and ion channels depending on the cell type and brain region.
Table 1.
Neurotensin-induced modulation of neuronal excitability
| Brain region | Neuron type | NT receptor(s) | Intracellular signaling | Action | Net effect | References |
|---|---|---|---|---|---|---|
| Amygdala (Basolateral) | Pyramidal | NTS1 | -- |
 glutamate/AMPA glutamate/AMPA |
Excite | Amano et al., 2008 |
|
| ||||||
| Basal forebrain | Cholinergic | -- | Ca2+ | Postsynaptic depolarization | Excite | Alonso et al., 1994 |
|
| ||||||
| Cerebral cortex | Pyramidal | NTS2 | -- |
 GABAA GABAA
|
Inhibit | Case et al., 2017 |
| -- |
 GABAB GABAB
|
|||||
| Fast spiking interneuron | -- | -- | Depolarization | Excite | Case et al., 2017 | |
|
| ||||||
| Cortex Layer 6b | Slow bursting | -- | -- | Postsynaptic depolarization | Excite | Combremont et al., 2016 |
|
| ||||||
| Dentate gyrus | Granule | NTS1 | Gαq/11 |
 TASK-3 K+conductance TASK-3 K+conductance |
Excite |
Zhang et al., 2015 Zhang et al., 2016 |
| Gαq/11, calmodulin, myosin light-chain kinase |
 glutamate/AMPA glutamate/AMPA |
|||||
|
| ||||||
| Diagonal band of Broca | Cholinergic | NTS1 | Ca2+ | Depolarization | Excite | Matthews et al., 1999 |
| Non-cholinergic | -- | -- | Depolarization | Excite | Jassar et al., 1999 | |
|
| ||||||
| Dorsal raphe nucleus | Serotonergic | NTS1 | -- | Depolarization | Excite | Jolas & Aghajanian, 1996 |
|
| ||||||
| Dorsolateral striatum | Medium spiny projection | NTS1 or NTS2 | -- |
 endo-cannabinoid endo-cannabinoid glutamate glutamate |
Excite | Yin et al., 2008 |
|
| ||||||
| Entorhinal cortex | Pyramidal | NTS1 | PLC, PKC |
 Trek-2 K+conductance Trek-2 K+conductance |
Excite | Xiao et al., 2014 |
|
| ||||||
| Globus pallidus | -- | NTS1 | PLC |
 glutamate glutamate |
Excite |
Chen et al., 2006 Chen et al., 2004 |
| -- |
 non-selective cation channel conductance non-selective cation channel conductance |
|||||
| -- |
 K+ conductance K+ conductance |
|||||
|
| ||||||
| Hippocampus CA1 | Pyramidal | NTS1 | PLC, PKC, IP3, Ca2+ |
 GABAA GABAA
|
Inhibit | Li et al., 2008 |
| Interneuron | NTS1 | -- | Depolarization | Excite | ||
|
| ||||||
| Hypothalamus | Orexin expressing | NTS1 or NTS2 | -- |
 non-selective cation channel conductance non-selective cation channel conductance |
Excite | Furutani et al., 2013 |
|
| ||||||
| Nucleus of the solitary tract | -- | NTS1 | -- |
 K+ conductance K+ conductance |
Excite or inhibit | Ogawa et al., 2005 |
| -- |
 GABAA GABAA
|
|||||
| -- |
 AMPA AMPA |
|||||
|
| ||||||
| Nucleus raphe magnus | Serotonin | NTS3 or NTS4 | Gαq/11, IP3, Ca2+ |
 non-selective cation channel conductance non-selective cation channel conductance |
Excite | Li et al., 2001b |
|
| ||||||
| Bed nucleus of the stria terminalis | -- | NTS1 or NTS2 | -- |
 GABAA GABAA
|
Inhibit |
Krawczyk et al., 2013 Normandeau et al., 2017 |
|
| ||||||
| Parabrachial nucleus | -- | NTS1 | -- |
 glutamate/AMPA glutamate/AMPA |
Excite | Saleh et al., 1997 |
|
| ||||||
| Periaqueductal gray | -- | NTS1 | -- | Depolarization | Excite |
Mitchel et al., 2009 Li et al., 2001a |
| -- |
 glutamate/mGlur5 glutamate/mGlur5 |
|||||
| -- |
 endo-cannabinoid endo-cannabinoid |
|||||
| -- |
 GABAA GABAA
|
|||||
| NTS3 or NTS4 | Gαq/11, IP3, Ca2+ | Depolarization | ||||
|
| ||||||
| Substantia nigra pars compacta | Dopamine | NTS1 | Gαq/11, IP3, Ca2+ |
 non-selective cation channel conductance non-selective cation channel conductance |
Excite or inhibit |
Shi & Bunney, 1992b Wu et al., 1995 Wu & Wang, 1995 Piccart et al., 2015 Gantz & Bean, 2017 Tschumi & Beckstead, 2018 |
| PKC |
 K+ conductance K+ conductance |
|||||
| PKA |
 GABAA GABAA
|
|||||
| NTS2 | -- |
 GABAB GABAB
|
||||
| -- |
 D2 D2 |
|||||
| -- | PKA |
 D2 D2 |
||||
| -- |
 2-arachidonyl-glycerol 2-arachidonyl-glycerol |
|||||
| -- |
 A-type K+ conductance A-type K+ conductance |
|||||
|
| ||||||
| Supra-chiasmatic nucleus | -- | NTS3 or NTS4 | -- | -- | Excite or inhibit | Coogan et al., 2001 |
|
| ||||||
| Superior colliculus | -- | -- | -- |
 glutamate sensitivity glutamate sensitivity |
Inhibit | Zhang et al., 1996 |
|
| ||||||
| Ventral tegmental area | Dopamine | NTS1 | Gαq/11, PLC |
 non-selective cation channel conductance non-selective cation channel conductance |
Excite or inhibit |
Shi & Bunney, 1992b Jiang et al., 1994 Chien et al., 1996 Kortleven et al., 2012 Kempadoo et al., 2013 Bose et al., 2015 Piccart et al., 2015 Stuhrman & Roseberry, 2015 |
| PLC |
 2-arachidonyl-glycerol 2-arachidonyl-glycerol |
|||||
| -- |
 glutamate glutamate |
|||||
| -- |
 glutamate/NMDA glutamate/NMDA |
|||||
| NTS2 or NTS3 or NTS4 | Ca2+ |
 glutamate/NMDA/AMPA glutamate/NMDA/AMPA |
||||
| -- | -- |
 GABAB GABAB
|
||||
| PKA |
 D2 D2 |
|||||
| Non-dopamine | NTS2 | -- |
 glutamate glutamate |
|||
NT also increases neuronal excitability through non-NTS1 receptors. In the periaqueductal gray and nucleus raphe magnus, like in the SNc and VTA, NT directly depolarizes neurons in a Gαq/11- and IP3-dependent manner (Li et al., 2001a, Li et al., 2001b). However, while direct depolarization is mediated by NTS1 in the SNc and VTA, in the periaqueductal gray and nucleus raphe magnus it is mediated by neither NTS1 or NTS2, suggesting a role for NTS3 and/or NTS4 (Li et al., 2001a, Li et al., 2001b). Similarly, neurons of the suprachiasmatic nucleus increase firing after NT application, and this effect persists in the presence of a NTS1 or non-selective NTS1/2 antagonist (Coogan et al., 2001). Reports also indicate inter-species differences in the NT-induced depolarization of dopamine neurons. For instance, NT depolarizes dopamine neurons in guinea pig midbrain in two distinct ways: a slow NTS1-mediated decrease in the afterhyperpolarization observed following an action potential, and a more rapid NTS2-mediated effect (Nalivaiko et al., 1998) that has not been reported in other species. Overall, NT can directly increase neuronal excitability through NTS1, NTS2, and likely NTS3 or NTS4 receptors.
Postsynaptic modulation of neurotransmitter signaling
In addition to directly depolarizing neurons, NT can also affect neuronal excitability by modulating signaling through other neurotransmitter systems. Most reports of this kind have described effects in the SNc and VTA, where NT remarkably modulates glutamate-, GABA-, and dopamine-mediated neurotransmission (Fig. 1). In the VTA, NT increases glutamatergic excitatory postsynaptic current (EPSC) amplitudes in dopamine neurons via NTS1 and non-dopamine neurons via NTS2 (Bose et al., 2015, Fig. 1). NT is also implicated in multiple extended forms of synaptic plasticity. In VTA dopamine neurons, low concentrations of NT induce long-term potentiation of NMDA receptor-mediated EPSCs through activation of NTS1 (Kempadoo et al., 2013, Fig. 1). At higher concentrations, NT induces long-term depression of both NMDA and AMPA receptor-mediated EPSCs, and these effects are not blocked by a NTS1 antagonist (Kempadoo et al., 2013, Fig. 1). Conversely, in basolateral amygdala pyramidal neurons, activation of NTS1 prevents a tetanus-induced long-term potentiation of AMPA receptor-mediated EPSCs. NT also decreases the amplitude of glutamatergic EPSCs postsynaptically in neurons of the superficial laminae of the superior colliculus (Zhang et al., 1996). Thus, NT may induce or prevent long-term potentiation of glutamate neurotransmission depending on the brain region, and even within a single cell type can variously enhance or depress glutamate signaling.
Figure 1.
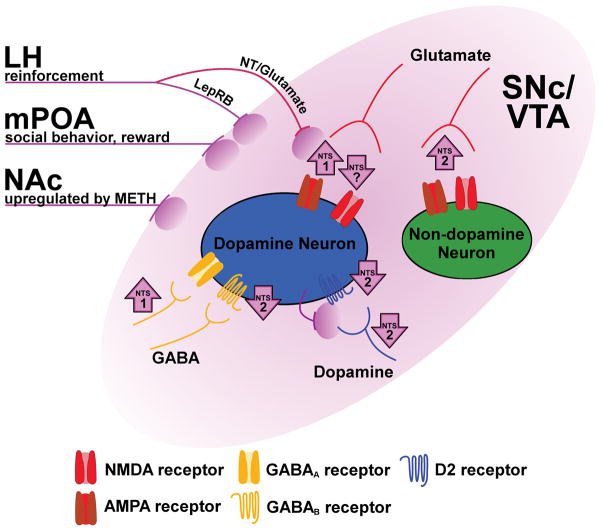
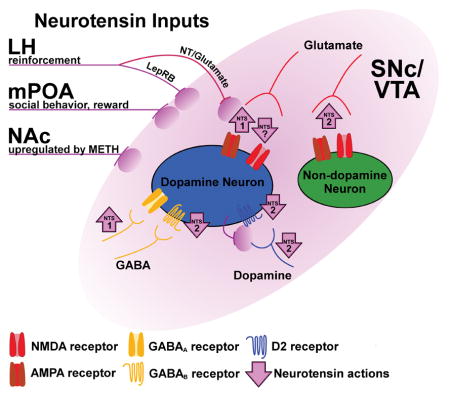
Diverse NT action in the substantia nigra pars compacta (SNc) and ventral tegmental area (VTA). This schematic indicates the architecture and behavioral roles of specific NT circuits, as well as NT-induced modulation of neurotransmitter signaling in the SNc/VTA. Purple lines and ovals represent sources of NT, including inputs from the LH that either co-express NT and glutamate (NT/glutamate) or express leptin receptors (LepRB). Purple arrows indicate an increase or decrease in signaling at a given synapse, including the responsible receptor subtype (if it is known). The location of the arrow indicates if the effect of NT occurs presynaptically (near the input), postsynaptically (adjacent to the receptor), or was not determined (between the input and receptor). NT release from dopamine neurons is putative and indicates possible retrograde transmission of NT at dendro-dendritic synapses between dopamine neurons.
In addition to glutamatergic effects, NT can also increase neuronal excitability of SNc and VTA dopamine neurons by decreasing D2 dopamine autoreceptor-mediated neurotransmission. D2 autoreceptors are G protein-coupled receptors located on dendrites, cell bodies, and terminals of mesencephalic dopamine neurons (Sesack et al., 1994; Robinson et al., 2017). In the SNc and VTA, these receptors are physiologically activated via dendro-dendritic synapses between dopamine neurons (Wilson et al., 1977; Groves & Linder, 1983), resulting in a powerful hyperpolarization through G protein-coupled inwardly rectifying potassium (GIRK) channels (Lacey et al., 1987; Davila et al., 2003; Beckstead et al., 2004). NT decreases D2 autoreceptor-mediated GIRK currents in both SNc and VTA dopamine neurons by decreasing presynaptic dopamine release as well as through a postsynaptic mechanism involving protein kinase A (Shi & Bunney, 1992b; Piccart et al., 2015; Stuhrman & Roseberry, 2015, Fig. 1). This NT-induced reduction of a hyperpolarizing GIRK channel conductance would be expected to increase excitability (i.e., produce disinhibition) of dopamine neurons. Unlike the IP3-dependent depolarization of dopamine neurons described above, this disinhibitory action of NT requires activation of NTS2 and persists in the absence of intracellular Ca2+ (Piccart et al., 2015, Stuhrman & Roseberry 2015, Fig. 1). Thus, NT excites dopamine neurons in part by blunting inhibitory D2 autoreceptor signaling, both pre- and postsynaptically.
Under physiological conditions in the SNc and VTA, D2-mediated inhibition of cellular excitability proceeds predominantly through synaptic receptors, with activation of extrasynaptic receptors largely culled by dopamine transporter function (Beckstead et al., 2004; Beckstead et al., 2007; Ford et al., 2010; Gantz et al., 2013; Courtney & Ford, 2014). However, extrasynaptic D2 receptors can be readily activated when dopamine transporter-mediated uptake is inhibited by drugs such as cocaine or methamphetamine (Beckstead et al., 2004; Beckstead et al., 2007; Branch & Beckstead, 2012; Courtney & Ford, 2014). Action at these functionally separate populations of D2 receptors can be explored by comparing D2 signaling elicited by iontophoresis of dopamine (presumably extrasynaptic D2 signaling) with stimulation-induced release of endogenous dopamine (D2 receptor-mediated inhibitory postsynaptic currents, or D2-IPSCs; Beckstead et al., 2004). Electrophysiological studies comparing currents obtained using these two methods of D2 receptor activation reveal divergent forms of regulation. For example, while low frequency electrical stimulation induces long-term depression of D2-IPSCs, currents elicited by iontophoresis of dopamine are not altered (Beckstead & Williams, 2007). NT can also induce a persistent depression of D2-IPSCs, but the effect on currents elicited by iontophoresis of dopamine is transient (Piccart et al., 2015, Fig. 1). Thus, NT is apparently able to differentially regulate functional compartments of D2 receptors (presumably synaptic and extrasynaptic) on dopamine neurons. Taken together, these studies reveal diverse NT-induced modulation of dopamine signaling.
Finally, recent work shows that NT postsynaptically increases dopamine neuron excitability in the SNc and VTA by decreasing GABA signaling at GABAB receptor synapses (Stuhrman & Roseberry, 2015; Tschumi & Beckstead, 2018, Fig. 1). This form of modulation is similar to NT-induced long-term depression of D2-IPSCs in that both proceed through NTS2 activation, however NT-induced depression of GABAB signaling is transient. Furthermore, while NT-induced depression of D2-IPSCs is not dependent on protein kinase C, data from our lab indicate that GABAB depression recovers in a protein kinase C-dependent manner (Tschumi & Beckstead, 2018, Fig. 1). GABAB receptors hyperpolarize dopamine neurons through a GIRK channel conductance that partially overlaps with that observed subsequent to activation of D2 receptors (Lacey et al., 1987; Beckstead & Williams, 2007), supporting the possibility that NT decreases GABAB and D2-mediated currents at the level of GIRKs (Stuhrman & Roseberry, 2015). Overall, growing evidence indicates that NT can postsynaptically modulate neuronal signaling through a diverse array of receptors, sometimes within a single brain region.
Presynaptic modulation of neurotransmitter release
While postsynaptic effects of NT are most often excitatory, the diversity of NT actions are perhaps best illustrated in reports of presynaptic modulation of neurotransmitter release. NT increases GABA release in several brain regions. In SNc dopamine neurons, NT increases the amplitude of GABAA receptor-mediated IPSCs by increasing the readily releasable pool of GABA vesicles in a process that requires NTS1 and PKA (Tschumi & Beckstead, 2018). Interestingly, enhanced GABA release is not evident when measuring GABAB receptor-mediated IPSCs in these neurons (Tschumi & Beckstead, 2018), suggesting that presynaptic effects of NT could be localized to specific GABA inputs. In the bed nucleus of the stria terminalis, NT presynaptically induces long-term potentiation of GABAA IPSCs by increasing the probability of release of GABA (Krawczyk et al., 2013; Normandeau et al., 2017). In the cerebral cortex, NT hyperpolarizes pyramidal cells by increasing GABA release, however this effect can be observed at GABAB receptor synapses and proceeds through NTS2 (Case et al., 2017). NT also increases GABA release in the hippocampal CA1 region, increasing the frequency of spontaneous IPSCs in a process that depends on NTS1, phospholipase C, IP3, and protein kinase C (Li et al., 2008). In the nucleus of the solitary tract, NT exhibits the somewhat counterintuitive effect of enhancing the release of both glutamate and GABA, increasing the frequency of spontaneous IPSCs and EPSCs through NTS1 activation (Ogawa et al., 2005). Thus, NT increases GABA release in several brain regions, but the intracellular proteins and specific alterations in synaptic signaling that mediate these effects can vary between regions.
NT also modulates the presynaptic release of glutamate and catecholamines. In the dentate gyrus, NT induces long-term potentiation of glutamate neurotransmission by increasing release probability and the number of readily releasable glutamate vesicles (Zhang et al., 2015). This long-term potentiation proceeds via activation of NTS1 resulting in influx of Ca2+ through L-type Ca2+ channels, and requires activation of calmodulin and myosin light-chain kinase (Zhang et al., 2015). In the parabrachial nucleus, NT increases the amplitude of evoked and spontaneous AMPA-receptor mediated EPSCs without altering currents elicited by bath application of AMPA, suggesting a presynaptic enhancement of glutamate release (Saleh et al., 1997). NT also depresses cerebellar Purkinje cell firing by increasing norepinephrine release from locus coeruleus-derived afferents (Marwaha et al., 1980). Finally, NT applied directly to the VTA increases dopamine neuron excitability by increasing a non-selective cation conductance and decreasing D2 autoreceptor-mediated inhibition, ultimately increasing DA release from dopamine neuron terminals in the nucleus accumbens (Kalivas & Duffy, 1990; Shi & Bunney, 1992a; Fawaz et al., 2009). Conversely, in the SNc and VTA, and using a stimulation protocol that is too rapid to engage presynaptic autoreceptors, NT modestly decreases somatodendritic dopamine release at dendro-dendritic D2 synapses (Piccart et al., 2015). Altogether, depending on the brain region, NT can alter the release of glutamate, GABA, norepinephrine, or dopamine (Table 1).
Effects on endocannabinoids
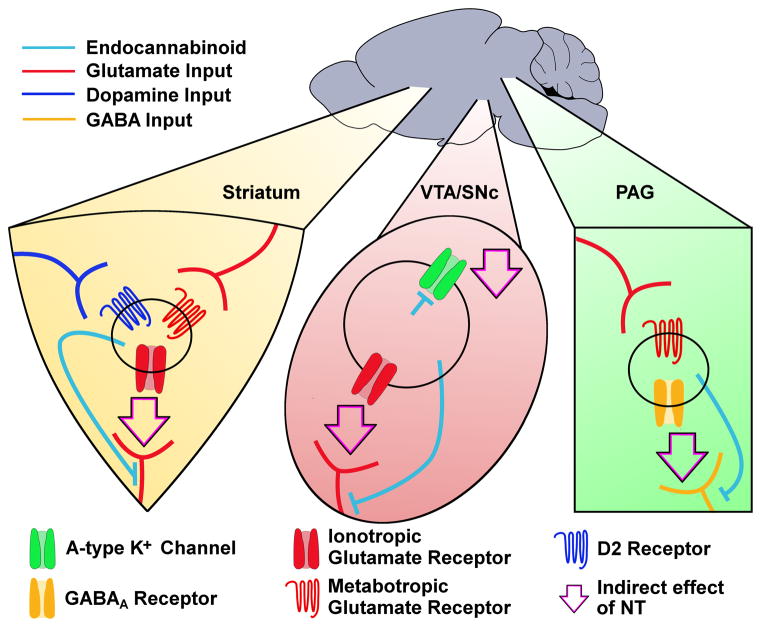
NT also modulates neuronal excitability, in the basal ganglia and elsewhere, through the release of endocannabinoids (Fig. 2). In medium spiny projection neurons of the dorsolateral striatum, NT induces endocannabinoid release in a process that depends on modulation of D2 and group I metabotropic glutamate (either type-1 or type-5) receptors, with endocannabinoid activity ultimately causing long-term depression of glutamate neurotransmission (Yin et al., 2008, Fig. 2). In the periaqueductal gray, NT decreases GABA neurotransmission through a chain of events: increased glutamate release activates the metabotropic glutamate type-5 receptor (mGluR5), causing the release of endocannabinoids and ultimately decreasing GABAA IPSC amplitudes (Mitchell et al., 2009, Fig. 2). In midbrain dopamine neurons, NT initiates the release of the endocannabinoid 2-arachidonoylglycerol (2-AG) through NTS1-mediated activation of phospholipase C (Kortleven et al., 2012, Fig. 2) which can modulate neuronal activity in two distinct ways. 2-AG acts directly on dopamine neurons to reduce A-type K+ current, resulting in increased firing rate (Gantz & Bean, 2017, Fig. 2). 2-AG also induces long-term depression of glutamate neurotransmission (Kortleven et al., 2012, Fig. 2). These two forms of modulation are seemingly contradictory in that one would be expected to increase dopamine neuron excitability through an intrinsic conductance while the other would decrease excitability by modifying glutamate input. In this case, the net effect of NT-induced 2-AG release may not be a blunt increase or decrease in firing, but rather a shift in priority toward intrinsically-driven firing and away from external signals. Interestingly, NT also enhances EPSCs in VTA dopamine neurons (Kempadoo et al., 2013; Bose et al., 2015), but the degree to which endocannabinoid signaling plays a role in this effect is unclear. Altogether, NT induces endocannabinoid release either by acting directly at postsynaptic NT receptors or indirectly by modulating signaling at D2 or metabotropic glutamate receptors.
Figure 2.
NT induces endocannabinoid release in multiple brain regions. This schematic indicates actions of NT mediated by endocannabinoid release in the striatum, VTA/SNc, and periaqueductal gray (PAG). In striatal medium spiny projection neurons, NT induces endocannabinoid release in a process that depends on D2 and/or metabotropic glutamate receptor activation. Released endocannabinoids then reduce glutamatergic EPSCs. In the VTA, released endocannabinoids also reduce glutamatergic EPSCs. In SNc dopamine neurons, production of 2-AG decreases conductance through A-type K+ channels. In the PAG, NT induces endocannabinoid release in a process that depends on metabotropic glutamate signaling. Released endocannabinoids then reduce GABAA IPSCs. Light blue lines represent paths of endocannabinoid signal. Colored lines represent glutamate (red), dopamine (dark blue), or GABA (orange) input. Arrows represent resultant endocannabinoid-induced reduction of neurotransmission or ion conductance (i.e., the indirect effect of NT receptor activation). Note that while the circles in each brain region represent postsynaptic cells in the context of endocannabinoid release, it is not clear if the D2 or metabotropic glutamate receptors necessary for NT-induced release of endocannabinoids are present on that same postsynaptic cell or if some other intermediary cell is involved.
So far, this review has covered a wide range of neuronal signals which are modulated by NT across many different brain regions. However, the net effect of physiological NT on excitability of neurons and consequent generation of behavior is less well understood and will require further investigation to determine the roles of endogenous NT signaling.
Endogenous NT signaling
Studies of NT signaling suggest that the localization and concentration of NT dictates its net effect on neuronal excitability. NT ligands applied to a single brain region can modulate neuronal activity both pre- and postsynaptically through diverse actions, and the concentration of NT ligand often dictates which of these actions predominates. For example, in two regions of the basal ganglia (the SNc and globus pallidus), exogenously applied NT ligands increase glutamate signaling at low concentrations and postsynaptically depolarize neurons at high concentrations (Chen et al., 2006, Chen et al., 2004, Kempadoo et al., 2013, Shi & Bunney, 1992a). Similarly, in the nucleus of the solitary tract, low concentrations of NT increase presynaptic GABA and glutamate release, while high concentrations directly depolarize neurons (Ogawa et al., 2005). Thus, exogenously-applied NT ligands can have competing effects on neuronal excitability -- even within a single brain region -- and interpreting the physiological relevance of these effects requires a comprehensive understanding of endogenous NT signaling at the level of the synapse. Anatomical studies of peptide and receptor localization suggest that NT may signal in either a synapse-localized or more diffuse, extrasynaptic fashion. Most NT receptors in the VTA are evenly dispersed across the cell membrane with only a few receptors localized in the synapse (Dana et al., 1989), suggesting that most NT signaling in this brain region occurs extrasynaptically. However, NT-containing dense-core vesicles are located in axon terminals targeting dopamine neuron perikarya (Bayer et al., 1991), supporting the possibility of synapse-localized NT action.
Electrophysiological studies also suggest that NT can have synapse-localized effects. Kempadoo et al. (2013) demonstrated that targeted stimulation of NT-containing afferents causes NT-induced plasticity of glutamatergic signaling in the VTA. Two key experiments from this study suggest that NT is physiologically released and acts exclusively at local synapses. An important detail in the interpretation of these experiments is that exogenous NT application bi-phasically modulates glutamate signaling, with a low concentration (10 nM) enhancing and a high concentration (≥ 100 nM) depressing NMDA receptor-mediated neurotransmission. First, in recordings from a single dopamine neuron, bath perfusion of NT (10 nM) either 1) depresses EPSCs generated by stimulation of inputs to the VTA from the NT-rich lateral hypothalamus, or 2) enhances EPSCs generated by stimulation of non-specific inputs near the neuron being recorded. This suggests that localized release of endogenous NT could seemingly shift the concentration-response of exogenously-applied NT toward depression at specific synapses. The fact that depression is observed only for EPSCs from lateral hypothalamic inputs suggests that NT must act extremely locally, as other glutamatergic synapses in the same cell are not similarly affected. The second key experiment in this study uses an NTS1 antagonist to further refine the inputs involved. The NTS1 antagonist SR48692 has no effect on NMDA EPSCs elicited by stimulation of non-specific inputs, but depresses NMDA EPSCs elicited by selective stimulation of lateral hypothalamic NT-expressing inputs. Thus, NT is released and enhances signaling at only a subset of glutamatergic synapses in VTA dopamine neurons, as this effect is occluded when non-specific glutamatergic inputs are stimulated. Together, these results show that NT can modulate highly localized synaptic activity within the VTA.
There is growing evidence that NT can also act as a retrograde synaptic messenger. Krawczyk and colleagues (2013) induced NT release in the bed nucleus of the stria terminalis by applying a depolarizing step lasting 100 milliseconds and repeated at a frequency of 2 Hz for 5 minutes to the cell being recorded. This release of NT resulted in long-term potentiation of GABAA IPSCs through an increase in the probability of release of GABA. This finding is consistent with NT acting as a retrograde messenger, as long-term plasticity is induced by depolarizing the postsynaptic cell and blocked by inhibition of vesicular release within that cell (Normandeau et al., 2017), but is expressed by an increase in presynaptic neurotransmitter release. Similarly, work from our lab suggests that low frequency electrical stimulation to the SNc causes NT release and long-term depression of D2-IPSCs in dopamine neurons (Piccart et al., 2015). This finding is also consistent with a putative retrograde effect of NT, as plasticity is blocked by chelating Ca2+ in the postsynaptic cell but expressed by a decrease in presynaptic neurotransmitter release. Anatomical evidence also supports NT action as a retrograde messenger, as NT is localized in cytoplasm and dense core vesicles in dopamine neuron dendrites in the VTA (Bayer et al., 1989). Investigations are underway to determine whether this phenomenon represents true retrograde neurotransmission or whether a local circuit is involved. Together, these studies provide fascinating new insights into the actions of endogenous NT signaling, and show that NT expression may influence both the projection targets of, and the local activity within, a variety of brain regions.
NT circuitry in behavior
The dense expression of NT neurons throughout the brain coupled with the diverse actions through which NT modulates neuronal excitability necessitate the use of circuit- and cell type-specific techniques to fully elucidate the role of NT signaling in behavior. Studies using classical techniques such as systemic and region-specific intracranial infusion of NT agonists throughout the brain, and particularly in structures of the basal ganglia, have laid the foundation for our growing understanding of NT physiology. These studies also reveal the importance of circuit-specific investigations of the role of NT signaling in behavior. One example comes from our ongoing work using an animal model of methamphetamine use disorder. In this model, mice are trained to self-administer methamphetamine through an intravenous catheter by responding operantly through a nose poke (Sharpe et al., 2014; Sharpe et al., 2017). We have observed that mice that are treated systemically with a NT receptor agonist or given an intra-VTA NT receptor antagonist self-administer less methamphetamine than controls (Sharpe et al., 2017; Dominguez-Lopez et al., 2017). Thus, NT in the VTA appears to increase, while NT in other brain regions may decrease, the reinforcing effects of methamphetamine. Conversely, methamphetamine can affect NT content in a brain region- and circuit-specific manner. For example, methamphetamine self-administration increases NT levels in the dorsal striatum, globus pallidus, nucleus accumbens shell, SNc, and VTA, but not the nucleus accumbens core or frontal cortex (Frankel et al., 2011; Hanson et al., 2012; Hanson et al., 2013). NT mRNA is upregulated in neurons that project from the nucleus accumbens shell to the VTA (Fig. 1), but not in any of the other eleven major NT-containing inputs to the VTA (Geisler & Zahm, 2006). This suggests that there may be differences in the contribution of NT to methamphetamine use disorder not only between the VTA and other brain regions but also between individual NT inputs to the VTA. However, the contribution of specific NT-containing inputs to the VTA in the reinforcing effects of methamphetamine have not yet been explored.
Outside of studies on methamphetamine, there is evidence to suggest that NT modulates reward-related behavior in different ways depending on which inputs to the VTA are activated. Neurons that contain NT often (if not always) express other neurotransmitters, including GABA, glutamate, and dopamine (Seroogy et al., 1988; Leinninger et al., 2011; Kempadoo et al., 2013). While the excitatory and inhibitory effects of the co-expressed neurotransmitters have not been well described, activation of NT-expressing inputs to the VTA can recapitulate the effects of intra-VTA infusions of NT agonists. For example, both intra-VTA infusion of NT and targeted (opto- or chemogenetic) stimulation of NT neurons projecting from the lateral hypothalamus to the VTA act as reinforcing stimuli and result in increased extracellular dopamine in the nucleus accumbens (Glimcher et al., 1987; Fawaz et al., 2009; Kempadoo et al., 2013; Patterson et al., 2015, Fig. 1). Thus, activation of even a small portion of the NT-expressing inputs to the VTA is sufficient to reproduce the behavioral effects of exogenous NT application. Interestingly, activation of even smaller subsets of these neurons produces different behaviors (Fig. 1). Within the VTA, a fraction of NT-expressing neurons projecting from the lateral hypothalamus also express the receptor for the anorexigenic hormone leptin, and while both NT and leptin receptors are expressed throughout the brain, they are co-localized only in these projections (Leinninger et al., 2011). Genetic ablation of leptin receptors from NT neurons results in increased feeding, altered sensitivity to amphetamine, and a decrease in the reinforcing effects of sucrose (Leinninger et al., 2011; Woodworth et al., 2017, Fig. 1). This suggests that NT is expressed in neuronal circuits that modulate reward-related behavior, although the degree to which changes in behavior are the result of altered NT signaling in the absence of leptin receptors is not yet clear. While the NT inputs to the VTA from the lateral hypothalamus have been characterized, a recent study suggests that other NT-inputs to the VTA also modulate reward-related behavior. Optogenetic stimulation of NT inputs from the medial preoptic area to the VTA results in increased time spent in social interaction zones and other changes in social behavior, particularly in male-female interactions (McHenry et al., 2017, Fig. 1). Again, it is not clear if these changes in social behavior are due to the action of NT or other neurotransmitters co-expressed in NT inputs. Although these studies provide new insights into how NT circuit architecture modulates behavior, there is still much to learn regarding the role of specific NT inputs to the VTA as well as other brain regions. Together, these studies suggest that even a small fraction of NT afferents to a brain region can produce substantial effects on behavior.
Summary and conclusions
NT and NT receptors are highly expressed in basal ganglia nuclei and throughout the brain. NT signaling can proceed through diverse pathways and mechanisms, resulting in modulation of both intrinsic conductances and synaptic transmission in a wide range of cell types. Endogenous NT signaling likely proceeds through synapse-localized signaling and can apparently proceed in an antero- or retrograde manner. It is now clear that NT alters neuronal excitability by diverse mechanisms of action and circuit architecture that allow for input-specific control of behavior. However, circuit-specific investigations into the synaptic and behavioral effects of NT are in their infancy, and improved technology will no doubt permit ever-increasing levels of precision in future studies of this remarkable neuropeptide.
The study of NT in many different brain regions and contexts makes it difficult to assign a unitary role to this myriad modulator of synaptic activity. NT may indeed act as an analgesic in the periphery and periaqueductal gray, a neuroleptic in the striatum, a feeding peptide in the lateral hypothalamus, a socialization signal in the medial preoptic area, and an anxiogenic signal in the bed nucleus of the stria terminalis (Kleczkowska & Lipkowski, 2013; Cáceda et al., 2006; Leinninger et al., 2011; McHenry et al., 2017; Normandeau et al., 2017). However, no study has yet determined a clear action of NT under basal (non-stimulated) conditions. For example, in rodent brain slices, antagonism of NT receptors alone almost never has any measurable effect. Only two of these studies cited in this review report any effect of NT antagonists in the absence of investigator-initiated stimulation, and those effects are minimal (Ogawa et al., 2005, Coogan et al., 2001). Furthermore, to our knowledge no study has reported a behavioral effect of a NT antagonist under non-stimulated conditions. It is possible that tonic NT signaling occurs at low enough levels to act in a synapse-localized fashion, modulating neuronal excitability and animal behavior at levels below the current level of detection. Perhaps more likely, the physiological role of endogenous NT in the brain may rely on phasic rather than tonic release of NT.
It is worth noting that NT signaling seems particularly well-suited to either punctuate or blunt the synaptic effects of salient stimuli in cases where fast neurotransmitter signaling is dysregulated. This statement is supported by two shared themes of NT physiology across multiple brain regions. First, NT can recruit multiple receptors and/or intracellular signaling cascades to modulate neuronal activity of single cell types. This is true in the dentate gyrus, globus pallidus, periaqueductal gray, SNc, and VTA where NT excites neurons by increasing a non-selective cation channel conductance and by either increasing glutamate or decreasing GABA signaling (See Table 1). Thus, if either ion channel expression or neurotransmitter signaling is dysregulated in a given cell, NT may yet have an excitatory effect through a backup mechanism. Second, repeated exposure to salient stimuli can upregulate NT signaling. For example, in the VTA, repeated exposure to psychostimulants results in increased expression of NT, which is itself reinforcing (Glimcher et al., 1987; Geisler & Zahm, 2006; Frankel et al., 2011; Hanson et al., 2012; Hanson et al., 2013). Also, in the bed nucleus of the stria terminalis, repeated exposure to either cocaine or unpredictable stress enhances NT-induced plasticity (Krawczyk et al., 2013, Normandeau et al., 2017). In this case the stimuli involved produce significant neuroadaptations, and it may be that upregulation of NT signaling is a form of homeostasis. Therefore, despite its many effects on ion channel conductances, the overall role of NT may not be to cause straightforward increases or decreases in excitability, but rather to either facilitate or combat neuronal activity or plasticity induced by salient stimuli. If a major function of NT signaling is to curb maladaptive neuronal activity, it follows that NT signaling is implicated in many disorders and highlights the importance of further investigations into NT physiology.
Acknowledgments
This work was supported by National Institute on Drug Abuse Grant R01 DA32701 (M.J.B.), as well as funds from the Presbyterian Health Foundation and the Oklahoma Center for Adult Stem Cell Research. The authors declare no competing financial interests.
List of Abbreviations
- 2-AG
2-arachidonoylglycerol
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- D2
dopamine D2 (receptor)
- EPSC
excitatory post-synaptic current
- GABA
γ-Aminobutyric acid
- GABAA
GABA type-A (receptor)
- GABAB
GABA type-B (receptor)
- GIRK
G protein-coupled inwardly-rectifying potassium (channel)
- IPSC
inhibitory post-synaptic current
- IP3
inositol triphosphate
- METH
methamphetamine
- mPOA
medial preoptic area
- NMDA
N-methyl-D-aspartate
- NT
neurotensin
- NTS1
neurotensin type-1 receptor
- NTS2
neurotensin type-2 receptor
- NTS3
neurotensin type-3 receptor
- NTS4
neurotensin type-4 receptor
- SNc
substantia nigra pars compacta
- TASK
TWIK-related acid-sensitive K+ channel
- TREK
TWIK-related K+ channel TREK-2
- TRP
transient receptor potential
- VTA
ventral tegmental area
Footnotes
Author Contributions:
C.W.T and M.J.B wrote the manuscript. M.J.B. funded the work.
References
- Alexander MJ, Leeman SE. Widespread expression in adult rat forebrain of mRNA encoding high-affinity neurotensin receptor. J Comp Neurol. 1998;402:475–500. [PubMed] [Google Scholar]
- Amano T, Wada E, Yamada D, Zushida K, Maeno H, Noda M, Wada K, Sekiguchi M. Heightened amygdala long-term potentiation in neurotensin receptor type-1 knockout mice. Neuropsychopharm. 2008;33:3135–3145. doi: 10.1038/npp.2008.38. [DOI] [PubMed] [Google Scholar]
- Bayer VE, Towle AC, Pickel VM. Ultrastructural localization of neurotensin-like immunoreactivity within dense core vesicles in perikarya, but not terminals, colocalizing tyrosine hydroxylase in the rat ventral tegmental area. J Comp Neurol. 1991;311:179–196. doi: 10.1002/cne.903110202. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Ford CP, Phillips PEM, Williams JT. Presynaptic regulation of dendrodendritic dopamine transmission. Eur J Neurosci. 2007;26:1479–1488. doi: 10.1111/j.1460-9568.2007.05775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–946. doi: 10.1016/j.neuron.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Williams JT. Long-term depression of a dopamine IPSC. J Neurosci. 2007;27:2074–2080. doi: 10.1523/JNEUROSCI.3251-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black PH. Stress and the inflammatory response: A review of neurogenic inflammation. Brain Behav Immun. 2002;16:622–653. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- Bose P, Rompré PP, Warren RA. Neurotensin enhances glutamatergic EPSCs in VTA neurons by acting on different neurotensin receptors. Peptides. 2015;73:43–50. doi: 10.1016/j.peptides.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Boudin H, Pelaprat D, Rostene W, Beaudet A. Cellular distribution of neurotensin receptors in rat brain: immunohistochemical study using an antipeptide antibody against the cloned high affinity receptor. J Comp Neurol. 1996;373:76–89. doi: 10.1002/(SICI)1096-9861(19960909)373:1<76::AID-CNE7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Branch SY, Beckstead MJ. Methamphetamine produces bidirectional, concentration-dependent effects on dopamine neuron excitability and dopamine-mediated synaptic currents. J Neurophysiol. 2012;108:802–809. doi: 10.1152/jn.00094.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraway R, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem. 1973;248:6854–6861. [PubMed] [Google Scholar]
- Cáceda R, Kinkead B, Nemeroff CB. Neurotensin: role in psychiatric and neurological diseases. Peptides. 2006;27:2385–2404. doi: 10.1016/j.peptides.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Case L, Lyons DJ, Broberger C. Desynchronization of the rat cortical network and excitation of white matter neurons by neurotensin. Cereb Cortex. 2017;27:2671–2685. doi: 10.1093/cercor/bhw100. [DOI] [PubMed] [Google Scholar]
- Chalon P, Vita N, Kaghad M, Guillemot M, Bonnin J, Delpech B, Le Fur G, Ferrara P, Caput D. Molecular cloning of a levocabastine-sensitive neurotensin binding site. FEBS letters. 1996;386:91–94. doi: 10.1016/0014-5793(96)00397-3. [DOI] [PubMed] [Google Scholar]
- Chen L, Yung KKL, Yung WH. Neurotensin depolarizes globus pallidus neurons in rats via neurotensin type-1 receptor. Neuroscience. 2004;125:853–859. doi: 10.1016/j.neuroscience.2004.02.031. [DOI] [PubMed] [Google Scholar]
- Chen L, Yung KKL, Yung WH. Neurotensin selectively facilitates glutamatergic transmission in globus pallidus. Neuroscience. 2006;141:1871–1878. doi: 10.1016/j.neuroscience.2006.05.049. [DOI] [PubMed] [Google Scholar]
- Chien PY, Farkas RH, Nakajima S, Nakajima Y. Single-channel properties of the nonselective cation conductance induced by neurotensin in dopaminergic-neurons. Proc Natl Acad Sci. 1996;93:14917–14921. doi: 10.1073/pnas.93.25.14917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan AN, Rawlings N, Luckman SM, Piggins HD. Effects of neurotensin on discharge rates of rat suprachiasmatic nucleus neurons in vitro. Neuroscience. 2001;103:663–672. doi: 10.1016/s0306-4522(00)00583-2. [DOI] [PubMed] [Google Scholar]
- Courtney NA, Ford CP. The timing of dopamine- and noradrenaline-mediated transmission reflects underlying differences in the extent of spillover and pooling. J Neurosci. 2014;34:7645–7656. doi: 10.1523/JNEUROSCI.0166-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana C, Vial M, Leonard K, Beauregard A, Kitabgi P, Vincent JP, Rostène W, Beaudet A. Electron microscopic localization of neurotensin binding sites in the midbrain tegmentum of the rat. I Ventral tegmental area and the interfascicular nucleus. J Neurosci. 1989;9:2247–2257. doi: 10.1523/JNEUROSCI.09-07-02247.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila V, Yan Z, Craciun LC, Logothetis D, Sulzer D. D3 dopamine autoreceptors do not activate g-protein-gated inwardly rectifying potassium channel currents in substantia nigra dopamine neurons. J Neurosci. 2003;23:5693–5697. doi: 10.1523/JNEUROSCI.23-13-05693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Lopez S, Piccart E, Lynch WB, Wollet MB, Sharpe AL, Beckstead MJ. Antagonism of neurotensin receptors in the ventral tegmental area decreases methamphetamine self-administration and methamphetamine seeking in mice. Int J Neuropsychopharmacol. 2017 doi: 10.1093/ijnp/pyx117. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elde R, Schalling M, Ceccatelli S, Nakanishi S, Hökfelt T. Localization of neuropeptide receptor mRNA in rat brain: initial observations using probes for neurotensin and substance P receptors. Neurosci letters. 1990;120:134–138. doi: 10.1016/0304-3940(90)90187-e. [DOI] [PubMed] [Google Scholar]
- Erwin VG, Su NC. Neurotensin and ethanol interactions on hypothermia and locomotor activity in LS and SS mice. Alcohol Clin Exp Res. 1989;13:91–94. doi: 10.1111/j.1530-0277.1989.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Erwin VG, Campbell AD, Myers R, Womer DE. Cross-tolerance between ethanol and neurotensin in mice selectively bred for ethanol sensitivity. Pharmacol Biochem Behav. 1995;51:891–899. doi: 10.1016/0091-3057(95)00070-d. [DOI] [PubMed] [Google Scholar]
- Erwin VG, Gehle VM, Davidson K, Radcliffe RA. Confirmation of correlations and common quantitative trait loci between neurotensin receptor density and hypnotic sensitivity to ethanol. Alcohol Clin Exp Res. 2001;25:1699–1707. [PubMed] [Google Scholar]
- Fassio A, Evans G, Grisshammer R, Bolam JP, Mimmack M, Emson PC. Distribution of the neurotensin receptor NTS1 in the rat CNS studied using an amino-terminal directed antibody. Neuropharmacology. 2000;39:1430–1442. doi: 10.1016/s0028-3908(00)00060-5. [DOI] [PubMed] [Google Scholar]
- Fawaz CS, Martel P, Leo D, Trudeau LE. Presynaptic action of neurotensin on dopamine release through inhibition of D2 receptor function. BMC Neurosci. 2009;10:96. doi: 10.1186/1471-2202-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro L, Tiozzo Fasiolo L, Beggiato S, Borelli AC, Pomierny-Chamiolo L, Frankowska M, Antonelli T, Tomasini MC, Fuxe K, Filip M. Neurotensin: A role in substance use disorder? J Psychopharmacol. 2016;30:112–127. doi: 10.1177/0269881115622240. [DOI] [PubMed] [Google Scholar]
- Ford CP, Gantz SC, Phillips PEM, Williams JT. Control of extracellular dopamine at dendrite and axon terminals. J Neurosci. 2010;30:6975–6983. doi: 10.1523/JNEUROSCI.1020-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel PS, Hoonakker AJ, Alburges ME, McDougall JW, McFadden LM, Fleckenstein AE, Hanson GR. Effect of methamphetamine self-administration on neurotensin systems of the basal ganglia. J Pharmacol Exp Ther. 2011;336:809–815. doi: 10.1124/jpet.110.176610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailly P, Najimi M, Hermans E. Evidence for the dual coupling of the rat neurotensin receptor with pertussis toxin-sensitive and insensitive G-proteins. FEBS letters. 2000;483:109–113. doi: 10.1016/s0014-5793(00)02095-0. [DOI] [PubMed] [Google Scholar]
- Gantz SC, Bean BP. Cell-autonomous excitation of midbrain dopamine neurons by endocannabinoid-dependent lipid signaling. Neuron. 2017;93:1375–1387. doi: 10.1016/j.neuron.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz SC, Bunzow JR, Williams JT. Spontaneous inhibitory synaptic currents mediated by a G protein-coupled receptor. Neuron. 2013;78:807–812. doi: 10.1016/j.neuron.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Bérod A, Zahm DS, Rostène W. Brain neurotensin, psychostimulants, and stress – emphasis on neuroanatomical substrates. Peptides. 2006;27:2364–2384. doi: 10.1016/j.peptides.2006.03.037. [DOI] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Neurotensin afferents of the ventral tegmental area in the rat:[1] re-examination of their origins and [2] responses to acute psychostimulant and antipsychotic drug administration. Eur J Neurosci. 2006;24:116–134. doi: 10.1111/j.1460-9568.2006.04928.x. [DOI] [PubMed] [Google Scholar]
- Glimcher PW, Giovino AA, Hoebel BG. Neurotensin self-injection in the ventral tegmental area. Brain Res. 1987;403:147–150. doi: 10.1016/0006-8993(87)90134-x. [DOI] [PubMed] [Google Scholar]
- Groves PM, Linder JC. Dendro-dendritic synapses in substantia nigra: descriptions based on analysis of serial sections. Exp Brain Res. 1983;49:209–217. doi: 10.1007/BF00238581. [DOI] [PubMed] [Google Scholar]
- Hanson GR, Hoonakker AJ, Alburges ME, McFadden LM, Robson CM, Frankel PS. Response of limbic neurotensin systems to methamphetamine self-administration. Neuroscience. 2012;203:99–107. doi: 10.1016/j.neuroscience.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson GR, Hoonakker AJ, Robson CM, McFadden LM, Frankel PS, Alburges ME. Response of neurotensin basal ganglia systems during extinction of methamphetamine self-administration in rat. J Pharmacol Exp Ther. 2013;346:173–181. doi: 10.1124/jpet.113.205310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst B, Holliday ND, Bach A, Elling CE, Cox HM, Schwartz TW. Common structural basis for constitutive activity of the ghrelin receptor family. J Biol Chem. 2004;279:53806–53817. doi: 10.1074/jbc.M407676200. [DOI] [PubMed] [Google Scholar]
- Jacobsen L, Madsen P, Jacobsen C, Nielsen MS, Gliemann J, Petersen CM. Activation and functional characterization of the mosaic receptor SorLA/LR11. J Biol Chem. 2001;276:22788–22796. doi: 10.1074/jbc.M100857200. [DOI] [PubMed] [Google Scholar]
- Jassar BS, Harris KH, Ostashewski PM, Jhamandas JH. Ionic mechanisms of action of neurotensin in acutely dissociated neurons from the diagonal band of Broca of the rat. J Neurophysiol. 1999;81:234–246. doi: 10.1152/jn.1999.81.1.234. [DOI] [PubMed] [Google Scholar]
- Jennes L, Stumpf WE, Kalivas PW. Neurotensin: Topographical distribution in rat brain by immunohistochemistry. J Comp Neurol. 1982;210:211–224. doi: 10.1002/cne.902100302. [DOI] [PubMed] [Google Scholar]
- Jiang ZG, Pessia M, North RA. Neurotensin excitation of rat ventral tegmental neurones. J Physiol. 1994;474:119–129. doi: 10.1113/jphysiol.1994.sp020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolas T, Aghajanian GK. Neurotensin excitation of serotonergic neurons in the dorsal raphe nucleus of the rat in vitro. Eur J Neurosci. 1996;8:153–161. doi: 10.1111/j.1460-9568.1996.tb01176.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Effect of acute and daily neurotensin and enkephalin treatments on extracellular dopamine in the nucleus accumbens. J Neurosci. 1990;10:2940–2949. doi: 10.1523/JNEUROSCI.10-09-02940.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempadoo KA, Tourino C, Cho SL, Magnani F, Leinninger GM, Stuber GD, Zhang F, Myers MG, Deisseroth K, de Lecea L, Bonci A. Hypothalamic neurotensin projections promote reward by enhancing glutamate transmission in the VTA. J Neurosci. 2013;33:7618–7626. doi: 10.1523/JNEUROSCI.2588-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleczkowska P, Lipkowski AW. Neurotensin and neurotensin receptors: characteristic, structure-activity relationship and pain modulation--a review. Eur J Pharmacol. 2013;716:54–60. doi: 10.1016/j.ejphar.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Kortleven C, Bruneau LC, Trudeau LE. Neurotensin inhibits glutamate-mediated synaptic inputs onto ventral tegmental area dopamine neurons through the release of the endocannabinoid 2-AG. Neuropharmacology. 2012;63:983–991. doi: 10.1016/j.neuropharm.2012.07.037. [DOI] [PubMed] [Google Scholar]
- Krawczyk M, Mason X, DeBacker J, Sharma R, Normandeau CP, Hawken ER, Di Prospero C, Chiang C, Martinez A, Jones AA, Doudnikoff É, Caille S, Bézard E, Georges F, Dumont ÉC. D1 Dopamine receptor-mediated LTP at gaba synapses encodes motivation to self-administer cocaine in rats. J Neurosci. 2013;33:11960–11971. doi: 10.1523/JNEUROSCI.1784-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol. 1987;392:397–416. doi: 10.1113/jphysiol.1987.sp016787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinninger GM, Opland DM, Jo YH, Faouzi M, Christensen L, Cappellucci LA, Rhodes CJ, Gnegy ME, Becker JB, Pothos EN, Seasholtz AF, Thompson RC, Myers MG. Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 2011;14:313–323. doi: 10.1016/j.cmet.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AH, Hwang HM, Tan PP, Wu T, Wang HL. Neurotensin excites periaqueductal gray neurons projecting to the rostral ventromedial medulla. J Neurophysiol. 2001a;85:1479–1488. doi: 10.1152/jn.2001.85.4.1479. [DOI] [PubMed] [Google Scholar]
- Li AH, Yeh TH, Tan PP, Hwang HM, Wang HL. Neurotensin excitation of serotonergic neurons in the rat nucleus raphe magnus: ionic and molecular mechanisms. Neuropharmacology. 2001b;40:1073–83. doi: 10.1016/s0028-3908(01)00030-2. [DOI] [PubMed] [Google Scholar]
- Li J, Song J, Zaytseva YY, Liu Y, Rychahou P, Jiang K, Starr ME, Kim JT, Harris JW, Yiannikouris FB, Katz WS, Nilsson PM, Orho-Melander M, Chen J, Zhu H, Fahrenholz T, Higashi RM, Gao T, Morris AJ, Cassis LA, Fan TWM, Weiss HL, Dobner PR, Melander O, Jia J, Evers BM. An obligatory role for neurotensin in high-fat-diet-induced obesity. Nature. 2016;533:411–415. doi: 10.1038/nature17662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Geiger JD, Lei S. Neurotensin enhances GABAergic activity in rat hippocampus CA1 region by modulating L-type calcium channels. J Neurophysiol. 2008;99:2134–2143. doi: 10.1152/jn.00890.2007. [DOI] [PubMed] [Google Scholar]
- Marcusson EG, Horazdovsky BF, Cereghino JL, Gharakhanian E, Emr SD. The sorting receptor for yeast vacuolar carboxypeptidase Y is encoded by the VPS10 gene. Cell. 1994;77:579–586. doi: 10.1016/0092-8674(94)90219-4. [DOI] [PubMed] [Google Scholar]
- Marwaha J, Hoffer B, Freedman R. Electrophysiological actions of neurotensin in rat cerebellum. Regul Pept. 1980;1:115–125. doi: 10.1016/0167-0115(80)90015-4. [DOI] [PubMed] [Google Scholar]
- Mazella J, Chabry J, Kitabgi P, Vincent JP. Solubilization and characterization of active neurotensin receptors from mouse brain. J Biol Chem. 1988;263:144–149. [PubMed] [Google Scholar]
- Mazella J, Botto JM, Guillemare E, Coppola T, Sarret P, Vincent JP. Structure, functional expression, and cerebral localization of the levocabastine-sensitive neurotensin/neuromedin N receptor from mouse brain. J Neurosci. 1996;16:5613–5620. doi: 10.1523/JNEUROSCI.16-18-05613.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazella J, Vincent JP. Functional roles of the NTS2 and NTS3 receptors. Peptides. 2006;27:2469–2475. doi: 10.1016/j.peptides.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Mazella J, Zsürger N, Navarro V, Chabry J, Kaghad M, Caput D, Ferrara P, Vita N, Gully D, Maffrand JP, Vincent JP. The 100-kDa neurotensin receptor is gp95/sortilin, a non-G-protein-coupled receptor. J Biol Chem. 1998;273:26273–26276. doi: 10.1074/jbc.273.41.26273. [DOI] [PubMed] [Google Scholar]
- McHenry JA, Otis JM, Rossi MA, Robinson JE, Kosyk O, Miller NW, McElligott ZA, Budygin EA, Rubinow DR, Stuber GD. Hormonal gain control of a medial preoptic area social reward circuit. Nat Neurosci. 2017;20:449–458. doi: 10.1038/nn.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell VA, Kawahara H, Vaughan CW. Neurotensin inhibition of GABAergic transmission via mGluR-induced endocannabinoid signaling in rat periaqueductal grey. J Physiol. 2009;587:2511–2520. doi: 10.1113/jphysiol.2008.167429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoi Y, Aizawa T, Haga S, Nakamura S, Namba Y, Ikeda K. Neuronal localization of a novel mosaic apolipoprotein E receptor, LR11, in rat and human brain. Brain Res. 1999;833:209–215. doi: 10.1016/s0006-8993(99)01542-5. [DOI] [PubMed] [Google Scholar]
- Nalivaiko E, Michaud JC, Soubrié P, Le Fur G. Electrophysiological evidence for putative subtypes of neurotensin receptors in guinea-pig mesencephalic dopaminergic neurons. Neuroscience. 1998;86:799–811. doi: 10.1016/s0306-4522(98)00084-0. [DOI] [PubMed] [Google Scholar]
- Nicot A, Rostene W, Berod A. Neurotensin receptor expression in the rat forebrain and midbrain: a combined analysis by in situ hybridization and receptor autoradiography. J Comp Neurol. 1994;341:407–419. doi: 10.1002/cne.903410310. [DOI] [PubMed] [Google Scholar]
- Nicot A, Rostene W, Bérod A. Differential expression of neurotensin receptor mRNA in the dopaminergic cell groups of the rat diencephalon and mesencephalon. J Neurosci Res. 1995;40:667–674. doi: 10.1002/jnr.490400512. [DOI] [PubMed] [Google Scholar]
- Normandeau CP, Ventura-Silva AP, Hawken ER, Angelis S, Sjaarda C, Liu X, Pêgo JM, Dumont ÉC. A key role for neurotensin in chronic-stress-induced anxiety-like behavior in rats. Neuropsychopharmacol. 2017 doi: 10.1038/npp.2017.134. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa WN, Baptista V, Aguiar JF, Varanda WA. Neurotensin modulates synaptic transmission in the nucleus of the solitary tract of the rat. Neuroscience. 2005;130:309–315. doi: 10.1016/j.neuroscience.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Patterson CM, Wong JMT, Leinninger GM, Allison MB, Mabrouk OS, Kasper CL, Gonzalez IE, Mackenzie A, Jones JC, Kennedy RT, Myers MG. Ventral tegmental area neurotensin signaling links the lateral hypothalamus to locomotor activity and striatal dopamine efflux in male mice. Endocrinology. 2015;156:1692–1700. doi: 10.1210/en.2014-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaprat D. Interactions between neurotensin receptors and G proteins. Peptides. 2006;27:2476–2487. doi: 10.1016/j.peptides.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Piccart E, Courtney NA, Branch SY, Ford CP, Beckstead MJ. Neurotensin induces presynaptic depression of D2 dopamine autoreceptor-mediated neurotransmission in midbrain dopaminergic neurons. J Neurosci. 2015;35:11144–11152. doi: 10.1523/JNEUROSCI.3816-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Pellino G, Fiorentino F, Rasheed S, Darzi A, Tekkis P, Kontovounisios C. A review of the role of neurotensin and its receptors in colorectal cancer. Gastroenterol Res Pract. 2017 doi: 10.1155/2017/6456257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson BG, Bunzow JR, Grimm JB, Lavis LD, Dudman JT, Brown J, Neve KA, Williams JT. Desensitized D2 autoreceptors are resistant to trafficking. Sci Rep. 2017;7:4379. doi: 10.1038/s41598-017-04728-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh TM, Kombian SB, Zidichouski JA, Pittman QJ. Cholecystokinin and neurotensin inversely modulate excitatory synaptic transmission in the parabrachial nucleus in vitro. Neuroscience. 1997;77:23–35. doi: 10.1016/s0306-4522(96)00463-0. [DOI] [PubMed] [Google Scholar]
- Sarret P, Beaudet A, Vincent JP, Mazella J. Regional and cellular distribution of low affinity neurotensin receptor mRNA in adult and developing mouse brain. J Comp Neurol. 1998;394:344–356. [PubMed] [Google Scholar]
- Sarret P, Krzywkowski P, Segal L, Nielsen MS, Petersen CM, Mazella J, Stroh T, Beaudet A. Distribution of NTS3 receptor/sortilin mRNA and protein in the rat central nervous system. J Comp Neurol. 2003;461:483–505. doi: 10.1002/cne.10708. [DOI] [PubMed] [Google Scholar]
- Sarret P, Perron A, Stroh T, Beaudet A. Immunohistochemical distribution of NTS2 neurotensin receptors in the rat central nervous system. J Comp Neurol. 2003;461:520–538. doi: 10.1002/cne.10718. [DOI] [PubMed] [Google Scholar]
- Seroogy K, Ceccatelli S, Schalling M, Hökfelt T, Frey P, Walsh J, Dockray G, Brown J, Buchan A, Goldstein M. A subpopulation of dopaminergic neurons in rat ventral mesencephalon contains both neurotensin and cholecystokinin. Brain Res. 1988;455:88–98. doi: 10.1016/0006-8993(88)90117-5. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Aoki C, Pickel VM. Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. J Neurosci. 1994;14:88–106. doi: 10.1523/JNEUROSCI.14-01-00088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe AL, Varela E, Beckstead MJ. Systemic PD149163, a neurotensin receptor 1 agonist, decreases methamphetamine self-administration in DBA/2J mice without causing excessive sedation. PloS One. 2017;12:e0180710. doi: 10.1371/journal.pone.0180710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe AL, Varela E, Bettinger L, Beckstead MJ. Methamphetamine self-administration in mice decreases GIRK channel-mediated currents in midbrain dopamine neurons. Int J Neuropsychopharmacol. 2014:18. doi: 10.1093/ijnp/pyu073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi WX, Bunney BS. Actions of neurotensin: a review of the electrophysiological studies. Ann N Y Acad Sci. 1992a;668:129–145. doi: 10.1111/j.1749-6632.1992.tb27345.x. [DOI] [PubMed] [Google Scholar]
- Shi WX, Bunney BS. Roles of intracellular cAMP and protein kinase A in the actions of dopamine and neurotensin on midbrain dopamine neurons. J Neurosci. 1992b;12:2433–2438. doi: 10.1523/JNEUROSCI.12-06-02433.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilling PD, Feifel D. The neurotensin-1 receptor agonist PD149163 blocks fear-potentiated startle. Pharmacol Biochem Behav. 2008;90:748–752. doi: 10.1016/j.pbb.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Gelais F, Legault M, Bourque MJ, Rompré PP, Trudeau LE. Role of calcium in neurotensin-evoked enhancement in firing in mesencephalic dopamine neurons. J Neurosci. 2004;24:2566–2574. doi: 10.1523/JNEUROSCI.5376-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhrman K, Roseberry AG. Neurotensin inhibits both dopamine- and GABA-mediated inhibition of ventral tegmental area dopamine neurons. J Neurophysiol. 2015;114:1734–1745. doi: 10.1152/jn.00279.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Masu M, Nakanishi S. Structure and functional expression of the cloned rat neurotensin receptor. Neuron. 1990;4:847–854. doi: 10.1016/0896-6273(90)90137-5. [DOI] [PubMed] [Google Scholar]
- Tschumi CT, Beckstead MJ. Neurotensin speeds inhibition of dopamine neurons through temporal modulation of GABAA and GABAB receptor-mediated synaptic input. Neuropharmacology. 2018 doi: 10.1016/j.neuropharm.2018.01.004. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vita N, Oury-Donat F, Chalon P, Guillemot M, Kaghad M, Bachy A, Thurneyssen O, Garcia S, Poinot-Chazel C, Casellas P, Keane P. Neurotensin is an antagonist of the human neurotensin NT 2 receptor expressed in Chinese hamster ovary cells. Eur J Pharmacol. 1998;360:265–272. doi: 10.1016/s0014-2999(98)00678-5. [DOI] [PubMed] [Google Scholar]
- Walker N, Lepee-Lorgeoux I, Fournier J, Betancur C, Rostene W, Ferrara P, Caput D. Tissue distribution and cellular localization of the levocabastine-sensitive neurotensin receptor mRNA in adult rat brain. Mol Brain Res. 1998;57:193–200. doi: 10.1016/s0169-328x(98)00074-6. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Groves PM, Fifková E. Monoaminergic synapses, including dendro-dendritic synapses in the rat substantia nigra. Exp Brain Res. 1977;30:161–174. doi: 10.1007/BF00237248. [DOI] [PubMed] [Google Scholar]
- Woodworth HL, Batchelor HM, Beekly BG, Bugescu R, Brown JA, Kurt G, Fuller PM, Leinninger GM. Neurotensin receptor-1 identifies a subset of ventral tegmental dopamine neurons that coordinates energy balance. Cell Rep. 2017;20:1881–1892. doi: 10.1016/j.celrep.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Li A, Wang HL. Neurotensin increases the cationic conductance of rat substantia nigra dopaminergic neurons through the inositol 1,4,5-trisphosphate-calcium pathway. Brain Res. 1995;683:242–250. doi: 10.1016/0006-8993(95)00379-5. [DOI] [PubMed] [Google Scholar]
- Wu T, Wang HL. Protein kinase C mediates neurotensin inhibition of inwardly rectifying potassium currents in rat substantia nigra dopaminergic neurons. Neurosci Lett. 1995;184:121–124. doi: 10.1016/0304-3940(94)11185-l. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Cilz NI, Kurada L, Hu B, Yang C, Wada E, Combs CK, Porter JE, Lesage F, Lei S. Activation of neurotensin receptor 1 facilitates neuronal excitability and spatial learning and memory in the entorhinal cortex: beneficial actions in an Alzheimer’s disease model. J Neurosci. 2014;34:7027–7042. doi: 10.1523/JNEUROSCI.0408-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Adermark L, Lovinger DM. Neurotensin reduces glutamatergic transmission in the dorsolateral striatum via retrograde endocannabinoid signaling. Neuropharmacology. 2008;54:79–86. doi: 10.1016/j.neuropharm.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Dong H, Cilz NI, Kurada L, Hu B, Wada E, Bayliss DA, Porter JE, Lei S. Neurotensinergic excitation of dentate gyrus granule cells via Gαq-coupled inhibition of TASK-3 channels. Cereb Cortex. 2016;26:977–990. doi: 10.1093/cercor/bhu267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Dong H, Lei S. Neurotensinergic augmentation of glutamate release at the perforant path-granule cell synapse in rat dentate gyrus: Roles of L-Type Ca2+ channels, calmodulin and myosin light-chain kinase. Neuropharmacology. 2015;95:252–260. doi: 10.1016/j.neuropharm.2015.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, DR, Bennett-Clarke CA, Rhoades RW. Effects of neurotensin on visual neurons in the superficial laminae of the hamster’s superior colliculus. Vis Neurosci. 1996;13:237–246. doi: 10.1017/s0952523800007471. [DOI] [PubMed] [Google Scholar]